Back to Journals » Neuropsychiatric Disease and Treatment » Volume 21
Human Neural Stem Cells Reduce Hypoxia-Ischemic Neurological Impairment Through the Regulation of Inflammatory M1/M2 Microglias in Rats
Authors Ding YB, Zhao Y , Chen BY, Wang Q, Wang ZY, Luan Z, Yi B
Received 3 October 2024
Accepted for publication 28 May 2025
Published 9 June 2025 Volume 2025:21 Pages 1173—1190
DOI https://doi.org/10.2147/NDT.S493431
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Taro Kishi
Ya-Bing Ding,1,2,* Yuan Zhao,1– 3,* Bing-Yu Chen,1 Qian Wang,1 Zhao-Yan Wang,1 Zuo Luan,1,2 Bin Yi4
1Department of Pediatrics, The Sixth Medical Center of PLA General Hospital, Beijing, People’s Republic of China; 2Department of Pediatrics, The Second School of Clinical Medicine, Southern Medical University, Guangzhou, People’s Republic of China; 3Department of Neonatology, Children’s Hospital of Shanxi, Women Health Centre of Shanxi, Taiyuan, People’s Republic of China; 4Department of Pediatrics, Gansu Provincial Maternity and Child-Care Hospital, Lanzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zuo Luan, Department of Peadiatric, The Sixth Medical Center of PLA General Hospital, Beijing, 100048, People’s Republic of China, Tel +86-18600310270, Fax +86-1066958303, Email [email protected] Bin Yi, Gansu Provincial Maternity and Child-care Hospital, Lanzhou, Gansu, 730000, People’s Republic of China, Email [email protected]
Objective: This study aims to investigate the therapeutic efficacy and migration of human neural stem cell (hNSC) therapy in a rat model of moderate to severe Hypoxic-ischemic encephalopathy (HIE).
Methods: hNSCs were transplanted into the brain ventricle of HIE-induced rats. The levels of vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor (BDNF), immunohistochemistry of M1 and M2 cells counts, as well as the apoptosis of hNSCs were conducted after 10 days transplantation. Furthermore, Hematoxylin-eosin (HE) staining and neurological function was assessed at 12 weeks post-implantation.
Results: After implantation, hNSCs survived for a duration of 12 weeks. After 10 days post-implantation, extensive migration of hNSCs was observed, with a higher concentration detected in the injured hemisphere of the brain. This study also noted an upregulation in the expression levels of VEGF and BDNF in rats treated with hNSCs (P < 0.05), along with a significant reduction in M1 microglia (P < 0.001), an increase in M2 microglia (P < 0.01), and a decrease in apoptotic cell numbers (P < 0.001). At 12 weeks post-implantation, approximately 30.68% ± 4.30% of hNSCs differentiated into mature neurons, while 23.50% ± 3.60% differentiated into oligodendrocytes and 14.00% ± 1.03% differentiated into astrocytes respectively. Compared to Sham-operated rats, histological examination revealed reduced brain injury following hNSC therapy (P < 0.05). Additionally, neurobehavioral assessments demonstrated significant cognitive function recovery through Morris water maze test as well as increased frequency of left forelimb usage during Cylinder test and improved movement coordination exhibited by enhanced claw performance using Catwalk test (All P < 0.05).
Conclusion: hNSCs exerted beneficial effects on brain damage recovery and apoptosis suppression via paracrine actions of VEGF and BDNF, M1/M2 microglial balance regulation, cell differentiation promotion. The study underlined the potential of hNSC implantation as a prospective neonatal HIE treatment strategy.
Keywords: hypoxic-ischemic encephalopathy, neural stem cell, VEGF, BDNF, migration
Introduction
Neonatal Hypoxic-ischemic encephalopathy (HIE) is a neurological function impairment caused by decreased oxygen and blood flow during the fetal period. It is one of the most common reasons for brain damage in term-infants or near-term infants, as well as the primary cause of mortality and long-term morbidity.1 Although there have been obvious improvements in obstetrical nursing and neonatal nursing in recent years, HIE incidence remains high even in developed countries. Moreover, in developing countries, HIE continues to be a significant health and economic burden on society.2 Research has shown that lack of oxygen and blood flow primarily affects the cerebral cortex, including the hippocampus, basal nucleus, and thalamus.3
In recent years, several studies have attempted to illustrate the mechanisms of HIE, achieving preliminary progress. The pathological process of HIE has been artificially divided into four stages: the Primary Phase, Latent Phase, Secondary Phase, and Chronic Tertiary Phase. These stages involve energetic failure, microglia activation, cascade inflammatory response, mitochondrial dysfunction, etc., ultimately triggering cell apoptosis and death.4 In brief, decreased cerebral blood flow causes dysfunction in glucose and oxygen supply. In this state, the reduced generation of ATP finally leads to energy failure. Energy failure affects the transmembrane transport of ions and water which results in cellular depolarization and induces the release of excitatory amino acids, ultimately causing excitation toxicity. Meanwhile, lactic acid accumulation due to anaerobic cellular respiration contributes to brain cell apoptosis and death.5
Until now, therapeutic hypothermia (TH) has been the only neurological protective method that has been proven to improve HIE prognosis. However, this method is limited by its narrow therapeutic time window and a lack of evidence from randomized control trials.6 Additionally, nearly half of moderate and severe HIE newborn patients who receive TH therapy still experience mortality or remain with moderate to severe neurological morbidity.7,8 Therefore, there is an urgent need to explore promising new therapy methods for improving HIE prognosis. Recently, stem cell therapy, based on stem cells or progenitor cells, has shown potential and promising neuroprotective and regenerative effects for damaged brain cells. This field has gained more attention in the context of central nervous system diseases.9
