Back to Journals » International Journal of Nanomedicine » Volume 20
How Effective are Key Phytocompound Carrying Polysaccharide Nanocarriers as Anti-Breast Cancer Therapy? A Comprehensive Review of the Literature
Authors Elsori D, Pandey P, Obaidur Rab S, Alharbi AM, Sahoo S, Pandey S, Srivastava M, Lakhanpal S, Saeed M , Khan F
Received 3 February 2025
Accepted for publication 26 May 2025
Published 27 June 2025 Volume 2025:20 Pages 8393—8413
DOI https://doi.org/10.2147/IJN.S520580
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Farooq A. Shiekh
Deena Elsori,1 Pratibha Pandey,2 Safia Obaidur Rab,3 Ahmed M Alharbi,4 Samir Sahoo,5 Shivam Pandey,6 Manish Srivastava,7 Sorabh Lakhanpal,8 Mohd Saeed,9 Fahad Khan10
1Faculty of Resilience, Rabdan Academy, Abu Dhabi, United Arab Emirates; 2Research and Innovation Cell, Rayat Bahra University, Mohali-140301, Punjab, India; 3Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia; 4Department of Medical Laboratory Science, College of Applied Medical Sciences, University of Ha’il, Hail, Saudi Arabia; 5Department of General Medicine, IMS and SUM Hospital, Siksha ‘O’ Anusandhan (Deemed to Be University), Bhubaneswar, Odisha, 751003, India; 6School of Applied and Life Sciences, Uttaranchal University, Dehradun, India; 7Department of Endocrinology, National Institute of Medical Sciences, NIMS University Rajasthan, Jaipur, India; 8School of Pharmaceutical Sciences, Lovely Professional University, Phagwara, Punjab, India; 9Department of Biology, College of Science, University of Hail, Hail, Saudi Arabia; 10Center for Global Health Research, Saveetha Medical College and Hospital, Saveetha Institute of Medical and Technical Sciences, Chennai, Tamil Nadu, India
Correspondence: Fahad Khan, Email [email protected]
Abstract: Breast cancer continues to be one of the most perilous diseases globally due to the challenges in identifying cost-effective and targeted targets for effective treatment strategies. In response to these unmet demands, much research has focused on investigating the anti-breast cancer properties of natural compounds due to their multi-target modes of action and favorable safety profiles. Numerous extracts of medicinal plants, essential oils, and natural bioactive substances have exhibited anticancer properties in preclinical breast cancer models. Nonetheless, the clinical utilization of therapies based on natural chemicals is constrained by challenges such as inadequate solubility and permeability. Innovative drug delivery systems utilizing nanoparticles are currently being investigated as adaptable solutions to overcome these limitations. Polysaccharide-based nanoparticles exhibit promising biodegradability and biocompatibility, making them suitable for targeted drug delivery systems among the diverse nanocarrier types. Many polysaccharide nanocarriers, including chitosan, hyaluronic acid, cellulose, starch, and complex polysaccharides, have been shown to enhance the bioavailability and therapeutic effectiveness of phytocompounds. This review examines the anticancer properties of phytocompounds and polysaccharide nanocarriers in breast cancer before focusing on the use of polysaccharide-based nanocarriers to deliver phytocompounds for the treatment of breast cancer.
Keywords: polysaccharide, phytocompounds, nanocarriers, drug delivery, breast cancer
Introduction
Breast cancer is a frequently diagnosed malignancy that affects approximately 2–2.5 million women globally each year.1 Breast cancer (BC) is characterized by the unregulated growth and spread of cells originating from the breast and adjacent tissues. It comprises of many subtypes, each of which is distinguished by a diverse array of clinical outcomes. An effective strategy for cancer prevention and therapy requires a comprehensive understanding of this variability.2,3 According to the World Health Organization (WHO), 170,000 women in India are projected to be affected by breast cancer, representing a 14% increase in the nation’s total cancer incidence.4 Although significant advancements have been made in understanding the molecular mechanisms of malignancy and the implementation of molecular-targeted therapeutics, breast cancer continues to be highly frequent and fatal worldwide.5 Premature breast cancer is generally managed with lumpectomy, partial or total mastectomy, radiation, and other adjuvant medicines, particularly cytotoxic chemotherapy and immunotherapy, which are notably linked to adverse effects, toxicities, and harm to healthy cells.6 To address these limitations, multiple research domains have concentrated on innovative diagnostic, preventive, and therapeutic approaches.7,8 Consequently, various natural and chemical substances have been proposed for medicinal purposes. More than half of these anticancer medications are derived from natural sources, particularly substances originating from plants.9,10 Phytocompounds have attracted the attention of cancer researchers because of their ability to complement regular drug dosages with minimal damage. Various herbs and their bioactive constituents play important roles in multiple cancer therapies.11 Recent preclinical and clinical studies have shown that various phytocompounds such as curcumin, hesperetin, rutin, EGCG, and naringenin, individually or in conjunction with other compounds or chemotherapeutic drugs, exhibit potential anticancer efficacy against breast carcinoma. These phytocompounds are associated with antiproliferative effects, apoptosis induction, anti-inflammatory properties, anti-angiogenic activity, anti-invasive and metastatic capabilities, cell cycle regulation, tumor suppression, and targeting of cancer stem cells in breast cancer.12,13
Notwithstanding the encouraging preclinical results, the physicochemical characteristics of these phytocompounds typically result in inadequate stability, water solubility, and bioavailability, which may impede their clinical utilization.14,15 Moreover, the clinical utilization of essential oils has been impeded by their significant volatility, pronounced susceptibility to environmental factors, poor stability, and high lipophilicity.16,17 Promising efforts have been made to address these constraints, particularly with the implementation of nanocarriers based drug delivery systems.18,19 Innovative anticancer systems have the potential to decrease toxicity, enhance therapeutic drug levels and bioaccessibility, and specifically target malignant tissues and cells directly. The primary aim in creating innovative drug delivery systems for targeted drug administration is to address the aforementioned significant limitations. Nanotechnology offers numerous advantages in treatment through site-specific drug delivery and targeted administration of precise medications in a regulated manner. Nanomedicine is pivotal in the advancement of precise therapies and drug formulations, as well as in the controlled release of drug delivery systems.20,21 The use of drug nanocarriers for therapeutic delivery presents a promising and innovative strategy to enhance cancer nanotherapy, owing to the unique advantages of nanocarriers in optimizing systemic circulation and therapeutic index while minimizing cytotoxic effects on normal cells.22,23 Nanomaterials have garnered significant interest because of their potential to address limitations in cancer immunotherapy, particularly in breast cancer.24–27 These nanoparticles can deliver and release various agents to specific locations, such as antigen-presenting cells (APCs), while maintaining outstanding structural integrity in serum. The encapsulation of antigens and immunostimulatory substances into nanoparticles, along with their efficient transport to lymph nodes by APCs, has been shown to significantly enhance T- and B-cell responses compared to soluble antigens and adjuvants.28 Research has been conducted using various cancer models to examine nanoparticle-based immunotherapy. For example, melittin-lipid nanoparticles, a new nanomaterial for melanoma immunotherapy, were recently introduced.29 The integration of immunomodulatory polysaccharides derived from natural herbs into nanocomposites has been shown to activate dendritic cells and increase cytokine production, as well as the proliferation of CD4+ and CD8+ T cells. Furthermore, the combination of these nanoparticles with the chemotherapeutic agent doxorubicin significantly reduced 4T1 tumor growth and lung metastasis while exhibiting no apparent toxicity.30
Nonetheless, there are still significant issues with drug delivery related to the biosafety and biocompatibility of nanocarrier-based delivery systems. Consequently, it is imperative to create secure nanocarriers for the delivery of phytocompounds. Nanoparticle carriers generated from polysaccharides exhibit remarkable features. Polysaccharides exhibit superior mucoadhesion, facilitate evasion from the reticuloendothelial system (RES), and exhibit anti-inflammatory characteristics.31 They can also be chemically manipulated to incorporate characteristics, such as tumor cell internalization and thermoresponsiveness.32,33 Polysaccharides, including Hyaluronic Acid (HA) and Chondroitin Sulfate, have inherent receptor-targeting qualities, particularly directing their action towards CD44 overexpressing tumor cells.34 Ulvan exhibits a significant affinity for P-selectin receptors, whereas polysaccharides from Angelica sinensis and pullulan show considerable potential for liver localization.35,36 Furthermore, polysaccharides from Gracilaria lemaneiformis have demonstrated significant efficacy towards αvβ3 integrin, which is upregulated in glioma cells.37 Their nanoparticles exhibit favorable surface characteristics, including substantial surface area, effective drug loading capacity, affinity for ligands and small molecules, surface charge, and hydrophilicity. These characteristics render them stable, targetable, and capable of modulating drug biodistribution. Consequently, polysaccharides have advantageous structural qualities that establish them as an exemplary foundational material for the construction of nanocarriers based delivery system.38
The key highlight of this review is to investigate the anticancer properties of polysaccharide-based phytocompound nanoparticles in relation to breast cancer. This study examined novel strategies in nanomedicine for breast cancer, focusing on active targeting and biomedically pertinent polysaccharide nanoparticles, including chitosan, hyaluronic acid, cellulose, starch, and complex polysaccharide nanoparticles. While numerous researchers have focused on the manufacturing of polysaccharide nanoparticles, only a limited number have reported the anti-cancer properties of these nanoparticles in relation to breast cancer.38,39 Polysaccharide nanoparticles hold great promise for future clinical imaging, diagnosis, and treatment. Ultimately, all of this knowledge will aid in the logical development of safe and biocompatible nanoparticles to fight severe diseases like cancer that were previously incurable, enhancing the healthcare system for present and future generations.
Phytocompounds as Chemopreventive Agents in Breast Cancer
Phytocompounds or phytochemicals confer several health benefits by directly interacting with key molecular and cellular targets, or by indirectly stabilizing conjugates that influence metabolic pathways.40 Liao et al (2013) reported that herbal compounds can effectively serve as adjuvants to conventional chemotherapy to overcome negative effects such as nausea, fatigue, mucositis, and anemia associated with conventional treatments such as chemotherapy.41 Numerous studies have demonstrated that various phytocompounds exhibit anticancer activity and can serve as treatment modalities through multiple mechanisms42–44 (Figure 1).
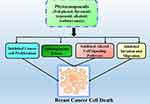 |
Figure 1 Different mechanism associated with phytocompounds based chemoprevention in breast cancer. |
One of the most significant strategies for controlling uncontrolled proliferation of breast cancer cells is the induction of apoptosis. Evidence indicates that natural products play a significant role in breast cancer treatment by inducing apoptosis. Over the past two decades, natural products have been extensively utilized in preclinical studies on breast cancer because of their abundant natural sources, safety, and effective therapeutic potential.45,46 Several apoptosis-linked pathways, including PI3K/AKT, MAPK, and p53, can be targeted to stimulate apoptosis in breast cancer cells.47,48 Natural products represent a significant resource for identifying potent drug molecule for the treatment of breast cancer.
Epigenetic disruption is predominantly observed alongside genetic alterations during the initial development and progression of cancer. Epigenetic modifications alter the cellular phenotype without altering the underlying DNA sequence. The mechanisms include DNA methylation, primarily at cytosines (resulting in 5-methylcytosine) adjacent to guanines (CpG dinucleotides), as well as acetylation, methylation, phosphorylation, and ubiquitination of histones and noncoding RNAs or miRNAs that influence mammalian gene expression.49 In breast carcinoma, aberrant histone modifications are linked to epigenetic shutdown of tumor suppressor genes and genome destabilization.50 Accumulating data indicated that phytocompounds, including secondary metabolites, may control epigenetic events and reverse epigenetic alterations prior to cancer onset.51,52 Phytochemicals reportedly influence epigenetic modifications by regulating key transcription elements, kinases, and growth factor receptor-mediated pathways, resulting in apoptotic induction, cell cycle arrest, and restoration of tumor suppressor genes.53 Chlebowski54 demonstrated the effective anticancer properties of tamoxifen and certain aromatase inhibitors, such as exemestane and anastrozole, in lowering down the breast cancer incidence in clinical trials. Nonetheless, some medications such as tamoxifen have positive side effects, which increases the risk of endometrial cancer. In contrast to synthetic drugs, phytocompounds exhibit considerable anticancer efficacy in breast cancer therapy, demonstrating reduced side effects and cytotoxicity in preclinical studies.55 Part of the cytochrome P450 enzyme family, aromatase, is a membrane-bound protein that causes the transformation of testosterone to estradiol (E2) and androstenedione to estrone (E1), and is crucial for the manufacture of estrogen.56 Recent studies have indicated that phytocompounds possess a chemical structure analogous to estrogen and can modify aromatase expression by directly suppressing its activity.57 Furthermore, evidences were indicated that phytocompounds have also demonstrated antitumor activities by targeting the arachidonic acid pathway, including the metabolic regulatory enzymes phospholipase A2s, cyclooxygenases, and lipoxygenases.58 Numerous studies have indicated that the arachidonic acid pathway is pivotal in both inflammation and cancer.59 A strong correlation was observed between elevated COX-2 levels and adverse prognosis, invasion, and proliferation of breast cancer cells.60 Ranger et al reported a positive link connection between the COX-2 expression levels and distant metastasis in breast cancer.61 In light of this, research was conducted, and it was ultimately shown that inhibiting COX-2 might reduce the metastatic activity of breast cancer cells in a mouse model.62 Several phytocompounds, including lycopene, curcumin, ginsetin, and apigenin, have been documented to decrease the formation of metabolic products thus being regarded as strong therapeutic options against breast cancer.9
In addition to their chemotherapeutic potential, some phytocompounds exhibit chemosensitizing effects in preclinical settings.63 Chemosensitization is a crucial clinical approach aimed at enhancing the effectiveness of chemotherapeutics by reducing dose-limiting effects, optimizing drug transport and activation, and overcoming chemoresistance in malignancies. Various mechanisms of chemoresistance in breast carcinoma have been recognized, which sufficiently hinder drug transport to and absorption within the cancer cell to achieve therapeutic effects.64 In breast cancer, combined chemotherapy procedures are employed to overcome drug resistance. However, clinical trials are also exploring chemosensitization strategies, including nutritional and exercise interventions, alongside novel and repurposed FDA-approved pharmacotherapies. Phytochemicals that exhibit chemosensitization potential include ursolic acid, betulinic acid, rutin, resveratrol, curcumin, and genistein.65–67 Nevertheless, they have multiple drawbacks such as nonspecific targeting, poor water solubility, and restricted therapeutic efficacy. An alternative approach involves employing biopolymeric nanocarriers, such as polysaccharides, which can provide effective targeted therapy when coupled with natural anticancer agents (phytocompounds).
