Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 20
How People with COPD Perceive and Communicate Exacerbations: A Multicountry Survey Study
Authors Franssen FME, Young R , van Boven JFM , Crooks MG , Eckerd M , Grobert M , Hurst JR , Hutchinson A , Linnell J , Stolz D, Winders T, Zhang J , El Khoury J , Nordon C
Received 28 January 2025
Accepted for publication 14 June 2025
Published 24 June 2025 Volume 2025:20 Pages 2035—2048
DOI https://doi.org/10.2147/COPD.S519772
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Zhang
Frits ME Franssen,1 Ruth Young,2 Job FM van Boven,3 Michael G Crooks,4 Marie Eckerd,5 Megan Grobert,2 John R Hurst,6 Ann Hutchinson,7 John Linnell,8 Daiana Stolz,9 Tonya Winders,10 Jing Zhang,11 Jad El Khoury,12 Clementine Nordon13
1Department of Respiratory Medicine, Maastricht University Medical Centre, Maastricht, the Netherlands; 2Ipsos Public Affairs, Washington, DC, USA; 3Department of Clinical Pharmacy and Pharmacology, Groningen Research Institute for Asthma and COPD (GRIAC), University Medical Center Groningen, University of Groningen, Groningen, the Netherlands; 4Respiratory Research Group, Hull University Teaching Hospitals NHS Trust, Hull, UK; 5Global Patient Engagement R&I, AstraZeneca, Wilmington, DE, USA; 6UCL Respiratory, University College London, London, UK; 7Hull York Medical School, University of Hull, Hull, UK; 8Patient Author, COPD Foundation, Washington, DC, USA; 9Clinic of Respiratory Medicine and Faculty of Medicine, University of Freiburg, Freiburg, Germany; 10Global Allergy & Airways Patient Platform, Vienna, Austria; 11Department of Pulmonary and Critical Care Medicine, Zhongshan Hospital, Shanghai Medical College, Fudan University, Shanghai, People’s Republic of China; 12Global Medical Affairs – Respiratory, BioPharmaceuticals Medical, AstraZeneca, Cambridge, UK; 13Evidence and Strategy – Respiratory, BioPharmaceuticals Medical, AstraZeneca, Cambridge, UK
Correspondence: Frits ME Franssen, Maastricht University Medical Centre, P. Debyelaan 25, 6229 HX, Maastricht, the Netherlands, Tel +31 43-3875044, Email [email protected]
Background and Objective: Exacerbations negatively impact quality of life of people living with chronic obstructive pulmonary disease (COPD) and can accelerate disease progression. Studies suggest that patients find it difficult to recognize exacerbations and, therefore, under-report exacerbations. We aimed to understand how people living with COPD perceive and communicate their experiences of exacerbations.
Methods: A cross-sectional survey including one open-ended question was developed using a targeted literature review, with input from patients and clinicians. People diagnosed with COPD were recruited from online consumer research panels in Brazil, China, France, Spain, UK, and USA and completed the survey. Responses were described overall and in specific subgroups; thematic analysis was used for the open-ended question.
Results: Of 857 respondents (median age 58 years; 50.5% male), 623 (72.7%) reported daily shortness of breath; 417 (48.7%) that daily symptoms changed “a little”. In the open-ended question, exacerbations were described through a narrative lens detailing subjective experiences of symptoms, their management, potential causes, and emotions felt during exacerbations, with no single preferred term. In the 671 (78.3%) respondents who reported ever having an exacerbation, these were identified as symptoms being “worse than usual” (52.8%) or because respondents had “more trouble than usual with daily activities” (50.1%).
Conclusion: While people living with COPD report confidence in their ability to identify exacerbations, there is a disconnect between their experience of exacerbations and the language and definitions used in clinical practice. A discussion guide emphasizing the use of plain language could improve communication between healthcare providers and patients.