The protective effect of mouse neural stem cells (NSCs) on the nervous system is mediated through the inhibition of NF-κB activation and suppression of neuron apoptosis, as well as the enhancement of vascular generation in HIE mouse models.10,11 However, there is still insufficient evidence regarding the potential therapeutic application of human NSCs (hNSCs) for neonatal HIE treatment.12 In comparison to allogeneic stem cells, hNSCs offer greater promise for clinical translation and are more suitable for human clinical trials. Some studies investigating NSC therapy for neonatal HIE have described a possible neurorestorative and neuroprotective effect within 3 weeks but without long-term regenerative effects.13–15
A comprehensive review of previous studies that specifically utilized hNSCs in HIE models indicates that although certain investigations have manifested short-term advantages, such as the transient amelioration of neurological function subsequent to transplantation without the employment of immunosuppressants, numerous aspects still necessitate in-depth exploration. For example, the paracrine, cell replacement, and inflammatory impacts of hNSCs transplantation within HIE rat models have been inadequately studied. Moreover, despite the significance of animal models in elucidating fundamental mechanisms, they frequently oversimplify the intricate diagnosis and treatment paradigms of human diseases.16–18 Consequently, the extrapolation of results from hNSCs in HIE animal models to human clinical applications is highly challenging. The current study is designed to probe the capabilities of hNSCs transplanted in HIE rats over a 12-week time frame. Specifically, it aims to ascertain whether the transplanted hNSCs can foster the regeneration and enhancement of neurobehavioral outcomes in a rat model of HIE in the long run and to explore the potential influence of the M1/M2 microglial balance during the process, especially in relation to cell replacement and inflammatory effects.
Materials and Methods
Animal Grouping and Model Preparation
Male Sprague-Dawley (SD) rats were housed in a specific pathogen-free (SPF)-level laboratory animal facility at Beijing Center for Physical and Chemical Analysis (SYXK-Beijing-2021-0014), with a controlled room temperature ranging from 20 to 24°C, room humidity maintained between 45% and 65%, and subjected to a regulated light/dark cycle of 12 hours each, while having unrestricted access to food and water.
The HIE-rat model was prepared using the Rice-Vannucci method.19 Postnatal day 7 rats weighing 15–17g underwent devascularization of the right common carotid artery under isoflurane anesthesia (4% concentration for induction and 2% for maintenance). Following a resting period of 3 hours, these rats were placed in a hypoxia incubator chamber containing 8% oxygen and 92% nitrogen for a duration exceeding 150 minutes. All experiments were followed the Guidelines for the Ethical Review of Laboratory Animal Welfare (GB/T 35892–2018) and conducted with approval from the institutional animal care and use committee (IACUC) of Beijing Center for Physical and Chemical Analysis (Approval No. 210520-SWDWF-001).
Using a random number table, the rats were randomly allocated into two groups: the Sham Group and the HIE Group. In the Sham Group, rats did not undergo artery devascularization and hypoxia incubation, while all other procedures were identical to those in the HIE Group. In the HIE Group, brain damage degree of surviving rats was assessed at 4 hours, 16 hours, and 24 hours after modeling using the Zea-Longa Scale (Table 1). Subsequently, rats with Zea-Longa scores20 of 2–3 (indicating moderate to severe HIE) were divided into two treatment groups: the HIE + PBS Group (HIE rats received phosphate buffer saline (PBS) treatment) and the HIE + hNSCs Group (HIE rats received hNSCs transplantation treatment).
 |
Table 1 Zea-Longa Scale to Assess Brain Impairment Degree of HIE Rats |
In the context of immunosuppressive agent utilization, should there be manifestations such as sluggish responsiveness, limb flaccidity, variations in respiratory rhythm, hair thinning, reddening and bleeding of the eye sockets, mouth, and nose, along with a substantial decrease in body weight, the dosage may be halved or administered on an alternate-day basis until the rat’s physiological state is completely recuperated.
hNSCs Preparation and Transplantation
With the approval of the Institutional Review Board (IRB No. 2015013) at the Sixth Medical Center of PLA General Hospital, as well as obtaining informed consent from the patient’s family members in accordance with the Helsinki Declaration, we isolated hNSCs from aborted fetal brain tissue. This procedure was also validated by review conducted by the Chinese National Institutes for Food and Drug Control (Report No. SH202200032). In brief, 5mL of hNSC culture medium was added to tissue culture flasks and pre-coated for 15 minutes, followed by incubation at 37°C for 5 minutes using accutase cell detachment solution. Adherent cells were then transferred to a specific cell medium and cultured in a CO2-containing atmosphere at 37°C in a cell incubator before cryopreservation.
After model building, on the 3rd day, transplantation of PBS and hNSCs was performed in HIE rats via the right ventricle of the brain. The transplants were conducted under isoflurane anesthesia. Bregma was used as the reference point for origin. The stereotaxic coordinates for the right ventricle were adjusted to Anterior-Posterior (AP) = 0mm, Median-Lateral (ML) = −1.2mm, and Depth Vernier (DV) = −2.5mm. A micro-injector was used to inject a 5μL PBS solution into rats in the HIE+PBS Group. Simultaneously, a suspension of 5×105 hNSCs (equivalent to 5μL) was injected into rats in the HIE+hNSCs Group. The injection velocity was set at 0.5μL per minute. 5 minutes after injection, the injector was removed and pressure applied with a cotton swab to compress the pinhole before careful suturing without any bleeding occurred. Rats were transferred to cages once they regained consciousness.