Emerging Trends of Polysaccharide Nanoparticles in Cancer Therapy
Polysaccharides are carbohydrate polymers composed of many identical and/or distinct monosaccharide components (Cn(H2O)n) linked by glycosidic linkages. Natural polysaccharides serve as abundant, renewable, sustainable, and ecofriendly biopolymers derived from various natural resources, including animals, plants, algae, and microbes.68,69 Typically, based on the principles of building block chemistry and modification methods, these polysaccharides display various chemical structures, including branched or linear forms, different charge states (neutral, negative, or positive charge), variable chemical makeup (α or β-glycosidic bonds), and a broad range of molecular weights. It is believed that about 90% of the substantial carbohydrate material in nature is employed in the synthesis of natural polysaccharides.70 Cellulose, starch, and chitin were the predominant and essential polysaccharides, respectively. Polysaccharides are a highly attractive choice for pharmaceutical and biomedical purposes because of their structural chemical variety, physicochemical and biological properties, safety, strong chemical reactivity, biological compatibility, and biodegradable properties. Their biological features and uses are enhanced by their low toxicity profile and excellent biocompatibility and biodegradability, while immunogenicity is dependent on particular structures.71
A variety of natural and manufactured water-soluble polymers have been chemically conjugated with medicinal molecules.72 Pharmacokinetic studies on drug-conjugating polysaccharides have demonstrated the significance of natural and sustainable polymers as effective drug delivery methods. In addition to synthetic water-soluble polymers, natural polymers such as chitosan, hyaluronic acid, dextran, and cellulose exhibit significant potential as drug carriers.73 The conjugation of hydrophobic doxorubicin (DOX) with an acid-cleavable hydrazone link produced DOX-chitosan nanoconjugates that exhibited pH-sensitive drug release.74 The research demonstrated that the chitosan-DOX conjugates with an acid-cleavable hydrazone link were stable at neutral pH and disintegrated at pH 5.0, thereby facilitating the release of DOX into cervical cancer HeLa cells. The prodrug nanoparticles were rapidly absorbed, demonstrating their potential for use in tumor-targeted therapeutics, resulting in significant accumulation and deposition of doxorubicin in HeLa cells.75 Basic carbohydrates, such as dextran, have been purportedly employed for the recombination of pharmaceuticals. Bacterial strains, such as Leuconostoc and Streptococcus, generate substantial quantities of dextran polysaccharides, encompassing both primary and secondary categories of dextran that provide potential therapeutic conjugation sites using specialized methodologies.76,77 The chemical modification of these materials purportedly enables selective and more efficient drug administration to tumors, according to the major amine and hydroxyl groups present in the chitosan framework.78 Skorik et al employed chitosan-based nanoparticles as nanocarriers to deliver of paclitaxel (PTX) and docetaxel (DTX). The drug-loaded succinyl and glutaryl chitosan nanoparticles exhibited significant cytotoxicity against gastrointestinal cell lines, hence enhancing their anticancer efficacy relative to free medicines.79 The revised carboxymethyl chitosan-based nanoparticles loaded with DOX and utilizing a tumor-homing ligand (3-carboxyphenylboronic acid) exhibited enhanced accumulation and penetration in mice with H22 lung metastases. This substantially diminished the mass of the H22 metastatic lung tumor by the enhanced infiltration and aggregation of nanoparticles at the tumor site.80 D-Glucuronic acid repeats and d-N-acetylglucosamine disaccharides combine to form hyaluronan through β-1,4- and β-1,3-glycosidic bonds.81 The hyaluronate framework consists of hydroxy and carboxyl groups conjugated to various medicinal compounds. Due to steric hindrance and the low reactivity of carboxyl groups, straight conjugation is not favored.82,83 HA-derived compounds, including functional head groups such as hydrazine, exhibit enhanced reactivity and drug conjugation effectiveness. HA derivatives requiring the substitution and functionalization of carboxylic acids exhibit enhanced drug loading while maintaining minimal modification of the polysaccharide structure.84 Paclitaxel (PTX) exhibited increased loading and less product toxicity than free PTX in conjugation with HA-deoxycholic acid linked to bio-reducible cysteamine. The binding properties of the drug persisted, even after considerable replenishment. Various techniques have been reported to address the limited solubility of HA in traditional organic solvents, which impedes cytotoxic conjugation reactions.85 These procedures encompass the utilization of polar solvent-water combinations, polyethylene dimethyl ether nanocomplexation, and ion formulations of long-chain aliphatic cations.86 The use of HA-drug conjugates provides specific targeting of upregulated CD44 receptors.87 HA-conjugated products such as HA-epirubicin, HA-mitomycin C, HA-butyrates, and HA-paclitaxel have significant efficacy in drug delivery. The histone deacetylase inhibitor exhibits increased apoptotic activity, leading to decreased in vivo tumor burden and suppression of in vitro cell proliferation upon conjugation with HA.88 The application of N, N′-dicyclohexylcarbodiimide (DCC) and cholic acid derivatives has facilitated the modification of sucrose and poly(d, l-lactic-co-glycolic acid) (PLSGA) via crosslinking. This signifies a promising advancement in a regulated drug delivery system for drugs with limited aqueous solubility.89 Various applications of composite hydrogels made from polysaccharides, such as chitin, chitosan, and nanocellulose have provided precise drug delivery systems, improved regenerative medicine, tissue engineering, wound care dressings, and water purification absorbents.90 Chitin composites can be synthesized into spherical nanogels by incorporating rhodamine 123 dye, which enhances medication dispersion and has potential in tissue engineering.91,92 Polysaccharides conjugated to folate and fluorescent characteristics exhibit an optimum medication loading and release profile, indicating possible uses for gene therapy targeting specific sites, including malignant cells.93 Recent advancements in the development of wound treatment solutions utilizing pectin in conjunction with cellulose and microfibrillated cellulose have yielded promising in vivo results. The results from animal models were promising; however, additional experimental studies were necessary for clinical success. Pectin serves as a medication carrier in therapeutic sprays for drug delivery. A pectin-based nasal spray formulation containing fentanyl alleviates cancer discomfort and enhances chemotherapy efficacy.94
Polysaccharide-Specific Delivery Mechanisms
Site-targeted drug administration should be considered during chemotherapy planning to maximize the therapeutic efficacy and minimize side effects. Nanotechnology-based approaches facilitate site-targeted drug delivery for physicians and researchers while also offering significant opportunities to investigate novel paths that can influence prognosis, diagnosis, and therapy. In the context of potent phytocompounds for breast cancer treatment, solubility and bioavailability present significant challenges; however, these issues may be mitigated through nanoparticle formulations.23,95–97 Currently, the therapeutic utilization of most nanoformulations depends on the accumulation of tumor nanoparticles through passive trans-endothelial pathways from blood vessels to tumor tissue, mostly via increased permeability and retention (EPR) mechanisms. Nonetheless, EPR-based formulations have many drawbacks, including nonspecific dispersion, inadequate tumor accumulation, and the heterogeneity of cancers and patients. Researchers have focused on evaluating updated nanoformulations with active targeting capabilities, including ligand-based cancer-targeting and stimuli-responsive drug delivery systems.98 Ligand-based targeting is accomplished by modifying the surfaces of nanocarriers with ligands that specifically engage with receptors or antigens overexpressed on tumor cells. This method augments the affinity of nanocarriers for cancer cell surfaces, thereby facilitating drug penetration. Furthermore, the absence of these receptors or antigens in normal cells inhibits nonspecific absorption in healthy cells and tissues. Tumor microenvironment (TME)-responsive delivery systems provide an on-demand drug release profile in response to alterations in the TME physiological characteristics, which are distinct from those of healthy tissues.99 TME is defined by acidic pH, hypoxia, enzymatic changes, modified redox conditions, increased reactive oxygen species (ROS), enhanced glutathione, adenosine triphosphate, and inflammatory mediators.44
Numerous nanoscale drug carriers effectively address these challenges and transport medications to target locations, thereby minimizing side effects. To circumvent the side effects of chemotherapy, researchers have developed a number of drug delivery methods, one of which is based on polysaccharides that specifically target cancer cells by active or passive targeting.100–104 Polysaccharides are safe, biodegradable, and hydrophilic biopolymers that can be readily chemically modified to enhance bioavailability and stability for the delivery of medicines to cancer tissues. Various polysaccharides, including chitosan, hyaluronic acid, alginates, cyclodextrin, dextran, guar gum, cellulose, and pectin, have been utilized in drug delivery systems for cancer therapy.105–110 Figure 2 and Table 1 summarizes the augmented antitumor efficacy of various phytocompounds based polysaccharide nanocarriers in breast cancer. The following section emphasizes the latest advancements in polysaccharide-based phytocompound nanocarriers for breast cancer therapy.
 |
Table 1 Phytocompounds Based Polysaccharide Nanoformulations and Their Associated Anticancer Mode of Action in Breast Cancer Therapy |
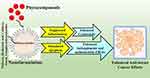 |
Figure 2 Enhanced anticancer effects of phytocompounds based polysaccharide nanocarriers in breast cancer therapy. |
How Chitosan is Acting as A Suitable Nanocarrier?
