Back to Journals » International Journal of Nanomedicine » Volume 19
How Nanoparticles Help in Combating Chronic Wound Biofilms Infection?
Authors Jing G, Hu C, Fang K, Li Y, Wang L
Received 6 July 2024
Accepted for publication 17 October 2024
Published 15 November 2024 Volume 2024:19 Pages 11883—11921
DOI https://doi.org/10.2147/IJN.S484473
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. RDK Misra
Gang Jing,1,* Chen Hu,2,* Keyi Fang,3 Yingying Li,3 Linlin Wang1
1Hainan General Hospital, Hainan Affiliated Hospital of Hainan Medical University, Haikou, Hainan, People’s Republic of China; 2Stomatological Hospital, School of Stomatology, Southern Medical University, Guangzhou, Guangdong, People’s Republic of China; 3School of Stomatology, Hainan Medical University, Haikou, Hainan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Linlin Wang, Hainan General Hospital, Hainan Affiliated Hospital of Hainan medical University, No. 19 Xiuhua Avenue, Haikou, Hainan, 570311, People’s Republic of China, Email [email protected]
Abstract: Chronic wound infection has become a global health problem, with bacterial biofilms, which are difficult to penetrate using traditional antibiotics, considered the primary cause of recurrent infection and delayed healing in chronic wounds. In recent years, the outstanding performance of nanomaterials in controlling biofilm infections has been widely acknowledged, and these materials are regarded as highly promising for chronic wound infection management. The formation and structure of chronic wound biofilms undergo complex dynamic changes. Therefore, a deep understanding of the underlying causes of repeated wound infections and the specific antibacterial mechanisms of nanomaterials at different stages of biofilm formation is crucial for effective “chronic wound infection management”. This review first reveals the relationship between biofilms, wound chronicity, and recurrent infections. Secondly, it focuses on the four stages of chronic wound biofilm formation: (1) adhesion stage, (2) aggregation and promotion stage, (3) maturation stage, and (4) regeneration and dissemination stage. It also comprehensively summarizes the specific antibacterial mechanisms of nanomaterials. This study analyzes essential factors affecting the control of chronic wound biofilms by nanoparticles from various perspectives, such as the material itself, the local wound environment, and the systemic host response. Finally, the limitations and potential future trends in current research are discussed. In summary, nanoparticles represent a promising strategy for combating chronic wound biofilm infections, and this review provides new insights for alternative adjuvant therapies in managing bacterial biofilm infections in chronic wounds.
Keywords: bacteria, nanomaterials, mechanism, wound management
Graphical Abstract:

Introduction
With the acceleration of aging in the global population, chronic wounds have become a significant public health threat and healthcare burden. A positive correlation was observed between the age of patients and the healing period of chronic wounds.1 Bacterial infection is the most common complication of chronic wounds, and most bacteria in chronic wounds occur as biofilm, which is a leading cause of delayed wound healing.2 Significant types of chronic wounds include diabetic ulcers, traumatic ulcers, pressure ulcers, and venous ulcers. Chronic non-healing wounds often deteriorate into multidrug-resistant (MDR) bacterial infections, producing a large amount of purulent discharge with malodor, presenting an urgent problem that requires clinical treatment.
The traditional treatment for bacterial biofilm removal in chronic wounds involves antibiotic therapies. Currently, studies have shown that the overuse of antibiotics significantly promotes the emergence of “multidrug-resistant bacteria”, which not only increases treatment costs but also can raise case fatality rates. The distribution of drug-resistant pathogens was identified as a significant global public health problem. Nanoparticles have been reported to exhibit high antimicrobial activity against “multidrug-resistant bacteria”, and due to multiple mechanisms acting simultaneously, it is difficult for new drug-resistant strains to emerge.3 Recent microbiological research has begun to focus on antibacterial nanoparticles, whose effectiveness in killing various pathogens in wounds, such as bacteria, fungi, and viruses, has been demonstrated in many laboratory studies.4
Recent studies have elucidated the mechanisms and advantages of nanoparticles in inhibiting bacterial growth and biofilm formation by comparing various types of nanomaterials—such as metal and polymer nanoparticles—regarding their antimicrobial properties.3 In addition, researchers have categorized these nanoparticles into four main types, each employing different mechanisms against biofilms. These mechanisms include the direct killing of bacteria within the biofilm, disruption of the biofilm’s structure, release of antimicrobial agents, and enhancement of immune function to combat infections5 However, many existing studies primarily focus on a single mechanism of action for nanoparticles against bacterial biofilms and lack a systematic analysis of the dynamic process of bacterial biofilm formation in chronic wounds.
In this paper, we explore the complex relationship between bacterial biofilms, non-healing chronic wounds, and recurrent infections. The formation of bacterial biofilms is a complex, multi-stage process. To effectively target these biofilms in chronic wounds, we have divided the mechanisms by which nanoparticles inhibit biofilm formation into four stages: (1) preventing the initial settlement and attachment of planktonic bacteria, (2) interfering with bacterial reproduction and colony formation, (3) disrupting mature bacterial biofilms within the chronic wound, and (4) reducing the regeneration and spread of bacteria on the surfaces of mature biofilms. It is important to note that these four stages are continuous and highly coordinated processes. Furthermore, we aim to summarize the factors influencing the efficacy of nanoparticles in inhibiting bacterial biofilm formation in chronic wounds, to guide rational design and promote the broader application of nanoparticles in treating chronic wound infections.
The Truth About Chronic Wound Infection
A chronic wound is defined as one that fails to achieve anatomical and functional integrity through a normal, orderly, and timely repair process. Key reasons contributing to delayed wound healing include infection, malnutrition, and poor coagulation function. The two typical clinical characteristics of chronic wounds are: (i) healing is delayed in infected wounds, with no healing tendency observed after more than one month, and (ii) repeated wound infections.6 Biofilms are inherently resistant to antimicrobial treatment, and the distinct bacterial state in the biofilm environment can promote a unique reinfection mechanism.
Wound healing is a refined tissue regeneration process that includes four distinct yet overlapping phases: hemostasis, inflammation, cell proliferation, and remodeling. A review indicates that due to the complexity of wounds, there is currently a lack of sufficient wound treatment materials that can systematically regulate the unique wound microenvironment. Hydrogels present significant advantages in wound therapy by allowing for spatiotemporal control of the healing process and tissue remodeling7 (Figure 1). For instance,Qi developed an “all-in-one” in situ injectable hydrogel (QOP) made from a polysaccharide matrix and polydopamine nanoparticles, which facilitates the sequential treatment of diabetic wounds. This hydrogel enhances diabetic wound repair by establishing a barrier that neutralizes inflammatory cytokines, inhibits bacterial growth, and eliminates reactive oxygen species, thereby accelerating the transition from the inflammatory to the proliferative phase. It also provides a three-dimensional network structure that supports cellular repair and tissue remodeling, ultimately being fully absorbed by the body.8 Therefore, chronic wound infections require smarter and more sophisticated designs to offer comprehensive and precise treatment.
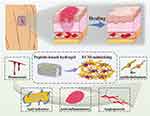 |
Figure 1 Schematic illustration of the influence of peptide-based self-assembling hydrogels on the wound healing process. Notes: Reproduced from Guan T, Li J, Chen C et al Self-Assembling Peptide Based Hydrogels for Wound Tissue Repair. Adv Sci (Weinh). 2022,9(10):e2104165. Creative Commons.7 |
Chronic Wound Infection: How Bacteria Become the Main Enemy?
Dynamic Synergistic Effect of the Abundance of Bacteria in Biofilms
Clinically, the abundant bacteria in the wound is crucial to clinical efficacy. Some studies have demonstrated that debridement can disrupt the diversity of wound bacterial communities due to the obstruction of anaerobic bacterial growth after epithelial tissue repair.9 Therefore, the relatively high abundance of bacteria in the biofilm can promote the chronicity of wound infections, especially with anaerobic ammonium oxidation bacteria.
The composition of bacterial diversity in biofilms undergoes a dynamic pattern of change over time. During the initial stage of wound formation, the normal skin flora dominates, consisting primarily of gram-positive aerobic bacteria. At this point, the biofilm is not pathogenic, and the activated host immune system can facilitate wound healing within weeks. With nutrient depletion, Corynebacterium and Staphylococcus begin colonizing the wound, resulting in a considerably different microbiome from the adjacent skin. Then, facultative anaerobic gram-negative bacteria begin to invade the wound, benefiting from aerobic bacteria that consume oxygen and create an anaerobic environment conducive to survival. The balance between microorganisms and the host’s innate immune system is threatened at this stage. Eventually, the wound becomes further infected, with specific bacteria causing stronger immune and inflammatory responses that affect deep tissues, such as muscle and bone. Simultaneously, blocked blood flow leads to local tissue ischemia, promoting the rapid growth of anaerobic microorganisms in a hypoxic environment, further delaying the healing of chronically infected wounds. Therefore, bacterial diversity and dynamic synergy are strongly associated with wound chronicity.10
Extracellular Polymeric Substance (EPS) Matrix Forms a Stable Three-Dimensional Protective Barrier
The EPS matrix surrounds the bacteria and displays a fibrous grid structure with high electron density, consisting of extracellular polysaccharides, proteins, lipids, and extracellular DNA (eDNA). This EPS matrix can prevent and delay the penetration of antibiotics into the biofilm, forming a protective barrier giving mature bacterial cells located deep in the matrix more time to develop resistance, resulting in the persistence of a chronic wound. Most studies have concluded that excessive EPS matrix harms the wound environment and can lead to non-healing chronic wounds. For example, membrane-attached lipoproteins can interact with eDNA in the matrix and promote biofilm formation associated with chronic wound infections.11
It is a matter of concern that the structure of EPS can change to adapt to the surrounding environment. Studies have shown that EPS matrix components are loosely adhered to the polystyrene surface in the in vitro pore plate model. As a result, enzymes quickly destroy biofilms and induce high levels of diffusion. However, in both microscopic and in vivo models, EPS exhibits tighter attachment and greater resistance to dispersion, possibly due to the activity of binding proteins. In addition, the expression of genes related to polysaccharide production in EPS is altered in different models.12 It has been fully confirmed that the biofilm EPS matrix plays a significant role in the occurrence and development of chronic wound infections. Therefore, changes in EPS stability and barrier function in different chronic wound infections are closely related to wound healing.
Lateral Transfer of Resistance Genes
The lateral transfer of resistance genes is an evolved survival strategy for bacteria. Resistance genes spread and exchange with different pathogenic bacterial strains through this mechanism. Increasing evidence demonstrates that acquiring exogenous DNA through lateral gene transfer (LGT) is a common phenomenon in bacteria, including transformation and conjugation. When resistance genes transfer horizontally, bacteria acquire intrinsic resistance to antibiotics, leading to chronic wound infections that are difficult to heal.
First, drug-resistant genes are widespread in bacterial biofilm communities of chronic wounds. In 2004, the first vancomycin-resistant Staphylococcus aureus was isolated by Cosgrove from the chronic wounds of patients receiving antibiotics.13 Similarly, a study on wound bacterial biofilm specimens to analyze the characteristics of antibiotic resistance genes found that more than 50% contained resistance genes for aminoglycosides (clindamycin), macrolides (erythromycin), and tetracyclines (minocycline), indicating that the presence of a wide range of resistance genes can be related to horizontal gene transfer.14 Further research confirmed that extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli colonizing burn wounds can transfer beta-lactamase genes to non-ESBL-producing Escherichia coli, which can rapidly dissolve β-lactam antibiotics. These plasmids carry various antibiotic resistance genes that play a predominant role in gene transfer.15 In addition, Kalan conducted a time-series shotgun metagenomics analysis of the microbial community (at the strain level) in chronic wound specimens from patients.16 The results indicated that S. aureus genomes associated with poor wound healing contained multiple antibiotic-resistance genes. These superantigens exacerbate the inflammatory response by non-specific stimulation of T cells, resulting in delayed wound healing.
Second, bacteria in chronic wound biofilms are in close contact with each other, facilitating lateral transfer between bacteria.17 The success rate of plasmid binding and transfer of antibiotic resistance genes is high, demonstrating a wide host range and expanding the antibiotic resistance spectrum of recipient bacteria.18 Some studies have shown that improper treatment of wounds infected with multiple bacteria can also cause the rapid transfer of drug-resistant genes to other pathogenic bacteria, which increases the treatment difficulty.19 These processes are interconnected and complementary. Therefore, the infection process of chronic wounds is related to the lateral transfer of resistance genes in bacterial biofilms.
Quorum Sensing (QS): Defense Against Host Immune Function and Epithelium Barrier Remodeling
Quorum sensing (QS) refers to the ability of bacteria to spontaneously produce and release specific signaling molecules, perceive their concentration changes, and regulate the group behavior of microorganisms in biofilms. QS is critical in electrical signaling, information propagation, and intercellular communication in chronic wound bacterial biofilms. A biofilm matrix structure that allows a high density of bacteria to inhabit a narrow space can enhance the signal transmission efficiency of QS. QS was identified as a critical factor in wound chronicity in recent years.
The QS system of bacterial biofilms can influence host mitochondria’s network morphology and energy characteristics, further regulating epithelial barrier properties, stimulating surrounding skin and mucous membranes, and delaying wound healing. Josephson et al found that QS molecules, such as 3-oxo-dodecyl-L-methoserine lactone (3O-C-HSL), can induce mitochondrial fragmentation, disrupt the inner mitochondrial membrane and cristae structure, alter mitochondrial respiratory enzymes and bioenergetics, and decrease mitochondrial membrane potential.20 This is mechanically accompanied by co-differential expression of the mitochondrial proteome and epithelial cell type-specific components, involving their structural organization, electron transport chain complexes, and stress responses.
The QS system of bacterial biofilms can also promote the chronicity of wounds by attenuating the host’s innate immune responses. The response of host immune cells to bacterial biofilms depends on the substantial state change in the QS system. A previous study showed that the QS system of Streptococcus can inhibit macrophage viability and decrease the expression levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6, interferon (IFN)-β, and nuclear factor (NF)-κB, which can lead to the occurrence of necrotizing skin disease.21
The QS system of bacterial biofilms also enables bacteria in chronic wounds to sense the presence of other bacteria by secreting and receiving chemical signals. Once the biofilm reaches a certain level of cell density or saturation, it will turn off the gene expressing EPS and reactivate the flagellum motility gene, allowing the bacteria to spread Planctomycetes to the surrounding area to find a new suitable environment, further expanding the chronic wound area.
Interestingly, He found that QS-defective mutants of bacterial biofilms, known as “cheaters”, are usually seen in chronic infectious wounds. Biofilms composed of QS-dysfunctional bacteria have an enhanced ability to kill human phagocytes, helping bacteria evade the immune system and making infection control more difficult.22 Similarly, earlier studies verified that the degree of infection of wild-type bacterial biofilms was lower than that of mutant bacterial biofilms in a mouse chronic burn wound model. QS receptors can utilize the signal molecules produced by others to increase the release of virulence factors, making bacteria more adaptive.23
Relation Between Bacterial Biofilm and Repetitive Wound Infection
Bacteria Pool
A biofilm continuously releases Planctomycetes as a bacterial repository. Regular antibiotic doses can eliminate Planctomycetes but cannot completely eradicate the bacteria in the biofilm. When the body’s defense system cannot control the bacterial biofilm, releasing bacteria from the biofilm to other parts of the body can cause reinfection, subsequently forming persistent foci and recurrent infections. Garcia analyzed the wounds of patients infected with Mucus using whole-genome sequencing techniques. The results showed that the outbreak of invasive wound Mucormycosis was primarily caused by biofilms with various Mucus strains in the environment rather than direct contact transmission between patients.24
“Dormant State” of Central Bacteria
Switching between a metabolically active and dormant state protects from environmental stresses and enables bacteria to overgrow under favorable conditions. Although a rapid increase in metabolically active bacteria can lead to complications, many dormant bacteria can also be problematic, as they tend to be more virulent and resistant to antibiotics. During the final phase of the biofilm’s life cycle, the structure of the biofilm is destroyed, and the dormant bacteria within the biofilm resuscitate and multiply swiftly, potentially causing recurrent infections in wounds. There are two main reasons for this: first, the dormant bacteria at the biofilm’s center exhibit strong drug resistance. Cellular dormancy triggered by toxin/antitoxin pairs (p)ppGpp, SOS response, and ATP levels are known to be the mechanistic basis of persistence (Figure 2).25 Conlon showed that the Planctomycetes isolated from the biofilm maintain a dormant phenotype, which can develop severe antibiotic resistance. Although the dormant bacteria in a biofilm are in a single free-floating state, their biological state remains unaltered.26 In addition, dormant cells in the biofilm can be activated under appropriate conditions and re-enter an active state, thus triggering the recurrence of the infection. This heterogeneity in the maturation process of biofilms, where cells differentiate to form two subpopulations of active and dormant cells, is the key to the high degree of drug resistance in biofilms.27 Second, the dormant bacteria in the center of the biofilm are characterized by low levels of inflammation. Thurlow’s study demonstrated that staphylococcal biofilms reduce inflammation in mouse macrophages by promoting the differentiation of these host immune cells compared to planktonic cells.28 Therefore, the immune evasion of bacterial biofilms is likely due primarily to the resulting lower inflammatory effects rather than the suppression of host immune defense mechanisms.
 |
Figure 2 Formation of bacterial persistence via cell dormancy. Various pathways could trigger bacterial cells into dormant state upon antibiotic stress. A considerable number of TA modules could free toxins upon stresses, which result in persister formation by inhibiting DNA replication, or transcription, or translation processes, or downregulating proton motive force (PMF) that is under the control of SOS response. Dormancy could be also triggered by accumulated alarmone molecules and decreased intracellular ATP levels. The gray layers represent the outer membrane and inner membrane, respectively, and the red square linkage represents the peptidoglycan and periplasmic space. Reproduced with permission from Zou J, Peng B, Qu J et al Are Bacterial Persisters Dormant Cells Only? Front Microbiol. 2021;12:708,580. Creative Commons.25 |
Interestingly, Bacterial persisters are not only dormant cells; studies have shown that metabolically active bacteria can maintain persistence by lowering intracellular antibiotic concentrations through efflux pumps. Cell wall-deficient bacteria (CWDB), including L-type and globular bodies produced by β-lactam antibiotics, are associated with antibiotic persistence.25
Antimicrobial Mechanism of Nanoparticles on Bacterial Biofilms in Chronic Wounds
Nanoparticles are considered safe and effective antibiotic alternatives due to their antibacterial activity, low toxicity, and difficulty producing drug-resistant bacteria. They have promising applications in the field of biomedicine. Skin cuts and abrasions are prone to infection, and it is crucial to swiftly eliminate microbes from festering wounds to accelerate healing and reduce morbidity. Numerous studies have applied nanoparticles to treat skin wounds, and the results indicate that nanoparticles are highly effective in treating chronic wounds.29 Nanotechnology offers a novel approach to treating chronic open wound infections. As a result, the antimicrobial mechanisms of nanoparticles have become a research focus. Scholars’ attention is mainly directed toward physical damage to bacterial cell membranes, the oxidative stress effect of reactive oxygen species (ROS), and the regulation of bacterial gene expression. However, these mechanisms do not account for bacterial biofilms’ dynamic formation and structure in chronic wounds, and their complex interactions remain unclear.