Use of Immunosuppressive Regimen
P7 rats from all three groups were administered subcutaneous cyclosporin A (10mg/kg/day, Sandimmun, Novartis) for 3 weeks starting 3 days prior to transplantation, followed by 100µg/mL in their drinking water until the endpoints.21 Adverse events such as dyspnea, orbital or nasal hemorrhage, and weight loss were closely monitored during cyclosporin A treatment. The dosage and administration schedule of cyclosporin A could be adjusted accordingly based on the actual situation.
Enzyme-Linked Immunosorbent Assay (ELISA)
After a period of 10 days post-implantation, six rats from each experimental group were selected for the quantification of vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF) expressions using an ELISA test.22 Following induction of anesthesia with isoflurane, all these rats were euthanized through brain dissection, and their bilateral brain tissues were extracted, cleansed, weighed, and homogenized. Subsequently, these brain samples underwent testing for VEGF and BDNF expression utilizing a VEGF/BDNF ELISA Kit in accordance with the manufacturer’s instructions.
Brain Section Preparations
At 10 days and 12 weeks post-implantation, six rats were selected from each group and underwent open-heart surgery under isoflurane anesthesia. Normal sodium and 4% paraformaldehyde were perfused into the left ventricle, with outflow from the open right atrial appendage. Following brain dissection, brain tissue was extracted and subjected to fixation, dehydration, embedding, and freezing. The tissue was then sliced into eight continuous segments based on the reference point of Bregma: Bregma = 2.52mm, 1.44mm, 0.48mm, −0.48mm, −1.44mm, −2.40mm, −3.36mm, −4.36 mm; each slice had a thickness of 8μm. The sections were stored at −80°C for future use.
TUNEL Assay
On day 10 post-implantation, the TUNEL assay was performed to measure cell apoptosis in the brain using an in situ apoptotic cells kit (Servicebio, Wuhan, China), following the manufacturer’s instructions.23 Three randomly chosen frozen brain sections from each group (Bregma = 2.52mm, 1.44mm, 0.48mm) were observed under a light microscope at a magnification of×40 in three views (cerebral cortex, corpus striatum, and periventricular white matter). Total TUNEL-positive stained cells as well as DAPI-positive stained cells were counted using Image-J software.
Immunohistochemical Staining
The frozen sections were blocked with 3% BSA /30% TritonX-100 PBS for 1 hour, followed by incubation with primary antibodies at the specified dilution ratios below under a temperature of 4°C: Anti-BDNF (1:100, rabbit polyclonal, Invitrogen), Anti-VEGF (1:200, rabbit monoclonal, Invitrogen), Anti-Arg1 (1:200, rabbit polyclonal, GeneTex), Anti-inducible nitric oxide synthase (iNOS) (1:100, rabbit polyclonal, Abcam), Anti-Stem 121 (1:500, mouse monoclonal,TakaRa), Anti-Map 2 (1:200, rabbit monoclonal, Abcam), Anti-GFAP (1:1000, rabbit monoclonal, Abcam), and Anti-MBP (1:100, rabbit polyclonal, Abcam). The sections were washed with PBS and then incubated in the dark at 37°C for 1 hour after adding an appropriate amount of Alexa Fluor 488 and 594 diluted in PBS. After another wash with PBS and DAPI staining, the slides were observed using a confocal laser scanning microscope to examine factor distribution and hNSCs migration.The quantification of microglia was performed by examining three sections (Bregma = 2.52mm, 1.44mm, 0.48mm) with three views under a light microscope at 40× magnification for each rat in each group. Subsequently, Image-J software was utilized to calculate both the parameters and the percentage of DAPI positive stained cells relative to total DAPI positive stained cells × 100%. Similar methods were employed to quantify the differentiation ability of hNSCs into mature neurons, oligodendrocytes, and astrocytes. In the HIE+ hNSCs Group rats’ sections, three sections (Bregma = −2.40mm, −3.36mm, −4.36mm) with three views under a light microscope at 40× magnification were examined for each rat. Then Image-J software was used to calculate both the parameters and the percentage of Stem 121 positive stained cells relative to total Stem 121 positive stained cells × 100%.
Hematoxylin-Eosin (HE) Staining
After 12 weeks post-transplantation, three rats were randomly selected from each group, and an additional section was chosen at random from each group for HE staining (ServiceBio Co., LTD., Wuhan, China). Following staining and sealing, pathological images of the brain tissue were observed and analyzed under a microscope. Three random fields of observation were selected for each section to conduct histopathological scoring as shown in Table 2.24,25
 |
Table 2 Histopathological Scoring Criteria |
Morris Water Maze Test
The Morris water maze test was conducted in the 12th week after hNSCs transplantation to assess cognitive function using a modified method.21 The test was performed four times per day, and the distance traveled by the rats was recorded with software. The average value for each rat was used for subsequent statistical analysis.
Cylinder Test
The Cylinder test was conducted at P53, P55, P57, P59, P61 and P63 to assess forelimb use preference, which was calculated as follows: (Impaired + 0.5 × Both) /(Non-impaired + Impaired + Both) ×100%. The average value for each rat was used for subsequent statistical analysis.
Catwalk Test
On the 12th week after hNSCs transplantation, the Catwalk system was utilized to evaluate the rats in each group. The gait parameters were automatically calculated by the analysis software. This experiment primarily assessed various gait parameters including Stand, Swing Speed, Print Length and Body Speed Variation regarding related behavioral changes.