Chitosan is a naturally occurring cationic polysaccharide that is extensively used in biomedical research. Chitosan is the primary derivative of chitin, located in the cell walls of fungi, mollusk shells, and exoskeletons of crustaceans. Chitosan is derived through the deacetylation of chitin under specific conditions, with a degree of deacetylation ranging from 60% to 100%. The molecular weight of commercially sourced chitosan ranges from 3,800 to 20,000 Daltons.143,144 Chitosan is a natural polysaccharide with limited solubility in water, although it dissolves in low pH solutions. Altered variants of chitin, including carboxymethyl chitin, sulfated glycol chitin, and fluorinated chitin, have been developed to enhance its aqueous solubility.145 Numerous chemical modifications have been implemented to produce various chitosan derivatives for controlled drug delivery systems.146,147 Chitosan exhibits antibacterial, antibiotic, and anticoagulant characteristics, and accelerates wound healing. Low molecular weight chitosan inhibits tumor growth and exhibits antitumor action with reduced toxicity to normal proliferating cells.136,137 Consequently, low-molecular-weight chitosan (LMWC) can elicit synergistic effects when used as a drug carrier. The cytotoxic effects of chitosan derivatives have been documented in several cancer cell lines, including MCF-7, HeLa, and HEK293 tumor cell lines.138 Chitosan nanoparticles have attracted considerable attention in cancer therapy owing to their distinctive qualities, such as biodegradability, biocompatibility, and mucoadhesiveness. These nanoparticles can transport therapeutic medicines to tumor locations via passive and active targeting methods. Chitosan-based nanocarriers in passive targeting use the increased permeability and retention (EPR) effect, enabling preferential accumulation in malignant tissues. This phenomenon results from the permeable vasculature and inadequate lymphatic drainage typically observed in tumors, allowing nanoparticles to infiltrate and persist within the tumor microenvironment.139 Active targeting utilizes ligand-receptor interactions to improve the specificity of medication delivery. Chitosan nanoparticles can be altered with targeted ligands, like folic acid, peptides, or antibodies, to selectively attach to cancer cells.140 Folate-linked chitosan nanocarriers can specifically target cancerous cells that overexpress folate receptors, enhancing the delivery of antitumor agents to the tumor location, while minimizing systemic toxicity. This strategy improves the therapeutic effectiveness of encapsulated medications while reducing their detrimental effects on normal tissues.141
Chitin and chitosan derivatives are promising candidates for use as polymeric carriers for anticancer drugs. The solubility and bioavailability of chitosan are enhanced by chemical modification via derivatization. Prior research has indicated that chemical modification of chitosan with an acetamido moiety and an amino group enhances the solubility of encapsulated pharmaceutical compounds.148 Certain cancer cells exhibit resistance to many anticancer agents, including docetaxel (DTX), methotrexate (MTX), cisplatin, and 5-fluorouracil.149 Presently, traditional chemodrugs have hazardous effects on various bodily systems, including the gonads, bone marrow, and gastrointestinal lining.150 LMWC, owing to its elevated positively charged amino group, has a strong attraction to the cancer cell membrane, which possesses a more significant negative charge than normal cells. Furthermore, chitosan has been shown to target cancer cells via electrostatic interactions with tumor cell membranes. Additionally, chitosan-drug nanoparticles serve as alternatives to traditional pharmaceuticals because of their specificity for cancer cells and biocompatibility.151,152 In a series of preclinical investigations, chitosan-based phytocompound nanocarriers have shown strong affinity and specificity for breast cancer cells, significant tumoricidal efficacy, and an effective inhibitory effect on metastasis.153
Lu et al developed hypoxia-responsive nanoparticles composed of carboxymethyl chitosan, encapsulating doxorubicin and Tanshinone IIA for breast cancer therapy. The findings indicated that these hypoxia-responsive nanoparticles not only augmented drug transport efficiency but also improved the therapeutic efficacy of DOX. The mean diameter of the nanoparticles was approximately 200–220 nm, and the optimal drug loading and encapsulation efficiency of TSIIA in DOX/TSIIA nanoparticles were 9.06% and 73.59%, respectively. Hypoxia-responsive behavior was documented in vitro, while synergistic efficacy was dramatically demonstrated in vivo, resulting in a tumor inhibitory rate of 85.87%. The in vitro anticancer assays confirmed that these nanoparticles produced a synergistic anti-tumor impact by blocking tumor fibrosis, reducing HIF-1α expression, and causing death in tumor cells.111 Awad et al developed chitosan nanoparticles loaded with anthocyanin and cisplatin, and evaluated their antitumor activity against MCF-7 breast cancer cells. These chitosan-based nanoparticles efficiently targeted breast cancer by triggering apoptosis in cancer cells, augmenting antioxidant defenses, and mitigating inflammation. They additionally impede tumor proliferation, migration and angiogenesis by downregulating MMP9 and VEGF, which highlights their therapeutic efficacy.112 In addition, chitosan nanoparticles infused with limonene and limonene-dominant essential oils have been developed for breast cancer therapy. These nanoparticles demonstrated improved antitumor efficacy against MDA-MB-468 breast cancer cells.113 Ghobadi-Oghaz et al developed chitosan-based nanoparticles for the co-delivery of curcumin (Cur) and berberine (Ber) in MDA-MB-231 breast cancer cells. Studies have demonstrated that Cur and Ber exhibit synergistic effects in various carcinomas. The resultant nanoparticles exhibited high entrapment effectiveness of approximately 75% for Cur and 60% for Ber. In vitro cytotoxicity test revealed that the co-encapsulated nanoparticles significantly increased cytotoxicity in MDA-MB-231 and A549 cancer cells. In vitro investigations using MDA-MB-231 cells revealed that these nanoparticles effectively enhanced cellular absorption and apoptosis while significantly inhibiting IL-8 pro-inflammatory cytokines compared to free Cur + Ber bioactive chemicals.114 Truong et al developed chitosan-coated nanostructured lipid carriers for the administration of tetrahydrocurcumin in breast cancer cells. The nanocarriers exhibited markedly improved in vitro skin permeation, cellular uptake, and notable cytotoxicity against MD-MBA-231 breast cancer cells compared to free THC, indicating Ch-NLCs as promising transdermal nanocarriers for THC in the treatment of breast cancer.115
A hydrogel nanocomposite comprising chitosan (CS), halloysite (HNT), and graphitic-carbon nitride (g-C3N4) was synthesized and incorporated with quercetin to provide a sustained release of quercetin and enhanced antiproliferative effects against MCF-7 breast cancer cells. The drug release profile demonstrated a targeted sustained-release and pH-sensitive release of quercetin over a 96-hour period. The MTT assay demonstrated significant cytotoxicity against breast cancer cells, specifically the MCF-7 cell line, in vitro, using the CS/HNT/g-C3N4 targeted delivery system compared to quercetin as a free medication.116
Nematollahi et al developed a quercetin-loaded chitosan/polyvinylpyrrolidone/γ-alumina nanocomposite to enhance drug loading and release efficiency in breast cancer. In vitro studies demonstrated that quercetin-loaded nanoparticles exhibited considerable cytotoxicity against MCF-7 breast cancer cells. The augmented apoptotic cell death corroborated the anticancer efficacy of this nanocomposite.117
Several studies have also shown the improved antitumor activity of chitosan-based curcumin nanoparticles. Abdouss et al produced a pH-sensitive curcumin nanocarrier composed of chitosan (CS), polyacrylic acid (PAA), and graphitic carbon nitride (g-C3N4) using water/oil/water (W/O/W) emulsification. The nanocarriers exhibited increased cytotoxicity and the highest apoptosis rate in MCF-7 breast cancer cells, indicating the superior efficacy of the nanocomposites in eradicating malignant cells.118 A separate study demonstrated that a curcumin-loaded chitosan/halloysite/carbon nanotube nanomixture induced apoptosis in MCF-7 cells and showed enhanced cytotoxicity of the drug-loaded nanocomposite relative to free curcumin.119
Kaur et al formulated ellagic acid (EA)-loaded chitosan nanoparticles and assessed their preclinical efficacy in both in vitro and in vivo breast cancer models. The results indicated that nanoformulations exhibited effective nanosized encapsulation of EA and demonstrated substantial drug entrapment and release capabilities. The nano-encapsulated EA demonstrated biocompatibility and exhibited greater cytotoxicity in vitro than EA alone. Likewise, markedly greater tumor regression was noted in mice treated with nano-EA compared to those receiving EA alone. Moreover, nanoformulations exhibited increased apoptosis in tumor tissues without notable damage in essential organs.120 Furthermore, Wang et al developed paclitaxel-loaded chitosan nanocarriers that showed enhanced cytotoxicity in breast cancer. Paclitaxel nanocarriers have demonstrated significant affinity and specificity for breast cancer cells, remarkable tumor cytotoxicity, and an effective inhibitory effect on metastasis. This advanced theranostic platform demonstrated significant inhibition of tumor growth and suppression of metastasis in an MDA-MB-231 mouse model, presenting therapeutic potential for enhancing anticancer combination therapy with substantial prospects for clinical application.121
How Hyaluronic Acid Play A Key Role As Nanocarrier?
Hyaluronic acid (HA) is a mucopolysaccharide composed of two saccharide units, glucuronic acid and N-acetylglucosamine. The presence of hydroxyl, carboxylic, and N-acetyl groups on HA makes it amenable to modification. Various medications can be directly associated with HA to form novel conjugates with enhanced anticancer efficacy. HA exhibits greater specificity for various tumor cells, especially tumor-initiating cells. Tumor cells exhibit overexpression of CD44 and LYVE-1 receptors, which are receptors that bind hyaluronic acid, but low expression was also observed on the surfaces of epithelial, hematopoietic, and brain cells.154 Owing to its biocompatibility, elevated viscoelasticity, and biodegradability, HA has been employed as a carrier in drug delivery systems, manifested as hydrogels and micelles. The intracellular absorption of HA-drug conjugates was promoted by CD44 caveolae-mediated endocytosis in tumor cells, hence improving the efficacy of targeted drug delivery.142,155,156 Hyaluronic acid-based drug nanocarriers have been employed in anticancer treatments. Agrawal et al157 synthesized LPT-HA-NCs by encapsulating lapatinib nanocrystals with HA to enhance their therapeutic efficacy against triple-negative breast cancer. The findings indicated that the nanoencapsulated drug carrier demonstrated enhanced anticancer efficacy compared to free medication and effectively impeded the spread of cancer cells to distant locations.158 Thus, we have provided an overview of the latest developments in the use of HA-based anticancer phytocompound nanocarriers for breast cancer treatment.
Gu et al created hyaluronic acid-SS-tetradecyl nano-carriers for sulforaphane to augment the inhibitory efficacy of the non-encapsulated phytocompound. The nanocarriers exhibited a high sulforaphane entrapment rate of 92.36% and a drug-loading efficiency of 33.64%. The carriers responded well to the mildly acidic and highly reducing tumor microenvironment, which caused the SFN-loaded nanodrug (SFN/M-HA-SS-TA) to quickly release SFN. The targeted recognition of CD44+ breast cancer cells by HA demonstrated the superior tumor-targeting capability of these nanocarriers. Furthermore, in comparison to free SFN, SFN/M-HA-SS-TA exhibited significantly enhanced suppression of BCSC-like characteristics, including self-renewal, invasiveness, and tumor formation both in vitro and in vivo.122 Bhattacharya et al developed HA encapsulated thymoquinone nanoparticles conjugated with Pluronic® P123 and F127 copolymer as a targeted drug delivery system for the anticancer phytochemical TQ to triple-negative breast cancer (TNBC) cells. These nanoparticles demonstrated significant cytotoxicity against TNBC cells while exhibiting no harmful effects on normal cells. Thorough examination has revealed its pro-apoptotic, anti-metastatic, and anti-angiogenic properties. Comprehensive mechanistic investigations revealed that HA-TQ-Nps inhibited TNBC cell migration by upregulating microRNA-361, which subsequently downregulated Rac1 and RhoA, thereby disrupting cancer cell migration influenced by the autocrine effect of VEGF-A. Furthermore, HA-TQ-Np therapy disrupted tumor-induced vascularization by diminishing the release of VEGF-A. The anti-metastatic and anti-angiogenic properties of HA-TQ-Nps were demonstrated in both MDA-MB-231 xenograft chick embryos and a 4T1 mammary solid tumor model in syngeneic mice.123 Sun et al (2023) synthesized amphiphilic hyaluronic acid polymers (dHAD) through the grafting of dodecylamine onto HA. dHAD self-assembled with quercetin to form drug-loaded micelles (dHAD-QT). Quercetin-loaded HA nanoparticles exhibited superior drug-loading capacities (75.9%) for QT and demonstrated markedly enhanced CD44 targeting relative to unmodified HA. These nanocarriers demonstrated significant cytotoxicity and apoptosis-inducing properties, attributed to their pH-sensitive nature, facilitating the rapid drug release of QT under acidic conditions. Significantly, in vivo tests demonstrated that dHAD-QT efficiently suppressed tumor growth in tumor-bearing mice, achieving a tumor suppression rate of 91.8%. Moreover, dHAD-QT extended the survival duration of tumor-bearing mice and mitigated the toxicity of the drug in normal tissues.124 Core-shell nanoparticles composed of zein and hyaluronic acid loaded with honokiol (HA-Zein-HNK) were designed for targeted delivery in breast cancer. The synthesized nanoparticles exhibited a mean diameter of 210.4 nm and negative surface charge. These nanoparticles demonstrated enhanced antiproliferative and pro-apoptotic effects on 4T1 cells. Wound healing and Transwell assays demonstrated that HA-loaded honokiol significantly impaired the migration and invasion of 4T1 cells. Mechanistic findings indicated that HA-Zein-HNK decreased vimentin expression and increased E-cadherin expression. An in vivo tissue distribution study demonstrated the superior tumor-targeting capability of HA-Zein.125 Luo et al created a multifunctional nanocomplex for the simultaneous delivery of paclitaxel (PTX) and STAT3 siRNA (siSTAT3) to decrease tumor growth and prevent metastasis in breast cancer cells. This nanocomplex exhibited increased cytotoxicity against tumor cells, which was attributed to the synergistic interaction between PTX and siSTAT3. Successful suppression of tumor metastasis was validated by using cell migration and invasion experiments in 4T1 cells. Significantly enhanced anticancer efficacy was noted in orthotopic 4T1 tumor-bearing mice, with no adverse effects, and lung metastasis was markedly suppressed in the 4T1 metastasis model.126 Yu et al produced nanoparticles of curcumin and HA encapsulated within a zeolitic imidazolate framework-8, utilizing a method predicated on the pH-dependent solubility of curcumin and the electrostatic interactions between zinc ions and the carboxyl groups of hyaluronic acid. These findings demonstrated that the breakdown of Cur during the synthesis of Cur@ZIF-8 was minimal. These nanocarriers exhibited a superior inhibitory effect on breast cancer compared to that of Cur@ZIF-8. The treatment of 4T1 cells with Cur@ZIF-8@HA resulted in increased cellular uptake and enhanced cytotoxicity, as evidenced by elevated lactate dehydrogenase release, G2/M phase cell cycle arrest, ROS generation, and apoptosis induction. In 4T1 tumor-bearing murine models, Cur@ZIF-8@HA demonstrated a more enhanced suppressive effect on tumor proliferation and lung metastasis.127
How Cellulose Play A Key Role As Nanocarrier?
Cellulose is a naturally existing linear polysaccharide with desirable properties, such as biodegradability and biocompatibility. Plant cell walls provide a limitless supply of biopolymers, which are among the most abundant naturally occurring substances.159 Cellulose is a complex carbohydrate that is composed of many cyclic glucose units. The origin of this biopolymer reveals a flat, ribbon-like structural shape with a chain of several hundred to thousands of β(1 → 4)-linked D-glucose molecules.160 Cellulose and its various modified derivatives are extensively utilized in drug delivery systems, particularly in cancer therapeutics, primarily to alter the solubility and/or gelation of different medicines, thereby regulating their release patterns. Cellulose nanocarriers are the subject of extensive research as prospective nanomaterials for targeted drug delivery, particularly in cancer treatment, due to their superior physical properties, including nanoscale dimensions, spindle-shaped morphology, prevalent surface hydroxyl groups, and biodegradability within living cells.161,162
Atallah et al synthesized carboxymethyl cellulose nanogels using lactoferrin (Lf) protein for the co-delivery of antimetabolite pemetrexed (PMT) and plant-derived polyphenol honokiol (HK). PMT/HK-loaded Lf-CMC NGs were effectively internalized by MDA-MB-231 breast cancer cells and exhibited enhanced in vitro cytotoxicity, as indicated by a modest combination index value (CI=0.17) and a larger dose reduction index (DRI) relative to free medicines. An in vivo antitumor investigation utilizing an Ehrlich ascites tumor (EAT) murine model demonstrated the significant efficacy of these cellulosic nanoparticles in inhibiting tumor proliferation, attributed to the diminished expression of VEGF-1, increased protein levels of caspase-3, and decreased Ki-67 protein levels in the tumor tissue.128 Quiñones et al encapsulated the phytocompound camptothecin into the inner core of cellulose nanoaggregates to achieve continuous release, while preserving its antiproliferative action. Camptothecin was encapsulated within cellulose nanoaggregates, attaining a concentration of 1.7–13.0 wt %. A prolonged release of camptothecin exceeding 150 h was observed under simulated physiological settings. A significant in vitro anticancer efficacy of camptothecin-loaded cellulose nanoparticles has been reported against MCF-7 breast cancer cells. The resulting cytotoxicity was analogous to that of free camptothecin at equivalent doses.129
How Starch Play A Key Role As Nanocarrier?