Keywords: chronic obstructive pulmonary disease, cough, quality of life, observational, symptoms
Introduction
Chronic obstructive pulmonary disease (COPD) poses a significant global health challenge, profoundly impacting the quality of life of an estimated 392 million people worldwide.1 COPD is characterized by day-to-day variation in symptoms and, in many patients, periods of exacerbation,2,3 defined as a deterioration in symptoms, over a period of less than 14 days and where other causes have been considered and excluded.4,5 Exacerbations are costly for health systems to treat, severely impact quality of life5–7 and escalate the risk of cardiopulmonary events, including subsequent exacerbations, cardiovascular events, and death, as well as other adverse medical outcomes. The prevention of COPD exacerbations is a key therapeutic objective, and prompt treatment of an exacerbation can reduce severity and accelerate recovery.8 This requires not only the accurate and early identification of exacerbations by patients but also the reporting of exacerbations to their healthcare providers (HCPs). However, COPD exacerbations are often poorly recognized and under-reported. Previous research suggests that one-third of people with COPD are aware when an exacerbation takes place,9,10 but less than one-third of exacerbations, when perceived, are reported.11 Several factors may contribute to the poor recognition and under-reporting of COPD exacerbations. Firstly, the major symptoms of COPD exacerbations are diverse and nonspecific.5 People living with COPD and other comorbidities may misattribute exacerbation symptoms, like breathlessness, to other conditions, such as heart failure or arrhythmia.5 Furthermore, the variation in symptoms associated with an exacerbation, and for some, the gradual worsening of symptoms,12 may make it difficult to distinguish between an exacerbation and daily symptom variation.13 Secondly, miscommunication between patients and their HCPs may lead to under-reporting of COPD exacerbations. For instance, people living with COPD often use nonmedical terms for an exacerbation (eg, “breathless”, “exhaustion”, or “dizziness”).9,14 Prior research has also indicated that patients and their clinicians rarely agree on what are the most concerning symptoms, exemplifying the potential communication gap between clinicians and people living with COPD.15 Improved understanding of how people living with COPD recognize and convey their experiences with exacerbations to HCPs is essential for developing strategies to enhance early detection, patient education, and overall management.16 Recognizing the challenges associated with identifying and reporting COPD exacerbations, few studies have explored how patients experience, recognize, and communicate exacerbations. Moreover, most studies were conducted with small sample sizes and in a limited number of Western countries.9,10,12–14 The present study aimed to explore how people living with COPD in multiple countries (1) experience day-to-day symptoms; (2) perceive, experience, and describe exacerbations; and (3) distinguish between day-to-day symptom variation and exacerbations.
Methods
This multicountry, observational, cross-sectional study used survey data collected from people living with COPD in Brazil, China, France, Spain, the UK, and the USA and is reported in accordance with the Consensus-Based Checklist for Reporting of Survey Studies (CROSS).17
Survey Development
A patients’ survey was developed based on a targeted literature review and guided by members of the “ACT on COPD” taskforce supported by AstraZeneca. This taskforce is an international, multidisciplinary research team with expertise in COPD comprising HCPs, researchers, and patient advocacy group representatives. Briefly, a targeted literature review identified peer-reviewed qualitative studies to better understand the nuances of patients’ experience and build a conceptual framework. Subsequently, a first version of the survey was created, and questions were checked for readability and comprehensiveness by five people living with COPD. The research team conducted several rounds of reviews until consensus was reached on the number and phrasing of questions (detailed methodology; Text S1, Tables S1 and S2).
The final version of the survey comprised 27 questions, including one open-ended question, and was organized into the following four sections: (1) socio-demographics and comorbidities; (2) perceptions and experiences with day-to-day symptoms of COPD, including the modified Medical Research Council (mMRC) dyspnea scale to assess severity of breathlessness;18 and (3) one open-ended question asking participants to describe “exacerbations” and aiming at understanding how respondents described exacerbations (if any) in their own words
Some people with COPD have times where their symptoms get worse than usual. During these times, people may do things to make themselves feel better, such as breathing exercises, using an inhaler, contacting their healthcare provider, taking antibiotics or steroids, or going to hospital. How would you describe these times when someone’s COPD symptoms are worse than usual?
All respondents contributed to the survey up until section (3), irrespective of whether they ever had an exacerbation of COPD. This allowed us to collect the respondents’ wording without having to formally define what was meant by an “exacerbation” in the survey and, thereby, influence their responses.19 For those who reported ever having an exacerbation, section (4) explored perceptions and experiences of, and actions taken during, COPD exacerbations (Figure S1).
The original survey was drafted in English then surveys were translated and back translated into the primary language for each country. Surveys were administered in Portuguese in Brazil, simplified Chinese in China, French in France, Spanish in Spain, and English in the UK and the USA. All questions required a response. The estimated time for completion was 10–12 minutes.
Source Population
To cover various countries, languages, and cultures, participants were recruited from Brazil, China, France, Spain, the UK, and the USA. For the first five countries, people were recruited from existing online consumer research panels run by m360.20 Panelists were invited via emails, banners, and loyalty programs. Panelists had to confirm their participation via email before being allowed to join the panels, following a double opt in.21 In the USA, panelists were recruited from Ipsos KnowledgePanel, a probability-based panel which recruits panelists through address-based sampling22 that can be used in, for example, public health research.23,24 US panelists who had previously self-reported that they were living with COPD were invited to take part in the survey. Both m360 and KnowledgePanel incentivize participation in surveys through vouchers. The study sponsor was unknown to respondents and was not involved in respondents’ identification, selection, or data collection.
Study Participants
All subjects gave their informed consent for inclusion before they participated in the study; this included consent to use de-identified responses for research and academic purposes.
Inclusion criteria were self-completed by respondents using screening questions, including age ≥35 years; COPD diagnosis provided by their HCP; living in Brazil, China, France, Spain, the UK, or the USA at the time of the survey; and willing and able to provide informed consent in the primary language of the country of residence. Regarding COPD, respondents were asked to identify their medical conditions from a group of possible conditions and did not know which one would qualify them for the study, to minimize the risk of self-selection.