Biofilms shield bacteria in chronic wounds, leading to and exacerbating the inflammatory response. Inhibitors tend to be unable to penetrate the biofilm, causing repeated infections of surrounding soft tissues and resulting in non-healing wounds. The life cycle of chronic wound biofilms consists of four stages: (1) the initial settlement and attachment of planktonic bacteria; (2) the reproduction and formation of early bacterial colonies; (3) the maturation of bacterial biofilms; and (4) the dissemination of bacteria on the biofilm surface. The types and states of bacteria vary significantly across these stages. Studies investigating how nanoparticles suppress biofilms in chronic wounds are still in the preliminary stages. Further exploration of the dynamic mechanisms between nanoparticles and chronic wound biofilms holds significant theoretical value. Therefore, this section primarily summarizes the role of nanoparticles in the different stages of chronic wound biofilms. Nanoparticles typically inhibit bacterial biofilm formation through multiple antimicrobial mechanisms. In most cases, the antimicrobial effect is not limited to a specific stage of the biofilm cycle but coincides in multiple phases (Figure 3).
 |
Figure 3 Antibacterial mechanism of nanoparticles on chronic wound biofilm. |
Preventing the Initial Settlement and Attachment of Planktonic Bacteria
Bacterial biofilms begin with planktonic bacteria near the wound surface, which neutralizes the electrostatic repulsion between the bacterial and wound surfaces, allowing initial settlement and attachment. Depending on the environment to which the wound is exposed, different types of biofilms are formed, and the initial attachment of bacteria is a dynamic selective process critical in shaping the composition of early colonies. A clinical study proved that while the removal of diseased tissue through surgical debridement successfully cleared biofilms from chronic wounds, the biofilms were reestablished within 48 hours after the initial debridement, and a substantial population of bacteria was detected in mature biofilms 72 hours post-debridement.30 This indicates a window of opportunity following debridement, during which the planktonic bacteria adhering to the wound surface are susceptible to therapeutic interventions. Therefore, effectively preventing initial bacterial attachment can hinder biofilm reformation.
Bacterial attachment to the wound involves the specific binding of bacterial surface adhesion molecules to particular protein receptors on the wound surface. Thus, bacterial adhesion can be inhibited by targeting bacterial adhesion molecules (protein structures31 and glycolipid components32) or by inhibiting the expression of adhesion-related genes. Disrupting the attraction between bacterial cells, closely associated with the bacteria’s surface charge, can prevent adhesion. The findings indicate that using nanoparticles to prevent initial bacterial attachment is crucial for exploring treatment strategies to regulate and control bacterial biofilm formation in chronic wounds.
Electrostatic Interaction
Due to the typically negative charge on bacterial surfaces within the milieu of chronic wounds, nanoparticles can influence bacterial adherence directly or indirectly, subsequently impacting the development of bacterial biofilms in such wounds.
Positively charged nanoparticles, for instance, have the potential to be drawn electrostatically towards the negatively charged bacterial cells present in the exudate of chronic wounds, reducing bacterial adhesion to the wound surface. Mei observed abundant adherence of positively charged nanomaterials to bacterial biofilms via electrostatic forces, a process that successfully eradicated bacteria and accelerated the healing of infected wounds in diabetic rats without inducing antibiotic resistance. The study suggests that the possible mechanism involves nanomaterials making close contact with the biofilm through electrostatic interaction, destroying the bacterial membrane, and inhibiting intracellular enzyme activity (Figure 4).33 Several other studies indicate that nano-carriers carrying a positive charge employ an identical mode of action involving the electrostatic attraction between the positively charged lipids in non-lamellar lyotropic liquid crystalline nanoparticles and the anionic bacterial biofilm. This interaction leads to the fusion of the bacterial membrane and subsequent disintegration of the biofilm’s architecture. Wang formulated mesoporous silica (MS) nanoparticles impregnated with tannic acid (TA), which were capable of creating a blood-clotting seal on injuries while also inhibiting bacterial adhesion via chemical bonds and electrostatic forces. These loaded MS nanoparticles demonstrated good antibacterial activity and can promote wound healing.34
 |
Figure 4 (A) Functional Silver Nanoparticle as a Benign Antimicrobial Agent That Eradicates Antibiotic-Resistant Bacteria and Promotes Wound Healing. The scanning electron microscopy (SEM) image shows that EPL-g-butyl@AgNPs was achieved by anchoring on the bacterial cell surface, damaging the cell walls as well as membranes, inhibiting intracellular biological functions, and finally leading to the cell death. Reproduced with permission. Copyright 2016, American Chemical Society. (B) TEM images of a thin section of (B−D) S. aureus and (E−G) P. aeruginosa (B and E) before and (C, D, F, and (G) after incubation with AgNPs@PDMAEMA-C4 for 30 min. (C) Optimization of synthesis conditions of AgNPs@PDMAEMA-C4. (H) The effect of a different alkyl chain on MIC value; (I and J) the effect of a different alkyl chain on the cytotoxicity effect of the nanoparticles with different concentrations (I and J are 1.0 and 2.0 μg/mL, respectively) against NIH3T3 cells; the effects of molar ratio of PDMAEMA-C4 and silver on (K) UV−vis spectrum, (L) size, Zeta potential, and (M) MIC value; the effects of volume of reducing agent on (N) UV−vis spectrum, (O) size, Zeta potential, and (P) MIC value; the effects of molecular weight of PDMAEMA-C4 on (Q) UV−vis spectrum and (S) MIC value; and (R) the GPC traces of PDMAEMA with different molecular weight. Reproduced with permission from Mei L, Lu Z, Zhang X, et al. Polymer-Ag nanocomposites with enhanced antimicrobial activity against bacterial infection. ACS Appl Mater Interfaces. 2014;6(18):15813–15821. Copyright 2014, American Chemical Society.33 |
In contrast, negatively charged nanoparticles can enhance electrostatic repulsion within the wound, disrupting bacterial cell attachment and serving an antimicrobial function. For example, silver nanoparticles (Ag-NPs) with reducing amino acids, which have a net negative surface charge, maximize electrostatic repulsion in the alkaline exudate environment of the wound and prevent the adhesion of drug-resistant bacteria.35 In addition, nanoparticles can be stabilized to prevent agglomeration by maintaining a negatively charged surface. For example, alginate silver nanoparticles exhibit potent germ-fighting capabilities, believed to result from the electrostatic repulsion of scattered nanoparticles coupled with an increase in surface area, enhancing interaction with bacterial cells in the biofilm. These nanoparticles also reduce the secretion of proinflammatory cytokines by macrophages, leading to a synergistic antibacterial effect.36
Prevent Bacterial Attachment
After bacterial adhesions (such as flagella, proteins, and others) attach to corresponding receptors on the exterior of host cells (such as extracellular matrix, glycoproteins, and others), bacteria can firmly adhere to the wound surface, causing wound infection.37,38
Initially, nanoparticles inhibit the production of adhesions, preventing bacteria from adhering to the wound matrix. For example, the adhesion of S. epidermidis primarily relies on the synthesis of polysaccharide intercellular adhesion (PIA) encoded by the ICAADBC locus, which can be suppressed by silver nanoparticles.39
Secondly, nanoparticles competitively bind to bacteria by mimicking bacterial adhesion receptors, effectively reducing bacterial binding to receptors in chronic wound biofilms. Halbus et al reported that MS silica nanoparticles with rough surfaces can form a reversible covalent bond with the diol group on the bacterial membrane, independent of electrostatic adhesion. This approach aimed to prevent bacteria from binding to receptors and maximize bacteriostatic efficacy (Figure 5).40
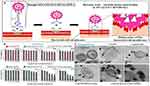 |
Figure 5 (A) The mechanism of self-grafting/covalent binding of the sugar groups expressed on the bacterial cell wall and boronic acid-functionalized surface-rough SiO2NPs. (B) The viability of the bacterial cells after treatment with surface-functionalized rough SiO2NPs pro-duced using two different catalysts, NH4OH (or NaOH), in the Stöber process, respectively. (C) SEM images show that the strong covalent interaction of the HPBA-functionalized CuONPs/SiO2 with the cell walls is probably the main contributor toward bacterial walls disruption and damage. Reproduced with permission from Halbus AF, Horozov TS, Paunov VN. “Ghost” Silica Nanoparticles of “Host”-Inherited Antibacterial Action. ACS Appl Mater Interfaces. 2019;11(42):38519–38530.. Copyright 2019, American Chemical Society.40 |
Ultimately, nanoparticles can modulate the expression of genes associated with bacterial adhesion. For example, with increasing selenium nanoparticle (SENP) content, the expression of protein genes (CSGA and CSGG) related to E. coli-mediated adhesion decreased gradually.41 In addition, membrane-related genes (OMPA and OMPF) were downregulated, while genes associated with producing destructive ROS (AHPF) were up-regulated. Similar correlations have been observed in multiple studies. Liu demonstrated that a Ti surface modified by AgNPs can regulate the gene expression linked to biofilm adhesion (ICAA and ICAR of S. epidermidis; FNBA and FNBB of S. aureus), preventing bacterial adhesion and biofilm formation.42 Shariati also confirmed that curcumin nanoparticles downregulated the transcription level of Pseudomonas aeruginosa adhesion genes.43
Interferes with Bacterial Reproduction and Colony Formation
Biofilm formation in chronic wounds differs from that observed in other infections. Mature large biofilms are less likely to be present in the chronic wound environment, whereas microcolony biofilm formation is more common. Certain species of bacteria, such as Pseudomonas aeruginosa, have been shown to exhibit already the effect of elevated levels of auto-inducing molecules (AIs) during the colony formation phase, and AIs can directly influence host cell immune function, so the presence of these colonies can promote wound chronicity.44 In addition, phagocytes cannot engulf larger colonies, and failed phagocytosis can result in the degranulation of phagocytes, ultimately leading to an overreaction of the immune system and disruption of the ecological balance in chronic wounds.45 Due to the increase in bacterial species and numbers, the primary goal of nanoparticles is to inhibit bacterial proliferation and aggregation during the early colony formation phase.
Direct Inhibition of Bacterial Proliferation
Metal or metal oxide nanoparticles exhibit synergistic antimicrobial properties against planktonic bacteria through mechanical perturbations (Figure 6)46 (encapsulation47 bacterial membrane insertion48 and bacterial membrane perforation)49 and oxidative stress (Figure 7),50 These mechanisms directly inhibit bacterial proliferation and interfere with bacterial micro-colony formation in wound areas.51 For example, a novel chitosan dressing impregnated with ZnO/N-halamine hybrid nanoparticles demonstrated rapid antimicrobial properties, reducing the bacterial cell proliferation rates of S. aureus and E. coli O157 by approximately 90% within 30 min of contact with wound colonies.52 Similarly, the scaffold composite of silk fibroin protein/chitin/AgNPs, with high antimicrobial activity, successfully inhibited the proliferation and aggregation of E. coli, S. aureus, and C. albicans in the wound area, preventing the growth of bacterial colonies.53
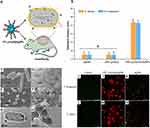 |
Figure 6 (A and B) The inhibition zone diameters of EPL-g-butyl@AgNPs (16.74 mm for S. aureus, 16.6 mm for P. aeruginosa) were significantly larger than those of EPL-g-butyl (9.06 mm, 9.05 mm) and AgNPs (9.1 mm, 9.1 mm), indicating the synergistic antibacterial effect of EPL-g-butyl and AgNPs. (C–H) The scanning electron microscopy (SEM) image shows that EPL-g-butyl@AgNPs was achieved by anchoring on the bacterial cell surface, damaging the cell walls as well as membranes, inhibiting intracellular biological functions, and finally leading to the cell death. (I–N) A live/dead staining test was performed on Pseudomonas aeruginosa and Staphylococcus aureus, with fluorescent micrographs of bacteria after 1 hour of antimicrobial drug treatment. Under the fluorescence microscope, live bacterial cells with intact membranes appeared green and dead bacterial cells with damaged membranes appeared red. In contrast to the green signal emitted by live bacteria (I and L), all cells died and aggregated after 1 h of EPL-g-butyl@AgNPs treatment (J and M). Figures (K) and (N) show that a large number of bacterial cells remained alive without any aggregation after 1 h of AgNPs treatment. epl -g-butyl ligands exhibited direct multivalent interactions with bacteria, giving the nanocomposites excellent antibacterial activity against both Gram-negative and Gram-positive bacteria. The antibacterial effect of EPL-g-butyl@AgNPs was confirmed. * p < 0.05. Reproduced with permission from Dai X, Guo Q, Zhao Y, et al. Functional Silver Nanoparticle as a Benign Antimicrobial Agent That Eradicates Antibiotic-Resistant Bacteria and Promotes Wound Healing. ACS Appl Mater Interfaces. 2016;8(39):25798–25807.. Copyright 2016, American Chemical Society.46 |
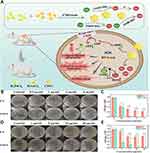 |
Figure 7 Continued. |
Although clinical strains exhibit diverse antimicrobial resistance profiles and continuously emerge with new drug resistance mechanisms, some scholars have reported that, in in vitro experiments, nanoparticles can inhibit the proliferation of laboratory standard bacteria and interfere with forming non-standard bacterial colonies isolated from clinical wounds. Amsari et al proposed that nanoparticles possess excellent anti-proliferative ability against clinical strains; their research demonstrated that ZnO-NPs inhibited the growth of colonies formed by clinical strains isolated from skin and soft tissue infected with methicillin-sensitive S. aureus (MSSA), methicillin-resistant S. aureus (MRSA), and methicillin-resistant S. epidermis (MRSE) clinical strains.54 In addition, Huang55 confirmed that Ag-NPs can prevent the rapid proliferation of P. aeruginosa isolated from chronic wounds and inhibit the formation of microflora in the wound environment, reducing the microbial load. Therefore, it is of great clinical significance to further study the inhibition of bacterial proliferation and colony formation by nanoparticles in vivo to disrupt bacterial biofilms in chronic wounds.