Statistical Analysis
Statistical analysis was conducted using SPSS software version 26.0 (SPSS Inc., Chicago, IL) and GraphPad Prism software version 8 (GraphPad Software, San Diego, CA). Data analysis between different groups was performed using t-test and one-way analysis of variance (ANOVA), followed by the Tukey multiple comparison post hoc test. The level of statistical significance between groups was defined as *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Each experiment was repeated at least three times, and the results were presented as mean±standard error of the mean (SEM).
Results
Implantation of hNSCs Enhanced VEGF and BDNF Expressions in HIE Rats
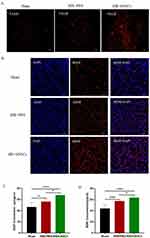
The paracrine effect of transplanted hNSCs within the brains of HIE rats was evaluated by conducting immunohistochemical staining on the striatum 10 days after transplantation, and levels of VEGF and BDNF were determined using ELISA. In comparison to the Sham Group (VEGF (46.63 ± 8.25) pg/mgprot, BDNF (44.04 ± 6.00) ng/gprot), the HIE + hNSCs Group exhibited significantly higher levels of VEGF ((67.96 ± 3.16) pg/mgprot) and BDNF ((63.69 ± 3.41)ng/gprot) when compared to the HIE + PBS Group (VEGF (56.25 ± 5.25) pg/mgprot, BDNF (57.54 ± 1.60) pg/mgprot) (Figure 1A–D, n = 6, P < 0.05).
Implantation of hNSCs Reduced M1 and Increase M2 Microglia Counts in HIE Rats
After 10 days transplantation, compared to the Sham Group (M1 microglia 34.66 ± 28.43, M2 microglia 31.16 ± 20.52), immunohistochemical staining for iNOS revealed that the count of M1 microglia in the HIE + hNSCs Group (67.16 ± 5.63) was significantly decreased than that in the HIE + PBS Group (230.83 ± 72.14) (n = 6, P < 0.001). Furthermore, immunohistochemical staining for Arginase-1 (Arg1) revealed that the count of M2 microglia was significantly higher in the HIE + hNSCs Group (249.33±109.93) than that in the HIE + PBS Group (110.50 ± 36.09) (n = 6, P < 0.01). It showed that the implantation of hNSCs resulted in a significant reduction of M1 microglia and an increase of M2 microglia within rat brains with HIE (Figure 2A–D).
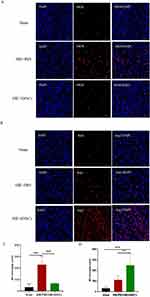
Implantation of hNSCs Reduced the Count of Apoptotic Cells in HIE Rats
To investigate the impact of hNSCs transplantation on brain apoptosis in HIE -rats, we quantified and analyzed the number of apoptotic cells in each group after 10 days of implantation. As shown in Figure 3, compared to the Sham Group (6.66 ± 1.36), hNSCs transplantation significantly reduced the count of apoptotic cells in HIE rats (HIE + hNSCs Group vs HIE + PBS Group (60.50 ± 8.01) vs (140.16 ± 26.56)) (P < 0.001).
Migration and Differentiation of Transplanted hNSCs in HIE Rats
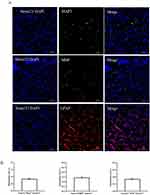
To evaluate the migration of hNSCs in HIE rats, immunohistochemical staining with Stem 121 revealed that hNSCs migrated in clusters along the pathways of the corpus callosum and cingulate gyrus. There was a higher concentration of hNSCs on the injured side of the brain and a longer migration distance after 10 days of implantation. On the 12th week post-implantation, hNSCs exhibited agglomerative migration along the pathways of corpus callosum and cingulated gyrus towards capsula externa. Furthermore, they were dispersed throughout the cerebral cortex and corpus striatum, with a continued higher concentration observed on the injured side. During this time period, hNSCs displayed diverse morphologies at different distributed locations (Figure 4).
At 12 weeks following transplantation in HIE rats, the differentiation of the transplanted cells was identified. Stem121, which represents human-derived cells, was double-stained with MAP2 (for neurons), MBP (for oligodendrocytes), and GFAP (for astrocytes) to ascertain the differentiation status of hNSCs. The results showed that MAP2 was co-expressed in 30.68% ± 4.30% of Stem121+ cells, which were distributed on both sides of the brain, with a greater number on the right side than on the left side, and mostly located in the cerebral cortex and striatum. For MBP, it was co-expressed in 23.50% ± 3.60% of Stem121+ cells, with more expression on the right side of the brain than on the left side. They were mainly present in white matter structures such as corpus callosum, cingulate gyrus, external capsule, as well as striatum. GFAP was co-expressed in 14.00% ± 1.03% of Stem121+ cells and had higher expression on the right side of the brain compared to the left side; it was found in both white matter and gray matter (P < 0.05) (Figure 5).
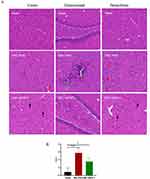
hNSCs Alleviated the Pathological Injury of Brain Tissue in HIE Rats
At 12 weeks, compared to the Sham Group, HE staining revealed significant abnormalities in the brain structure of rats within the HIE + PBS Group, characterized by decreased neuronal count and disordered arrangement in specific areas of the cortex, degeneration of some neurons, shrinkage and intense staining of nuclei, along with basophilic cells. Additionally, there was evident deposition of calcium salts in the brain parenchyma. However, hNSCs therapy significantly mitigated pathological injury in the brain resulting in a notable reduction in pathological score compared to that of HIE + PBS Group (P < 0.05) (Figure 6A and B).
hNSCs Mitigated Neurobehavioral Deficits in HIE Rats
The Morris water maze test, Cylinder test, and Catwalk test were conducted to assess neurobehavioral deficits in HIE rats treated with hNSCs at 12 weeks post-transplantation. Results from the Morris water maze test showed that compared to the Sham Group, the HIE rats exhibited significantly decreased latency in locating the navigation platform and reduced crossing times over the platform during probe trial testing. However, rats treated with hNSCs demonstrated a greater advantage in terms of latency to find the platform and number of platform crossings compared to HIE rats (P < 0.05) (Figure 7A). To evaluate spontaneous movement after hNSCs transplantation, we used the Cylinder test to measure left forelimb usage rates across all groups. Both HIE+hNSCs Group and HIE+PBS Group displayed significantly lower left forelimb usage rates compared to rats in Sham Group. However, hNSCs treatment increased it for HIE rats (P < 0.05) (Figure 7B).