Starch is a polymeric carbohydrate that consist of many glucose units connected by glycosidic linkages. It is frequently present as a carbohydrate source in the human diet. This substance is a component of several basic foods including rice, manioc, wheat, corn, and potatoes. Unadulterated plant starch can be transformed into a white, aqueous, and efflorescent powder. The powder consisted of fine granules, with a diameter ranging from 2 to 100 μm and a thickness of approximately 1.5 μm. The fundamental polymeric formula is (C6H10O5)n, and the glucose monomer is referred to as α-d-glycose (or α-d-glycopyranose). The unique physicochemical characteristics and functional attributes of starch for diverse biomedical and pharmacological applications are derived from multiple botanical sources including maize, potato, rice, and wheat.163 Starch can potentially be used as a carrier for drug delivery and other bioactive substances. Chemically modified starch with increased reactive chemical sites serves as a beneficial biocompatible carrier that is easily digestible in the human body.164 Starch-based nanoparticles utilize a complex method for cancer therapy. These nanoparticles, engineered to utilize the Enhanced Permeability and Retention (EPR) effect, are selectively concentrated in neoplastic tissues. Upon entering the tumor microenvironment, cancer cells infiltrate via diverse cellular absorption pathways. These nanoparticles can transport therapeutic agents, such as chemotherapeutic medicines or nucleic acids, which are released intracellularly in response to external stimuli, such as pH variations. While causing minimal harm to healthy tissues, antitumor agents carry out their anticancer activities by interfering with cellular processes, reducing gene expression, or modifying the immune response. Starch-derived nanoparticles facilitate targeted medication administration, minimize systemic toxicity, and ensure regulated and prolonged release.165 Their biodegradability guarantees eventual elimination from the body, whereas integrated imaging agents provide real-time assessment of treatment effectiveness. This adaptable method facilitates customized cancer treatments, potentially enhancing patient outcomes and reducing adverse effects.166
Pourmadadi et al developed a pH-sensitive drug delivery system utilizing a nanocomposite of polyacrylic acid, starch, and titanium dioxide as a carrier for the anti-cancer agent curcumin targeting breast cancer cells. The findings indicated that this nanocomplex of curcumin exhibited superior efficacy by enhancing bioavailability and facilitating the regulated release of the drug in comparison to free curcumin. Additionally, the stimulation of apoptosis, which signifies the cell death of cancer cells and the great efficacy of the designed nanocarrier, was the primary mechanism by which cancer cells were destroyed in the presence of this nanocomposite as opposed to free curcumin.130 Mariadoss et al created p-coumaric acid-loaded aptamer-conjugated starch nanoparticles (Apt-p-CA-AStNPs) for efficient treatment of triple-negative breast cancer MDA-MB-231 cells. The functionalized starch-based p-coumaric acid nanomaterial exhibited an optimal diameter with significant polydispersity (0.299 ± 0.05), potentially enhancing the drug delivery mechanism in MDA-MB-231 cells, along with a surface charge of (−29.23 ± 1.35 mV) and sustained release characteristics of nanoparticles (up to 42 h). Conjugated starch nanoparticles increased cytotoxicity in MDA-MB-231 cells via ROS generation, nuclear damage, mitochondrial membrane potential disruption, and altered expression of apoptosis-related proteins. Overall, these data demonstrated that these nanoparticles effectively suppressed MDA-MB-231 cells by modulating apoptosis.131 Saikia et al created thiolated starch-coated iron oxide nanoparticles infused with curcumin and examined their cytotoxic effects on MCF-7 breast cancer cells. Nanoparticles with a 5% polymer covering demonstrated a drug encapsulation effectiveness of up to 78%, while the loading efficiency exceeded 80%. Curcumin-loaded nanoparticles exhibited dose-dependent cytotoxicity in breast cancer cells. The cell viability decreased in dose-responsive manner of free curcumin and curcumin-loaded nanoparticles. The cytotoxicity of the curcumin-loaded nanoparticles was markedly greater than that of free curcumin at all doses. This could be explained by curcumin’s improved dispersibility in the thiolated coated iron oxide magnetic nanoparticles.132
How Other Complex Polysaccharides Play A Key Role As Nanocarrier?
In addition to the aforementioned polysaccharide nanoformulations of phytocompounds, certain complex polysaccharide combinations have been shown to enhance the anticancer activity of these phytocompounds in the management of breast cancer.167 Afzali et al produced chitosan-β-cyclodextrin-TPP-folic acid/alginate nanoparticles and encapsulated curcumin. The encapsulation of curcumin into nanoparticles resulted in a nearly spherical morphology with an average particle size of 155 nm. An in vitro cytotoxicity study demonstrated a dose-dependent response against breast cancer cells after 24 h of incubation. Conversely, an in vitro cell uptake investigation demonstrated the active targeting of curcumin nanocarriers into spheroids. CXCR4 expression was approximately 30 times lower than that of curcumin alone. These nanoparticles suppressed proliferation and enhanced apoptosis of spheroid human breast cancer cells.133 Guo et al created hyaluronic acid/dextran-based polymeric micelles for the simultaneous administration of ursolic acid (UA) and doxorubicin (DOX) for combinatorial multidrug-resistant treatment. The micelles exhibited a spherical morphology, restricted size distribution (approximately 140 nm), and satisfactory drug co-loading capacity (DOX: 8.41%, UA: 9.06%). Following hyaluronic acid (HA)-mediated endocytosis, lysosomal hyaluronidase facilitated the breakdown of the HA layer, thereby exposing the positively charged triphenylphosphine groups, which markedly increased the mitochondrial accumulation of nano micelles. Consequently, DOX and UA were selectively delivered to mitochondria in response to endogenous ROS, resulting in significant mitochondrial damage through ROS generation, mitochondrial membrane potential depolarization, and energy supply disruption, thereby reinstating the susceptibility of MCF-7/ADR cells to chemotherapeutic agents. Significantly, these polysaccharide-based micelles exhibited strong anticancer activity without notable toxicity in the MDR tumor-bearing nude mice model.134
Sampath et al developed surface-modified curcumin-embedded poly(lactic-co-glycolic acid) (PLGA) nanoparticles utilizing several capping agents, including chitosan, dextran, poly(ethylene glycol), and emulsifiers. These compounds have been investigated to mitigate the solubility deficiencies and inadequate bioavailability of curcumin. All nanoformulations exhibited high loading efficiencies ranging from 54% to 89%. The loading efficiency of emulsifier-modified nanoparticles far surpassed that of nanoparticles lacking emulsifiers. The MTT assay indicated that curcumin-encapsulated PLGA nanospheres were more efficient at inhibiting cancer cell proliferation than free curcumin. The in vitro anticancer efficacy of PLGA nanoparticles infused with curcumin and other capping agents in MCF-7 cells demonstrated superior effectiveness in inhibiting cell proliferation. Cellular absorption of emulsified dextran-capped curcumin-encapsulated PLGA nanoparticles significantly exceeded that of PLGA nanoparticles with alternative capping agents, emulsifiers, and free curcumin. Curcumin selectively promotes apoptosis in rapidly growing cells, with a more significant effect observed in cancer cells compared to normal cells.135
Limitations of Polysaccharide Based Nanocarriers
In general, employing nanoparticles for medication delivery may be regarded as unsafe because smaller particle sizes are associated with increased reactivity and toxicity.168 However, the shape, chemistry, hydrophobicity/hydrophilicity, and surface charge of nanomaterials are some of the additional variables that may contribute to this behavior.169 The creation of novel materials and the alteration of current materials are essential elements in the construction of effective carriers. Natural polysaccharides that exhibit excellent biocompatibility and distinctive physicochemical features are regarded as optimal for drug delivery applications. Simultaneously, the selection of appropriate polysaccharides for tumor-targeted drug delivery presents numerous obstacles. The diverse functional groups in polysaccharides provide chemical modifications and assist in the conjugation or loading of targeted medicinal molecules. The structure of naturally occurring polysaccharides is challenging to ascertain because their molecular weight and chemical nature fluctuate with seasonal and environmental conditions.170 The molecular weight of polysaccharides influences the particle size distribution in drug delivery systems. Regulating the molecular weight of polysaccharides significantly increases their production cost. Moreover, the chemical alteration of polysaccharides must preserve their inherent features, particularly their biocompatibility. During the preparation procedure, it is essential to investigate whether polysaccharides can fulfill their intended roles. Nonetheless, the inadequate mechanical strength and unpredictable hydration rate of natural polysaccharides restrict their applications in some delivery systems. Moreover, polysaccharide-based carriers can be readily conjugated with targeting ligands to facilitate drug delivery to affected locations while preserving healthy cells. Drug leakage into the bloodstream, resistance, and extended circulation are critical issues to consider when developing carriers.171 Moreover, the carrier must ensure high encapsulation efficiency and sustained drug release to achieve a synergistic effect. Currently, the quantity of polysaccharides utilized in commercial formulations is minimal, with the majority concentrated on the research objectives.172 The clinical efficacy of polysaccharide-based drug delivery methods is intrinsically limited. The structures of polysaccharides, which differ based on their source and processing, affect activities such as their anticancer effects. Unclear structure-function interactions limit productivity and use.173 Although drug conjugation to polysaccharides enhances the solubility of water-insoluble drugs in aqueous solutions, safeguards against enzymatic and chemical degradation, and reduces elimination rates due to elevated molecular weight, prolonged systemic circulation, improved biodistribution, and augmented bioavailability, the pharmacokinetics of these drug carriers are affected by the molecular weight, charge, polydispersity, and structural variations of the polysaccharide-drug conjugate. Consequently, achieving uniformity, reproducibility, and scalability in polysaccharide-conjugate systems is challenging.174 This necessitates investigation of the optimal commercial transformation of various polysaccharides. Despite the aforementioned limitations and problems, polysaccharides possess significant potential for development.
The Future Prospective
Globally, there is a growing focus on phytocompounds, owing to their remarkable anticancer therapeutic advantages in preclinical applications. Consequently, identifying suitable formulations or technologies for their delivery via multiple routes presents the greatest difficulty, owing to issues such as inadequate solubility, low bioavailability, and instability. Many polysaccharide nanoparticles have been identified as suitable for the encapsulation or loading of diverse phytocompounds to improve their bioavailability and medicinal efficiency. Polysaccharide materials are of substantial importance in biomedicine because of their excellent biocompatibility, biodegradability, and chemical modification. Researchers have created diverse polysaccharide-based carriers for distinct tumor types. As predicted, polysaccharide nanomedicines loaded with phytocompounds exhibited targeted tumor specificity and improved anticancer efficacy in breast cancer. Altogether, the integration of polysaccharide nanocarriers with phytocompounds demonstrates a significant potential for the treatment of breast cancer. It is anticipated that more phytocompound-based polysaccharide nanomedicines could be employed in the clinical setting to treat breast cancer. Researchers should aim for therapy options that are cost-effective, highly efficacious, and exhibit minimal toxicity. In the future, these phytocompound-based polysaccharide nanocarriers will soon be used in a wider range of clinical settings, beyond cancer therapy.
Acknowledgment
The authors are thankful to the Deanship of Research and Graduate Studies, King Khalid University, Abha, Saudi Arabia, for financially supporting this work through the Large Research Group Project under Grant no. R.G.P.2/410/46.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Leena Panigrahi L, Samal P, Ranjan Sahoo S. et al. Nanoparticle-mediated diagnosis, treatment, and prevention of breast cancer. Nanoscale Adv. 2024;6(15):3699–3713. PMID: 39050943; PMCID: PMC11265592. doi:10.1039/d3na00965c.
2. Fahad Ullah M. Breast Cancer: current Perspectives on the Disease Status. Adv Exp Med Biol. 2019;1152:51–64. PMID: 31456179. doi:10.1007/978-3-030-20301-6_4.
3. Kerr AJ, Dodwell D, McGale P, et al. Adjuvant and neoadjuvant breast cancer treatments: a systematic review of their effects on mortality. Cancer Treat Rev. 2022;105:102375. Epub 2022 Mar 4. PMID: 35367784; PMCID: PMC9096622. doi:10.1016/j.ctrv.2022.102375.
4. Jain V, Kumar H, Anod HV, et al. A review of nanotechnology-based approaches for breast cancer and triple-negative breast cancer. J Control Release. 2020;326:628–647. Epub 2020 Jul 10. PMID: 32653502. doi:10.1016/j.jconrel.2020.07.003.