The in vivo antibacterial activity of different nanoparticles was reported. For example, P. aeruginosa is a common pathogenic bacterium in burn wounds. In mice with non-treated chronic wounds, Ag-NPs significantly reduced the incidence of burn wound infections caused by P. aeruginosa colonies, effectively treated the chronic wounds, and led to complete wound healing after four weeks.56 Studies have demonstrated that nanoparticles can inhibit the proliferation of P. aeruginosa in a mouse model of excised wounds. One possible explanation is that when nanoparticles bind to glutathione in vivo, they form the nitroso intermediate S-nitroso glutathione (GSNO), which inhibits the high-density growth of P. aeruginosa in the wound area.57
Indirect Inhibition of Bacterial Proliferation
Monocytes and macrophages are critical immune cells involved in regulating skin wound healing. These cells play a crucial role at the onset of bacterial colony formation. Some researchers have proposed the theory of “macrophage polarization” to explain the role of nanoparticles in stimulating macrophage-mediated inhibition of bacterial proliferation.58 Huang et al used an in vivo rat model of S. aureus-infected skin wounds and demonstrated that i Cu(2+) release and material surface characteristics of Cu-containing micro/nano-topographical coating could activate distinct signaling pathways in macrophages. The activated M1 macrophages can reprogram macrophages to exhibit inhibitory activity against bacterial proliferation59 They reported that IONPs, in combination with the triggering of the Fenton reaction, significantly enhance the role of macrophages in inhibiting S. aureus colony formation in wounds by inducing the polarization of M1 macrophages to stimulate ROS production. Saleh et al supported this theory and developed hyaluronan-based nano-hydrogels to regulate wound skin tissue macrophages during the polarization of the healing process. The results showed increased anti-inflammatory Arg-1 gene expression and decreased proinflammatory markers (including TNF-α, β, and IL-1-6). This provides further evidence that nanoparticle hydrogels have strong potential to combat biofilm-related infections and accelerate healing.60 More excitingly, in order to achieve a harmonious balance of proinflammatory and anti-inflammatory properties in the infected area, Deng proposed a “combinatorial” strategy utilizing azithromycin (AZM)-hybrid nanocomposite termed GOx@FexSy/AZM for the on-demand treatment of diabetic wounds. On-demand treatment of diabetic wounds showed that these components inhibit the activity of proinflammatory transcription factors in macrophages, promoting macrophage polarization towards a reparative M2 phenotype and facilitating tissue remodeling. A rational transition from the inflammatory to the reparative phase is accelerated by meeting the requirements of different stages of wound healing.61
Some studies believe that the indirect immune effect of nanoparticles on biofilms in chronic wounds cannot be explained solely by stimulating macrophage polarization and that there can also be an influence on the gene expression of immune cells. Rahimi investigated the inhibitory effect of gold nanoparticles on Candida albicans in infected burn wounds. Their results and theoretical evidence indicate that nano-complexes can significantly reduce the gene expression level in macrophages when treating chronic infectious wounds, playing an immunomodulatory role, reducing the release of cytokines by macrophages, and thus inhibiting bacterial infection.62 Dai also confirmed that the ε-polylysine/AgNP nanocomposite (EPL-G-Butyl@AgNPs) can regulate the relative gene expression levels of CD3 T cells and CD68 macrophages, inhibiting bacterial proliferation and effectively promoting the healing of infected wounds in rats.46
Although the theories explaining the mechanisms by which nanoparticles regulate the immune response to chronically infected wounds are incomplete, the studies above indicate that mononuclear macrophages exert an indirect effect in nanoparticle-mediated bacterial proliferation inhibition and play a key role in promoting the healing of skin wound infections.
Induction of Bacterial-Specific Aggregation
The induction of specific bacterial aggregation is an essential and intriguing bacteriostatic mechanism. Many researchers have gone beyond the conventional bacteriostatic approach and demonstrated that when ethylene glycol chitosan-conjugated nanoparticles are located at the site of acidity-related focal infection, the surface charge transfer causes the pH-responsive GCS surface to become positively charged. These nanoparticles and the negatively charged MRSA cell wall induce bacterial aggregation through strong electrostatic interactions. Subsequently, near-infrared (NIR) light thermal activation, which enables direct and specific heating of bacterial colonies, can further maximize bacteriostatic activity.63 Further, Yang et al demonstrated that TS4-NP can encapsulate Staphylococcus aureus (S. aureus) cells by binding tightly to the bacterial surface through hydrophobic and electrostatic interactions and inducing TS4-S formation. S. aureus aggregation exerts membrane disruption and reactive oxygen species (ROS) production effects, leading to intracellular DNA breaks and bacterial death (Figure 8).64 Similar results were observed when GO-quaternary ammonium salt (GO-QAS) nanocomposites were applied to E. coli.65,66 Researchers have also found that nanoparticles can induce specific aggregation to eliminate infections caused by a single microbial community dominated by one bacterium and play this role in complex multi-microbial infection communities.67 These findings show that nanoparticles can be utilized to induce specific bacterial aggregation in the acidic environment of focal infections. However, when exposed to host fibroblasts in the physiological environment, the nanoparticles exhibit a net neutral charge and do not adhere to the negatively charged adjacent host cells, resulting in minimal photothermal damage to healthy tissues. This protects the surrounding skin while inhibiting bacterial growth and promoting wound healing. Therefore, nanoparticles can induce specific bacterial aggregation, competitively bind to bacteria in the wound area, and inhibit bacterial proliferation and colony formation.
 |
Figure 8 (A)TS NPs prepared with a TEOS/Si-QAC molar ratio of 4:1 (the corresponding products are called TS4 NPs) can kill Gram-positive Staphylococcus aureus (S. aureus) bacteria by an “adhesion-aggregation-disruption” mechanism: NPs can tightly bind to the surface of S. aureus by hydrophobic and electrostatic interactions (“adhesion”) and cover the entire bacterial cell. NPs can tightly bind to the surface of S. aureus through hydrophobic and electrostatic interactions, covering the entire bacterial cell (“adhesion”). (B) After treatment with TS4 NPs, (E) coli cells were only partially adsorbed by TS4 NPs, whereas S. aureus cells could be completely surrounded by NPs, which may limit S. aureus growth, propagation and metabolism. (C and D) Only ts4-treated S. aureus cells exhibited strong green fluorescence, indicating intracellular ROS production. (E) DNA extracted from untreated Staphylococcus aureus cells showed a clear bright band, whereas a weaker band appeared in the TS4 group of Staphylococcus aureus cells, suggesting that reactive oxygen species caused severe damage to DNA. (F) TS4-mediated bacterial aggregation was observed by confocal microscopy imaging within 5 min after the addition of TS4 to S. aureus suspensions, and TS4-induced bacterial cell aggregation was also observed at other time points (30 min, 2 h, and 6 h). Bacterial aggregation was not evident in the control group not treated with TS4. (G) DLS results also confirmed the presence of micrometer-sized aggregates in the TS4 group. Used with permission of The Royal Society of Chemistry from Yang J, Zhu YX, Lu P, et al. One-step synthesis of quaternized silica nanoparticles with bacterial adhesion and aggregation properties for effective antibacterial and antibiofilm treatments. J Mater Chem B. 2022;10(16):3073–3082. Copyright 2022; permission conveyed through Copyright Clearance Center, Inc.64 |
Cleavage of Mature Bacterial Biofilms in the Chronic Wound
Mature biofilms can form within 10 h in chronic wounds and persist in open wounds. Microbial behavior in high-viscosity wound environments resembles biofilms, while behavior in low-viscosity wound environments resembles bacterioplankton. Many studies have shown systemic antimicrobial agents are ineffective against bacteria in chronic wounds.68,69 Previously, researchers often attributed this to insufficient blood supply. However, many studies have found that heavy exudative wounds can present with infection and inflammation, even when the soft tissue near the wound has an abundant blood supply. This is presumably related to the presence of microorganisms with biofilm phenotypes. The evaporation of moisture in heavily exudative wounds leads to increased extracellular fluid viscosity, making microorganisms in this environment more prone to exhibit biofilm phenotypes, including reduced sensitivity to antimicrobials, resulting in difficult-to-control infections. These findings can have far-reaching implications for the treatment of chronic wounds. As a result, nanoparticles must employ multiple mechanisms to disrupt the development of mature biofilms.
Buffer Weak Alkalinity of Wound Microenvironment
Normal tissues typically exhibit pH values between 5.0 and 7.0, with a mildly acidic character on the surface. The skin wound microenvironment plays a critical role in inhibiting pathogenic microorganisms. Studies have shown that the pH of chronic infection wounds increases to 6.5–8.5, with the alkaline environment likely resulting from necrotic tissue, bacteria, and bacterial byproducts such as ammonia, which create favorable conditions for bacterial growth. In addition, mature bacterial biofilms in chronic wounds can inactivate skin keratinocytes and fibroblasts, accelerating their apoptosis and reducing epithelial keratinocyte migration, impairing wound healing. Thus, adjusting the wound’s pH can alter the bacterial growth environment’s microecology and regulate the biofilm’s pathogenic effects to accelerate wound healing. Nanoparticles can adjust the biofilm’s pH to enhance the wound’s defensive buffering capacity. On the one hand, nanoparticles can counteract alkali production and reduce the pH in chronic wound regions by slowly releasing phosphate or bicarbonate, disrupting mature biofilms. For example, AgNPs exhibit significant bactericidal activity and can promote wound healing in a chronic wound model in infected rats due to their high oxidative stress effect, acidic pH, and ROS-stimulated release of silver ions (Ag) and AMPs70 (Figure 9). On the other hand, Nanoreactors can intelligently activate multienzyme cascade reactions at high sugar levels to produce acidic substances such as gluconic acid (GA) and hydrogen peroxide, leading to the release of nitric oxide (NO), which can combat bacterial infection and inflammation in chronic wounds of diabetes.71
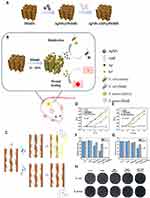 |
Figure 9 (A) Preparation process of AgNPs-AMP@PSiMPs. (B) AgNPs-AMP@PSiMPs as a synergistic antibacterial platform for wound disinfection and healing. (C–H) Mechanisms of ROS- and H+-stimulated release of Ag+ and AMPs. The in vitro antibacterial activity testing results verified that AgNPs-AMP@PSiMPs displayed the highest antibacterial activity among the three types of bactericidal agents. Reproduced with permission from Jin Y, Yang Y, Duan W, et al. Synergistic and On-Demand Release of Ag-AMPs Loaded on Porous Silicon Nanocarriers for Antibacteria and Wound Healing. ACS Appl Mater Interfaces. 2021;13(14):16127–16141. Copyright 2021, American Chemical Society.70 |
“Digestion” of Biofilm EPS Matrix
The EPS matrix surrounding biofilms acts as a physical barrier, protecting bacteria from bacteriostatic agents and immune cells. Nanoparticles can induce the degradation of the EPS matrix through various mechanisms.72
Previous studies have shown that nanoparticles can inhibit the production of EPS components and mediate EPS degradation, causing biofilms’ “physical” collapse. In this scenario, bacteria shed from the biofilm become exposed and lose their inherent protection. Ultimately, the connections between bacteria are disrupted, rendering them susceptible to drugs. Nanoparticles further exert a bacteriostatic effect on biofilms, eventually killing the bacteria.73 Ali demonstrated that nanoparticles attached to the EPS surface of wound biofilms inhibit the generation of extracellular polysaccharides, migrate within the EPS matrix, and infiltrate bacterial cell walls, inhibiting early biofilm growth via intracellular ROS activity.74
Subsequent research has shown that metal nanoparticles can interact with the EPS composition. Hiebner et al demonstrated that both the charge and surface functional groups of silica NPs (Si-NPs) significantly influence the degree of interaction and localization between Si-NPs and EPS macromolecules (including proteins, polysaccharides, and DNA) by evaluating the affinities of different functionalized Si-NPs and EPS macromolecules in biofilms.75 Weldrick demonstrated that protease-functionalized nano-carriers can “digest” EPS substrates, cross bacterial biofilms, reach bacteria formerly protected by the biofilm, and deliver antibiotics directly to bacterial cell walls, enhancing the clearance of bacterial biofilms formed in six chronic wounds76 (Figure 10).
 |
Figure 10 (A–F and J–M) Diagram of the mechanism of action of the Carbopol Aqua SF1−Alcalase 2.4 L FG nano-gel particles on biofilms adhered to a substrate. Fluorescent images and tapping mode atomic force microscopy of S. aureus biofilm formation show that Alcalase-nanogel Particles can digested the biofilm matrix but left the cells largely untouched. Reproduced with permission. (G) It shows that after seeding a membrane with 1×105 CFUmL−1 for 24 h at 37 °C, the growth control continued to increase in cell density after a further 1, 6, and 24 h of growth. (H) It shows that there is a correlation between the increase of the concentration of free ciprofloxacin in the presence of an equivalent amount of Alcalase−Carbopol NPs and the residual bacterial cell viability after treatment. (I) It shows the results on the concentration dependence of the cell viability on the antibiotic treatment in experiments where the ciprofloxacin was encapsulated within the Carbopol nanogel, rather than being delivered separately as a co-treatment. **p < 0.01, ***p < 0.001. Reproduced with permission from Weldrick PJ, Hardman MJ, Paunov VN. Enhanced Clearing of Wound-Related Pathogenic Bacterial Biofilms Using Protease-Functionalized Antibiotic Nanocarriers. ACS Appl Mater Interfaces. 2019;11(47):43902–43919. Copyright 2019, American Chemical Society.76 |
Further studies indicate that nanoparticles can adsorb and compromise the integrity of EPS through physical action. For instance, AgNPs can pierce the EPS, forming pores that destroy its structure, facilitating the action of antimicrobials in combating bacterial biofilms in wounds.77 Consistent with these findings, Dong confirmed that nanoparticles can adsorb to the surface layer of biofilms through electrostatic interactions, disrupt the biofilm matrix, and diffuse throughout the entire membrane.78 Further research indicates that QAS@CM adsorbs onto the biofilm surface via multiple charge interactions, penetrating the EPS and diffusing the film into nanoparticles. By responding to the biofilm acid/lipase microenvironment, these nanoparticles will further break down into quaternary ammonium oligomers and act as multivalent inhibitors, disrupting the established biofilm and killing the corresponding bacteria79 (Figure 11). In addition, nanoparticles can target components of EPS, such as lipoproteins, polysaccharides and eDNA, damaging the integrity of biofilm structures and creating pathways for antimicrobial agents.80
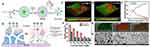 |
Figure 11 (A–H) Quaternary Ammonium Salt-Based Cross-Linked Micelle (QAS@CM) to Combat Biofilm. CLSM images and SEM images show that the QAS@CM readily penetrated and dispersed throughout the biofilm. Reproduced with permission from Liu F, He D, Yu Y, et al. Quaternary Ammonium Salt-Based Cross-Linked Micelles to Combat Biofilm. Bioconjug Chem. 2019;30(3):541–546. Copyright 2019, American Chemical Society.79 |
Accordingly, regardless of whether nanoparticles can degrade EPS in mature biofilms, it can be concluded that nanoparticles can inhibit biofilm formation by acting on the EPS, making them potential agents for managing chronic wound pathogenic biofilms in clinical practice.
Blocking QS Signaling of the Biofilm
Quorum sensing (QS) is a significant information transfer mode between bacteria in biofilms through chemical signaling molecules. The methods of signal transmission in biofilms are diverse. Currently, the QS signaling system of P. aeruginosa is the most well-understood. Pseudomonas aeruginosa can use QS to orchestrate the production of multiple virulence phenotypes at high bacterial densities, particularly in immunocompromised and hospitalized patients at high risk of chronic wound MDR infections. Recent experimental and theoretical studies have shown that nanoparticles can inhibit QS in Pseudomonas aeruginosa. For example, silica nanoparticles (Si-NP) can target QS signaling molecules, such as acylserine lactone (HSL), to prevent bacterial communication, effectively silencing and isolating bacterial cells and exerting a bacteriostatic effect.81 Miller demonstrated that nanoparticles can inhibit virulence factor secretion by downregulating QS regulatory genes LASR, RHI, RHLR, PQSA, and PQSR, thus reducing the production of virulence factors in P. aeruginosa (rhamnolipids, pyoverdin, hemolysin, elastase, and protease).82 Other studies have indicated that the ability of AgNP to up-regulate the expression of QS-related genes is due to its binding to QS-related proteins in Pseudomonas aeruginosa.83 These findings demonstrate that nanoparticles exhibit significant QS activity against P. aeruginosa. In addition, there is a subtle relationship between the normal flora and pathogenic microbes in chronic wounds. Once the normal flora on the skin’s surface enters the wound, it can also lead to infection. For instance, the genus Staphylococcus, which forms part of the commensal skin microbiota, includes the pathogen S. aureus, which can cause purulent skin infections. S. aureus primarily uses small-molecule polypeptides (ALP) as QS signaling molecules. Studies have confirmed that nanoparticles can also disrupt the QS signaling transmission system of S. aureus and regulate mature biofilm activity.84
Nanoparticles can influence long-distance signal transduction between different bacterial species. Autoinducer-2 (AI-2) is the only known signaling molecule capable of transmitting cross-species, long-distance communication between bacteria in biofilms.85 Gomez-Gomez et al demonstrated that nanoparticles can block interspecies QS, interrupt signal transmissions within biofilms, and disrupt complex multi-bacterial biofilms.86 Further research revealed that magnetic nanoparticles can capture and target cells using magnetic mobility, synthesize the “universal” bacterial QS signaling molecule AI-2, and deliver it to the surface of E. coli to manipulate the QS of bacterial biofilms.87,88 Therefore, nanoparticles target pathogenic bacteria and regulate the signal transduction systems of bacterial biofilms as QS inhibitors, offering a novel approach to chronic wound infections.