Additionally, compared to rats in the Sham Group, HIE rats exhibited significantly longer standing time and slower swing speed within the HIE+PBS Group. However, transplantation of hNSCs significantly improved both standing time and swing speed (Figure 7C and D). Moreover, relative to rates observed in the Sham Group, HIE rats showed much lower print length and body speed variation. Nevertheless, transplantation of hNSCs enhanced both print length and body speed variation (Figure 7E and F) (All P < 0.05).
Discussion
The present study (Figure 8) aimed to investigate the long-term fate and efficacy of transplanted hNSCs in a rat model of HIE, which is a common clinical syndrome in term or near-term infants resulting from neonatal asphyxia. Effective treatments for moderate and severe HIE are still lacking. P7 rats were chosen as they are considered equivalent to newborn infants due to their peak stage of brain development. The mechanisms underlying brain damage during this phase resemble perinatal asphyxia in term infants, making P7 rats suitable for developing an HIE model that mimics humans.24,26–28 Previous studies have demonstrated that unilateral occlusion of the common carotid artery combined with hypoxia in neonatal rats leads to infarction and worsened functional outcomes.29–31 In this study, the HIE rat model exhibited thinning of the cerebral cortex, atrophy of white matter, and broadening of lateral ventricles on the injured side. The presence of various neurological sequelae is commonly observed in infants with severe HIE, and our 12-week HIE rat model also exhibited similar pathological and functional impairments, encompassing motor and cognitive dysfunctions, which aligns more closely with the clinical requirements.
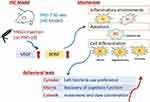 |
Figure 8 Schematic illustration of current study. |
HIE can lead to compromised blood flow, resulting in tissue infarction and damage to vascular integrity. This can cause damage to both gray and white matter, leading to impaired neural network formation, delayed myelin development, neuronal loss, and other manifestations that trigger a cascade of inflammatory reactions. VEGF has the potential to promote vascular growth, restore blood flow, and directly exert neuroprotective effects on neurons. BDNF levels were found to be elevated in the ipsilateral hippocampus and cerebral cortex of HIE rats at 6 hours post-injury, along with upregulation of enzymes/receptors involved in regulating neurological function. These factors play a central role in the recovery process of the central nervous system by inducing brain damage both internally and externally through migration and differentiation of neural cells into specific areas.32–34 In this study, we observed a significant increase in VEGF and BDNF expressions in rats treated with hNSCs, suggesting that hNSCs exert their effects on brain damage recovery through paracrine factors such as VEGF-BDNF signaling pathway as well as autosecretion mechanisms associated with transplanted hNSCs, which is consistent with previous research findings.35
Reactive oxygen species (ROS)-induced oxidative stress plays a crucial role in the pathogenesis and progression of HIE. Excessive ROS can lead to modifications or degeneration of membranes, proteins, lipids, and DNA, resulting in an inflammatory response cascade and protease secretion. These byproducts participate in complex interactions among multiple pathways that ultimately lead to brain injury.36 Microglia are innate immune cells within the brain and serve as the primary site for NLRP3 inflammasome expression, playing an essential role in neurological inflammation. M1 microglia can exacerbate brain injury, while M2 microglia can alleviate brain damage and enhance recovery. In infants with HIE, there is an observed increase in the number of M1 microglia and a decrease in the number of M2 microglia.37,38 In this study, on the 10th day after implantation, we observed a significant decrease in M1 microglia and a notable increase in M2 microglia with hNSCs transplantation. This balance between M1 and M2 microglia seemed as a key aspect of our research as it contributes to maintaining a normal brain environment and promoting recovery from HIE-induced brain damage.39 Additionally, cell apoptosis is a complex process regulated by caspase-3 and its family members, and neuronal death resulting from reduced oxygen and blood flow is closely associated with cellular apoptosis. In the context of immature HIE brains, cell apoptosis plays a pivotal role in disease progression.40,41 The anti-apoptotic properties of stem cells are widely recognized worldwide, and our study also revealed that hNSCs transplantation on the 10th day after grafting may led to a reduction in cell apoptosis by maintaining the balance of M1/M2 microglia as well as enhancing the secreting of VEGF and BDNF within the HIE-rats brain.