5. Subhan MA, Muzibur Rahman M. Recent Development in Metallic Nanoparticles for Breast Cancer Therapy and Diagnosis. Chem Rec. 2022;22(7):e202100331. Epub 2022 Feb 10. PMID: 35146897. doi:10.1002/tcr.202100331.
6. Leon-Ferre RA, Goetz MP. Advances in systemic therapies for triple negative breast cancer. BMJ. 2023;381:e071674. PMID: 37253507. doi:10.1136/bmj-2022-071674.
7. Mirzaei-Parsa MJ, Najafabadi MRH, Haeri A, et al. Preparation, characterization, and evaluation of the anticancer activity of artemether-loaded nano-niosomes against breast cancer. Breast Cancer. 2020;27(2):243–251. Epub 2019 Oct 16. PMID: 31621052. doi:10.1007/s12282-019-01014-w.
8. Ahmadi S, Seraj M, Hosseini S, Akbarzadeh I, Saffar S, Mostafavi E. In vitro Development of Controlled-Release Nanoniosomes for Improved Delivery and Anticancer Activity of Letrozole for Breast Cancer Treatment. Int J Nanomedicine. 2022;17:6233–6255. PMID: 36531115; PMCID: PMC9753765. doi:10.2147/IJN.S384085.
9. Mitra S, Dash R. Natural Products for the Management and Prevention of Breast Cancer. Evid Based Complement Alternat Med. 2018;2018:8324696. PMID: 29681985; PMCID: PMC5846366. doi:10.1155/2018/8324696.
10. Adel M, Akbarzadeh A, Rabiee N, et al. Chemotherapeutic effects of Apigenin in breast cancer: preclinical evidence and molecular mechanisms; enhanced bioavailability by nanoparticles. Biotechnol Rep. 2022;34:e00730. PMID: 35686000; PMCID: PMC9171451. doi:10.1016/j.btre.2022.e00730.
11. González S. Dietary Bioactive Compounds and Human Health and Disease. Nutrients. 2020;12(2):348. PMID: 32013067; PMCID: PMC7071229. doi:10.3390/nu12020348.
12. Ganesan K, Du B, Chen J. Effects and mechanisms of dietary bioactive compounds on breast cancer prevention. Pharmacol Res. 2022;178:105974. Epub 2021 Nov 21. PMID: 34818569. doi:10.1016/j.phrs.2021.105974.
13. Naeem M, Iqbal MO, Khan H, et al. A Review of Twenty Years of Research on the Regulation of Signaling Pathways by Natural Products in Breast Cancer. Molecules. 2022;27(11):3412. PMID: 35684353; PMCID: PMC9182524. doi:10.3390/molecules27113412.
14. Aung TN, Qu Z, Kortschak RD, Adelson DL. Understanding the Effectiveness of Natural Compound Mixtures in Cancer through Their Molecular Mode of Action. Int J Mol Sci. 2017;18(3):656. PMID: 28304343; PMCID: PMC5372668. doi:10.3390/ijms18030656.
15. Hasan-Abad AM, Atapour A, Motedayyen H, ArefNezhad R. Plant-Based Anticancer Compounds With a Focus on Breast Cancer. Cancer Rep (Hoboken). 2024;7(10):e70012. PMID: 39453820; PMCID: PMC11506041. doi:10.1002/cnr2.70012.
16. Sharifi-Rad J, Sureda A, Tenore GC, et al. Biological Activities of Essential Oils: from Plant Chemoecology to Traditional Healing Systems. Molecules. 2017;22(1):70. PMID: 28045446; PMCID: PMC6155610. doi:10.3390/molecules22010070.
17. Pavoni L, Perinelli DR, Bonacucina G, Cespi M, Palmieri GF. An Overview of Micro- and Nanoemulsions as Vehicles for Essential Oils: formulation, Preparation and Stability. Nanomaterials (Basel). 2020;10(1):135. PMID: 31940900; PMCID: PMC7023169. doi:10.3390/nano10010135.
18. Jabir M, Sahib UI, Taqi Z, et al. Linalool-Loaded Glutathione-Modified Gold Nanoparticles Conjugated with CALNN Peptide as Apoptosis Inducer and NF-κB Translocation Inhibitor in SKOV-3 Cell Line. Int J Nanomedicine. 2020;15:9025–9047. PMID: 33235450; PMCID: PMC7680166. doi:10.2147/IJN.S276714.
19. Yap KM, Fuloria S, Wu YS, et al. Drug Delivery of Natural Products Through Nanocarriers for Effective Breast Cancer Therapy: a Comprehensive Review of Literature. Int J Nanomedicine. 2021;16:7891–7941. PMID: 34880614; PMCID: PMC8648329. doi:10.2147/IJN.S328135.
20. Lv Y, Jiang H, Liu Y, Cao J, Lu W, Feng Y. Nano-Drug Delivery Systems Based on Natural Products. Int J Nanomedicine. 2024;19:541–569. PMID: 38260243; PMCID: PMC10802180. doi:10.2147/IJN.S443692.
21. Carrion CC, Nasrollahzadeh M, Jaleh B, Soufi GJ, Iravani S. Lignin, lipid, protein, hyaluronic acid, starch, cellulose, gum, pectin, alginate and chitosan-based nanomaterials for cancer nanotherapy: challenges and opportunities. Int J Biol Macromol. 2021;178:193–228. Epub 2021 Feb 22. PMID: 33631269. doi:10.1016/j.ijbiomac.2021.02.123.
22. Wang Y, Deng T, Liu X, et al. Smart Nanoplatforms Responding to the Tumor Microenvironment for Precise Drug Delivery in Cancer Therapy. Int J Nanomedicine. 2024;19:6253–6277. PMID: 38911497; PMCID: PMC11193972. doi:10.2147/IJN.S459710.
23. Pandey P, Verma M, Lakhanpal S, et al. An Updated Review Summarizing the Anticancer Potential of Poly(Lactic-co-Glycolic Acid) (PLGA) Based Curcumin, Epigallocatechin Gallate, and Resveratrol Nanocarriers. Biopolymers. 2025;116(1):e23637. Epub 2024 Oct 17. PMID: 39417679. doi:10.1002/bip.23637.
24. Guo LJ, Wu J, Lu W, et al. Nanoparticles Modulating the Immune Microenvironment in Breast Cancer Treatment. Int J Nanomedicine. 2025;20:1367–1382. PMID: 39917056; PMCID: PMC11799854. doi:10.2147/IJN.S492713.
25. Ning S, Shangguan P, Zhu X, et al. Pyridinium Rotor Strategy toward a Robust Photothermal Agent for STING Activation and Multimodal Image-Guided Immunotherapy for Triple-Negative Breast Cancer. J Am Chem Soc. 2025;147(9):7433–7444. Epub 2025 Feb 20. PMID: 39977833; PMCID: PMC11887044. doi:10.1021/jacs.4c15534.
26. Chen Z, Zhou Y, Rao K, et al. Bionic aggregation-induced emission photosensitizer for enhanced cancer immunotherapy. Mater Today Bio. 2024;28:101217. PMID: 39285944; PMCID: PMC11402640. doi:10.1016/j.mtbio.2024.101217.
27. Ning S, Lyu M, Zhu D, et al. Type-I AIE Photosensitizer Loaded Biomimetic System Boosting Cuproptosis to Inhibit Breast Cancer Metastasis and Rechallenge. ACS Nano. 2023;17(11):10206–10217. Epub 2023 May 15. PMID: 37183977. doi:10.1021/acsnano.3c00326.
28. Alharthi S, Alrashidi AA, Ziora Z, Ebrahimi Shahmabadi H, Alavi SE. Innovative PEGylated chitosan nanocarriers for co-delivery of doxorubicin and CpG in breast cancer therapy: preparation, characterization, and immunotherapeutic potential. Med Oncol. 2025;42(5):176. PMID: 40266471. doi:10.1007/s12032-025-02714-4.
29. Yu X, Dai Y, Qi S, et al. Melittin-lipid nanoparticles target to lymph nodes and elicit a systemic anti-tumor immune response. Nat Commun. 2020;11(1):1110. PMID: 32111828; PMCID: PMC7048802. doi:10.1038/s41467-020-14906-9.
30. Zhang S, Pang G, Chen C, et al. Effective cancer immunotherapy by Ganoderma lucidum polysaccharide-gold nanocomposites through dendritic cell activation and memory T cell response. Carbohydr Polym. 2019;205:192–202. Epub 2018 Oct 13. PMID: 30446095. doi:10.1016/j.carbpol.2018.10.028.
31. Yang W, Wang L, Fang M, et al. Nanoparticle Surface Engineering with Heparosan Polysaccharide Reduces Serum Protein Adsorption and Enhances Cellular Uptake. Nano Lett. 2022;22(5):2103–2111. Epub 2022 Feb 15. PMID: 35166110. PMCID: PMC9540343. doi:10.1021/acs.nanolett.2c00349.
32. Li L, Zhang P, Li C, Guo Y, Sun K. In vitro/vivo antitumor study of modified-chitosan/carboxymethyl chitosan “boosted” charge-reversal nanoformulation. Carbohydr Polym. 2021;269:118268. Epub 2021 May 31. PMID: 34294300. doi:10.1016/j.carbpol.2021.118268.
33. Otto S, Marina PF, Zhou F, Blencowe A. Thermoresponsive polysaccharides with tunable thermoresponsive properties via functionalisation with alkylamide groups. Carbohydr Polym. 2021;254:117280. Epub 2020 Oct 24. PMID: 33357856. doi:10.1016/j.carbpol.2020.117280.
34. Liu YS, Chiu CC, Chen HY, Chen SH, Wang LF. Preparation of chondroitin sulfate-g-poly(ε-caprolactone) copolymers as a CD44-targeted vehicle for enhanced intracellular uptake. Mol Pharm. 2014;11(4):1164–1175. Epub 2014 Mar 12. PMID: 24592868. doi:10.1021/mp400607h.
35. Forero Ramirez LM, Gobin E, Aid-Launais R, et al. Gd(DOTA)-grafted submicronic polysaccharide-based particles functionalized with fucoidan as potential MR contrast agent able to target human activated platelets. Carbohydr Polym. 2020;245:116457. Epub 2020 May 25. PMID: 32718599. doi:10.1016/j.carbpol.2020.116457.
36. Wang K, Xu J, Liu Y, et al. Self-assembled Angelica sinensis polysaccharide nanoparticles with an instinctive liver-targeting ability as a drug carrier for acute alcoholic liver damage protection. Int J Pharm. 2020;577:118996. Epub 2020 Jan 3. PMID: 31904402. doi:10.1016/j.ijpharm.2019.118996.
37. Jiang W, Fu Y, Yang F, et al. Gracilaria lemaneiformis polysaccharide as integrin-targeting surface decorator of selenium nanoparticles to achieve enhanced anticancer efficacy. ACS Appl Mater Interfaces. 2014;6(16):13738–13748. Epub 2014 Aug 8. PMID: 25073123. doi:10.1021/am5031962.
38. Kareemi AF, Likhitkar S. Applications and advancements of polysaccharide-based nanostructures for enhanced drug delivery. Colloids Surf B Biointerfaces. 2024;238:113883. Epub 2024 Mar 30. PMID: 38615389. doi:10.1016/j.colsurfb.2024.113883.
39. Jiang Y, Yan C, Li M, et al. Delivery of natural products via polysaccharide-based nanocarriers for cancer therapy: a review on recent advances and future challenges. Int J Biol Macromol. 2024;278(Pt 4):135072. Epub 2024 Aug 25. PMID: 39191341. doi:10.1016/j.ijbiomac.2024.135072.
40. Saldanha SN, Tollefsbol TO. The role of nutraceuticals in chemoprevention and chemotherapy and their clinical outcomes. J Oncol. 2012;2012:192464. Epub 2011 Dec 7. PMID: 22187555; PMCID: PMC3236518. doi:10.1155/2012/192464.
41. Liao GS, Apaya MK, Shyur LF. Herbal medicine and acupuncture for breast cancer palliative care and adjuvant therapy. Evid Based Complement Alternat Med. 2013;2013:437948. Epub 2013 Jun 12. PMID: 23840256; PMCID: PMC3694462. doi:10.1155/2013/437948.
42. Chimento A, D’Amico M, De Amicis F, Pezzi V. The Involvement of Natural Polyphenols in Molecular Mechanisms Inducing Apoptosis in Tumor Cells: a Promising Adjuvant in Cancer Therapy. Int J Mol Sci. 2023;24(2):1680. PMID: 36675194; PMCID: PMC9863215. doi:10.3390/ijms24021680.
43. Giménez-Bastida JA. Dietary Bioactive Compounds and Breast Cancer. Int J Mol Sci. 2023;24(11):9731. PMID: 37298682; PMCID: PMC10253700. doi:10.3390/ijms24119731.
44. Bozzuto G, Calcabrini A, Colone M, Dupuis ML, Pellegrini E, Stringaro A. Phytocompounds and Nanoformulations for Anticancer Therapy: a Review. Molecules. 2024;29(16):3784. PMID: 39202863; PMCID: PMC11357218. doi:10.3390/molecules29163784.
45. Yuan L, Cai Y, Zhang L, Liu S, Li P, Li X. Promoting Apoptosis, a Promising Way to Treat Breast Cancer With Natural Products: a Comprehensive Review. Front Pharmacol. 2022;12:801662. PMID: 35153757; PMCID: PMC8836889. doi:10.3389/fphar.2021.801662.