Antimicrobial agents more easily eradicate biofilms formed by bacteria with defective QS genes. Although the mechanism was clarified, QS has become an attractive target for anti-biofilm therapy. Chen et al prepared curcumin (Cur) loaded clustered nanoparticles with active targeting ability to inhibit QS in order to enhance the selective accumulation of Cur in biofilms, and the QS inhibitor, Cur, utilized the active targeting ability of its cd54, which enhanced the Cur-da NPs’ biofilm permeability, significantly improving their therapeutic efficacy.89 In addition, blocking QS with QSI alone was recognized as insufficient to prevent biofilm formation because it lacks bactericidal properties. Hence, through the co-assembly of quercetin and copper ions, QC NPs underwent catabolism to release copper ions and quercetin in the low pH microenvironment where bacterial colonies accumulate. The former eliminated bacteria by disrupting the integrity of cell membranes, and the latter disrupted the QS process responsible for biofilms by down-regulating the expression of specific genes, effectively preventing Gram-negative Pseudomonas aeruginosa and Gram-positive Staphylococcus aureus from forming biofilms90(Figure 12).
 |
Figure 12 (A) Representative SEM images of the individual bacteria illustrating the bacterial cell membranes inside P. aeruginosa and S. aureus biofilms with different treatments. (B) Intracellular components of the bacterial cells released under different treatments, indicated by the absorbance at 260 nm. Schematic illustration of the anti-QS mechanism of Qe against (C) P. aeruginosa and (D) S. aureus. The normalized QS-related gene expression of (E) P. aeruginosa and (F) S. aureus assessed by RT-qPCR. (G) Schematic illustration depicting the anti-biofilm mechanism of the QC NPs by killing bacteria and inhibiting QS. In summary, QC NPs with dual functions (bactericidal and anti-qs) exhibited significant antibiofilm activity against Pseudomonas aeruginosa and Staphylococcus aureus. Therefore, the potential antibiotic membrane mechanism is shown in this figure. When bacteria begin to proliferate and form small colonies, they release certain organic acids to create a mildly acidic microenvironment. This acidity causes the QC NPs to break down and release Cu2+ ions and Qe. Subsequently, the released Cu2+ ions cause severe damage to the bacterial membranes, leading to bacterial elimination. *p < 0.05, **p < 0.01, ***p < 0.001. Used with permission of The Royal Society of Chemistry from Cheng J, Zhang H, Lu K, et al. Bi-functional quercetin/copper nanoparticles integrating bactericidal and anti-quorum sensing properties for preventing the formation of biofilms. Biomater Sci. 2024;12(7):1788–1800. Copyright 2024; permission conveyed through Copyright Clearance Center, Inc.90 |
Intrawound Lysis of the Bacterial Biofilm
Lysis is an essential stage in developing bacterial biofilms in chronic wounds. First, nanoparticles can simulate the “dart” to physically destroy the membrane structure in multiple directions and cleave the biofilm of chronic wounds.
Second, nanoparticles can dissolve the substrate through enzymatic activity, further enhancing EPS penetration to disperse the biofilm, leaving the bacteria in a free-floating state once removed from the biofilm.91 Since surface properties greatly influence the catalytic properties of nanomaterials, gold nanoparticles (AuNPs) with different surface modifications exhibited glucose oxidase, peroxidase, superoxide dismutase, and catalase mimetic activities, respectively.92 Chen et al indicated that AuNPs immobilized on biofunctionalized mesoporous silica exhibit intrinsic oxidase mimetic activity, which can directly cleave bacterial biofilms under mild conditions (Figure 13).93
 |
Figure 13 (A) Schematic illustration of the construction of a light-controlled “Band-Aid”-like hydrogel containing multiple enzyme-mimic CeO2 nanoparticles for programmable infected wound healing. (B) Photoresponsive processes inside the hydrogels. (C) Optical density at 600 nm (OD600 nm) of the bacterial suspension after incubating with different hydrogels under different conditions for 4h. Data are expressed as mean±SD (n=3). (D–G) Fluorescence microscopy images of 2H11 cells (SV40 transferred murine endothelial cells) that are control (D) and incubated with the suspension of bacteria (E) and MGCB-GO-CeO2 (F) and MGCB-GO (G) containing hydrogel treated bacteria; the mixture was treated with ultraviolet light irradiation before transfer. Reproduced with permission from Chen Z, Wang Z, Ren J, et al. Enzyme Mimicry for Combating Bacteria and Biofilms. Acc Chem Res. 2018;51(3):789–799. Copyright 2018 and 2017, American Chemical Society.93 |
Finally, nanoparticles can induce the automatic disintegration of wound biofilms by blocking signal channels and sending incorrect signals to bacterial populations. Ilk found that chitosan nanoparticles can inhibit QS-related processes by regulating AI-mediated QS. When bacteria disperse from the wound biofilm, they lose their inherent protection, and signal transduction between bacteria is blocked. Planktonic bacteria are less sensitive to antimicrobial agents, making healing chronic wounds easier.94
Lessens Regeneration and Spread of Bacteria on Mature Biofilm Surfaces
Scarcity of nutrients and accumulation of antimicrobial agents or cellular waste will lead to biofilm disintegration. Subsequently, viable bacteria leave the biofilm to spread. Disassembly of the biofilm often involves enzymes destroying the substrate composition and releasing surviving bacterial cells from the biofilm’s surface. Studies have shown that after bacteria are released from the biofilm surface, biofilms in chronic wounds can regenerate rapidly, most of which are related to residual bacteria in the biofilm. It is estimated that residual bacteria constitute only ~15% of the biofilm volume. These bacteria develop resistance to antibiotics due to phenotypic changes. Therefore, if residual bacteria can be targeted by nanoparticles at this stage, the regeneration and spread of the biofilm can be effectively reduced.95
Downregulate the Pathogenicity of Residual Bacteria in the Wound
The dormant state of pathogenic bacteria is a highly regulated strategy to survive environmental extremes. This dormant subclass of bacteria is known as persisters. For example, the persistence of P. aeruginosa in diseased tissues can lead to the recurrence of chronic wound infections when the body’s resistance is low.96 Although essential differences exist between drug-resistant bacteria formed by genetic mutation and persisters, the latter remain alive after antibiotic treatment and are not genetically altered. Recent studies have shown that drug-resistant bacteria exhibit significant retention phenomena, and persisters themselves can serve as an intermediate state leading to the development of drug-resistant bacteria.97 Evidence confirmed the ability of nanoparticles to inhibit the activity of persisters.98 Mei et al demonstrated that CuO/ZnO nanoparticles can prevent biofilm formation and inhibit persistent pathogenic bacteria. They also revealed that the nanoparticles have no significant killing effect on the residual bacteria compared to their static state. Therefore, dynamic residual bacteria in vivo were less sensitive to bacteriostatic agents than static residual bacteria.99 Kalita et al reported that the local application of surface-functionalized gold nanoclusters eliminated MRSA infection in refractory nondiabetic wounds in rats and accelerated the healing process of chronic wounds. The potential mechanism is that nanoparticles reduce the β-lactamase level of MRSA, then bind to the surface of residual bacteria, permeabilizing the bacterial membrane and reducing the pathogenicity of residual bacteria.99
Intervene with the Resuscitation of Residual Bacteria
Recent studies have shown that the dormancy depth of bacterial biofilms of the same species treated with different bacteriostatic agents varies, primarily manifested by differences in the recovery time of persisters. The metabolism of residual bacteria remains stagnated in the dormant state, allowing resistance to antimicrobial agents. To diminish the tolerance state of residual bacteria, scholars have explored several methods:
First, altering the metabolic status of bacteria without promoting increased activity reduces persisters.100 For example, Gao developed a new type of antimicrobial material, magnetic Fe4O3 nanomaterials loaded with CS, which are highly permeable and can effectively disrupt the structure of biofilm and activate the holding bacteria, thus achieving complete sterilization.101 The MRSA-caused infection was effectively treated by the HBPL-crosslinked HMP hydrogel in vivo, and thereby the wound closure at inflammatory phase was promoted significantly. The reason is that HBPL inhibited bacterial quorum sensing (QS) system, downregulated virulent genes, and interfered bacterial metabolism. 100 Second, decreasing the resuscitation proportion of residual bacteria by interfering with the de-polymerization of protein aggregates during resuscitation has proven effective. One key indicator of dormancy depth is endogenous protein aggregation. The reduction of cellular ATP levels promotes dormancy. Protein aggregates must be eliminated and proteostasis restored for cells to recover from dormancy. Pu et al found that the lag time for bacterial regeneration was determined by the ability to recruit a functional DnaK-ClpB mechanism that promotes protein breakdown in an ATP-dependent manner.102 However, resuscitation intervention of residual bacteria in chronic wounds using nanoparticles has not yet been studied. Nonetheless, nanoparticles with controlled designs provide new inspiration for inhibiting bacteria in intractable chronic wounds.
Influencing Factors
Factors Related to Nanoparticles
Size Effect
The size of nanoparticles can affect their antibacterial activity. Increased surface activity is achieved by reducing particle size to a certain level, causing the electronic energy levels to shift from a continuous to a discrete state, enlarging the energy gap, which is crucial for nanoparticle-bacteria interactions. Although some reports indicate that size is an influential factor in the antimicrobial properties of nanoparticles, the results are not entirely consistent. Two main perspectives exist. Most studies indicate that smaller nanoparticles have a greater surface area and thus exhibit a stronger ability to inhibit biofilm formation than larger particles. For instance, in terms of water solubility and biological activity, high-molecular-weight chitosan outperforms low-molecular-weight chitosan, as Peng reported. The study suggested that low-molecular-weight chitosan (LMWC) can serve as an ideal stabilizer for nanoparticles, and LMWC-coated AgNPs can effectively control bacterial biofilm infection in MRSA wounds in mice.103 A recent study showed that multifunctional ultrafine Ag-NP hydrogels can effectively remove bacterial biofilms from infected wounds and optimize the regulation of inflammatory responses to promote wound healing by increasing cell proliferation and re-epithelialization.104 These results align with other studies showing a size-dependent effect of AgNPs on bacterial biofilms. Compared to larger spherical nanoparticles, smaller nanoparticles exhibit better antibacterial activity against biofilms due to their larger surface-area-to-volume ratio.105
In contrast, another study using a splinted mouse wound model found that tannic acid (TA)-modified, larger-sized Ag-NPs have stronger bacterial infection-inhibiting abilities than unmodified or smaller-sized Ag-NPs. It was further confirmed that 13-nm Ag-NPs lacked effective antimicrobial properties, but they can cause a strong inflammatory response on damaged skin.106 The direct interaction of nanoparticles with bacterial membranes and intracellular proteins, along with the release of bactericidal silver ions from the nanoparticle surface, can contribute to the antibacterial properties of Ag-NPs.107 Truong confirmed that larger GO-Ag-NPs exhibited higher antimicrobial biofilm activity than smaller GO-Ag-NPs.108
In conclusion, nanoparticles can regulate the formation of bacterial biofilms in chronic wounds through size effects, promoting wound healing(Table 1)
 |
Table 1 Influencing factors related to nanoparticles |
Surface Zeta Potential
The ability of nanoparticles to attract bacteria, reduce bacterial adhesion in chronic wound areas, and inhibit biofilm formation is attributed to their generally positive surface zeta potential. Nie et al demonstrated in vivo skin wound healing experiments that changes in the ratio of Ag (-)/Ag (+) nanoclusters to Ag nanoparticles (+) can be utilized to control the zeta potential of Ag (-)/Ag (+) nanoclusters. When nanoclusters are converted to positively charged nanoparticles of small size, they can effectively treat and prevent chronic wound infections.109 Many studies have yielded consistent results. For example, Mi et al showed that composite nanoparticles, specifically positively charged nanoparticles, can target bacterial cell walls and further inhibit the formation of bacterial biofilms.110 A study have shown that reported that lipid-polymer hybrid nanoparticles with a positively charged surface can target bacterial cell walls by providing a moist environment and preventing bacterial infection.110
However, other studies have drawn opposite conclusions. Wehling et al argued that the antimicrobial activity of nanodiamond particles is related to the negative charge on their surfaces, especially those containing anhydride groups. This result can be explained by the anisotropic negative charge distribution on the nanodiamond surface, which prevents bacteria from attaching to chronic wound surfaces. That is, the zeta-negative potential of the nanoparticles enhances their activity in preventing biofilm formation in chronic wounds.111 Salvioni et al demonstrated that negatively charged silver nanoparticles exhibit strong antimicrobial activity and lower toxicity than the currently available positively charged Ag+.112 Two main reasons are proposed for this: primarily, although the cation pump generally controls the uptake of Ag+ ions, Ag-NPs can be taken up by non-specifically interacting bacteria, resulting in higher Ag content on bacterial walls in biofilms.113 Secondly, Ag atoms form a favorable redox potential on the nanoparticle surface, generating many free radicals, which produce ROS. Gold nanoparticles with a negative surface charge have shown the highest specific activity against infected S. aureus biofilms and can promote wound healing.114
The greater the zeta potential difference between the bacterial biofilm and the nano-ions, the better the internalization of particles and the superior the antibacterial performance.115 For example, ZnTiO3/Fe3+is a nano crystal with a nano porous structure, and the actual impedance of the nano structure decreases with the increase of Fe3+ions, thereby improving the antibacterial performance of the nano structure.116 Therefore, the zeta surface charge of nanoparticles plays a crucial role in inhibiting bacterial adhesion and biofilm formation in chronic wounds.5
Three-Dimensional Structure
The anti-biofilm activity of nanoparticles depends more on their structure, specifically surface morphology and crystal structure, which exhibit shape-dependent bacteriostatic properties. The surface morphology of the nanoparticles is a crucial factor. One study evaluated the anti-biofilm activity of AgNPs with different shapes. The results demonstrated that spherical Ag-NPs > discoidal AgNPs > triangular plate-shaped AgNPs, and the difference in silver ion release was utilized to explain the morphology-dependent antimicrobial activity of AgNPs.5 Hameed et al also found that Pseudomonas aeruginosa biofilm, a common pathogen in chronic wounds, is susceptible to gold nanoparticles, which exhibit significant physical cleavage and antimicrobial activity against it at low concentrations.117 Another study assessed the effects of nanoparticle shape on biofilm activity after eco-friendly biosynthesis of spherical, rectangular, pentagon, and hexagon AgNPs of different sizes. The results indicated that pentagon and hexagon AgNPs are the most resistant to bacterial biofilm formation. Hence, the surface morphology of nanoparticles is a significant factor affecting their properties.118
Li et al also demonstrated that the crystal structure of nanoparticles can influence their interactions with biofilm components. A theoretical simulation showed that the crystal structure of nanoparticles alters microbial community structure in a structure-dependent manner.119 Based on an analysis of biofilm bacteria, Cha et al showed that combining ZnO nanoparticles with specific crystal geometries interfered with the conformational reorganization of enzymes. The parameters strongly depend on the crystal geometry structure, which enzymes need to exert their antibacterial-related catalytic activity. Therefore, the surface morphology of nanoparticles is an essential factor influencing the properties of nanoparticles (Table 1).120
Aggregation State
In addition to the factors above, the anti-biofilm activity of nanoparticles is also influenced by their aggregation and dispersion states. Cheng et al demonstrated that chitosan-AgNPs prepared via the green method can be uniformly dispersed in the matrix and significantly impact combating chronic wound infections and promoting healing.121 Houshyar reported that nano-crystalline diamond (ND) dispersed in polyε-caprolactone (PCL)-based nanofiber scaffolds can treat chronic wounds by enhancing epithelial cell proliferation and inhibiting microbial activity.122 ZnO nanoparticles dispersed in ionic solutions exhibit high antimicrobial biofilm efficacy against skin-specific bacteria, possibly due to the enhanced dispersion in ionic solutions and ROS production, leading to bacterial cell lysis.123
However, most current studies do not strictly control or unify other factors when investigating specific ones. For instance, when studying the effect of size, other factors such as surface morphology or polymerization state must be strictly controlled to eliminate their influence. Therefore, conducting more comprehensive and in-depth investigations to control interfering factors is essential. Besides the intrinsic properties of nanoparticles, their ability to resist biofilm formation is also affected by the surrounding environment, including the local wound microenvironment and the host’s body (Table 1).
 |
Table 2 Influencing factors related to locally infected wounds |
Factors Related to Locally Infected WoundsTable 2
Bacteria
Different wound microenvironments have unique bacterial communities(Table 2). Bacteria specific to chronic wounds include S. aureus, P. aeruginosa, Streptococcus, and Corynebacterium, among others.131 Different bacterial populations colonize chronic wounds and can contribute directly or indirectly to the chronic wound phenotype. MRSA can act as a pathogen in diabetic foot ulcers and extend the wound-healing process to twice the duration compared to ulcers without MRSA.132 Several bacteria are isolated from patients with venous leg ulcers (VLU), but S. aureus and P. aeruginosa are identified as the primary wound pathogens. Most acute and chronic wound infections contain both aerobic and anaerobic bacteria. Gangrene, necrotizing fasciitis, and cellulitis of the skin and soft tissues are frequently caused by a combination of anaerobic and aerobic bacteria, leading to infection.128 These bacteria are typically part of the normal microbiota. Mixtures of microorganisms are often involved in many skin lesions, and biofilms formed by these pathogenic microorganisms appear on acute wounds, eventually transforming into chronic infections.
