Back to Journals » International Journal of Nanomedicine » Volume 19
Human Serum Albumin/Selenium Complex Nanoparticles Protect the Skin from Photoaging Injury
Authors Yao K, Peng Y, Tang Q, Liu K, Peng C
Received 26 October 2023
Accepted for publication 11 June 2024
Published 6 September 2024 Volume 2024:19 Pages 9161—9174
DOI https://doi.org/10.2147/IJN.S446090
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. RDK Misra
Kai Yao,1,* Yongbo Peng,2,* Qiyu Tang,3 Kaixuan Liu,3 Cheng Peng3
1Department of Vascular Surgery, The Third Xiangya Hospital of Central South University, Changsha, Hunan, People’s Republic of China; 2College of Pharmacy, Chongqing Medical University, Chongqing, People’s Republic of China; 3Department of Plastic Surgery, The Third Xiangya Hospital of Central South University, Changsha, Hunan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Cheng Peng, Email [email protected]
Introduction: Photoaging-induced skin damage leads to appearance issues and dermatoma. Selenium nanoparticles (SeNPs) possess high antioxidant properties but are prone to inactivation. In this study, human serum albumin/SeNPs (HSA-SeNPs) were synthesized for enhanced stability.
Methods: HSA-SeNPs were prepared by self-assembling denatured human serum albumin and inorganic selenite. The cytotoxicity of HSA-SeNPs was assessed using the MTT method. Cell survival and proliferation rates were tested to observe the protective effect of HSA-SeNPs on human skin keratinocytes against photoaging. Simultaneously, ICR mice were used for animal experiments. H&E and Masson trichromatic staining were employed to observe morphological changes in skin structure and collagen fiber disorders after UVB irradiation. Quantitative RT-PCR was utilized to measure changes in mRNA expression levels of factors related to collagen metabolism, inflammation, oxidative stress regulation, and senescence markers.
Results: The HSA-SeNPs group exhibited significantly higher survival and proliferation rates of UVB-irradiated keratinocytes than the control group. Following UVB irradiation, the back skin of ICR mice displayed severe sunburn with disrupted collagen fibers. However, HSA-SeNPs demonstrated superior efficacy in alleviating these symptoms compared to SeNPs alone. In a UVB-irradiated mice model, mRNA expression of collagen type I and III was dysregulated while MMP1, inflammatory factors, and p21 mRNA expression were upregulated; concurrently Nrf2 and Gpx1 mRNA expression were downregulated. In contrast, HSA-SeNPs maintained the mRNA expression of those factors to be stable In addition, the level of SOD decreased, and MDA elevated significantly in the skin after UVB irradiation, but no significant differences in SOD and MDA levels between the HSA-SeNPs group with UVB irradiation and the UVB-free untreated group.
Discussion: HSA-SeNPs have more anti-photoaging effects on the skin than SeNPs, including the protective effects on skin cell proliferation, cell survival, and structure under photoaging conditions. HSA-SeNPs can be used to protect skin from photoaging and repair skin injury caused by UVB exposure.
Keywords: selenium nanoparticles, human serum albumin, skin photoaging, senescence, SOD
Introduction
Skin aging is commonly categorized into intrinsic and extrinsic aging.1 The latter primarily arises from environmental factors, such as ultraviolet (UV) radiation and harmful chemicals, with UV radiation being the predominant contributor. Skin photoaging, also known as skin aging caused by UV radiation,2,3 encompasses both morphological and physiological alterations in the skin following prolonged exposure to UV radiation. This process not only gives rise to a range of aesthetic concerns but also exhibits a close association with dermatoma occurrence.4,5
Traditionally, UV radiation can be categorized into three groups: UVA (320–400 nm), UVB (280–320 nm), and UVC (100–280 nm). Among these, UVB radiation is primarily responsible for skin photoaging damage.3 Photoaging not only manifests as wrinkles and pigmentation in human skin but also results in a decrease in the number of skin cells and abnormal connective tissue composition, particularly the loss of collagen type I.6 Additionally, photoaged human skin exhibits induction of matrix metalloproteinases (MMPs) and interleukin-1 (IL-1),7,8 which play a crucial role in the injury caused by skin photoaging. The stimulation of UVB triggers MMP expression in skin tissue through both membrane receptor-dependent and -independent pathways.9 In the progression of skin photoaging, matrix metalloproteinases (MMPs) function by degrading collagen fibers. Specifically, exposure to low doses of UVB radiation on human skin induces the upregulation of activating protein-1 (AP-1), resulting in heightened expression of MMP-1, 9kd gelatinase, and stromal lysin-1 at both mRNA and protein levels.10 This cascade contributes to the degradation of collagen fibers, playing a crucial role in skin photoaging.11 In vitro studies found that reactive oxygen species (ROS) could double the mRNA expression of MMP-2 in fibroblasts, suggesting that ROS may cause photoaging damage by increasing MMP-2 and degrading related matrix.12 Additionally, studies have documented an excessive deposition of MMP-1, MMP-3, and MMP-10 in UVB-irradiated skin.13 Consequently, MMP-1, MMP-3, and MMP-10 may be implicated in the diminished collagen fibers observed in solar-exposed skin. In contemporary research, there is mounting evidence emphasizing the significant role of reactive oxygen species (ROS) in UVB-induced skin photoaging.14–16 The skin harbors numerous natural UV chromophores, which upon absorbing UV photon energy, become excited and subsequently engage with molecular oxygen to generate ROS, facilitated by various enzymes and transition metal ions. Apart from direct ROS induction, UVB exposure triggers ROS accumulation in the skin through indirect pathways. Notably, studies have identified CD11b+ leukocytes infiltrating the skin post-UV irradiation as the primary contributors to ROS production. ROS orchestrate the UVB-induced activation of transcription factors such as AP-1 and nuclear factor-kappa B (NF-κB), thereby enhancing the secretion of matrix metalloproteinases (MMPs).17 Furthermore, ROS possess the capacity to directly impair normal collagen fibers, thereby exacerbating the process of skin photoaging.18