46. Zhang J, Wu Y, Yang X, Xia G, Wang G. Natural products and derivatives for breast cancer treatment: from drug discovery to molecular mechanism. Phytomedicine. 2024;129:155600. Epub 2024 Apr 7. PMID: 38614043. doi:10.1016/j.phymed.2024.155600.
47. Li Q, Ye Z, Wang G, Chen Y, Deng J. Natural Products as Novel Therapeutic Agents for Triple-Negative Breast Cancer: current Evidence, Mechanisms, Challenges, and Opportunities. Molecules. 2025;30(6):1201. PMID: 40141978; PMCID: PMC11944566. doi:10.3390/molecules30061201.
48. Tu SN, Hu F, Zhang J, Cai H, Yang J. Research progress on the signaling pathway mechanism of terpenoids against breast cancer. Discov Oncol. 2025;16(1):433. PMID: 40163255; PMCID: PMC11958888. doi:10.1007/s12672-025-01881-0.
49. Khan A, Khan A, Khan MA, et al. Phytocompounds targeting epigenetic modulations: an assessment in cancer. Front Pharmacol. 2024;14:1273993. PMID: 38596245; PMCID: PMC11002180. doi:10.3389/fphar.2023.1273993.
50. Yin J, Gu T, Chaudhry N, Davidson NE, Huang Y. Epigenetic modulation of antitumor immunity and immunotherapy response in breast cancer: biological mechanisms and clinical implications. Front Immunol. 2024;14:1325615. PMID: 38268926; PMCID: PMC10806158. doi:10.3389/fimmu.2023.1325615.
51. Shankar E, Kanwal R, Candamo M, Gupta S. Dietary phytochemicals as epigenetic modifiers in cancer: promise and challenges. Semin Cancer Biol. 2016;40–41:82–99. Epub 2016 Apr 23. PMID: 27117759; PMCID: PMC5067170. doi:10.1016/j.semcancer.2016.04.002.
52. Khan SI, Aumsuwan P, Khan IA, Walker LA, Dasmahapatra AK. Epigenetic events associated with breast cancer and their prevention by dietary components targeting the epigenome. Chem Res Toxicol. 2012;25(1):61–73. Epub 2011 Oct 28. PMID: 21992498. doi:10.1021/tx200378c.
53. Landis-Piwowar KR, Milacic V, Dou QP. Relationship between the methylation status of dietary flavonoids and their growth-inhibitory and apoptosis-inducing activities in human cancer cells. J Cell Biochem. 2008;105(2):514–523. PMID: 18636546; PMCID: PMC2574743. doi:10.1002/jcb.21853.
54. Chlebowski RT. Current concepts in breast cancer chemoprevention. Pol Arch Med Wewn. 2014;124(4):191–199. Epub 2014 Mar 10. PMID: 24618912. doi:10.20452/pamw.2190.
55. Ko EY, Moon A. Natural Products for Chemoprevention of Breast Cancer. J Cancer Prev. 2015;20(4):223–231. Epub 2015 Dec 30. PMID: 26734584; PMCID: PMC4699749. doi:10.15430/JCP.2015.20.4.223.
56. Maggiolini M, Bonofiglio D, Pezzi V, et al. Aromatase overexpression enhances the stimulatory effects of adrenal androgens on MCF7 breast cancer cells. Mol Cell Endocrinol. 2002;193(1–2):13–18. PMID: 12160997. doi:10.1016/s0303-7207(02)00091-6.
57. Lephart ED. Modulation of Aromatase by Phytoestrogens. Enzyme Res. 2015;2015:594656. Epub 2015 Dec 21. PMID: 26798508; PMCID: PMC4699002. doi:10.1155/2015/594656.
58. Yarla NS, Bishayee A, Sethi G, et al. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin Cancer Biol. 2016;40–41:48–81. Epub 2016 Feb 4. PMID: 26853158. doi:10.1016/j.semcancer.2016.02.001.
59. Yang H, Rothenberger E, Zhao T, et al. Regulation of inflammation in cancer by dietary eicosanoids. Pharmacol Ther. 2023;248:108455. Epub 2023 May 29. PMID: 37257760. doi:10.1016/j.pharmthera.2023.108455.
60. Sahu A, Raza K, Pradhan D, Jain AK, Verma S. Cyclooxygenase-2 as a therapeutic target against human breast cancer: a comprehensive review. WIREs Mech Dis. 2023;15(3):e1596. Epub 2023 Mar 28. PMID: 36978255. doi:10.1002/wsbm.1596.
61. Ranger GS, Thomas V, Jewell A, Mokbel K. Elevated cyclooxygenase-2 expression correlates with distant metastases in breast cancer. Anticancer Res. 2004;24(4):2349–2351. PMID: 15330183.
62. Stasinopoulos I, Dr O, Wildes F, Glunde K, Bhujwalla ZM. Silencing of cyclooxygenase-2 inhibits metastasis and delays tumor onset of poorly differentiated metastatic breast cancer cells. Mol Cancer Res. 2007;5(5):435–442. PMID: 17510310. doi:10.1158/1541-7786.MCR-07-0010.
63. Prasad NR, Muthusamy G, Shanmugam M, Ambudkar SV. South Asian Medicinal Compounds as Modulators of Resistance to Chemotherapy and Radiotherapy. Cancers (Basel). 2016;8(3):32. PMID: 26959063; PMCID: PMC4810116. doi:10.3390/cancers8030032.
64. Ji X, Lu Y, Tian H, Meng X, Wei M, Cho WC. Chemoresistance mechanisms of breast cancer and their countermeasures. Biomed Pharmacother. 2019;114:108800. Epub 2019 Mar 25. PMID: 30921705. doi:10.1016/j.biopha.2019.108800.
65. Sartaj A, Baboota S, Ali J. Assessment of Combination Approaches of Phytoconstituents with Chemotherapy for the Treatment of Breast Cancer: a Systematic Review. Curr Pharm Des. 2021;27(45):4630–4648. PMID: 34477513. doi:10.2174/1381612827666210902155752.
66. Chavda VP, Nalla LV, Balar P, et al. Advanced Phytochemical-Based Nanocarrier Systems for the Treatment of Breast Cancer. Cancers (Basel). 2023;15(4):1023. PMID: 36831369; PMCID: PMC9954440. doi:10.3390/cancers15041023.
67. Zunica ERM, Axelrod CL, Kirwan JP. Phytochemical Targeting of Mitochondria for Breast Cancer Chemoprevention, Therapy, and Sensitization. Int J Mol Sci. 2022;23(22):14152. PMID: 36430632; PMCID: PMC9692881. doi:10.3390/ijms232214152.
68. Meng Q, Zhong S, Xu L, et al. Review on design strategies and considerations of polysaccharide-based smart drug delivery systems for cancer therapy. Carbohydr Polym. 2022;279:119013. Epub 2021 Dec 15. PMID: 34980356. doi:10.1016/j.carbpol.2021.119013.
69. Sun Y, Jing X, Ma X, Feng Y, Hu H. Versatile Types of Polysaccharide-Based Drug Delivery Systems: from Strategic Design to Cancer Therapy. Int J Mol Sci. 2020;21(23):9159. PMID: 33271967; PMCID: PMC7729619. doi:10.3390/ijms21239159.
70. Tudu M, Samanta A. Natural polysaccharides: chemical properties and application in pharmaceutical formulations. European Polymer Journal. 2023;184:111801.
71. Shokrani H, Shokrani A, Sm S, et al. Polysaccharide-based nanocomposites for biomedical applications: a critical review. Nanoscale Horiz. 2022;7(10):1136–1160. PMID: 35881463. doi:10.1039/d2nh00214k.
72. Pang X, Yang X, Zhai G. Polymer-drug conjugates: recent progress on administration routes. Expert Opin Drug Deliv. 2014;11(7):1075–1086. Epub 2014 Apr 23. PMID: 24758250. doi:10.1517/17425247.2014.912779.
73. Yang J, Han S, Zheng H, Liu J. Preparation and application of micro/nanoparticles based on natural polysaccharides. Carbohydr Polym. 2015;123:53–66. Epub 2015 Jan 22. PMID: 25843834. doi:10.1016/j.carbpol.2015.01.029.
74. Chen C, Zhou JL, Han X, Song F, Wang XL, Wang YZ. A prodrug strategy based on chitosan for efficient intracellular anticancer drug delivery. Nanotechnology. 2014;25(25):255101. Epub 2014 Jun 4. PMID: 24896540. doi:10.1088/0957-4484/25/25/255101.
75. Chen CK, Huang PK, Law WC, Chu CH, Chen NT, Lo LW. Biodegradable Polymers for Gene-Delivery Applications. Int J Nanomedicine. 2020;15:2131–2150. PMID: 32280211; PMCID: PMC7125329. doi:10.2147/IJN.S222419.
76. Siddiqui NN, Aman A, Silipo A, Qader SA, Molinaro A. Structural analysis and characterization of dextran produced by wild and mutant strains of Leuconostoc mesenteroides. Carbohydr Polym. 2014;99:331–338. Epub 2013 Aug 7. PMID: 24274515. doi:10.1016/j.carbpol.2013.08.004.
77. Sadahiro J, Mori H, Saburi W, Okuyama M, Kimura A. Extracellular and cell-associated forms of Gluconobacter oxydans dextran dextrinase change their localization depending on the cell growth. Biochem Biophys Res Commun. 2015;456(1):500–505. Epub 2014 Dec 6. PMID: 25490393. doi:10.1016/j.bbrc.2014.11.115.
78. Taghipour-Sabzevar V, Sharifi T, Moghaddam MM. Polymeric nanoparticles as carrier for targeted and controlled delivery of anticancer agents. Ther Deliv. 2019;10(8):527–550. Epub 2019 Sep 9. PMID: 31496433. doi:10.4155/tde-2019-0044.
79. Skorik YA, Golyshev AA, Kritchenkov AS, et al. Development of drug delivery systems for taxanes using ionic gelation of carboxyacyl derivatives of chitosan. Carbohydr Polym. 2017;162:49–55. Epub 2017 Jan 6. PMID: 28224894. doi:10.1016/j.carbpol.2017.01.025.
80. Wang X, Wei B, Cheng X, Wang J, Tang R. 3-Carboxyphenylboronic acid-modified carboxymethyl chitosan nanoparticles for improved tumor targeting and inhibitory. Eur J Pharm Biopharm. 2017;113:168–177. Epub 2017 Jan 13. PMID: 28089786. doi:10.1016/j.ejpb.2016.12.034.
81. Longinotti C. The use of hyaluronic acid based dressings to treat burns: a review. Burns Trauma. 2014;2(4):162–168. PMID: 27602379; PMCID: PMC5012021. doi:10.4103/2321-3868.142398.
82. Mero A, Pasqualin M, Campisi M, Renier D, Pasut G. Conjugation of hyaluronan to proteins. Carbohydr Polym. 2013;92(2):2163–2170. Epub 2012 Dec 7. PMID: 23399272. doi:10.1016/j.carbpol.2012.11.090.
83. Starigazdová J, Nešporová K, Čepa M, et al. In vitro investigation of hyaluronan-based polymeric micelles for drug delivery into the skin: the internalization pathway. Eur J Pharm Sci. 2020;143:105168. Epub 2019 Nov 26. PMID: 31783157. doi:10.1016/j.ejps.2019.105168.
84. Smejkalová D, Nešporová K, Huerta-Angeles G, et al. Selective in vitro anticancer effect of superparamagnetic iron oxide nanoparticles loaded in hyaluronan polymeric micelles. Biomacromolecules. 2014;15(11):4012–4020. Epub 2014 Oct 13. PMID: 25268047. doi:10.1021/bm501065q.
85. Pushpamalar J, Veeramachineni AK, Owh C, Loh XJ. Biodegradable Polysaccharides for Controlled Drug Delivery. Chempluschem. 2016;81(6):504–514. Epub 2016 Jun 2. PMID: 31968918. doi:10.1002/cplu.201600112.
86. Goodarzi N, Varshochian R, Kamalinia G, Atyabi F, Dinarvand R. A review of polysaccharide cytotoxic drug conjugates for cancer therapy. Carbohydr Polym. 2013;92(2):1280–1293. Epub 2012 Oct 22. PMID: 23399156. doi:10.1016/j.carbpol.2012.10.036.
87. Khan AR, Yang X, Du X, et al. Chondroitin sulfate derived theranostic and therapeutic nanocarriers for tumor-targeted drug delivery. Carbohydr Polym. 2020;233:115837. Epub 2020 Jan 11. PMID: 32059890. doi:10.1016/j.carbpol.2020.115837.
88. Arpicco S, Milla P, Stella B, Dosio F. Hyaluronic acid conjugates as vectors for the active targeting of drugs, genes and nanocomposites in cancer treatment. Molecules. 2014;19(3):3193–3230. PMID: 24642908; PMCID: PMC6271549. doi:10.3390/molecules19033193.
89. Crucho CIC, Barros MT. Surfactant-free polymeric nanoparticles composed of PEG, cholic acid and a sucrose moiety. J Mater Chem B. 2014;2(25):3946–3955. Epub 2014 May 19. PMID: 32261646. doi:10.1039/c3tb21632b.
90. Huang KX, Zhou LY, Chen JQ, et al. Applications and perspectives of quaternized cellulose, chitin and chitosan: a review. Int J Biol Macromol. 2023;242(Pt 3):124990. Epub 2023 May 19. PMID: 37211070. doi:10.1016/j.ijbiomac.2023.124990.