A target number of 540 respondents with COPD and a history of exacerbations was determined based on the 20-to-1 sample-to-item rule of thumb.25 However, it was anticipated that 60–70% of people living with COPD would have ever experienced an exacerbation;26,27 the target sample size was increased to ≥800 participants with COPD.
Because this was an online survey, several quality checks were conducted namely to identify potential bots and avoid multiple entries from the same respondent; this used the time taken to complete the survey, straight lining of survey responses and nonsensical responses to questions. Surveys completed by suspected bots were discarded, and additional participants were included to reach the target population size.28
All surveys were administered online between March 10, 2023 and May 2, 2023.
Ethics
This study was conducted in accordance with the Declaration of Helsinki. This study was deemed exempt by the Pearl Institutional Review Board (IRB number: 00007772; Study: 23-IPSO-179) according to FDA 45 CFR 46.104(d)(2).
Analysis
Data were de-identified by the consumer research panel holders (eg, email address) prior to transfer to the data analysis team, translated into English, and then analyzed using quantitative and qualitative methods. Findings were then integrated to identify common themes.
Quantitative Analysis
Respondents’ demographics and survey responses were described overall. To understand how respondents who completed the entire survey may differ from those who completed the first parts (never exacerbated), socio-demographic and clinical characteristics were described by lifetime exacerbation status (ever/never exacerbated). Social and cultural differences were also explored through stratified analyses. The terms used to describe an exacerbation and “ways of identifying an exacerbation” were explored by region and country. Items related to emotions (“feelings associated with day-to-day symptoms of COPD”) were described by sex. “Ways of identifying an exacerbation” were described by sex and age (≥60 years / <60 years), and perceived “easiness of identification of an exacerbation” was described by dyspnea severity score. No inferential statistical tests were conducted. R version 4.2.2 and Microsoft Excel were used for analysis and data visualizations.
Qualitative Analysis
The open-ended question was analyzed using thematic analysis.29 Coding of responses used an iterative and collaborative process involving three members of the research team30 who provided an initial codebook, which was refined manually. The final codebook was applied to all remaining transcripts, with any additional emergent codes being added based on group discussion. To determine subsequent themes, the team discussed which themes were more prominent in the data29 (Text S2).
Results
In total, 857 individuals completed the survey, of whom 671 (78.3%) reported ever having an exacerbation in their lifetime (Figure 1). Respondents’ socio-demographics and comorbid diseases are summarized in Table 1. Overall, median age was 58 years; 50.5% of participants were male, and 31.5% reported comorbid asthma. Compared with those who never exacerbated, respondents who did were younger (median age 56 and 65 years old, respectively) and more likely to have comorbid asthma (35.6% and 16.7%, respectively) and to report an mMRC grade ≥2 (52.8% and 31.7%, respectively). Results by country are provided in Table S3.
 |
Table 1 Socio-Demographic Characteristics and Comorbid Diseases of Respondents, Overall and by Lifetime Exacerbation Status, [N = 857] |
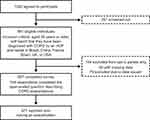 |
Figure 1 Flowchart of respondents. Brazil, China, France, Spain and the UK used opt-in panels, whereas the USA used a recruitment-based panel. An additional 70 respondents completed the survey while it was closing and were excluded. aData issues were identified based on the time taken to complete a survey, straight lining of survey responses and nonsensical responses to survey questions.28 Abbreviation: COPD, chronic obstructive pulmonary disease. |
Perception and Experience of Daily Symptoms
Daily symptoms most commonly reported were “shortness of breath” (72.7%), “tiredness” (57.8%), and “cough” (48.5%). In 755 (88.1%) respondents, the number of daily symptoms was ≥2 (Table S4). The most common pairwise combinations of symptoms were shortness of breath + tiredness (47.1%; 404/857) and shortness of breath + cough (38.3%; 328/857) (Table S5 and Figure S2).
Regarding variability of daily symptoms, the most common responses were that daily symptoms changed “a little” (48.7%; 417/857) and “always the same” (45.3%; 388/857); only 5.5% (47/857) of respondents reported that their symptoms changed “a lot”. COPD symptoms impacted daily lives “a lot” or “a great deal” for 35.0% (300/857) of respondents. Table S6 summarizes emotions associated with daily symptoms: 49.0% felt “worried”, 44.9% “frustrated”, and 34.5% “depressed”. Males felt more frequently “frustrated”, while females felt more frequently “depressed” or “panicked”.