The same nanoparticles exhibit different effects on various types of bacterial biofilms. Ferreres compared the antimicrobial effect of metal nanoparticles on Gram-positive and Gram-negative bacteria. The results indicated that Gram-positive bacteria were more sensitive to nanoparticles than Gram-negative bacteria. Peptidoglycan and acidic polysaccharides, including phosphate walls, are the main components of the cell wall of Gram-positive bacteria, and the overall surface of the cell wall is negatively charged.133 Therefore, the diversity of wound microbiota complicates patient treatment, mainly when many drug-resistant microorganisms are present in chronic wound biofilms. When colonizing bacteria such as Staphylococcus epidermidis and Coccidioides live on the skin, they typically prevent the colonization of pathogenic bacteria.129 Nevertheless, chronic wounds are associated with pathogenic bacteria colonizing the wound bed. Most chronic wounds harbor more than one type of bacteria, which work synergistically, causing previously non-toxic bacteria to become harmful and damage the host.
Current research on chronic wound infection primarily uses the following three types of bacterial biofilm models to study the bacteriostatic effects of agents. The first model employs a single biofilm of pathogenic bacteria that infect wounds. Selecting a single strain to construct a research biofilm is relatively simple, with easy standardization, substantial control, and good repeatability. However, it has the limitation of not simulating clinical chronic wound bacterial biofilms. Hasan et al employed MRSA biofilms to simulate chronic wounds in diabetic patients and demonstrated that chitosan nanoparticles strongly bind to MRSA biofilm substrates, significantly enhancing anti-biofilm activity.2
Ghaseminezhad et al also employed a single S. aureus biofilm to verify the Ag/FeO nano-complex in treating wound infection in an in vitro chronic wound model.127 Nour et al studied the effect of AgNPs on chronic wound biofilm infections using a single P. aeruginosa biofilm model.56 The bacterial biofilm of chronic wounds comprises several types of bacteria, typically 3–10 dominant types, and hundreds of other microbes in varying densities. Different types of wounds contain different types of bacterial biofilms. Another approach is to use double or multiple strains to construct in vitro biofilms for research. In 2009, Trivedi et al generated multi-microbial chronic wound biofilms within 24 h, which included several vital anaerobic bacteria. All selected strains were associated with chronic wounds: Streptococcus anaerobicus, Anaerobicus lactocolysis, S. megacolossus, and E. coli. This study is distinctive in that it does not adhere to the traditional modeling view that aerobic and anaerobic bacteria cannot coexist in standard liquid culture due to a lack of spatial balance. Instead, it creates a microenvironment for the growth of multi-bacterial biofilms, establishing favorable conditions for subsequent studies.130 There are scholars evaluated the anti-biofilm properties of nanoparticles using various bacterial biofilms containing S. aureus, P. aeruginosa, and A. baumannii. Dual-species and multispecies biofilms are more complex than single bacterial community models and better reflect the actual presence of bacterial biofilms in chronic wounds.134 Another approach is to construct bacterial biofilms by obtaining bacterial strains directly from chronic wounds in animals or humans and culturing them in vitro, better mimicking the bacterial biofilms that infect wound surfaces and reflecting clinical realities. After evaluating the effects of ZnO and AgNPs on biofilms of clinical isolates from standard MRSA and burn-resistant wound infections, respectively.134 Sánchez et al indicated that there is no significant difference between the two in terms of inhibiting the formation of these biofilms.134 Alvarado et al also studied the effect of clinical isolates of bacterial biofilms on the inhibition of AgNPs in chronic wound infection.4 This model also enables interactions between biofilm bacteria, which can further influence the host’s immune response. Hence, many factors cannot be controlled.
Inflammatory Reaction
The inflammatory response in chronic wounds is a protective mechanism of the body to remove the noxious stimulus while also initiating the healing process (Table 2). Not only does the inflammatory response help defend against harmful stimuli, but the degree of this response can influence nanoparticles’ ability to combat bacterial biofilm-infected wounds. This is mainly reflected in the following aspects: (1) Chronic wound exudate affects the aggregation state of nanoparticles in chronic wounds. It is well known that nanoparticle aggregation impacts their antimicrobial properties. Most nanoparticles are distributed in the wound area, not in pure water solutions but within wound exudates. Brown et al found that exposure of low-dose Si-NPs to different mediators (normal saline, bovine serum protein, body exudate) can increase neutrophils and inflammatory response, and the dispersive mediators can influence the extent of this response.4 (2) Extracellular neutrophilic traps (NETS) in inflammatory exudates, whose unique morphological structure can limit the diffusion of bacteria in chronic wound biofilms, are conducive to eliminating pathogenic microorganisms by nanoparticles. These NETS consist of a combination of DNA fibers and granular proteins, and the formation of NETS is a critical factor in innate immune defense. In 2004, Brinkmann demonstrated that central granulocytes can kill bacteria and prevent their spread in the body by forming a net structure with their DNA as the skeleton outside the cell, which adsorbs bactericidal proteins and hydrolases contained in various granules.135 Likewise, Wang et al discovered that TTD-loaded macrophages (TLMs) could precisely capture and eradicate bacteria while being activated toward the proinflammatory and antibacterial M1 phenotype upon light illumination. In addition to mediating direct bactericidal activity within phagolysosomes and inducing a mesh formation, nanoparticles can also help treat infected skin wounds.136 Complement and antibodies in inflammatory exudates synergize with nanoparticles against pathogenic bacteria in the biofilm. Studies have confirmed that bacterial infectious diseases can activate the classical complement pathway in the body.137 Many studies have also shown that protein can prevent bacterial biofilm infection in burn wound models and disrupt biofilm formation by activating the antimicrobial activity of macrophages.138
Wound Environment (pH Value, Temperature)
The wound microenvironment plays an essential role in the complex process of wound infection healing (Table 2). Studies have demonstrated that the wound microenvironment, including factors such as action time, temperature, pH, and oxygen concentration, can influence the structure of bacterial biofilms. Many studies have indicated that the primary host factors present in wound beds include damaged tissue, red blood cells, and plasma. Variations in wound conditions can lead to differences in the resistance of bacterial biofilms to antimicrobial agents.139
First, the duration of biofilm model culture in this study varied significantly, ranging from 1 to 70 days. Zhang found that the epidemiology of wound infections evolves over time.140 During the first week after injury, low-pathogenic gram-positive microorganisms are the most common bacterial isolates from wounds. As wound healing progresses, gram-negative bacilli increase in number while gram-positive bacilli decrease.141 Therefore, to identify and understand the different growth stages of each bacterial biofilm model, comprehensive growth measurements of selected bacterial biofilm models should be conducted before initiating studies on chronic wound bacterial biofilms. In addition, blank control and biofilm models derived from chronic wounds should be used for comparative studies where possible.
Second, the temperature of chronic wounds is commonly considered 37°C in many studies. For example, Wright’s study maintained the bacterial incubation temperature in a wound environment at a constant 37°C, showing that this temperature is optimal for bacterial growth in biofilms.142 However, in actual human or animal chronic wounds, the temperature fluctuates with ambient and physiological conditions and is not constant. Therefore, when culturing bacterial biofilms from chronic wounds, the temperature should vary between 36.2°C and 37.5°C to closely mimic human skin conditions. On the other hand, Real time monitoring of wound status by quantifying specific biomarkers such as pH, temperature, and tissue oxygenation can significantly assist in the treatment and care of chronic infected wounds.124
Chronic wound pH plays a critical role in regulation, although it remains a topic of debate. Most researchers propose that chronic wound microenvironment pH can affect the antibacterial properties of nanoparticles. One study found that in the acidic environment of skin wounds, charge reversal of Ag- occurs, and the large size of Ag-/Ag+ clusters is replaced by small, positively charged nanoparticles, which can easily adhere to negatively charged bacteria, enhancing their antibacterial properties.143 In addition, functionalizing silver nanoparticles with catechol in aqueous solution can stabilize the nanoparticles and enhance their ability to inhibit bacterial biofilm formation.144 However, other researchers argue that bacterial biofilms’ metabolites reverse-regulate chronic wounds’ pH, affecting healing.145
Dead tissue within wounds can provide nutrients for bacterial biofilms, and different bacteria have varying nutritional requirements. Chronic wound areas contain more nutrients than any other healthy tissue, supporting the growth of most bacteria. Wahid et al proposed the Bacteria may cause infection during the wound healing process, thereby affecting the healing process. In this case, the presence of bacteria may consume nutrients around the wound, affecting the speed and quality of wound healing.146 Trivedi suggested that incorporating laked blood and chopped meat-based media can better simulate physiological levels of iron, carbohydrates, and host proteins, which should all be considered when analyzing antimicrobial tolerance and sensitivity in chronic wounds. The microenvironment of chronic wound infections can also be altered by cytokines and toxins released due to the coexistence of different bacteria following treatment with bacteriostatic agents.130 Therefore, the media selected for chronic wounds should be capable of supporting and promoting the normal formation of the wound bacterial biofilms under investigation.
Systemic Factors of the Host
Age
One of the critical factors affecting the healing of chronic wound infections is age. Studies have shown that patients with chronic skin and soft tissue wounds are predominantly middle-aged and elderly. In addition, patients with chronic wounds tend to have multiple concurrent underlying diseases and longer disease durations. The percentage of healed patients is also lower compared to those with acute wounds.145
Clinically, local thickness burns in older individuals are more likely to convert into full-thickness skin injuries than in younger individuals. Farinas et al reported that this clinical phenomenon and the mediated immune system are closely related to the different local damage responses. Studies have demonstrated that at least two kinds of cytokines, CCL5 (RANTES) and EGF, respond differently in young and elderly burn victims. This further speculates that the local proinflammatory environment can increase conversion rates from partial to full burns.147 Jackson’s study also drew a similar conclusion: the increase of baseline serum eotaxin in the elderly after an injury can trigger their local wound overreaction and lead to poor clinical efficacy.148
Immune Status
Chronic wound infections caused by bacterial biofilms often result in low immune function, disproportionate quantities of T cell subsets, reduced cytokine activity, and partial deficiencies in immunoglobulin or other subclasses. The immune system plays a significant role in the anti-infection process of nanoparticles. T cells are critical components of cellular immunity and immune regulation. Numerous studies have confirmed that nanoparticles can stimulate changes in T cell subsets, increase lymphocyte numbers, induce immune regulation, and improve cellular immune function, collectively inhibiting the formation of bacterial biofilms in the wound area.126,149 Macrophages are essential for timely and effective wound healing. They have been shown to modulate inflammation and promote the migration and proliferation of regenerating cells, enhancing tissue deposition and wound closure. Studies have demonstrated that applying α-gal nanoparticles to wounds can increase macrophage invasion, stimulate an immediate inflammatory response, and inhibit bacterial infection.150 Yu et al also confirmed that iron oxide nanoparticles induced macrophage polarization, which counteracted intracellular S. aureus, stimulated reactive oxygen species (ROS) production, and increased macrophage ability to inhibit S. aureus biofilms.151 Studies have also suggested that enhanced immune function can contribute to the process by which nanoparticles combat bacterial biofilms in chronic wounds and promote wound repair. Cheng et al showed that the uptake of large quantities of inorganic nanoparticles induces vacuolation in macrophages, enhancing the immune response.152 They demonstrated that macrophages exhibit stronger inhibitory effects on S. aureus biofilm following activation.59
Systemic Diseases
The most important characteristic of chronic wound biofilms is that the microenvironment in which they grow is inevitably affected by the host’s systemic disease. Various systemic diseases can have diverse effects on bacterial biofilms’ formation, type, and structure in chronic wound areas. These diseases include, but are not limited to, diabetes mellitus, cancer, tuberculosis, and vitamin deficiency, with diabetes mellitus being the most closely related to chronic wound development. High levels of oxidative stress in wounds are associated with diabetes, and the redox state is critical for forming chronic wound biofilms.153 In addition, patients with diabetes exhibit abnormal inflammation and immune responses, making them more vulnerable to chronic wound infections. Nguyen found that the expressions of TLR 2, TLR 4, interleukin 1β, and tumor necrosis factor-α in diabetic wound biofilms were significantly lower than those in wild-type wounds, and the ability of diabetic wounds to recognize bacterial infection via the TLR pathway declined. This decline resulted in insufficient stimulation of the host by cytokines, potentially underlying the mechanisms of diabetes-related susceptibility to chronic wound infections.154 Some studies have also dynamically described the bacterial biofilms of breast cancer-related malignant wounds, and the results showed that 70% of the confirmed strict anaerobic strain biofilms were present in these wounds. Therefore, tumors can affect the bacterial biofilm load and cause chronic wound microflora imbalance.155
The combined effects of systemic disease should be fully considered when studying the interaction between nanoparticles and bacterial biofilms in chronic wounds. In other words, the effects of different nanoparticles should be compared after ensuring consistency between test groups regarding systemic disease. Systemic diseases can also interfere with or synergize with nanomaterials’ anti-chronic wound bacterial biofilm effects.
Conclusions and Outlook
In conclusion, nanoparticles represent a promising class of antimicrobial materials for chronic wound management, which can effectively inhibit a range of bacterial biofilms, including those formed by antibiotic-resistant strains such as Staphylococcus aureus, Pseudomonas aeruginosa, and Klebsiella pneumoniae. Future research should emphasize their potential applications in antimicrobial therapies for chronic wounds. To fully harness the efficacy of nanoparticles in these therapies, the following areas warrant further investigation.
The mechanisms by which nanoparticles inhibit bacterial biofilms in chronic wounds require more in-depth investigation
Currently, much of the understanding remains at a superficial cellular level, while research into the underlying molecular mechanisms has stagnated. This limitation arises from several factors.5 Firstly, the performance of conventional electron microscopes, commonly used to analyze bacterial biofilm morphology, restricts observation capabilities. Higher-resolution laser scanning confocal microscopes are necessary to fully visualize the three-dimensional structures of intracellular membranes, alongside specialized imaging techniques to distinguish between bacterial cells and nanoparticles at the nanoscale.156 Secondly, existing in vitro chronic infection models are suboptimal. While numerous studies have explored the inhibitory mechanisms of nanoparticles against common pathogens like Staphylococcus aureus and Pseudomonas aeruginosa, they often utilize single-strain biofilm models that overlook bacterial interactions. This approach diverges significantly from the clinical reality of mixed bacterial infections in wound biofilms and limits the applicability of findings. Comprehensive experimental evaluations of diverse bacterial biofilms, therefore, are essential to differentiate their structures and functions, identify common mechanisms of action, and enhance therapeutic strategies.156–159 Thirdly, in vivo research on bacterial biofilm inhibition is underdeveloped. Such studies can address the limitations of in vitro approaches, validate the mechanisms of action of nanoparticles in a human-like environment, and demonstrate their effectiveness beyond laboratory conditions.
Nanoparticles have the potential to achieve significant breakthroughs in combating infections in chronic wounds.
Firstly, advances in the controllable design and synthesis of nanomorphic structures enable researchers to inhibit bacterial biofilm formation by tailoring the structure and surface properties of nanoparticles to target critical components of biofilms in the skin. For instance, Tan et al utilized positively charged chitosan nanoparticles as carriers for deoxyribonuclease I (DNAase I), which targets extracellular DNA in biofilms, and oxacillin to combat Staphylococcus aureus biofilms. After 24 hours of treatment, these polymeric nanoparticles eradicated 70% and 100% of mature Staphylococcus aureus biofilms at oxacillin concentrations of 0.0625 and 2 µg/mL, respectively.160 Compared to traditional antibiotics, nanoparticles enable precise targeting of chronic wound biofilms, minimize damage to surrounding tissues, and facilitate precision medicine. However, research on these nanomaterials is still in its infancy, and additional biofilm-targeting factors remain to be explored. Secondly, nanocarriers are widely employed in drug delivery systems, with a key advantage being their ability to selectively transport therapeutic agents to the wound site in response to specific stimuli. This capability allows for controlled drug release and enhances therapeutic outcomes while reducing side effects. For example, biodegradable disulfide-bridged mesoporous silica nanoparticles (MSNs) have been developed to co-deliver silver nanoparticles and chlorhexidine (CHX) for biofilm inhibition. The resulting Ag-MSNs@CHX exhibited redox- and pH-responsive release of CHX and silver ions, driven by a redox-triggered matrix degradation mechanism in a biomimetic biofilm microenvironment.161 Thirdly, “smart” nanoparticles can be designed to respond to various microenvironmental cues, such as acidic pH, specific enzymes, and bacterial toxins, as well as external stimuli like light and heat. Incorporating these responsive nanoparticles into wound dressings can enhance wound healing.48,162 However, it is important to address safety concerns; conventional photochemical or chemical-responsive systems may inadvertently harm healthy tissues. It is thus essential to research safer and more sensitive response systems. For example, phototherapy-based nanoparticles utilize photodynamic or photothermal therapies to target microorganisms by generating reactive oxygen species or localized heat. Although these methods avoid reliance on reactive oxygen species, the high temperatures required may damage surrounding cells, and the emergence of heat-resistant bacteria poses additional challenges.5 Finally, the emerging concept of “shifting the focus from bacteria to the host” offers new directions for designing antibacterial nanomaterials. Chronic wound infections are closely linked to the body’s immune response and the realization of immunomodulatory functions opens new avenues for the development and clinical application of antibacterial nanomaterials. For instance, silver nanoparticles (AgNPs) exhibit immunomodulatory properties that can activate inflammatory vesicles, thereby promoting the production and release of neutrophils and neutrophil extracellular traps (NETs) to combat bacterial infections.53
Several significant obstacles and limitations hinder the transition of antimicrobial nanomaterials from laboratory research to clinical application, which warrants further exploration.