Altered plasticity is commonly observed in patients with HIE after brain injury. For cell migrations, at the 12-week mark post-implantation, we observed that hNSCs migrated in clusters along the pathways of the corpus callosum and cingulate gyrus towards the external capsule.42 Additionally, they were scattered throughout the cerebral cortex and corpus striatum, with a higher concentration on the injured side. In this study, we found variations in hNSC morphology across different distributed locations, which we speculate are influenced by altered plasticity. Moreover, for cell differentiation purposes, HIE-induced brain injury can be repaired through cellular differentiation. However, only an estimated 0.2% of injured neurons could be replaced by differentiated cells. Therefore, increasing the number of replaced cells for injured neurons may aid in brain damage recovery. Daadi et al reported a poor differentiation rate of implanted hNSCs in a rat model of middle cerebral artery occlusion.16,43,44 However, our study demonstrated a satisfactory differentiation rate in an HIE rat model following hNSC implantation. As shown by our results after 12 weeks of implantation: 30.68% ± 4.30% hNSCs differentiated into matured neurons; 23.50% ± 3.60% hNSCs differentiated into oligodendrocytes; and 14.00% ± 1.03% hNSCs differentiated into astrocytes. The higher rate of differentiation can primarily be attributed to our utilization of an immunosuppressive regimen and the employment of highly active and pure hNSCs. As demonstrated by Serrenho et al,30 in the realm of behavioral tests undertaken to assess the impact of hNSC implantation on the recovery from HIE brain damage, we employed cylinder tests, in conjunction with Morris water maze and catwalk tests. Remarkably, all three of these tests yielded positive results, thereby further validating the therapeutic potential of hNSCs. The uniqueness of this study lies in its foundation on previous regimens. Moreover, it continuously observes the neurological sequelae of HIE even after 12 weeks of hypoxia-ischemia, providing valuable insights into the long-term effects and potential therapeutic strategies for this debilitating condition.
Stem cell therapies, with a particular emphasis on neural stem cell therapies, have proffered a glimmer of hope to patients beleaguered by devastating diseases.45 However, as is the case with any nascent and innovative treatment modality, it is not without its attendant drawbacks. In the context of clinical translation, this therapy is beleaguered by a constellation of challenges and limitations. For example, ethical quandaries in clinical translation loom large, given the nature of neural stem cell sources that may involve human embryos or fetal tissues, thereby sparking intense debates regarding the definition of human life and the rights and interests of embryos and fetuses. Additionally, the criteria for the judicious utilization of neural stem cells in the heterogeneous landscape of individual neurological diseases remain elusive. The current state-of-the-art in the techniques requisite for isolating and culturing neural stem cells, as well as in securing reliable and abundant cell sources, is rife with inadequacies.46 These issues are likely to require an extensive temporal investment for their resolution. Preclinical animal studies have furnished evidence attesting to the clinical benefits of neural stem cell (NSC) transplantation. However, a yawning chasm of knowledge persists regarding the actual in vivo fate of transplanted NSCs in the human body. The immunogenicity of neural stem cells presents a formidable hurdle that demands surmounting in the realm of research. Anti-non gal antibodies have been implicated in mediating the acute and chronic immune rejection responses of the graft.47 Alarmingly, the current immunosuppressive regimens that are customarily deployed to thwart allograft rejection may prove inadequate in suppressing the production of anti-non gal antibodies. To address the immunogenicity conundrum of neural stem cells, a two-pronged approach is warranted. On the one hand, concerted efforts can be channeled into the development of novel immunosuppressive drugs. The objective here is to adroitly inhibit the production of anti-non gal antibodies while preserving, to a certain extent, the immune system’s innate ability to fend off pathogens. On the other hand, exploration of strategies for inducing immune tolerance to a diverse array of xenogeneic or allogeneic antigens holds significant promise.
Conclusion
In conclusion, the implantation of hNSCs has shown potential in alleviating brain damage and suppress apoptosis through paracrine actions involving VEGF/BDNF, regulation of M1/M2 microglia to improve inflammatory environments as well as by promoting cell differentiation for functional recovery. These effects have been validated through three behavioral tests. Therefore, hNSC implantation holds promise as an effective long-term treatment strategy for neonatal HIE in the future. Further research is essential to comprehensively evaluate the prospects of hNSC implantation. Specifically, exploring the immunological responses elicited by hNSC transplantation would provide valuable insights into its safety profile and potential adverse effects within the body. Moreover, optimizing the dosage regimen of hNSCs holds the potential to substantially augment the therapeutic effectiveness and guarantee more reproducible and beneficial clinical outcomes.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by the National Key R&D Programme of China with Ministry of Science and Technology of the People’s Republic of China (grant number 2017YFA0104200, and 2018YFA0108601). This research was also supported by National Key Clinical Specialty Project.
Disclosure
The authors declare that they have no conflict of interest.