Describing COPD Exacerbations in Their Own Words
Among the 857 respondents, 749 (87.4%) provided a reply to the open-ended question (“How would you describe these times when someone’s COPD symptoms are worse than usual?”). The large number of open-ended responses coupled with the brevity of responses meant that while saturation was reached for the themes, these were direct and brief rather than detailed. Respondents described an “exacerbation” in terms of their symptoms experienced, actions taken to treat or alleviate symptoms, and triggers of and the emotions that come with experiencing new, worse, or prolonged symptoms (Table 2). Exacerbations were described through a narrative lens detailing overall experiences. Only a minority of open-ended responses (1.3%; 10/749) used a single term or a couple of words like “exacerbation”.
 |
Table 2 Categories of Describers for an Exacerbation, Using Free Text (N = 749) |
Among the list of possible terms to describe “times when someone’s COPD symptoms are worse than usual”, a great diversity of terms were selected (Table 3), “feeling worse than usual” (40.1%), “COPD attack” (29.4%), and “cold” (26.3%) being the most commonly selected. “Flare-up” (19.8%) and “exacerbation” (17.7%) were used less frequently. There were variations by region: 61.8% of respondents in Brazil preferred “crisis” and 58.1% in China preferred “COPD attack”. One in four respondents from the USA did not use any of the terms listed and did not suggest an alternative phrase. A variety of terms were used in Spain, France, and the UK, without one clear dominant term across or within countries (Figure 2).
 |
Table 3 Terms Used to Describe an Exacerbation, Overall and by Region, [N = 857] |
Perception and Experience of Exacerbations
Of the 671 respondents who ever exacerbated, 78.5% (527/671) had their latest exacerbation in the past year. Exacerbation symptoms were considered to impact them a “great deal” or “a lot” in 54.1% (363/671) of respondents. In terms of how exacerbations impacted respondents, 73.5% (493/671) described greater difficulty than usual “doing everyday activities” and 48.1% (323/671) reported that they stayed “at home more than usual”.
Symptoms considered most alarming during an exacerbation were “shortness of breath” in 47.4% (318/671). Other symptoms were selected by less than 10% of respondents (eg, “chest tightness”, “cough”, or “difficulty doing everyday activities”). Regarding emotions, respondents reported feeling “worried” (57.2%; 384/671) or “frustrated” (40.8%; 274/671) during an exacerbation.
According to respondents, common causes of exacerbations were colds or lung infections (60.8%; 408/671) or changes in the weather (51.1%; 343/671), with 5.2% (35/671) not knowing what caused COPD exacerbations.
Distinguishing Between an Exacerbation and Daily Symptom Variation
The majority of respondents reported that recognizing an exacerbation was either “very easy” or “fairly easy” (“very easy”: 16.4% [110/671]; “fairly easy”: 35.3% [237/671]; “somewhat easy”: 37.4% [251/671]; “not easy” for 10.7% [72/671]). Recognizing an exacerbation was less frequently considered as “very” to “fairly” easy in individuals with a mMRC grade <2 versus grade ≥2 (46.3% and 56.2%, respectively) (Table S7).
Figure 3 describes the way respondents identify exacerbations either based on changes in daily symptoms, difficulties in daily activities, or by relying on other people or personal past experiences: 70.3% (472/671) of respondents identified exacerbations by noticing changes in daily symptoms (thus, 29.7% did not use symptoms to identify an exacerbation), including 52.8% (354/671) who recognized exacerbations as symptoms being “worse than usual”. In 50% (336/671) of respondents, exacerbations were identified because they had “more trouble than usual with daily activities”. Over half of the respondents (60.4%; 405/671) used two or more methods to identify an exacerbation. The most common combinations were using change in symptoms coupled with their ability to do daily activities (“activities”), described by 22.5% (106/472). There was no observable difference in ways to identify an exacerbation based on age, sex, or country. Changes in symptoms and trouble with daily activities were the most common ways to detect exacerbations across subgroups (Tables S8.1 and S8.2).
Discussion
In this multicountry study encompassing six nations, a comprehensive survey was conducted with a large sample of people living with COPD to gain insights into their perspectives on exacerbations.
Key Findings
Daily symptoms of COPD were common and varied between respondents. Most respondents did not experience significant day-to-day variation in symptoms. Strong emotions were associated with daily symptoms: more than one-third felt frustrated, depressed, or worried by daily symptoms, which significantly impacted daily lives for 35% of respondents. The majority of respondents felt somewhat confident they were able to tell when an exacerbation was about to occur. Most respondents identified an exacerbation through new, worsening, or prolonged symptoms, often in combination with other indicators like changes in daily activities. In contrast, one-third of respondents relied on changes in their daily activities, prior exacerbations, or other people telling them. A small proportion did not know how to distinguish between exacerbations and daily symptoms.
A second finding of this study is the apparent disconnect between respondents’ experience of exacerbations and the symptom-centric language used by HCPs in clinical practice. A range of terms was used by respondents, who conceptualized exacerbations beyond symptoms describing their feelings or changes in their daily activities. Functional definitions, such as difficulties doing daily activities, were often used to identify exacerbations. In contrast, clinical literature focuses on the duration and type of symptoms experienced and objective signs of clinical deterioration.5 In addition, strong emotions were reported in relation to exacerbations and are rarely used by clinicians to refer to these events (eg, being worried, frightened). To describe an exacerbation, plain language like “feeling worse than usual” was preferred to “exacerbation” or “flare-up”. These results indicate that while most respondents were confident that they could recognize an exacerbation, they often describe them in ways that differ from clinical guidelines,5 which may make it challenging to communicate their experiences to care providers.