Firstly, the effectiveness of treatments for chronic wounds is influenced by a complex interplay of factors, including not only bacterial infection but also chronic inflammation and oxidative stress. Consequently, there is a need for multi-targeted therapeutic nanosystems that not only eliminate bacteria but also regulate oxidative stress, thereby facilitating the transition from the inflammatory to the reparative phase of wound healing.8 Moreover, the rise of smart devices and the Internet has opened avenues for remote monitoring and performance adjustments of antimicrobial systems and allows for personalized treatments tailored to specific scenarios. For instance, smart wound dressings can gather data (such as temperature, reactive oxygen species, pH, glucose, bacteria, and uric acid) from a patient’s wound site and relay this information to a smart terminal. This enables medical staff to provide more accurate and timely chronic wound care advice through remote real-time monitoring.163 Secondly, biosafety is a critical concern. The safety of nanomaterials can be enhanced through appropriate design strategies while preserving their antimicrobial efficacy. However, the development and purification of nanoparticles present challenges, particularly because raw materials often include metal salts. Employing green fabrication processes may improve their biosafety. In addition, issues related to toxicity from ion release and reactive oxygen species generation during bacteriostasis have garnered significant attention, which necessitates a thorough evaluation of these factors to ensure the biosafety of nano-antimicrobial materials. Thirdly, bacterial resistance also represents a concern. While nanomaterials may reduce the risk of resistance through multiple antibacterial mechanisms compared to conventional antibiotics,164 they can also facilitate the transfer of antibiotic resistance genes (ARGs).165 Finally, the development of new smart nanomaterials for antimicrobial applications faces hurdles that impede stable diffusion and industrialization. High costs, significant technological barriers, low production yields, and challenges in sterilization mean that many of these materials are limited to laboratory-scale production.
The interaction among skin microorganisms, the host, and the external environment forms the skin’s microecological balance.
Both a stable ecological environment and microbial diversity play crucial roles in maintaining the skin barrier and fostering a symbiotic relationship with the host. Therefore, when evaluating any antimicrobial material for controlling skin biofilm-related diseases, it is essential to move beyond a narrow focus on its antimicrobial properties and consider its potential impact on skin microecology. The effects of nanoparticles are dual-sided. On one hand, unintended side effects of nanoparticles can disrupt the skin’s microecological balance. Research indicates that microbial diversity within skin biofilms positively influences skin health. Antimicrobial agents that compromise this diversity can upset the balance and potentially lead to secondary infections. Some nanoparticles with potent antimicrobial properties may lack specificity for pathogenic bacteria (eg, Pseudomonas aeruginosa) and can act similarly to broad-spectrum antibiotics, resulting in the unintended death of beneficial bacteria (eg, Staphylococcus epidermidis) and causing dysbiosis in the skin’s microecological balance. On the other hand, nanoparticles have the potential to modulate skin microecology. Unlike conventional antibiotics, nanoparticles can be engineered for specific purposes, allowing for targeted prevention and treatment of skin biofilm infections through microecological regulation. For instance, by targeting dominant bacteria or key virulence factors in chronic wound biofilms, specially designed nanoparticles can help preserve beneficial skin bacteria while effectively combating pathogens.166 In addition, nanoparticles may affect the ecological balance of the host’s individual environment. For example, they could disrupt the ecological balance of intestinal microecology and impact the composition and abundance of intestinal bacterial communities.167 Moreover, the ecological impact of nanomaterials on the surrounding environment must be considered. It has been proposed that organic materials—such as plants, microorganisms, bacteria, fungi, and viruses—can be utilized through biosynthesis techniques to create ecologically sound nanoparticles. The use of biosynthesized selenium nanoparticles (SeNPs), for instance, aims to minimize the environmental harm associated with chemical synthesis.168 Therefore, it is necessary to understand the environmental fate of these materials in order to mitigate their ecological risks.
While this paper focuses on the mechanisms of action of nanoparticles in chronic wound biofilms, it is essential to consider how other novel nanostructures contribute to the treatment of these infections.
Several types of nanomaterials warrant attention: (1) Microneedle Patches: These patches feature tiny tips that easily penetrate and disrupt biofilm barriers, allowing antimicrobial drugs to directly reach infected wounds and significantly enhancing treatment efficacy. Notably, microneedle patches that incorporate nano-motor technology offer a groundbreaking approach to combat bacterial biofilm infections and provide a novel strategy for wound management.169 (2) 2D Nanosheets: These materials inhibit bacteria through direct physical interactions with bacterial membranes. Larger nanosheets engage in surface-area-mediated interactions to encapsulate and segregate bacterial cells, which impedes nutrient transfer and can lead to reversible bacterial inactivation. Conversely, smaller nanosheets, with their sharp edges, can puncture bacterial membranes and create holes that compromise membrane integrity and induce bacterial death.170 (3) Surface Nanopatterns: These patterns can physically trap bacteria or disrupt biofilms and address superficial bacterial infections. Incorporating nanoscale patterns into wound dressings not only guides the directional growth of cells but also promotes the formation of vascular networks, thereby effectively accelerating the wound-healing process.171 (4) Metal-Organic Frameworks (MOFs): MOFs operate through various mechanisms, including cell membrane penetration, DNA damage, disruption of the electron transport system, and reactive oxygen species production. Their unique properties—such as a high number of active sites, large specific surface area, and high porosity—make them promising candidates for biofilm treatment.172 (5) Photodynamic Therapy Nanostructures: These are currently a hot research topic.173 However, on the one hand, their efficacy depends on the presence of light and oxygen, which may limit their use in deeper or more shielded biofilms. On the other hand, although nanoparticles used for photothermal therapy (PTT) do not rely on ROS, the required high temperature may damage cells, and the emergence of heat-resistant bacteria complicates their effectiveness.162 They hold promise for treating chronic wound infections, but further investigation is necessary to fully understand their mechanisms, properties, and potential applications, especially regarding their long-term safety and environmental impact.
Abbreviations
MDR, multidrug-resistant; EPS, Exopolymeric substance; eDNA, extracellular DNA; ESBL, extended spectrum beta-lactamase; QS, Quorum sensing; 3O-C-HSL, 3-oxo-dodecyl-L-methoserine lactone; TNF, tumor necrosis factor; IL, interleukin; IFN, interferon; NF, nuclear factor; TA, tannic acid; MS, mesoporous silica; Ag-NPs, silver nanoparticles; SENP, selenium nanoparticle; MSSA, methicillin-sensitive S. aureus; MRSA, methicillin-resistant S. aureus; MRSE, methicillin-resistant S. epidermis; GSNO, S-nitroso glutathione; IONPs, iron oxide nanoparticles; NIR, near infrared; Si-NPS, silica NPs; HSL, acyl-serine lactone; ALP, molecule polypeptides; AI-2, Autoinducer-2; LMWC, low-molecular weight chitosan; PCL, polyε-caprolactone; ND, nanocrystalline diamond; VLU, venous leg ulcers; NETS, Extracellular neutrophilic traps; IL-33, interleukins; WLM, wound-like medium model.
Consent for Publication
All authors consent to publication.
Acknowledgments
We acknowledge the financial support by Hainan Provincial Natural Science Foundation of China (822QN449), the National Natural Science Fund Cultivating 530 Project of Hainan General Hospital (2021QNXM02), the Courtyard’s Level Program of Hainan General Hospital(QN202012) and the Education Department of Hainan Province(Hnky2023-28).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was funded by the Hainan Provincial Natural Science Foundation of China (822QN449), the National Natural Science Fund Cultivating 530 Project of Hainan General Hospital(2021QNXM02), the Courtyard’s Level Program of Hainan General Hospital(QN202012) and the Education Department of Hainan Province(Hnky2023-28).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. T Vos, RM Barber, B Bell, A Bertozzi-Villa, S Biryukov. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi:10.1016/S0140-6736(15)60692-4
2. Hasan N, Cao J, Lee J, et al. PEI/NONOates-doped PLGA nanoparticles for eradicating methicillin-resistant staphylococcus aureus biofilm in diabetic wounds via binding to the biofilm matrix. Mater Sci Eng C. 2019;103:109741. doi:10.1016/j.msec.2019.109741
3. Gkartziou F, Giormezis N, Spiliopoulou I, et al. Nanobiosystems for Antimicrobial Drug-Resistant Infections. Nanomaterials. 2021;11(5):1075. doi:10.3390/nano11051075
4. Alvarado-Gomez E, Martinez-Castanon G, Sanchez-Sanchez R, et al. Evaluation of anti-biofilm and cytotoxic effect of a gel formulation with Pluronic F-127 and silver nanoparticles as a potential treatment for skin wounds. Mater Sci Eng C. 2018;92:621–630. doi:10.1016/j.msec.2018.07.023
5. Sedighi O, Bednarke B, Sherriff H, et al. Nanoparticle-Based Strategies for Managing Biofilm Infections in Wounds: a Comprehensive Review. ACS Omega. 2024;9(26):27853–27871. doi:10.1021/acsomega.4c02343
6. Leaper D, Assadian O, Edmiston CE. Approach to chronic wound infections. Br J Dermatol. 2015;173(2):351–358. doi:10.1111/bjd.13677
7. Guan T, Li J, Chen C, et al. Self-Assembling Peptide-Based Hydrogels for Wound Tissue Repair. Adv Sci. 2022;9(10):e2104165. doi:10.1002/advs.202104165
8. Qi X, Xiang Y, Cai E, et al. All-in-one: harnessing multifunctional injectable natural hydrogels for ordered therapy of bacteria-infected diabetic wounds. Chem Eng J. 2022;439:135691. doi:10.1016/j.cej.2022.135691
9. Sun F, Wei Y, Li S, et al. Shift in the submucosal microbiome of diseased peri-implant sites after non-surgical mechanical debridement treatment. Front Cell Infect Microbiol. 2022;12:1091938. doi:10.3389/fcimb.2022.1091938
10. Versey Z, Da Cruz Nizer WS, Russell E, et al. Biofilm-innate immune interface: contribution to chronic wound formation. Front Immunol. 2021;12:648554. doi:10.3389/fimmu.2021.648554
11. Kavanaugh JS, Flack CE, Lister J, et al. Identification of Extracellular DNA-Binding Proteins in the Biofilm Matrix. mBio. 2019;10(3). doi:10.1128/mBio.01137-19.
12. Redman WK, Welch GS, Rumbaugh KP. Differential Efficacy of Glycoside Hydrolases to Disperse Biofilms. Front Cell Infect Microbiol. 2020;10:379. doi:10.3389/fcimb.2020.00379
13. Passalacqua KD, Satola SW, Crispell EK, Read TD. A mutation in the PP2C phosphatase gene in a Staphylococcus aureus USA300 clinical isolate with reduced susceptibility to vancomycin and daptomycin. Antimicrob Agents Chemother. 2012;56(10):5212–5223. doi:10.1128/AAC.05770-11
14. Mudrik-Zohar H, Carasso S, Gefen T, et al. Microbiome Characterization of Infected Diabetic Foot Ulcers in Association With Clinical Outcomes: traditional Cultures Versus Molecular Sequencing Methods. Front Cell Infect Microbiol. 2022;12:836699. doi:10.3389/fcimb.2022.836699
15. Smillie C, Garcillan-Barcia MP, Francia MV, et al. Mobility of plasmids. Microbiol Mol Biol Rev. 2010;74(3):434–452. doi:10.1128/MMBR.00020-10
16. Kalan LR, Meisel JS, Loesche MA, et al. Strain- and Species-Level Variation in the Microbiome of Diabetic Wounds Is Associated with Clinical Outcomes and Therapeutic Efficacy. Cell Host Microbe. 2019;25(5):641–655.
17. Gillings MR. Lateral gene transfer, bacterial genome evolution, and the Anthropocene. Ann N Y Acad Sci. 2017;1389(1):20–36. doi:10.1111/nyas.13213
18. Botelho J, Grosso F, Peixe L. Antibiotic resistance in Pseudomonas aeruginosa - Mechanisms, epidemiology and evolution. Drug Resist Updat. 2019;44:100640. doi:10.1016/j.drup.2019.07.002
19. Li P, Luo W, Xiang TX, et al. Horizontal gene transfer via OMVs co-carrying virulence and antimicrobial-resistant genes is a novel way for the dissemination of carbapenem-resistant hypervirulent Klebsiella pneumoniae. Front Microbiol. 2022;13:945972. doi:10.3389/fmicb.2022.945972
20. Josephson H, Ntzouni M, Skoglund C, et al. Pseudomonas aeruginosa N-3-Oxo-Dodecanoyl-Homoserine Lactone Impacts Mitochondrial Networks Morphology, Energetics, and Proteome in Host Cells. Front Microbiol. 2020;11:1069. doi:10.3389/fmicb.2020.01069
21. Rahbari KM, Chang JC, Federle MJ. A Streptococcus Quorum Sensing System Enables Suppression of Innate Immunity. mBio. 2021;12(3). doi:10.1128/mBio.03400-20
22. He L, Le KY, Khan BA, et al. Resistance to leukocytes ties benefits of quorum sensing dysfunctionality to biofilm infection. Nat Microbiol. 2019;4(7):1114–1119. doi:10.1038/s41564-019-0413-x
23. Rumbaugh KP, Diggle SP, Watters CM, et al. Quorum sensing and the social evolution of bacterial virulence. Curr Biol. 2009;19(4):341–345. doi:10.1016/j.cub.2009.01.050
24. Garcia-Hermoso D, Criscuolo A, Lee SC, et al. Outbreak of invasive wound mucormycosis in a burn unit due to multiple strains of mucor circinelloides f. circinelloides resolved by whole-genome sequencing. Chowdhary A. editor. mBio. 2018; Vol. 9(2):e00573–18
25. Zou J, Peng B, Qu J, et al. Are Bacterial Persisters Dormant Cells Only? Front Microbiol. 2021;12:708580. doi:10.3389/fmicb.2021.708580
26. Conlon BP, Nakayasu ES, Fleck LE, et al. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature. 2013;503(7476):365–370. doi:10.1038/nature12790
27. Fisher RA, Gollan B, Helaine S. Persistent bacterial infections and persister cells. Nat Rev Microbiol. 2017;15(8):453–464. doi:10.1038/nrmicro.2017.42
28. Thurlow LR, Joshi GS, Richardson AR. Peroxisome Proliferator-Activated Receptor gamma Is Essential for the Resolution of Staphylococcus aureus Skin Infections. Cell Host Microbe. 2018;24(2):261–270. doi:10.1016/j.chom.2018.07.001
29. Sathiyaseelan A, Saravanakumar K, Wang MH. Bimetallic silver-platinum (AgPt) nanoparticles and chitosan fabricated cotton gauze for enhanced antimicrobial and wound healing applications. Int J Biol Macromol. 2022;220(220):1556–1569. doi:10.1016/j.ijbiomac.2022.09.045
30. Nakagami G, Schultz G, Kitamura A, et al. Rapid detection of biofilm by wound blotting following sharp debridement of chronic pressure ulcers predicts wound healing: a preliminary study. Int Wound J. 2020;17(1):191–196. doi:10.1111/iwj.13256
31. Alvarado-Gomez E, Perez-Diaz M, Valdez-Perez D, et al. Adhesion forces of biofilms developed in vitro from clinical strains of skin wounds. Mater Sci Eng C. 2018;82:336–344. doi:10.1016/j.msec.2017.08.028
32. Ponnuvel S, Sankar S, Ponnuraj K. Analyzing the adhesion mechanism of FnBPA, a surface adhesin from Staphylococcus aureus on its interaction with nanoparticle. Microb Pathog. 2020;146:104239. doi:10.1016/j.micpath.2020.104239
33. Mei L, Lu Z, Zhang X, et al. Polymer-Ag nanocomposites with enhanced antimicrobial activity against bacterial infection. ACS Appl Mater Interfaces. 2014;6(18):15813–15821. doi:10.1021/am502886m
34. Wang C, Zhou H, Niu H, et al. Tannic acid-loaded mesoporous silica for rapid hemostasis and antibacterial activity. Biomater Sci. 2018;6(12):3318–3331. doi:10.1039/C8BM00837J