91. Jayakumar R, Nair A, Rejinold NS, Maya S, Nair SV. Doxorubicin-loaded pH-responsive chitin nanogels for drug delivery to cancer cells. Carbohydrate Polymers. 2012;87(3):2352–2356.
92. Rejinold NS, Nair A, Sabitha M, et al. Synthesis, characterization and in vitro cytocompatibility studies of chitin nanogels for biomedical applications. Carbohydr Polym. 2012;87(1):943–949. Epub 2011 Aug 24. PMID: 34663060. doi:10.1016/j.carbpol.2011.08.044.
93. Sivakumar B, Aswathy RG, Nagaoka Y, et al. Multifunctional carboxymethyl cellulose-based magnetic nanovector as a theragnostic system for folate receptor targeted chemotherapy, imaging, and hyperthermia against cancer. Langmuir. 2013;29(10):3453–3466. Epub 2013 Feb 27. PMID: 23409925. doi:10.1021/la305048m.
94. Bossi P, Locati L, Bergamini C, et al. Fentanyl pectin nasal spray as treatment for incident predictable breakthrough pain (BTP) in oral mucositis induced by chemoradiotherapy in head and neck cancer. Oral Oncol. 2014;50(9):884–887. Epub 2014 Jul 4. PMID: 25001894. doi:10.1016/j.oraloncology.2014.06.013.
95. Ghadi R, Dand N. BCS class IV drugs: highly notorious candidates for formulation development. J Control Release. 2017;248:71–95. Epub 2017 Jan 11. PMID: 28088572. doi:10.1016/j.jconrel.2017.01.014.
96. Imam SS, Alshehri S, Ghoneim MM, et al. Recent Advancement in Chitosan-Based Nanoparticles for Improved Oral Bioavailability and Bioactivity of Phytochemicals: challenges and Perspectives. Polymers (Basel). 2021;13(22):4036. PMID: 34833334; PMCID: PMC8617804. doi:10.3390/polym13224036.
97. Kumar G, Virmani T, Sharma A, Pathak K. Codelivery of Phytochemicals with Conventional Anticancer Drugs in Form of Nanocarriers. Pharmaceutics. 2023;15(3):889. PMID: 36986748; PMCID: PMC10055866. doi:10.3390/pharmaceutics15030889.
98. Giri PM, Banerjee A, Layek B. A Recent Review on Cancer Nanomedicine. Cancers (Basel). 2023;15(8):2256. PMID: 37190185; PMCID: PMC10136552. doi:10.3390/cancers15082256.
99. Tiwari H, Rai N, Singh S, et al. Recent Advances in Nanomaterials-Based Targeted Drug Delivery for Preclinical Cancer Diagnosis and Therapeutics. Bioengineering (Basel). 2023;10(7):760. PMID: 37508788; PMCID: PMC10376516. doi:10.3390/bioengineering10070760.
100. Mukherjee D, Raikwar S. Recent Update on Nanocarrier(s) as the Targeted Therapy for Breast Cancer. AAPS PharmSciTech. 2024;25(6):153. PMID: 38961013. doi:10.1208/s12249-024-02867-x.
101. Herdiana Y, Sriwidodo S, Sofian FF, Wilar G, Diantini A. Nanoparticle-Based Antioxidants in Stress Signaling and Programmed Cell Death in Breast Cancer Treatment. Molecules. 2023;28(14):5305. PMID: 37513179; PMCID: PMC10384004. doi:10.3390/molecules28145305.
102. Solanki H, Zhai BT, Fan Y, et al. Targeted Drug Delivery Systems for Curcumin in Breast Cancer Therapy. Int J Nanomedicine. 2023;18:4275–4311. PMID: 37534056; PMCID: PMC10392909. doi:10.2147/IJN.S410688.
103. Solanki R, Bhatia D. Stimulus-Responsive Hydrogels for Targeted Cancer Therapy. Gels. 2024;10(7):440. PMID: 39057463; PMCID: PMC11275390. doi:10.3390/gels10070440.
104. Kim K. Hybrid Systems of Gels and Nanoparticles for Cancer Therapy: advances in Multifunctional Therapeutic Platforms. Gels. 2025;11(3):170. PMID: 40136875; PMCID: PMC11941994. doi:10.3390/gels11030170.
105. Wang J, Wu X, Chen J, Gao T, Zhang Y, Yu N. Traditional Chinese medicine polysaccharide in nano-drug delivery systems: current progress and future perspectives. Biomed Pharmacother. 2024;173:116330. Epub 2024 Feb 28. PMID: 38422656. doi:10.1016/j.biopha.2024.116330.
106. Song M, Aipire A, Dilxat E, et al. Research Progress of Polysaccharide-Gold Nanocomplexes in Drug Delivery. Pharmaceutics. 2024;16(1):88. PMID: 38258099; PMCID: PMC10820823. doi:10.3390/pharmaceutics16010088.
107. Jamroży M, Kudłacik-Kramarczyk S, Drabczyk A, Krzan M. Advanced Drug Carriers: a Review of Selected Protein, Polysaccharide, and Lipid Drug Delivery Platforms. Int J Mol Sci. 2024;25(2):786. PMID: 38255859; PMCID: PMC10815656. doi:10.3390/ijms25020786.
108. Raveendran S, Giram A, Elmi M, et al. Combinatorial Therapy: targeting CD133+ Glioma Stem-like Cells with a Polysaccharide-Prodrug Complex Functionalised Gold Nanocages. Biomedicines. 2024;12(5):934. PMID: 38790896; PMCID: PMC11117750. doi:10.3390/biomedicines12050934.
109. Cui M, Tian Y, et al. A highly therapeutic and selective delivery system for curcumin based on nanocellulose and folic acid. Cellulose. 2023;30:5113–5126. doi:10.1007/s10570-023-05122-x
110. Babaeenezhad E, Rashidipour M, Jangravi Z, Moradi Sarabi M, Shahriary A. Cytotoxic and epigenetic effects of berberine-loaded chitosan/pectin nanoparticles on AGS gastric cancer cells: role of the miR-185-5p/KLF7 axis, DNMTs, and global DNA methylation. Int J Biol Macromol. 2024;260(Pt 2):129618. Epub 2024 Jan 20. PMID: 38253156. doi:10.1016/j.ijbiomac.2024.129618.
111. Lu M, Ma L, Li J, et al. Construction of carboxymethyl chitosan-based nanoparticles of hypoxia response for co-loading doxorubicin and tanshinone IIA. Int J Biol Macromol. 2023;244:125362. Epub 2023 Jun 15. PMID: 37330079. doi:10.1016/j.ijbiomac.2023.125362.
112. Awad MG, Hanafy NA, Ali RA, El-Monem DD, El-Shafiey SH, El-Magd MA. Unveiling the therapeutic potential of anthocyanin/cisplatin-loaded chitosan nanoparticles against breast and liver cancers. Cancer Nanotechnology. 2024;15(1):57.
113. Alipanah H, Farjam M, Zarenezhad E, Roozitalab G, Osanloo M. Chitosan nanoparticles containing limonene and limonene-rich essential oils: potential phytotherapy agents for the treatment of melanoma and breast cancers. BMC Complement Med Ther. 2021;21(1):186. PMID: 34215240; PMCID: PMC8254332. doi:10.1186/s12906-021-03362-7.
114. Ghobadi-Oghaz N, Asoodeh A, Mohammadi M. Fabrication, characterization and in vitro cell exposure study of zein-chitosan nanoparticles for co-delivery of curcumin and berberine. Int J Biol Macromol. 2022;204:576–586. Epub 2022 Feb 11. PMID: 35157902. doi:10.1016/j.ijbiomac.2022.02.041.
115. Truong TH, Alcantara KP, Bulatao BPI, et al. Chitosan-coated nanostructured lipid carriers for transdermal delivery of tetrahydrocurcumin for breast cancer therapy. Carbohydr Polym. 2022;288:119401. Epub 2022 Mar 24. PMID: 35450653. doi:10.1016/j.carbpol.2022.119401.
116. Sabzini M, Pourmadadi M, Yazdian F, Khadiv-Parsi P, Rashedi H. Development of chitosan/halloysite/graphitic-carbon nitride nanovehicle for targeted delivery of quercetin to enhance its limitation in cancer therapy: an in vitro cytotoxicity against MCF-7 cells. Int J Biol Macromol. 2023;226:159–171. Epub 2022 Nov 23. PMID: 36435458. doi:10.1016/j.ijbiomac.2022.11.189.
117. Nematollahi E, Pourmadadi M, Yazdian F, Fatoorehchi H, Rashedi H, Nigjeh MN. Synthesis and characterization of chitosan/polyvinylpyrrolidone coated nanoporous γ-Alumina as a pH-sensitive carrier for controlled release of quercetin. Int J Biol Macromol. 2021;183:600–613. Epub 2021 Apr 28. PMID: 33932424. doi:10.1016/j.ijbiomac.2021.04.160.
118. Abdouss H, Pourmadadi M, Zahedi P, et al. Green synthesis of chitosan/polyacrylic acid/graphitic carbon nitride nanocarrier as a potential pH-sensitive system for curcumin delivery to MCF-7 breast cancer cells. Int J Biol Macromol. 2023;242(Pt 3):125134. Epub 2023 May 29. PMID: 37257532. doi:10.1016/j.ijbiomac.2023.125134.
119. Farokh A, Pourmadadi M, Rashedi H, Yazdian F, Navaei-Nigjeh M. Assessment of synthesized chitosan/halloysite nanocarrier modified by carbon nanotube for pH-sensitive delivery of curcumin to cancerous media. Int J Biol Macromol. 2023;237:123937. Epub 2023 Mar 5. PMID: 36882143. doi:10.1016/j.ijbiomac.2023.123937.
120. Kaur H, Ghosh S, Kumar P, Basu B, Nagpal K. Ellagic acid-loaded, tween 80-coated, chitosan nanoparticles as a promising therapeutic approach against breast cancer: in-vitro and in-vivo study. Life Sci. 2021;284:119927. Epub 2021 Sep 4. PMID: 34492262. doi:10.1016/j.lfs.2021.119927.
121. Wang P, Peng Z, Zhang Y, et al. A chitosan-camouflaged nanomedicine triggered by hierarchically stimuli to release drug for multimodal imaging-guided chemotherapy of breast cancer. Carbohydr Polym. 2024;335:122073. Epub 2024 Mar 18. PMID: 38616095. doi:10.1016/j.carbpol.2024.122073.
122. Gu HF, Ren F, Mao XY, Du M. Mineralized and GSH-responsive hyaluronic acid based nano-carriers for potentiating repressive effects of sulforaphane on breast cancer stem cells-like properties. Carbohydr Polym. 2021;269:118294. Epub 2021 Jun 5. PMID: 34294320. doi:10.1016/j.carbpol.2021.118294.
123. Bhattacharya S, Ghosh A, Maiti S, et al. Delivery of thymoquinone through hyaluronic acid-decorated mixed Pluronic® nanoparticles to attenuate angiogenesis and metastasis of triple-negative breast cancer. J Control Release. 2020;322:357–374. Epub 2020 Mar 31. PMID: 32243981. doi:10.1016/j.jconrel.2020.03.033.
124. Sun J, Li M, Lin K, et al. Delivery of quercetin for breast cancer and targeting potentiation via hyaluronic nano-micelles. Int J Biol Macromol. 2023;242(Pt 2):124736. Epub 2023 May 5. PMID: 37148944. doi:10.1016/j.ijbiomac.2023.124736.
125. Zhang Q, Wang J, Liu D, et al. Targeted delivery of honokiol by zein/hyaluronic acid core-shell nanoparticles to suppress breast cancer growth and metastasis. Carbohydr Polym. 2020;240:116325. Epub 2020 Apr 28. PMID: 32475585. doi:10.1016/j.carbpol.2020.116325.
126. Luo K, Gao Y, Yin S, et al. Co-delivery of paclitaxel and STAT3 siRNA by a multifunctional nanocomplex for targeted treatment of metastatic breast cancer. Acta Biomater. 2021;134:649–663. Epub 2021 Jul 18. PMID: 34289420. doi:10.1016/j.actbio.2021.07.029.
127. Yu S, Wang S, Xie Z, et al. Hyaluronic acid coating on the surface of curcumin-loaded ZIF-8 nanoparticles for improved breast cancer therapy: an in vitro and in vivo study. Colloids Surf B Biointerfaces. 2021;203:111759. Epub 2021 Apr 10. PMID: 33892283. doi:10.1016/j.colsurfb.2021.111759.
128. Atallah MA, Ma S, Ma A, et al. Green self-assembled lactoferrin carboxymethyl cellulose nanogels for synergistic chemo/herbal breast cancer therapy. Colloids Surf B Biointerfaces. 2022;217:112657. Epub 2022 Jun 24. PMID: 35803031. doi:10.1016/j.colsurfb.2022.112657.
129. Quiñones JP, Mardare CC, Hassel AW, Brüggemann O. Testosterone- and vitamin-grafted cellulose ethers for sustained release of camptothecin. Carbohydr Polym. 2019;206:641–652. Epub 2018 Nov 19. Erratum in: Carbohydr Polym. 2019;208:323-327. doi: 10.1016/j.carbpol.2018.12.085. PMID: 30553368. doi:10.1016/j.carbpol.2018.11.047.
130. Pourmadadi M, Tajiki A, Abdouss M. A green approach for preparation of polyacrylic acid/starch incorporated with titanium dioxide nanocomposite as a biocompatible platform for curcumin delivery to breast cancer cells. Int J Biol Macromol. 2023;242(Pt 1):124785. Epub 2023 May 9. PMID: 37169052. doi:10.1016/j.ijbiomac.2023.124785.