A third result is the predominance and impact of both breathlessness and fatigue as daily symptoms. “Shortness of breath” was the most reported daily symptom, and breathlessness was considered as the “most alarming” symptom associated with COPD exacerbations. Almost half of respondents describing breathlessness as a daily symptom also reported tiredness and over one-third reported a cough. The prevalence of the reports of tiredness aligns with prior evidence indicating that fatigue is a common occurrence among patients with severe respiratory disease;31 however, the results of this population study indicate that fatigue is not restricted to patients with severe disease but may also be experienced by patients with mild or moderate COPD.
Implications for Clinical Practice
In contrast to prior studies, respondents did not report significant variation in daily COPD symptoms and were relatively confident that they could identify an exacerbation.5,9,11,32 Identifying an exacerbation does not mean that exacerbations are reported to HCPs.11 Our results suggest reasons why under-reporting of exacerbations may persist despite some confidence in recognizing them. In line with prior studies, the survey results showed that respondents identified exacerbations through their functional impact, associated emotions, and using nontechnical terms to describe an exacerbation.9,33 The discrepancy between the perspectives of HCPs and patients’ perspectives may lead to patients under-reporting exacerbations even when asked about their occurrence. This lack of shared language and a common understanding of what a COPD exacerbation is makes it more difficult for patients and clinicians to communicate, potentially leading to poorer patient satisfaction, understanding, and outcomes.34 Based on the study findings, several ways to reduce the communication gap between clinicians and people living with COPD are suggested. First, less technical terms and multiple lines of questioning may need to be used by clinicians to ascertain if someone has had an exacerbation. Asking how individuals felt may also help to identify periods of exacerbation. Additionally, open-ended questions and probes focused on actions taken, symptoms, times that they could not do their normal activities, or times that they felt fatigued may allow patients to describe changes in their COPD symptoms, which in turn could facilitate identification of an exacerbation by a clinician. Such techniques are part of narrative medicine practices, which can enhance clinical analysis and facilitate more accurate diagnoses, prognoses, and treatment plans by immersing HCPs in the patient’s world and allowing them to view COPD from the patient’s perspective.35,36 To help implement narrative medicine techniques, a discussion guide could be developed for (and by) HCPs and patients, providing example questions and prompts for HCPs and illustrating the variety of ways people living with COPD conceptualize their exacerbations, to support earlier diagnosis of COPD exacerbations. A discussion guide could also be coupled with educational programs on the importance of early recognition, prevention, and treatment of COPD exacerbations by providing a shared understanding and common language to describe exacerbations and thereby facilitate discussions between clinicians and patients.
Regarding breathlessness, one way to improve patient wellbeing would be for HCPs to acknowledge breathlessness as a concern and to incorporate patient concerns around breathlessness within pulmonary rehabilitation (PR) programs. PR has been shown to improve the clinical outcomes and wellbeing for those living with COPD.5,37,38 However, PR programs are highly variable, ranging in length, services offered, and comprehensiveness of the program.37 There is some evidence that PR programs that have a longer period of follow up and are more comprehensive, such as offering breathlessness support services, may be more effective at improving patient outcomes.38–41 Therefore, incorporating education focused on self-management of breathlessness may be effective. However, most PR programs do not consider dyspnea as the most important outcome.42 It is unclear which components of PR are the most effective in improving dyspnea, and future research could focus on understanding which interventions provide the most benefit to breathlessness.
Study Limitations
This study relied on respondents to self-report of COPD diagnosis, which may lead to selection bias.43 For instance, nearly one-third of respondents also reported asthma, indicating a possible misdiagnosis of asthma as COPD. More than 60% of respondents reported an exacerbation in the past year compared with an expected proportion of 30–50% given prior studies.44,45 Respondents could have misidentified exacerbations, especially since uncontrolled asthma and cardiovascular disease may present with similar symptoms to COPD exacerbations.46 It is also unclear whether this study population consists of people with severe COPD (73% reported shortness of breath and 53% had an mMRC grade ≥2) or if their ability to detect exacerbations is superior to the average. However, to minimize self-selection of individuals with more severe COPD, invited panelists were not told that the survey would focus on COPD. Another source of selection is the use of the online survey, which requires a high-level of digital literacy.47 Prior research has suggested that greater digital literacy is associated with higher interest in health and access to information.48 As a result, the study population may have over-selected respondents who are more engaged and involved in their health and thus more confident and/or able to detect COPD exacerbations. Finally, responses provided to the open-ended question were brief, so themes derived from qualitative analysis were not as rich or as descriptive.49 As a result, the findings were presented using a mixed-methods approach.
Conclusions
This study suggests that people living with COPD feel confident that they can detect exacerbations, but they experience and describe exacerbations in a variety of ways. This may contribute to a communication gap with clinicians. Clinicians should use plain language when educating patients on ways to identify and manage exacerbations. A discussion guide could facilitate patient-clinician discussions around recognizing and managing exacerbations.