35. Shu M, He F, Li Z, et al. Biosynthesis and Antibacterial Activity of Silver Nanoparticles Using Yeast Extract as Reducing and Capping Agents. Nanoscale Res Lett. 2020;15(1):14. doi:10.1186/s11671-019-3244-z
36. Seo SY, Lee GH, Lee SG, et al. Alginate-based composite sponge containing silver nanoparticles synthesized in situ. Carbohydr Polym. 2012;90(1):109–115. doi:10.1016/j.carbpol.2012.05.002
37. Zhu H, Bao B, Zheng X. Is NS-EDTA Effective in Clearing Bacteria From Infected Wounds in a Rat Model? Clin Orthop Relat Res. 2018;476(5):1083–1090. doi:10.1007/s11999.0000000000000232
38. Bur S, Preissner KT, Herrmann M, et al. The Staphylococcus aureus extracellular adherence protein promotes bacterial internalization by keratinocytes independent of fibronectin-binding proteins. J Invest Dermatol. 2013;133(8):2004–2012. doi:10.1038/jid.2013.87
39. Qin H, Cao H, Zhao Y, et al. In vitro and in vivo anti-biofilm effects of silver nanoparticles immobilized on titanium. Biomaterials. 2014;35(33):9114–9125. doi:10.1016/j.biomaterials.2014.07.040
40. Halbus AF, Horozov TS, Paunov VN. ”Ghost” Silica Nanoparticles of ”Host”-Inherited Antibacterial Action. ACS Appl Mater Interfaces. 2019;11(42):38519–38530. doi:10.1021/acsami.9b14403
41. Hu C, Wang L, Lin Y, et al. Nanoparticles for the treatment of oral biofilms: current state, mechanisms, influencing factors, and prospects. Adv Healthcare Mater. 2019;8(24):1901301. doi:10.1002/adhm.201901301
42. Liu W, Golshan NH, Deng X, et al. Selenium nanoparticles incorporated into titania nanotubes inhibit bacterial growth and macrophage proliferation. Nanoscale. 2016;8(34):15783–15794. doi:10.1039/C6NR04461A
43. Shariati A, Asadian E, Fallah F, et al. Evaluation of Nano-curcumin effects on expression levels of virulence genes and biofilm production of multidrug-resistant Pseudomonas aeruginosa isolated from burn wound infection in Tehran, Iran. Infect Drug Resist. 2019;Volume 12(12):2223–2235. doi:10.2147/IDR.S213200
44. Biswas NN, Kutty SK, Barraud N, et al. Indole-based novel small molecules for the modulation of bacterial signalling pathways. Org Biomol Chem. 2015;13(3):925–937. doi:10.1039/C4OB02096K
45. Gierlikowska B, Stachura A, Gierlikowski W, et al. Phagocytosis, Degranulation and Extracellular Traps Release by Neutrophils-The Current Knowledge, Pharmacological Modulation and Future Prospects. Front Pharmacol. 2021;12:666732. doi:10.3389/fphar.2021.666732
46. Dai X, Guo Q, Zhao Y, et al. Functional Silver Nanoparticle as a Benign Antimicrobial Agent That Eradicates Antibiotic-Resistant Bacteria and Promotes Wound Healing. ACS Appl Mater Interfaces. 2016;8(39):25798–25807. doi:10.1021/acsami.6b09267
47. Jena P, Bhattacharya M, Bhattacharjee G, et al. Bimetallic gold-silver nanoparticles mediate bacterial killing by disrupting the actin cytoskeleton MreB. Nanoscale. 2020;12(6):3731–3749. doi:10.1039/C9NR10700B
48. Rai A, Pinto S, Velho TR, et al. One-step synthesis of high-density peptide-conjugated gold nanoparticles with antimicrobial efficacy in a systemic infection model. Biomaterials. 2016;85:99–110. doi:10.1016/j.biomaterials.2016.01.051
49. Thomason HA, Lovett JM, Spina CJ, et al. Silver oxysalts promote cutaneous wound healing independent of infection. Wound Repair Regen. 2018;26(2):144–152.21qag. doi:10.1111/wrr.12627
50. Wen Y, Chen W, Wu R, et al. Chitosan‐stabilized PtAu nanoparticles with multienzyme‐like activity for mixed bacteria infection wound healing and insights into its antibacterial mechanism. Small Structures. 2024;5(6):2300553. doi:10.1002/sstr.202300553
51. Di Z, Shi Z, Ullah MW, et al. A transparent wound dressing based on bacterial cellulose whisker and poly(2-hydroxyethyl methacrylate). Int J Biol Macromol. 2017;105(Pt 1):638–644. doi:10.1016/j.ijbiomac.2017.07.075
52. Ma W, Li L, Lin X, et al. Novel ZnO/N-halamine-Mediated Multifunctional Dressings as Quick Antibacterial Agent for Biomedical Applications. ACS Appl Mater Interfaces. 2019;11(34):31411–31420. doi:10.1021/acsami.9b10857
53. Qi M, Zhu X, Yu X, et al. Preparation of W/O hypaphorine–chitosan nanoparticles and its application on promoting chronic wound healing via alleviating inflammation block. Nanomaterials. 2021;11(11):2830. doi:10.3390/nano11112830
54. Wang F, Fang RH, Luk BT, et al. Nanoparticle-Based Antivirulence Vaccine for the Management of Methicillin-Resistant Staphylococcus aureus Skin Infection. Adv Funct Mater. 2016;26(10):1628–1635. doi:10.1002/adfm.201505231
55. Huang Y, Bai L, Yang Y, et al. Biodegradable gelatin/silver nanoparticle composite cryogel with excellent antibacterial and antibiofilm activity and hemostasis for Pseudomonas aeruginosa-infected burn wound healing. J Colloid Interface Sci. 2022;608(Pt 3):2278–2289. doi:10.1016/j.jcis.2021.10.131
56. Nour EDS, El-Tayeb TA, Abou-Aisha K, et al. In vitro and in vivo antimicrobial activity of combined therapy of silver nanoparticles and visible blue light against Pseudomonas aeruginosa. Int J Nanomed. 2016;11(11):1749–1758. doi:10.2147/IJN.S102398
57. De Marchi J, Jornada DS, Silva FK, et al. Triclosan resistance reversion by encapsulation in chitosan-coated-nanocapsule containing α-bisabolol as core: development of wound dressing. Int J Nanomed. 2017;Volume 12(12):7855–7868. doi:10.2147/IJN.S143324
58. Aurore V, Caldana F, Blanchard M, et al. Silver-nanoparticles increase bactericidal activity and radical oxygen responses against bacterial pathogens in human osteoclasts. Nanomedicine. 2018;14(2):601–607. doi:10.1016/j.nano.2017.11.006
59. Huang Q, Ouyang Z, Tan Y, et al. Activating macrophages for enhanced osteogenic and bactericidal performance by Cu ion release from micro/nano-topographical coating on a titanium substrate. Acta Biomater. 2019;100(100):415–426. doi:10.1016/j.actbio.2019.09.030
60. Saleh B, Dhaliwal HK, Portillo-Lara R, et al. Local Immunomodulation Using an Adhesive Hydrogel Loaded with miRNA-Laden Nanoparticles Promotes Wound Healing. Small. 2019;15(36):e1902232. doi:10.1002/smll.201902232
61. Deng S, Ou K, Zhang C, et al. A one-two punch strategy for diabetic wound management based on an antibiotic-hybrid biomineralized iron sulfide nanoparticle. Acta Biomater. 2024;181:333–346. doi:10.1016/j.actbio.2024.04.027
62. Rahimi H, Roudbarmohammadi S, Delavari HH, et al. Antifungal effects of indolicidin-conjugated gold nanoparticles against fluconazole-resistant strains of Candida albicans isolated from patients with burn infection. Int J Nanomed. 2019;14:5323–5338. doi:10.2147/IJN.S207527
63. Korupalli C, Huang CC, Lin WC, et al. Acidity-triggered charge-convertible nanoparticles that can cause bacterium-specific aggregation in situ to enhance photothermal ablation of focal infection. Biomaterials. 2017;116(116):1–9. doi:10.1016/j.biomaterials.2016.11.045
64. Yang J, Zhu YX, Lu P, et al. One-step synthesis of quaternized silica nanoparticles with bacterial adhesion and aggregation properties for effective antibacterial and antibiofilm treatments. J Mater Chem B. 2022;10(16):3073–3082. doi:10.1039/D1TB02830H
65. Qian W, Yan C, He D, et al. pH-triggered charge-reversible of glycol chitosan conjugated carboxyl graphene for enhancing photothermal ablation of focal infection. Acta Biomater. 2018;69(69):256–264. doi:10.1016/j.actbio.2018.01.022
66. Qing G, Zhao X, Gong N, et al. Thermo-responsive triple-function nanotransporter for efficient chemo-photothermal therapy of multidrug-resistant bacterial infection. Nat Commun. 2019;10(1):4336. doi:10.1038/s41467-019-12313-3
67. Liu T, Liu Y, Liu M, et al. Synthesis of graphene oxide-quaternary ammonium nanocomposite with synergistic antibacterial activity to promote infected wound healing. Burns Trauma. 2018;6:16. doi:10.1186/s41038-018-0115-2
68. Pradeep A, Ashok N, Priya V, et al. Colistimethate sodium-chitosan hydrogel for treating Gram-negative bacterial wound infections. Int J Biol Macromol. 2022;214:610–616. doi:10.1016/j.ijbiomac.2022.06.113
69. Cheng C, Zhong H, Zhang Y, et al. Bacterial responsive hydrogels based on quaternized chitosan and GQDs-epsilon-PL for chemo-photothermal synergistic anti-infection in diabetic wounds. Int J Biol Macromol. 2022;210:377–393. doi:10.1016/j.ijbiomac.2022.05.008
70. Jin Y, Yang Y, Duan W, et al. Synergistic and On-Demand Release of Ag-AMPs Loaded on Porous Silicon Nanocarriers for Antibacteria and Wound Healing. ACS Appl Mater Interfaces. 2021;13(14):16127–16141. doi:10.1021/acsami.1c02161
71. Li G, Huang Y, Zhao L, et al. Targeting and Microenvironment-Activated Nanoreactor for Diabetic Chronic Wound Healing via Multienzyme Cascade Reactions. ACS Appl Mater Interfaces. 2024;16(5):6315–6326. doi:10.1021/acsami.3c12427
72. Fulaz S, Vitale S, Quinn L, et al. Nanoparticle-Biofilm Interactions: the Role of the EPS Matrix. Trends Microbiol. 2019;27(11):915–926. doi:10.1016/j.tim.2019.07.004
73. Gao X, Middepogu A, Deng R, et al. Adsorption of extracellular polymeric substances from two microbes by TiO2 nanoparticles. Sci Total Environ. 2019;694:133778. doi:10.1016/j.scitotenv.2019.133778
74. Ali K, Ahmed B, Khan MS, Musarrat J. Differential surface contact killing of pristine and low EPS pseudomonas aeruginosa with aloe vera capped hematite (α-Fe2O3) nanoparticles. J Photochem Photobiol B. 2018;188:146–158. doi:10.1016/j.jphotobiol.2018.09.017
75. Hiebner DW, Barros C, Quinn L, et al. Surface functionalization-dependent localization and affinity of SiO2 nanoparticles within the biofilm EPS matrix. Biofilm. 2020;2:100029. doi:10.1016/j.bioflm.2020.100029
76. Weldrick PJ, Hardman MJ, Paunov VN. Enhanced Clearing of Wound-Related Pathogenic Bacterial Biofilms Using Protease-Functionalized Antibiotic Nanocarriers. ACS Appl Mater Interfaces. 2019;11(47):43902–43919. doi:10.1021/acsami.9b16119
77. More PR, Pandit S, Filippis A, et al. Silver Nanoparticles: bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms. 2023;11(2):369. doi:10.3390/microorganisms11020369
78. Dong F, Zhou Y. Differential transformation and antibacterial effects of silver nanoparticles in aerobic and anaerobic environment. Nanotoxicology. 2019;13(3):339–353. doi:10.1080/17435390.2018.1548667
79. Liu F, He D, Yu Y, et al. Quaternary Ammonium Salt-Based Cross-Linked Micelles to Combat Biofilm. Bioconjug Chem. 2019;30(3):541–546. doi:10.1021/acs.bioconjchem.9b00010
80. Wang X, Wang D, Lu H, et al. Strategies to Promote the Journey of Nanoparticles Against Biofilm-Associated Infections. Small. 2024;20(10):e2305988. doi:10.1002/smll.202305988
81. Boursier ME, Moore JD, Heitman KM, et al. Structure-Function Analyses of the N-Butanoyl l-Homoserine Lactone Quorum-Sensing Signal Define Features Critical to Activity in RhlR. ACS Chem Biol. 2018;13(9):2655–2662. doi:10.1021/acschembio.8b00577
82. Miller KP, Wang L, Chen YP, et al. Engineering nanoparticles to silence bacterial communication. Front Microbiol. 2015;6:189. doi:10.3389/fmicb.2015.00189
83. Saeki EK, Martins HM, Camargo LC, et al. Effect of Biogenic Silver Nanoparticles on the Quorum-Sensing System of Pseudomonas aeruginosa PAO1 and PA14. Microorganisms. 2022;10(9):1755. doi:10.3390/microorganisms10091755
84. Hu C, He G, Yang Y, et al. Nanomaterials Regulate Bacterial Quorum Sensing: applications, Mechanisms, and Optimization Strategies. Adv Sci. 2024;11(15):e2306070. doi:10.1002/advs.202306070
85. Ascenso OS, Torcato IM, Miguel AS, et al. Synthesis and biological activity of a potent optically pure autoinducer-2 quorum sensing agonist. Bioorg Chem. 2019;85:75–81. doi:10.1016/j.bioorg.2018.12.022
86. Gomez-Gomez B, Arregui L, Serrano S, et al. Unravelling mechanisms of bacterial quorum sensing disruption by metal-based nanoparticles. Sci Total Environ. 2019;696:133869. doi:10.1016/j.scitotenv.2019.133869
87. Guan Y, Tsao CY, Quan DN, et al. Focusing quorum sensing signalling by nano-magnetic assembly. Environ Microbiol. 2018;20(7):2585–2597. doi:10.1111/1462-2920.14284
88. Fernandes R, Tsao CY, Hashimoto Y, et al. Magnetic nanofactories: localized synthesis and delivery of quorum-sensing signaling molecule autoinducer-2 to bacterial cell surfaces. Metab Eng. 2007;9(2):228–239. doi:10.1016/j.ymben.2006.11.004
89. Chen Y, Gao Y, Huang Y, et al. Inhibiting Quorum Sensing by Active Targeted pH-Sensitive Nanoparticles for Enhanced Antibiotic Therapy of Biofilm-Associated Bacterial Infections. ACS Nano. 2023;17(11):10019–10032. doi:10.1021/acsnano.2c12151
90. Cheng J, Zhang H, Lu K, et al. Bi-functional quercetin/copper nanoparticles integrating bactericidal and anti-quorum sensing properties for preventing the formation of biofilms. Biomater Sci. 2024;12(7):1788–1800. doi:10.1039/D4BM00034J
91. Xu C, Bing W, Wang F, et al. Versatile Dual Photoresponsive System for Precise Control of Chemical Reactions. ACS Nano. 2017;11(8):7770–7780. doi:10.1021/acsnano.7b01450
92. Shan J, Li X, Yang K, et al. Efficient Bacteria Killing by Cu(2)WS(4) Nanocrystals with Enzyme-like Properties and Bacteria-Binding Ability. ACS Nano. 2019;13(12):13797–13808. doi:10.1021/acsnano.9b03868
93. Chen Z, Wang Z, Ren J, et al. Enzyme Mimicry for Combating Bacteria and Biofilms. Acc Chem Res. 2018;51(3):789–799. doi:10.1021/acs.accounts.8b00011
94. Ilk S, Saglam N, Ozgen M, et al. Chitosan nanoparticles enhances the anti-quorum sensing activity of kaempferol. Int J Biol Macromol. 2017;94(Pt A):653–662. doi:10.1016/j.ijbiomac.2016.10.068
95. Saito A, Miyazaki H, Fujie T, et al. Therapeutic efficacy of an antibiotic-loaded nanosheet in a murine burn-wound infection model. Acta Biomater. 2012;8(8):2932–2940. doi:10.1016/j.actbio.2012.04.019
96. Fleming D, Niese B, Redman W, et al. Contribution of Pseudomonas aeruginosa Exopolysaccharides Pel and Psl to Wound Infections. Front Cell Infect Microbiol. 2022;12:835754. doi:10.3389/fcimb.2022.835754
97. Jamil B, Imran M. Factors pivotal for designing of nanoantimicrobials: an exposition. Crit Rev Microbiol. 2018;44(1):79–94. doi:10.1080/1040841X.2017.1313813
98. Zalis EA, Nuxoll AS, Manuse S, et al. Stochastic Variation in Expression of the Tricarboxylic Acid Cycle Produces Persister Cells. mBio. 2019;10(5). doi:10.1128/mBio.01930-19.
99. Mei L, Lu Z, Zhang W, et al. Bioconjugated nanoparticles for attachment and penetration into pathogenic bacteria. Biomaterials. 2013;34(38):10328–10337. doi:10.1016/j.biomaterials.2013.09.045
100. Tu C, Lu H, Zhou T, et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials. 2022;286:121597. doi:10.1016/j.biomaterials.2022.121597
101. Gao Y, Wu J, Shen J, et al. Chitosan modified magnetic nanocomposite for biofilm destruction and precise photothermal/photodynamic therapy. Int J Biol Macromol. 2024;259(Pt 2):129402. doi:10.1016/j.ijbiomac.2024.129402
102. Pu Y, Li Y, Jin X, et al. ATP-Dependent Dynamic Protein Aggregation Regulates Bacterial Dormancy Depth Critical for Antibiotic Tolerance. Mol Cell. 2019;73(1):143–156. doi:10.1016/j.molcel.2018.10.022
103. Peng Y, Song C, Yang C, et al.Low molecular weight chitosan-coated silver nanoparticles are effective for the treatment of MRSA-infected wounds. Int J Nanomed. 2017;(12):295–304. doi:10.2147/IJN.S122357
104. Haidari H, Bright R, Strudwick XL, et al. Multifunctional ultrasmall AgNP hydrogel accelerates healing of S. aureus infected wounds. Acta Biomater. 2021;128(128):420–434. doi:10.1016/j.actbio.2021.04.007
105. Zille A, Fernandes MM, Francesko A, et al. Size and Aging Effects on Antimicrobial Efficiency of Silver Nanoparticles Coated on Polyamide Fabrics Activated by Atmospheric DBD Plasma. ACS Appl Mater Interfaces. 2015;7(25):13731–13744. doi:10.1021/acsami.5b04340
106. Orlowski P, Zmigrodzka M, Tomaszewska E, et al. Tannic acid-modified silver nanoparticles for wound healing: the importance of size. Int J Nanomed. 2018;Volume 13(13):991–1007. doi:10.2147/IJN.S154797
107. Huma ZE, Javed I, Zhang Z, et al. Nanosilver Mitigates Biofilm Formation via FapC Amyloidosis Inhibition. Small. 2020;16(21):e1906674.