References
1. Wu YW, Comstock BA, Gonzalez FF, et al. Trial of erythropoietin for hypoxic-ischemic encephalopathy in newborns. N Engl J Med. 2022;387(2):148–159. doi:10.1056/NEJMoa2119660
2. Wang Z, Zhang P, Zhou W, et al. Neonatal hypoxic-ischemic encephalopathy diagnosis and treatment: a national survey in china. BMC Pediatr. 2021;21(1):261. doi:10.1186/s12887-021-02737-6
3. Martin LJ, Brambrink A, Koehler RC, et al. Primary sensory and forebrain motor systems in the newborn brain are preferentially damaged by hypoxia-ischemia. J Comp Neurol. 1997;377(2):262–285. doi:10.1002/(SICI)1096-9861(19970113)377:2<262::AID-CNE8>3.0.CO;2-1
4. Rodríguez M, Valez V, Cimarra C, et al. Hypoxic-ischemic encephalopathy and mitochondrial dysfunction: facts, unknowns, and challenges. Antioxid Redox Signal. 2020;33(4):247–262. doi:10.1089/ars.2020.8093
5. Ranjan AK, Gulati A. Advances in therapies to treat neonatal hypoxic-ischemic encephalopathy. J Clin Med. 2023;12(20):6653. doi:10.3390/jcm12206653
6. Finder M, Boylan GB, Twomey D, et al. Two-year neurodevelopmental outcomes after mild hypoxic ischemic encephalopathy in the era of therapeutic hypothermia. JAMA Pediatr. 2020;174(1):48–55. doi:10.1001/jamapediatrics.2019.4011
7. Li B, Dasgupta C, Huang L, et al. MiRNA-210 induces microglial activation and regulates microglia-mediated neuroinflammation in neonatal hypoxic-ischemic encephalopathy. Cell Mol Immunol. 2020;17(9):976–991. doi:10.1038/s41423-019-0257-6
8. Victor S, Rocha-Ferreira E, Rahim A, et al. New possibilities for neuroprotection in neonatal hypoxic-ischemic encephalopathy. Eur J Pediatr. 2022;181(3):875–887. doi:10.1007/s00431-021-04320-8
9. Pimentel-Coelho PM, Mendez-Otero R. Cell therapy for neonatal hypoxic-ischemic encephalopathy. Stem Cells Dev. 2010;19(3):299–310. doi:10.1089/scd.2009.0403
10. Wang L, Jiang F, Li Q, et al. Mild hypothermia combined with neural stem cell transplantation for hypoxic-ischemic encephalopathy: neuroprotective effects of combined therapy. Neural Regen Res. 2014;9(19):1745–1752. doi:10.4103/1673-5374.143417
11. Zheng XR, Zhang SS, Yin F, et al. Neuroprotection of VEGF-expression neural stem cells in neonatal cerebral palsy rats[J]. Behav Brain Res. 2012;230(1):108–115. doi:10.1016/j.bbr.2012.01.026
12. B LY, Wang Y, Tang JP, et al. Neuroprotective effects of ginsenoside Rg1-induced neural stem cell transplantation on hypoxic-ischemic encephalopathy. Neural Regen Res. 2015;10(5):753–759. doi:10.4103/1673-5374.156971
13. Shinoyama M, Ideguchi M, Kida H, et al. Cortical region-specific engraftment of embryonic stem cell-derived neural progenitor cells restores axonal sprouting to a subcortical target and achieves motor functional recovery in a mouse model of neonatal hypoxic-ischemic brain injury. Front Cell Neurosci. 2013;7:128. doi:10.3389/fncel.2013.00128
14. Otani T, Ochiai D, Masuda H, et al. The neurorestorative effect of human amniotic fluid stem cells on the chronic phase of neonatal hypoxic-ischemic encephalopathy in mice. Pediatr Res. 2019;85(1):97–104. doi:10.1038/s41390-018-0131-8
15. Lu S, Li K, Yang Y, et al. Optimization of an Intranasal Route For The Delivery Of Human Neural Stem Cells To Treat A Neonatal Hypoxic-Ischemic Brain Injury Rat Model. Neuropsychiatr Dis Treat. 2022;18:413–426. doi:10.2147/NDT.S350586
16. Daadi MM, Davis AS, Arac A, et al. Human neural stem cell grafts modify microglial response and enhance axonal sprouting in neonatal hypoxic-ischemic brain injury. Stroke. 2010;41(3):516–523. doi:10.1161/STROKEAHA.109.573691
17. Huang L, Wong S, Snyder EY, Hamblin MH, Lee JP. Human neural stem cells rapidly ameliorate symptomatic inflammation in early-stage Ischemic-reperfusion cerebral injury. Stem Cell Res Ther. 2014;5(6):129. doi:10.1186/scrt519
18. Ji G, Liu M, Zhao XF, et al. NF-κB signaling is involved in the effects of intranasally engrafted human neural stem cells on neurofunctional improvements in neonatal rat hypoxic-ischemic encephalopathy. CNS Neurosci Ther. 2015;21(12):926–935. doi:10.1111/cns.12441
19. Jiang L, Xu Z, Li H, et al. TAK‑242 exerts a neuroprotective effect via suppression of the TLR4/MyD88/TRIF/NF‑κB signaling pathway in a neonatal hypoxic‑ischemic encephalopathy rat model. Mol Med Rep. 2020;22(2):1440–1448. doi:10.3892/mmr.2020.11220
20. Xue LL, Wang F, Niu RZ, et al. Offspring of rats with cerebral hypoxia-ischemia manifest cognitive dysfunction in learning and memory abilities. Neural Regen Res. 2020;15(9):1662–1670. doi:10.4103/1673-5374.276359
21. Wang X, Zang J, Yang Y, et al. Transplanted human oligodendrocyte progenitor cells restore neurobehavioral deficits in a rat model of preterm white matter injury. Front Neurol. 2021;12:749244. doi:10.3389/fneur.2021.749244
22. Zheng Z, Zhang L, Qu Y, et al. Mesenchymal stem cells protect against hypoxia-ischemia brain damage by enhancing autophagy through brain derived neurotrophic factor/mammalin target of rapamycin signaling pathway. Stem Cells. 2018;36(7):1109–1121. doi:10.1002/stem.2808
23. Wu G, Chen Z, Wang P, et al. Hydrogen inhalation protects hypoxic-ischemic brain damage by attenuating inflammation and apoptosis in neonatal rats. Exp Biol Med. 2019;244(12):1017–1027. doi:10.1177/1535370219855399
24. Min YJ, Ling EA, Li F. Immunomodulatory mechanism and potential therapies for perinatal hypoxic-ischemic brain damage. Front Pharmacol. 2020;11:580428. doi:10.3389/fphar.2020.580428
25. Zhang S, Li W, Xu Y, et al. Alpha1-antitrypsin protects the immature mouse brain following hypoxic-ischemic injury. Front Cell Neurosci. 2023;17:1137497. doi:10.3389/fncel.2023.1137497