Abbreviations
COPD, Chronic obstructive pulmonary disease; CROSS, Consensus-Based Checklist for Reporting of Survey Studies; HCP, Healthcare provider; mMRC, Modified Medical Research Council; PR, Pulmonary rehabilitation.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author.
Human Ethics Approval Declaration
This study was deemed exempt by the Pearl Institutional Review Board (IRB number: 00007772; Study: 23-IPSO-179).
Artificial Intelligence Disclosure
The authors declare that they have not used any type of generative artificial intelligence for the writing of this manuscript, nor for the creation of images, graphics, tables, or their corresponding captions.
Acknowledgments
The authors would like to thank the survey respondents and members of AstraZeneca’s Patient Partnership Program who took the time to review the survey for comprehension and relevance. We would like to thank Katie Russo, Camille Hoy, and Jade Huynh-Pring of Ipsos Public Affairs, Washington DC, USA, for their contributions to the analysis and data collection process. Subsets of data were presented at IPCRG, Athens, Greece, 9–11 May 2024 and ERS, Vienna, Austria, September 7–11, 2024.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Funding was provided by AstraZeneca for the development and conduct of the study survey and the data analysis.
Disclosure
Job FM van Boven has received consultancy fees and/or grants from Aardex, ALK Abello, AstraZeneca, Chiesi, the European Commission Cooperation in Science and Technology (COST; COST Action 19,132), GSK, Novartis, Pfizer, Pill Connect, Teva, Trudell Medical, and Vertex. Michael G Crooks has received grants from Asthma + Lung UK, AstraZeneca, Boehringer Ingelheim, Chiesi, the National Institute for Health and Care Research (NIHR), Pfizer, and Phillips; consultancy fees from AstraZeneca, Chiesi, Orion, Sanofi and Synairgen; support for meeting attendance from AstraZeneca, Boehringer Ingelheim, Chiesi, and GSK; and honoraria and/or nonfinancial support from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, and Pfizer. Frits ME Franssen has received speaker/consultancy fees, support for meeting attendance or travel, receipt of equipment, drugs, gifts, or other services and/or grants from AstraZeneca, Chiesi, GSK, Merck Sharp & Dohme, Novartis, Pfizer, Sanofi and Verona Pharma. John R Hurst has received speaker/consultancy fees, payments/honoraria and/or grants from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, and Takeda. Ann Hutchinson has received grants, honoraria, and support for meeting attendance from AstraZeneca. John Linnell reports compensation for services spent in consulting activities from AstraZeneca, Mylan/Theravance, and Verona Pharma, and from the COPD Foundation Patient-Powered Research Network, Johns Hopkins University, and the University of Illinois Chicago; payment/honoraria from Chiesi; support for meeting attendance from the American Lung Association; participation on a data safety monitoring board for Johns Hopkins School of Medicine, all in the capacity of presenting the patient perspective within the COPD patient community, as well as holding stock options in Ansible Health. Daiana Stolz reports grants from the Swiss National Foundation (SNF 189280) and unrestricted grants from AstraZeneca, Boston Scientific, and Curetis AG, as well as honoraria from, and participation on data safety monitoring boards, in talks, or on advisory boards for AstraZeneca, Berlin-Chemie/Menarini, Boehringer Ingelheim, Chiesi, CSL Behring, Curetis AG, GSK, Merck, Merck Sharp & Dohme, Novartis, Sanofi, and Vifor. She is the current Global Initiative for Chronic Obstructive Lung Disease (GOLD) representative for Switzerland, the immediate past Education Council Chair of the European Respiratory Society (ERS), and past President of the Education Committee of the Swiss Respiratory Society. Tonya Winders has received speaker/consultancy fees and/or honoraria from Amgen, AstraZeneca, GSK, Novartis, Sanofi Regeneron and Roche. Jing Zhang has received speaker/consultancy fees from AstraZeneca, Boehringer Ingelheim, GSK, and Pfizer. Marie Eckerd, Jad El Khoury, and Clementine Nordon are employees of AstraZeneca and hold shares and stock options in the company. Megan Grobert and Ruth Young are employees of Ipsos Public Affairs, Washington DC. AstraZeneca contracted Ipsos for the development, fielding and analysis of study survey and medical writing support. The authors report no other conflicts of interest in this work.
References
1. Adeloye D, Song P, Zhu Y, et al. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med. 2022;10(5):447–458. doi:10.1016/S2213-2600(21)00511-7
2. Lopez-Campos JL, Calero C, Quintana-Gallego E. Symptom variability in COPD: a narrative review. Int J Chron Obstruct Pulmon Dis. 2013;8:231–238. doi:10.2147/COPD.S42866
3. Kim V, Aaron SD. What is a COPD exacerbation? Current definitions, pitfalls, challenges and opportunities for improvement. Eur Respir J. 2018;52(5):1801261. doi:10.1183/13993003.01261-2018
4. Celli BR, Fabbri LM, Aaron SD, et al. An updated definition and severity classification of chronic obstructive pulmonary disease exacerbations: the Rome proposal. Am J Respir Crit Care Med. 2021;204(11):1251–1258. doi:10.1164/rccm.202108-1819PP
5. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease report. 2024. Available from: https://goldcopd.org/2024-gold-report/.