108. Truong T, Kumar SR, Huang YT, et al. Size-Dependent Antibacterial Activity of Silver Nanoparticle-Loaded Graphene Oxide Nanosheets. Nanomaterials. 2020;10(6):1207. doi:10.3390/nano10061207
109. Nie X, Gao F, Wang F, et al. Charge-reversal silver clusters for targeted bacterial killing. J Mater Chem B. 2021;9(19):4006–4014. doi:10.1039/D1TB00378J
110. Mi G, Shi D, Wang M, et al. Reducing Bacterial Infections and Biofilm Formation Using Nanoparticles and Nanostructured Antibacterial Surfaces. Adv Healthc Mater. 2018;7(13):e1800103. doi:10.1002/adhm.201800103
111. Wehling J, Dringen R, Zare RN, et al. Bactericidal activity of partially oxidized nanodiamonds. ACS Nano. 2014;8(6):6475–6483. doi:10.1021/nn502230m
112. Salvioni L, Galbiati E, Collico V, et al. Negatively charged silver nanoparticles with potent antibacterial activity and reduced toxicity for pharmaceutical preparations. Int J Nanomed. 2017;Volume 12(12):2517–2530. doi:10.2147/IJN.S127799
113. Elnaggar MG, Jiang K, Eldesouky HE, et al. Antibacterial nanotruffles for treatment of intracellular bacterial infection. Biomaterials. 2020;262:120344. doi:10.1016/j.biomaterials.2020.120344
114. Tang S, Zheng J. Antibacterial Activity of Silver Nanoparticles: structural Effects. Adv Healthc Mater. 2018;7(13):e1701503. doi:10.1002/adhm.201701503
115. Mandal D, Kumar DS, Das B, et al. Bio-fabricated silver nanoparticles preferentially targets Gram positive depending on cell surface charge. Biomed Pharmacother. 2016;83(83):548–558. doi:10.1016/j.biopha.2016.07.011
116. El Nahrawy AM, Hemdan BA, Abou Hammad AB. Morphological, impedance and terahertz properties of zinc titanate/fe 3 + nanocrystalline for suppression of pseudomonas aeruginosa biofilm. Nano-Struct Nano-Objects. 2021;26:100715. doi:10.1016/j.nanoso.2021.100715
117. Hameed S, Wang Y, Zhao L, et al. Shape-dependent significant physical mutilation and antibacterial mechanisms of gold nanoparticles against foodborne bacterial pathogens (Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus) at lower concentrations. Mater Sci Eng C. 2020;108:110338. doi:10.1016/j.msec.2019.110338
118. Kumari M, Pandey S, Giri VP, et al. Tailoring shape and size of biogenic silver nanoparticles to enhance antimicrobial efficacy against MDR bacteria. Microb Pathog. 2017;105(105):346–355. doi:10.1016/j.micpath.2016.11.012
119. Li K, Qian J, Wang P, et al. Responses of freshwater biofilm formation processes (from colonization to maturity) to anatase and rutile TiO2 nanoparticles: effects of nanoparticles aging and transformation. Water Res. 2020;182:115953. doi:10.1016/j.watres.2020.115953
120. Cha SH, Hong J, McGuffie M, et al. Shape-Dependent Biomimetic Inhibition of Enzyme by Nanoparticles and Their Antibacterial Activity. ACS Nano. 2015;9(9):9097–9105. doi:10.1021/acsnano.5b03247
121. Cheng X, Pei X, Xie W, et al. pH‐Triggered Size‐Tunable Silver Nanoparticles: targeted Aggregation for Effective Bacterial Infection Therapy. Small. 2022;18(22). doi:10.1002/smll.202200915.
122. Houshyar S, Kumar GS, Rifai A, et al. Nanodiamond/poly-epsilon-caprolactone nanofibrous scaffold for wound management. Mater Sci Eng C. 2019;100:378–387. doi:10.1016/j.msec.2019.02.110
123. Aditya A, Chattopadhyay S, Jha D, et al. Zinc Oxide Nanoparticles Dispersed in Ionic Liquids Show High Antimicrobial Efficacy to Skin-Specific Bacteria. ACS Appl Mater Interfaces. 2018;10(18):15401–15411. doi:10.1021/acsami.8b01463
124. Youssef K, Ullah A, Rezai P, et al. Recent advances in biosensors for real time monitoring of pH, temperature, and oxygen in chronic wounds. Mater Today Bio. 2023;22:100764. doi:10.1016/j.mtbio.2023.100764
125. Cheon JY, Kim SJ, Rhee YH, et al. Shape-dependent antimicrobial activities of silver nanoparticles. Int J Nanomed. 2019;Volume 14(14):2773–2780. doi:10.2147/IJN.S196472
126. Han Y, Pan H, Li W, et al. T Cell Membrane Mimicking Nanoparticles with Bioorthogonal Targeting and Immune Recognition for Enhanced Photothermal Therapy. Adv Sci. 2019;6(15):1900251. doi:10.1002/advs.201900251
127. Ghaseminezhad SM, Shojaosadati SA, Meyer RL. Ag/Fe3O4 nanocomposites penetrate and eradicate S. aureus biofilm in an in vitro chronic wound model. Colloids Surf B. 2018;163:192–200. doi:10.1016/j.colsurfb.2017.12.035
128. Da SJ, Leal EC, Carvalho E. Bioactive Antimicrobial Peptides as Therapeutic Agents for Infected Diabetic Foot Ulcers. Biomolecules. 2021;11(12):1.
129. Cau L, Williams MR, Butcher AM, et al. Staphylococcus epidermidis protease EcpA can be a deleterious component of the skin microbiome in atopic dermatitis. J Allergy Clin Immunol. 2021;147(3):955–966. doi:10.1016/j.jaci.2020.06.024
130. Trivedi U, Madsen JS, Rumbaugh KP, et al. A post-planktonic era of in vitro infectious models: issues and changes addressed by a clinically relevant wound like media. Crit Rev Microbiol. 2017;43(4):453–465. doi:10.1080/1040841X.2016.1252312
131. A Uberoi, A McCready-Vangi, EA Grice. The wound microbiota: microbial mechanisms of impaired wound healing and infection. Nat Rev Microbiol. 2024;2024:1.
132. Silva V, Peirone C, Amaral JS, et al. High Efficacy of Ozonated Oils on the Removal of Biofilms Produced by Methicillin-Resistant Staphylococcus aureus (MRSA) from Infected Diabetic Foot Ulcers. Molecules. 2020;25(16):3601. doi:10.3390/molecules25163601
133. Ferreres G, Bassegoda A, Hoyo J, et al. Metal-Enzyme Nanoaggregates Eradicate Both Gram-Positive and Gram-Negative Bacteria and Their Biofilms. ACS Appl Mater Interfaces. 2018;10(47):40434–40442. doi:10.1021/acsami.8b14949
134. Sanchez MC, Toledano-Osorio M, Bueno J, et al. Antibacterial effects of polymeric PolymP-n Active nanoparticles. An in vitro biofilm study. Dent Mater. 2019;35(1):156–168. doi:10.1016/j.dental.2018.11.015
135. Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi:10.1126/science.1092385
136. Wang P, Wu B, Li M, et al. Lysosome-Targeting Aggregation-Induced Emission Nanoparticle Enables Adoptive Macrophage Transfer-Based Precise Therapy of Bacterial Infections. ACS Nano. 2023;17(11):10365–10375. doi:10.1021/acsnano.3c00796
137. Reis ES, Mastellos DC, Hajishengallis G, Lambris JD. New insights into the immune functions of complement. Nat Rev Immunol. 2019;19(8):503–516. doi:10.1038/s41577-019-0168-x
138. Maltz-Matyschsyk M, Melchiorre CK, Knecht DA, Lynes MA. Bacterial metallothionein, PmtA, a novel stress protein found on the bacterial surface of Pseudomonas aeruginosa and involved in management of oxidative stress and phagocytosis. Johnson MDL. editor. mSphere. 2024; Vol. 9(5):e00210–24
139. Raafat D, Otto M, Reppschlager K, et al. Fighting Staphylococcus aureus Biofilms with Monoclonal Antibodies. Trends Microbiol. 2019;27(4):303–322. doi:10.1016/j.tim.2018.12.009
140. Zhang K, Li Y, He J, et al. Therapeutic Effect of Epidermal Growth Factor Combined With Nano Silver Dressing on Diabetic Foot Patients. Front Pharmacol. 2021;12:627098. doi:10.3389/fphar.2021.627098
141. Lin Z, Wu T, Wang W, et al. Biofunctions of antimicrobial peptide-conjugated alginate/hyaluronic acid/collagen wound dressings promote wound healing of a mixed-bacteria-infected wound. Int J Biol Macromol. 2019;140(140):330–342. doi:10.1016/j.ijbiomac.2019.08.087
142. Wright E, Neethirajan S, Weng X. Microfluidic wound model for studying the behaviors of Pseudomonas aeruginosa in polymicrobial biofilms. Biotechnol Bioeng. 2015;112(11):2351–2359. doi:10.1002/bit.25651
143. Nie X, Gao F, Wang F, et al. Charge-reversal silver clusters for targeted bacterial killing. J Mater Chem B. 2021;9(19):46–414.
144. Choudhury R, Majumdar M, Biswas P, Khan S, Misra TK. Kinetic study of functionalization of citrate stabilized silver nanoparticles with catechol and its anti-biofilm activity. Nano-Struct Nano-Objects. 2019;19:100326. doi:10.1016/j.nanoso.2019.100326
145. Dalisson B, Barralet J. Bioinorganics and Wound Healing. Adv Healthc Mater. 2019;8(18):e1900764. doi:10.1002/adhm.201900764
146. Wahid F, Zhao X, Zhao X, et al. Fabrication of Bacterial Cellulose-Based Dressings for Promoting Infected Wound Healing. ACS Appl Mater Interfaces. 2021;13(28):32716–32728. doi:10.1021/acsami.1c06986
147. Franco AC, Aveleira C, Cavadas C. Skin senescence: mechanisms and impact on whole-body aging. Trends Mol Med. 2022;28(2):97–109. doi:10.1016/j.molmed.2021.12.003
148. Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6(265):265sr6. doi:10.1126/scitranslmed.3009337
149. Abd EN, Ahmed EA, Abd-Ellatief RB, et al. Metoclopramide nanoparticles modulate immune response in a diabetic rat model: association with regulatory T cells and proinflammatory cytokines. Int J Nanomed. 2019;14:2383–2395. doi:10.2147/IJN.S196842
150. Galili U, Goldufsky JW, Schaer GL. alpha-Gal Nanoparticles Mediated Homing of Endogenous Stem Cells for Repair and Regeneration of External and Internal Injuries by Localized Complement Activation and Macrophage Recruitment. Int J Mol Sci. 2022;23(19):11490. doi:10.3390/ijms231911490
151. Yu B, Wang Z, Almutairi L, et al. Harnessing iron-oxide nanoparticles towards the improved bactericidal activity of macrophage against Staphylococcus aureus. Nanomedicine. 2020;24:102158. doi:10.1016/j.nano.2020.102158
152. Cheng J, Zhang Q, Fan S, et al. The vacuolization of macrophages induced by large amounts of inorganic nanoparticle uptake to enhance the immune response. Nanoscale. 2019;11(47):22849–22859. doi:10.1039/C9NR08261A
153. Graves N, Phillips CJ, Harding K. A narrative review of the epidemiology and economics of chronic wounds. Br J Dermatol. 2022;187(2):141–148. doi:10.1111/bjd.20692
154. Li D, Wu M. Pattern recognition receptors in health and diseases. Signal Transduction Target Ther. 2021;2021:1.
155. Fromantin I, Seyer D, Watson S, et al. Bacterial floras and biofilms of malignant wounds associated with breast cancers. J Clin Microbiol. 2013;51(10):3368–3373. doi:10.1128/JCM.01277-13
156. Singh NB, Kumar B, Usman UL, Susan M. Nano revolution: exploring the frontiers of nanomaterials in science, technology, and society. Nano-Struct Nano-Objects. 2024;39:101299. doi:10.1016/j.nanoso.2024.101299
157. Buch PJ, Chai Y, Goluch ED. Treating Polymicrobial Infections in Chronic Diabetic Wounds. Clin Microbiol Rev. 2019;32(2). doi:10.1128/CMR.00091-18
158. Wang C, Gu B, Liu Q, et al.Combined use of vancomycin-modified Ag-coated magnetic nanoparticles and secondary enhanced nanoparticles for rapid surface-enhanced Raman scattering detection of bacteria. Int J Nanomed. 2018;(13):1159–1178. doi:10.2147/IJN.S150336
159. Qin S, Xiao W, Zhou C, et al. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct Target Ther. 2022;7(1):199. doi:10.1038/s41392-022-01056-1
160. Tan Y, Ma S, Leonhard M, et al. Enhancing antibiofilm activity with functional chitosan nanoparticles targeting biofilm cells and biofilm matrix. Carbohydr Polym. 2018;200(200):35–42. doi:10.1016/j.carbpol.2018.07.072
161. Lu MM, Ge Y, Qiu J, et al. Redox/pH dual-controlled release of chlorhexidine and silver ions from biodegradable mesoporous silica nanoparticles against oral biofilms. Int J Nanomed. 2018;Volume 13(13):7697–7709. doi:10.2147/IJN.S181168
162. Jiang L, Loo SCJ. Intelligent Nanoparticle-Based Dressings for Bacterial Wound Infections. ACS Appl Bio Mater. 2021;4(5):3849–3862. doi:10.1021/acsabm.0c01168
163. Qi X, Xiang Y, Cai E, et al. Inorganic–organic hybrid nanomaterials for photothermal antibacterial therapy. Coord. Chem. Rev. 2023;496:215426. doi:10.1016/j.ccr.2023.215426
164. Makabenta J, Nabawy A, Li CH, et al. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat Rev Microbiol. 2021;19(1):23–36. doi:10.1038/s41579-020-0420-1
165. Lu J, Wang Y, Jin M, et al. Both silver ions and silver nanoparticles facilitate the horizontal transfer of plasmid-mediated antibiotic resistance genes. Water Res. 2020;169:115229. doi:10.1016/j.watres.2019.115229
166. Zhang Y, Mortimer M, Guo LH. Interplay between engineered nanomaterials and microbiota. Environ Sci. 2020;7(9):2454–2485.
167. Lamas B, Martins BN, Houdeau E. Impacts of foodborne inorganic nanoparticles on the gut microbiota-immune axis: potential consequences for host health. Part Fibre Toxicol. 2020;17(1):19. doi:10.1186/s12989-020-00349-z
168. Senthamarai MD, Hillary VE, Rajan MR, Ceasar SA. Biosynthesis of selenium nanoparticles and its biological applications: a systematic review. Nano-Struct Nano-Objects. 2024;39:101261. doi:10.1016/j.nanoso.2024.101261
169. Zhu W, Mei J, Zhang X, et al. Photothermal Nanozyme-Based Microneedle Patch against Refractory Bacterial Biofilm Infection via Iron-Actuated Janus Ion Therapy. Adv Mater. 2022;34(51):e2207961. doi:10.1002/adma.202207961
170. Roy S, Mondal A, Yadav V, et al. Mechanistic Insight into the Antibacterial Activity of Chitosan Exfoliated MoS(2) Nanosheets: membrane Damage, Metabolic Inactivation, and Oxidative Stress. ACS Appl Bio Mater. 2019;2(7):2738–2755. doi:10.1021/acsabm.9b00124
171. Linklater DP, Baulin VA, Juodkazis S, et al. Mechano-bactericidal actions of nanostructured surfaces. Nat Rev Microbiol. 2021;19(1):8–22. doi:10.1038/s41579-020-0414-z
172. Furukawa H, Cordova KE, O’Keeffe M, Yaghi OM. The chemistry and applications of metal-organic frameworks. Science. 2013;341(6149):1230444. doi:10.1126/science.1230444
173. Qi X, Huang Y, You S, et al. Engineering Robust Ag-Decorated Polydopamine Nano-Photothermal Platforms to Combat Bacterial Infection and Prompt Wound Healing. Adv Sci. 2022;9(11):e2106015. doi:10.1002/advs.202106015
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.