26. Papazian O. Neonatal hypoxic-ischemic encephalopathy. Medicina. 2018;78(Suppl 2):36–41. PMID: 30199363.
27. Fang CZ, Yang YJ, Wang QH, et al. Intraventricular injection of human dental pulp stem cells improves hypoxic-ischemic brain damage in neonatal rats. PLoS One. 2013;8(6):e66748. doi:10.1371/journal.pone.0066748
28. Edwards AB, Feindel KW, Cross JL, et al. Modification to the Rice-Vannucci perinatal hypoxic-ischaemic encephalopathy model in the P7 rat improves the reliability of cerebral infarct development after 48 hours. J Neurosci Methods. 2017;288:62–71. doi:10.1016/j.jneumeth
29. Yao K, Yang Q, Li Y, et al. MicroRNA-9 mediated the protective effect of ferulic acid on hypoxic-ischemic brain damage in neonatal rats. PLoS One. 2020;15(5):e0228825. doi:10.1371/journal.pone.0228825
30. Serrenho I, Rosado M, Dinis A, et al. Stem cell therapy for neonatal hypoxic-ischemic encephalopathy: a systematic review of preclinical studies. Int J Mol Sci. 2021;22(6):3142. doi:10.3390/ijms22063142
31. Archambault J, Moreira A, McDaniel D, et al. Therapeutic potential of mesenchymal stromal cells for hypoxic ischemic encephalopathy: a systematic review and meta-analysis of preclinical studies. PLoS One. 2017;12(12):e0189895. doi:10.1371/journal.pone.0189895
32. Jin K, Zhu Y, Sun Y, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99(18):11946–11950. doi:10.1073/pnas.182296499
33. Xiong LL, Chen J, Du RL, et al. Brain-derived neurotrophic factor and its related enzymes and receptors play important roles after hypoxic-ischemic brain damage. Neural Regen Res. 2021;16(8):1453–1459. doi:10.4103/1673-5374.303033
34. Chavez-Valdez R, Miller S, Spahic H, et al. Therapeutic hypothermia modulates the relationships between indicators of severity of neonatal hypoxic ischemic encephalopathy and serum biomarkers. Front Neurol. 2021;12:748150. doi:10.3389/fneur.2021.748150
35. Rosenkranz K, Kumbruch S, Tenbusch M, et al. Transplantation of human umbilical cord blood cells mediated beneficial effects on apoptosis, angiogenesis and neuronal survival after hypoxic-ischemic brain injury in rats. Cell Tissue Res. 2012;348(3):429–438. doi:10.1007/s00441-012-1401-0
36. Zhao M, Zhu P, Fujino M, et al. Oxidative stress in hypoxic-ischemic encephalopathy: molecular mechanisms and therapeutic strategies. Int J Mol Sci. 2016;17(12):2078. doi:10.3390/ijms17122078
37. Bachiller S, Jiménez-Ferrer I, Paulus A, et al. Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response. Front Cell Neurosci. 2018;12:488. doi:10.3389/fncel.2018.00488
38. Aryanpour R, Pasbakhsh P, Zibara K, et al. Progesterone therapy induces an M1 to M2 switch in microglia phenotype and suppresses NLRP3 inflammasome in a cuprizone-induced demyelination mouse model[J]. Int Immunopharmacol. 2017;51:131–139. doi:10.1016/j.intimp.2017.08.007
39. Jaworska J, Zalewska T, Sypecka J, et al. Effect of the HDAC inhibitor, sodium butyrate, on neurogenesis in a rat model of neonatal hypoxia-ischemia: potential mechanism of action. Mol Neurobiol. 2019;56(9):6341–6370. doi:10.1007/s12035-019-1518-1
40. Diao M, Qu Y, Liu H, et al. Effect of carbamylated erythropoietin on neuronal apoptosis in fetal rats during intrauterine hypoxic-ischemic encephalopathy. Biol Res. 2019;52(1):28. doi:10.1186/s40659-019-0234-7
41. He Y, Tang J, Zhang M, et al. Human placenta derived mesenchymal stem cells transplantation reducing cellular apoptosis in hypoxic-ischemic neonatal rats by down-regulating semaphorin 3a/neuropilin-1. Neuroscience. 2024;536:36–46. doi:10.1016/j.neuroscience.2023.11.007
42. Parry SM, Peeples ES. The impact of hypoxic-ischemic brain injury on stem cell mobilization, migration, adhesion, and proliferation. Neural Regen Res. 2018;13(7):1125–1135. doi:10.4103/1673-5374.235012
43. Keleher F, Lindsey HM, Kerestes R, et al. Multimodal analysis of secondary cerebellar alterations after pediatric traumatic brain injury. JAMA Network Open. 2023;6(11):e2343410. doi:10.1001/jamanetworkopen.2023.43410
44. Williamson MR, Jones TA, Drew MR. Functions of subventricular zone neural precursor cells in stroke recovery. Behav Brain Res. 2019;376:112209. doi:10.1016/j.bbr.2019.112209
45. Huang L, Zhang L. Neural stem cell therapies and hypoxic-ischemic brain injury. Prog Neurobiol. 2019;173:1–17. doi:10.1016/j.pneurobio.2018.05.004
46. Bonner JF, Haas CJ, Fischer I. Preparation of neural stem cells and progenitors: neuronal production and grafting applications. Methods Mol Biol. 2013;1078:65–88. doi:10.1007/978-1-62703-640-5_7
47. Ramos-Zúñiga R, González-Pérez O, Macías-Ornelas A, et al. Ethical implications in the use of embryonic and adult neural stem cells. Stem Cells Int. 2012;2012:470949. doi:10.1155/2012/470949
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.