6. Andersson F, Borg S, Jansson SA, et al. The costs of exacerbations in chronic obstructive pulmonary disease (COPD). Respir Med. 2002;96(9):700–708. doi:10.1053/rmed.2002.1334
7. Desikan R, Mason HL, Rupp MT, Skehan M. Health-related quality of life and healthcare resource utilization by COPD patients: a comparison of three instruments. Qual Life Res. 2002;11(8):739–751. doi:10.1023/a:1020836719321
8. Wilkinson TM, Donaldson GC, Hurst JR, Seemungal TA, Wedzicha JA. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169(12):1298–1303. doi:10.1164/rccm.200310-1443OC
9. Kessler R, Ståhl E, Vogelmeier C, et al. Patient understanding, detection, and experience of COPD exacerbations: an observational, interview-based study. Chest. 2006;130(1):133–142. doi:10.1378/chest.130.1.133
10. Doward L, Svedsater H, Whalley D, et al. Salford lung study in chronic obstructive pulmonary disease (SLS COPD): follow-up interviews on patient-centred outcomes. NPJ Prim Care Respir Med. 2017;27(1):66. doi:10.1038/s41533-017-0066-2
11. Langsetmo L, Platt RW, Ernst P, Bourbeau J. Underreporting exacerbation of chronic obstructive pulmonary disease in a longitudinal cohort. Am J Respir Crit Care Med. 2008;177(4):396–401. doi:10.1164/rccm.200708-1290OC
12. Robinson K, Lucas E, van den Dolder P, Halcomb E. Living with chronic obstructive pulmonary disease: the stories of frequent attenders to the emergency department. J Clin Nurs. 2018;27(1–2):48–56. doi:10.1111/jocn.13842
13. Korpershoek Y, Vervoort S, Nijssen L, Trappenburg J, Schuurmans MJ. Factors influencing exacerbation-related self-management in patients with COPD: a qualitative study. Int J Chron Obstruct Pulmon Dis. 2016;11:2977–2990. doi:10.2147/COPD.S116196
14. Adams R, Chavannes N, Jones K, Ostergaard MS, Price D. Exacerbations of chronic obstructive pulmonary disease – a patients’ perspective. Prim Care Respir J. 2006;15(2):102–109. doi:10.1016/j.pcrj.2006.01.003
15. Miravitlles M, Ferrer J, Baró E, Lleonart M, Galera J. Differences between physician and patient in the perception of symptoms and their severity in COPD. Respir Med. 2013;107(12):1977–1985. doi:10.1016/j.rmed.2013.06.019
16. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa0909883
17. Sharma A, Minh Duc NT, Luu Lam Thang T, et al. A consensus-based checklist for reporting of survey studies (CROSS). J Gen Intern Med. 2021;36(10):3179–3187. doi:10.1007/s11606-021-06737-1
18. American Thoracic Society (ATS). Surveillance for respiratory hazards in the occupational setting. Am Rev Respir Dis. 1982;126(5):952–956.
19. Cooper H, Coutanche MN, McMullen LM, et al. APA Handbook of Research Methods in Psychology. Volume 1: Foundations, Planning, Measures, and Psychometrics.
20. m360 Research. Global healthcare community. 2024. Available from: https://www.m360research.com/global-healthcare-community/.
21. Baker R, Blumberg SJ, Brick JM, et al. Research synthesis: AAPOR report on online panels. Public Opin Q. 2010;74(4):711–781. doi:10.1093/poq/nfq048