131. Mariadoss AVA, Saravanakumar K, Sathiyaseelan A, Karthikkumar V, Wang MH. Smart drug delivery of p-Coumaric acid loaded aptamer conjugated starch nanoparticles for effective triple-negative breast cancer therapy. Int J Biol Macromol. 2022;195:22–29. Epub 2021 Dec 1. PMID: 34861273. doi:10.1016/j.ijbiomac.2021.11.170.
132. Saikia C, Das MK, Ramteke A, Maji TK. Controlled release of curcumin from thiolated starch-coated iron oxide magnetic nanoparticles: an in vitro evaluation. International Journal of Polymeric Materials and Polymeric Biomaterials. 2017;66(7):349–358.
133. Afzali E, Eslaminejad T, Yazdi Rouholamini SE, Shahrokhi-Farjah M, Ansari M. Cytotoxicity Effects of Curcumin Loaded on Chitosan Alginate Nanospheres on the KMBC-10 Spheroids Cell Line. Int J Nanomedicine. 2021;16:579–589. PMID: 33531802; PMCID: PMC7846832. doi:10.2147/IJN.S251056.
134. Guo Y, Yang X, Zhang Y, et al. Hyaluronic acid/dextran-based polymeric micelles co-delivering ursolic acid and doxorubicin to mitochondria for potentiating chemotherapy in MDR cancer. Carbohydr Polym. 2024;332:121897. Epub 2024 Feb 6. PMID: 38431408. doi:10.1016/j.carbpol.2024.121897.
135. Sampath M, Pichaimani A, Kumpati P, Sengottuvelan B. The remarkable role of emulsifier and chitosan, dextran and PEG as capping agents in the enhanced delivery of curcumin by nanoparticles in breast cancer cells. Int J Biol Macromol. 2020;162:748–761. Epub 2020 Jun 23. PMID: 32585267. doi:10.1016/j.ijbiomac.2020.06.188.
136. Park JK, Chung MJ, Choi HN, Park YI. Effects of the molecular weight and the degree of deacetylation of chitosan oligosaccharides on antitumor activity. Int J Mol Sci. 2011;12(1):266–277. PMID: 21339986; PMCID: PMC3039952. doi:10.3390/ijms12010266.
137. Lee ET, Song J, Lee JH, Goo BG, Park JK. Analysis of molecular structure and topological properties of chitosan isolated from crab shell and mushroom. Int J Biol Macromol. 2024;266(Pt 2):131047. Epub 2024 Mar 22. PMID: 38521325. doi:10.1016/j.ijbiomac.2024.131047.
138. Adhikari HS, Yadav PN. Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action. Int J Biomater. 2018;2018:2952085. PMID: 30693034; PMCID: PMC6332982. doi:10.1155/2018/2952085.
139. Sachdeva B, Negi P, Ghosh A, et al. Chitosan Nanoparticles-Based Cancer Drug Delivery: application and Challenges. Mar Drugs. 2023;21(4):211. PMID: 37103352; PMCID: PMC10142570. doi:10.3390/md21040211.
140. Ma C, Cheng Z, Tan H, et al. Nanomaterials: leading immunogenic cell death-based cancer therapies. Front Immunol. 2024;15:1447817. PMID: 39185425; PMCID: PMC11341423. doi:10.3389/fimmu.2024.1447817.
141. Afonin G, Lutfi Ismaeel G, Al-Hussainy AF, et al. Enhanced Tumor Targeting: curcumin-Loaded Chitosan Nanoparticles for Precision Drug Delivery: a Review. Journal of Nanostructures. 2024;14(1):291–300.
142. Yin S, Huai J, Chen X, et al. Intracellular delivery and antitumor effects of a redox-responsive polymeric paclitaxel conjugate based on hyaluronic acid. Acta Biomater. 2015;26:274–285. Epub 2015 Aug 20. PMID: 26300335. doi:10.1016/j.actbio.2015.08.029.
143. Zając A, Sąsiadek W, Dymińska L, et al. Chitosan and Its Carboxymethyl-Based Membranes Produced by Crosslinking with Magnesium Phytate. Molecules. 2023;28(16):5987. PMID: 37630242; PMCID: PMC10459599. doi:10.3390/molecules28165987.
144. Seidi F, Khodadadi Yazdi M, Jouyandeh M, et al. Chitosan-based blends for biomedical applications. Int J Biol Macromol. 2021;183:1818–1850. Epub 2021 May 7. PMID: 33971230. doi:10.1016/j.ijbiomac.2021.05.003.
145. Chitin SU. Characteristic, Sources, and Biomedical Application. Curr Pharm Biotechnol. 2020;21(14):1433–1443. PMID: 32503407. doi:10.2174/1389201021666200605104939.
146. Alvarado Tenorio G, Espinosa Neira R, Orta CA Á, Romero Zúñiga GY, Ortega Ortiz H. Synthesis and Characterization of Iodinated Chitosan Nanoparticles and Their Effects on Cancer Cells. Int J Biomater. 2024;2024:3850286. PMID: 39376508; PMCID: PMC11458297. doi:10.1155/2024/3850286.
147. Kumar S, Dutta J, Dutta PK. Preparation and characterization of N-heterocyclic chitosan derivative based gels for biomedical applications. Int J Biol Macromol. 2009;45(4):330–337. Epub 2009 Aug 7. PMID: 19665475. doi:10.1016/j.ijbiomac.2009.08.002.
148. Quiñones JP, Peniche H, Peniche C. Chitosan Based Self-Assembled Nanoparticles in Drug Delivery. Polymers (Basel). 2018;10(3):235. PMID: 30966270; PMCID: PMC6414940. doi:10.3390/polym10030235.
149. Miao T, Wang J, Zeng Y, Liu G, Chen X. Polysaccharide-Based Controlled Release Systems for Therapeutics Delivery and Tissue Engineering: from Bench to Bedside. Adv Sci (Weinh). 2018;5(4):1700513. PMID: 29721408; PMCID: PMC5908359. doi:10.1002/advs.201700513.
150. Andreadis C, Vahtsevanos K, Sidiras T, Thomaidis I, Antoniadis K, Mouratidou D. 5-Fluorouracil and cisplatin in the treatment of advanced oral cancer. Oral Oncol. 2003;39(4):380–385. PMID: 12676258. doi:10.1016/s1368-8375(02)00141-0.
151. Basak D, Arrighi S, Darwiche Y, Deb S. Comparison of Anticancer Drug Toxicities: paradigm Shift in Adverse Effect Profile. Life (Basel). 2021;12(1):48. PMID: 35054441; PMCID: PMC8777973. doi:10.3390/life12010048.
152. Herdiana Y, Wathoni N, Gozali D, Shamsuddin S, Muchtaridi M. Chitosan-Based Nano-Smart Drug Delivery System in Breast Cancer Therapy. Pharmaceutics. 2023;15(3):879. PMID: 36986740; PMCID: PMC10051865. doi:10.3390/pharmaceutics15030879.
153. Hossen S, Hossain MK, Mk B, Mia MNH, Rahman MT, Uddin MJ. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: a review. J Adv Res. 2018;15:1–18. PMID: 30581608; PMCID: PMC6300464. doi:10.1016/j.jare.2018.06.005.
154. Jong A, Wu CH, Gonzales-Gomez I, et al. Hyaluronic acid receptor CD44 deficiency is associated with decreased Cryptococcus neoformans brain infection. J Biol Chem. 2012;287(19):15298–15306. doi:10.1074/jbc.M112.353375
155. Widjaja LK, Bora M, Chan PN, Lipik V, Wong TT, Venkatraman SS. Hyaluronic acid-based nanocomposite hydrogels for ocular drug delivery applications. J Biomed Mater Res A. 2014;102(9):3056–3065. Epub 2013 Oct 9. Erratum in: J Biomed Mater Res A. 2015;103(6):2201. PMID: 24124098. doi:10.1002/jbm.a.34976.
156. Yadav N, Francis AP, Priya VV, et al. Polysaccharide-Drug Conjugates: a Tool for Enhanced Cancer Therapy. Polymers (Basel). 2022;14(5):950. PMID: 35267773; PMCID: PMC8912870. doi:10.3390/polym14050950.
157. Agrawal S, Dwivedi M, Ahmad H, et al. CD44 targeting hyaluronic acid coated lapatinib nanocrystals foster the efficacy against triple-negative breast cancer. Nanomedicine. 2018;14(2):327–337. Epub 2017 Nov 9. PMID: 29129754. doi:10.1016/j.nano.2017.10.010.
158. Fu CP, Cai XY, Chen SL, et al. Hyaluronic Acid-Based Nanocarriers for Anticancer Drug Delivery. Polymers (Basel). 2023;15(10):2317. PMID: 37242892; PMCID: PMC10224391. doi:10.3390/polym15102317.
159. Mali P, Sherje AP. Cellulose nanocrystals: fundamentals and biomedical applications. Carbohydr Polym. 2022;275:118668. Epub 2021 Sep 14. PMID: 34742407. doi:10.1016/j.carbpol.2021.118668.
160. Lugoloobi I, Maniriho H, Jia L, Namulinda T, Shi X, Zhao Y. Cellulose nanocrystals in cancer diagnostics and treatment. J Control Release. 2021;336:207–232. Epub 2021 Jun 5. PMID: 34102221. doi:10.1016/j.jconrel.2021.06.004.
161. Bhandari J, Mishra H, Pk M, Wimmer R, Ahmad FJ, Talegaonkar S. Cellulose nanofiber aerogel as a promising biomaterial for customized oral drug delivery. Int J Nanomedicine. 2017;12:2021–2031. PMID: 28352172; PMCID: PMC5359002. doi:10.2147/IJN.S124318.
162. Sheikhi A, Hayashi J, Eichenbaum J, et al. Recent advances in nanoengineering cellulose for cargo delivery. J Control Release. 2019;294:53–76. Epub 2018 Nov 27. PMID: 30500355; PMCID: PMC6385607. doi:10.1016/j.jconrel.2018.11.024.
163. Tabasum S, Younas M, Zaeem MA, et al. A review on blending of corn starch with natural and synthetic polymers, and inorganic nanoparticles with mathematical modeling. Int J Biol Macromol. 2019;122:969–996. Epub 2018 Oct 18. PMID: 30342145. doi:10.1016/j.ijbiomac.2018.10.092.
164. Lee CS, Hwang HS. Starch-Based Hydrogels as a Drug Delivery System in Biomedical Applications. Gels. 2023;9(12):951. PMID: 38131937; PMCID: PMC10743264. doi:10.3390/gels9120951.
165. Sarder R, Piner E, Rios DC, et al. Copolymers of starch, a sustainable template for biomedical applications: a review. Carbohydr Polym. 2022;278:118973. Epub 2021 Dec 4. PMID: 34973787. doi:10.1016/j.carbpol.2021.118973.
166. Pei J, Yan Y, Jayaraman S, et al. A review on advancements in the application of starch-based nanomaterials in biomedicine: precision drug delivery and cancer therapy. Int J Biol Macromol. 2024;265(Pt 1):130746. Epub 2024 Mar 10. PMID: 38467219. doi:10.1016/j.ijbiomac.2024.130746.
167. Choudhury PD, Ikbal AMA, Saha S, et al. Recent Advances in Multifaceted Drug Delivery Using Natural Polysaccharides and Polyacrylamide-Based Nanomaterials in Nanoformulation. Curr Top Med Chem. 2024. Epub ahead of print. PMID: 39473113. doi:10.2174/0115680266316522241015143856.
168. Ai J, Biazar E, Jafarpour M, et al. Nanotoxicology and nanoparticle safety in biomedical designs. Int J Nanomedicine. 2011;6:1117–1127. Epub 2011 May 31. PMID: 21698080; PMCID: PMC3118686. doi:10.2147/IJN.S16603.
169. Elmowafy M, Shalaby K, Elkomy MH, et al. Polymeric Nanoparticles for Delivery of Natural Bioactive Agents: recent Advances and Challenges. Polymers (Basel). 2023;15(5):1123. PMID: 36904364; PMCID: PMC10007077. doi:10.3390/polym15051123.
170. Barclay TG, Day CM, Petrovsky N, Garg S. Review of polysaccharide particle-based functional drug delivery. Carbohydr Polym. 2019;221:94–112. Epub 2019 May 26. PMID: 31227171; PMCID: PMC6626612. doi:10.1016/j.carbpol.2019.05.067.
171. Prasher P, Sharma M, Mehta M, et al. Current-status and applications of polysaccharides in drug delivery systems. Colloid and Interface Science Communications. 2021;42:100418.
172. Xu BW, Li SS, Ding WL, et al. From structure to function: a comprehensive overview of polysaccharide roles and applications. Food Frontiers. 2025;6(1):15–39.
173. Hsu CY, Allela OQB, Hussein AM, et al. Recent advances in polysaccharide-based drug delivery systems for cancer therapy: a comprehensive review. Artif Cells Nanomed Biotechnol. 2024;52(1):564–586. Epub 2024 Dec 5. PMID: 39639430. doi:10.1080/21691401.2024.2436350.
174. Gupta A, Sood A, Fuhrer E, Djanashvili K, Agrawal G. Polysaccharide-Based Theranostic Systems for Combined Imaging and Cancer Therapy: recent Advances and Challenges. ACS Biomater Sci Eng. 2022;8(6):2281–2306. Epub 2022 May 5. PMID: 35513349. doi:10.1021/acsbiomaterials.1c01631.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