22. Ipsos. KnowledgePanel. 2024. Available from: https://www.ipsos.com/en-us/solutions/public-affairs/knowledgepanel.
23. Wintemute GJ, Crawford A, Robinson SL, et al. Firearm ownership and support for political violence in the United States. JAMA Network Open. 2024;7(4):e243623. doi:10.1001/jamanetworkopen.2024.3623
24. Do VV, Spears CA, Ling PM, et al. Racial/ethnic disparities in exposure to e-cigarette advertising among U.S. youth. Public Health. 2024;230:89–95. doi:10.1016/j.puhe.2024.02.011
25. Costello AB, Osborne J. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. PARE. 2005;10(1):1–9. doi:10.7275/jyj1-4868
26. Kerkhof M, Voorham J, Dorinsky P, et al. The long-term burden of COPD exacerbations during maintenance therapy and lung function decline. Int J Chron Obstruct Pulmon Dis. 2020;15:1909–1918. doi:10.2147/COPD.S253812
27. Memon M, Ting H, Cheah JH, Ramayah T, Chuah F, Cham TH. Sample size for survey research: review and recommendations. JASEM. 2020;4(2):i–xx. doi:10.47263/JASEM.4(2)01
28. Goodrich B, Fenton M, Penn J, Bovay J, Mountain T. Battling bots: experiences and strategies to mitigate fraudulent responses in online surveys. Appl Econ Perspect Policy. 2023;45(2):762–784. doi:10.1002/aepp.13353
29. Braun V, Clarke V. Thematic analysis. In: APA Handbook of Research Methods in Psychology, Vol 2. Research Designs: Quantitative, Qualitative, Neuropsychological, and Biological. APA Handbooks in Psychology®. American Psychological Association; 2012:57–71. doi:10.1037/13620-004
30. MacQueen K, McLellan-Lemal E, Kay K, Milstein B. Codebook development for team-based qualitative analysis. CAM. 1998;10(2):31–36. doi:10.1177/1525822X980100020301
31. Houben-Wilke S, Deng Q, Janssen DJ, Franssen FM, Spruit MA. Symptom burden and its associations with clinical characteristics in patients with COPD: a clustering approach. ERJ Open Res. 2024;10(4):01052–02023. doi:10.1183/23120541.01052-2023
32. Kessler R, Partridge MR, Miravitlles M, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J. 2011;37(2):264–272. doi:10.1183/09031936.00051110
33. Hutchinson A, Russell R, Cummings H, et al. Exploring the experiences, understandings, and expectations of exacerbations of patients with COPD and their carers: an interview study. BJGP Open. 2024:
34. King A, Hoppe RB. “Best practice” for patient-centered communication: a narrative review. J Grad Med Educ. 2013;5(3):385–393. doi:10.4300/JGME-D-13-00072.1
35. Fioretti C, Mazzocco K, Riva S, Oliveri S, Masiero M, Pravettoni G. Research studies on patients’ illness experience using the narrative medicine approach: a systematic review. BMJ Open. 2016;6(7):e011220. doi:10.1136/bmjopen-2016-011220
36. Charon R. Narrative Medicine Honoring the Stories of Illness.
37. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;2015(2):CD003793. doi:10.1002/14651858.CD003793.pub3
38. Gordon CS, Waller JW, Cook RM, Cavalera SL, Lim WT, Osadnik CR. Effect of pulmonary rehabilitation on symptoms of anxiety and depression in COPD: a systematic review and meta-analysis. Chest. 2019;156(1):80–91. doi:10.1016/j.chest.2019.04.009
39. Higginson IJ, Bausewein C, Reilly CC, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Respir Med. 2014;2(12):979–987. doi:10.1016/S2213-2600(14)70226-7
40. Qian MY, Politis J, Thompson M, et al. Individualized breathlessness interventions may improve outcomes in patients with advanced COPD. Respirology. 2018;23(12):1146–1151. doi:10.1111/resp.13324
41. Silva L, Maricoto T, Costa P, Berger-Estilita J, Padilha JM. A meta-analysis on the structure of pulmonary rehabilitation maintenance programmes on COPD patients’ functional capacity. NPJ Prim Care Respir Med. 2022;32(1):38. doi:10.1038/s41533-022-00302-x
42. Spruit MA, Pitta F, Garvey C, et al. Differences in content and organisational aspects of pulmonary rehabilitation programmes. Eur Respir J. 2014;43(5):1326–1337. doi:10.1183/09031936.00145613
43. Barr RG, Herbstman J, Speizer FE, Camargo CA. Validation of self-reported chronic obstructive pulmonary disease in a cohort study of nurses. Am J Epidemiol. 2002;155(10):965–971. doi:10.1093/aje/155.10.965
44. Han MK, Quibrera PM, Carretta EE, et al. Frequency of exacerbations in COPD: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619–626. doi:10.1016/S2213-2600(17)30207-2
45. Whittaker H, Van Ganse E, Dalon F, et al. Differences in severe exacerbations rates and healthcare utilisation in COPD populations in the UK and France. BMJ Open Respir Res. 2022;9(1):e001150. doi:10.1136/bmjresp-2021-001150
46. Chen H, Luo X, Du Y, et al. Association between chronic obstructive pulmonary disease and cardiovascular disease in adults aged 40 years and above: data from NHANES 2013-2018. BMC Pulm Med. 2023;23(1):318. doi:10.1186/s12890-023-02606-1
47. Milanti A, Chan DN, Parut AA, So WK. Determinants and outcomes of eHealth literacy in healthy adults: a systematic review. PLoS One. 2023;18(10):e0291229. doi:10.1371/journal.pone.0291229
48. Eysenbach G, Wyatt J. Using the internet for surveys and health research. J Med Internet Res. 2002;4(2):E13. doi:10.2196/jmir.4.2.e13
49. LaDonna KA, Taylor T, Lingard L. Why open-ended survey questions are unlikely to support rigorous qualitative insights. Acad Med. 2018;93(3):347–349. doi:10.1097/ACM.0000000000002088
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