Since ROS plays an important role in the occurrence of skin photoaging, the antioxidant therapy with free radical scavenger as the main component can prevent skin photoaging injury. Previous results showed that topical application of antioxidants could effectively inhibit the formation of sunburn cells, lipid peroxidation and polyamide production.19 Notable antioxidants encompass vitamin C, Na2SeO3, β-carotene, catalase, superoxide dismutase (SOD), among others.20 Selenium, a trace mineral found in soil and water, plays a crucial role in maintaining human health. It is an essential nutrient that our bodies require in small amounts for various physiological functions. Selenium acts as a powerful antioxidant, protecting our cells from damage caused by harmful free radicals. This mineral also supports the proper functioning of the immune system, helping to defend against infections and diseases. Previous study showed that selenium had the characteristics of scavenging free radicals and improving the antioxidant capacity of the body with a very narrow margin between minimum acceptable intake and toxicity.21 Compared with selenium salt (Na2SeO3), selenium nanoparticles (SeNPs) have the advantages of lower toxicity and higher absorption rate.22,23 The effect of SeNPs on scavenging free radicals is stronger than Na2SeO3, and the semi-clearance concentration of SeNPs is also lower.24 SeNPs have the ability to induce phosphorylation of Jun N-terminal kinase (JNK), thereby preventing a decrease in mitochondrial membrane potential and inhibiting activation of the mitochondrial pathway.25,26 Consequently, this leads to inhibition of ROS production and attainment of anti-aging effects through their antioxidant properties.
Nevertheless, SeNPs are subjected to aggregation and inactivation, because of nano elemental selenium (Se0) self-aggregation effect to form insoluble Se and loss their solubility in the solution, which need the dispersant and stabilizer to carry the nano elemental selenium to stop neonatal Se0 atoms self-adhesive form insoluble Se.27 In this study, to attach better stability and water solubility to selenium nanoparticles, a form of human serum albumin/selenium nanoparticles were prepared by self-assembly of denatured human serum albumin and inorganic selenite, hereinafter termed as HSA-SeNPs (Figure 1), due to the negative charge dispersant effects and the unique hydrophobic spatial structure of the albumin, which is easier to control when it is made in pilot production.28,29 As for the protein carrier, denatured human serum albumin was selected in this study to be better applied to human body, facilitating the absorption rate as well as reducing toxicity and side effects.30 MTT method was used to detect the cytotoxicity of HSA-SeNPs. Then cell survival and proliferation rates were tested to observe the effect of HSA-SeNPs on protecting human skin keratinocytes from photoaging. Simultaneously, ICR mice were used for animal experiments. H&E and Masson trichromatic staining were used to observe the structural changes of skin morphologically and the disorders of collagen fibers after UVB irradiation. Quantitative RT-PCR was used to measure the mRNA expression changes of collagen metabolism-related factors (collagen type I gene col1a1, collagen type III gene col3a3 and MMP1), inflammatory factors (Tumor necrosis factor-α, TNF-α; and IL-1β), oxidative stress regulators (nuclear factor erythroid 2-related factor 2, Nrf2; and glutathione peroxidase 1, Gpx1) and senescence markers (p16 and p21) after UVB exposure. Ultimately, it is concluded that HSA-SeNPs have more anti-photoaging effects than the traditional SeNPs.
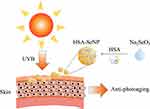 |
Figure 1 The schematic diagram for the synthesis and anti-photoaging effects of HSA-SeNPs. |
Results
Synthesis and Characterization of HSA-SeNPs
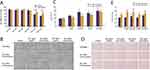
Based on the HSA-SeNPs design, HSA was mixed with sodium selenite in ethanol first to form HSA/Se0 complex nanoparticles constructer using a desolvation/reducing complex method through reducing agent ascorbic acid. Different from the previous preparation method of SeNPs, HSA is not only as hyperdispersant for red nano Se in HSA-SeNPs system, and denatured HSA is the carrier for nano Se produce. Transmission electron microscopy (TEM) images show that the final HSA-SeNPs were spherical with a uniform size of about 21 nm (Figure 2A). Dynamic light scattering (DLS) analysis showed that the hydrodynamic diameter of HSA-SeNPs was approximately 40 ± 8.6 nm, larger than that of HSA alone (7 ± 2.5 nm) (Figure 2B). And the nano size of SeNPs was obtained as well as HSA-SeNPs (Figure 2C and D). The ζ-potential analysis in Figure 2E showed that HSA-SeNPs had more negative charge (−38.5 mV) than HSA (−29.3 mV), which possibly help HSA-SeNPs penetrate through keratinocytes to dermal mucus with negative interaction and thus facilitating skin anti-UV damage ability.31
Referring to the reported determined method,32) the compared content determination of Se and HSA components between HSA-SeNPs and SeNPs was to be confirmed that HSA-SeNPs is the complex of HSA and Se with the molar ratio of ~10:1, but no obvious HSA components in SeNPs. These results proved the proof of concept of HSA/Se0 complex nanoparticles constructer in HSA-SeNPs. The circular dichroism (CD) spectra analysis was conducted to examine the characteristic of HSA in HSA-SeNP’s secondary structures (Figure 2F). The results illustrated that the absorbance of HSA-SeNPs in the range from 195 to 250 nm was almost same as natural HSA, indicating that the secondary structuring of HSA in HSA-SeNPs, including α helix, β sheet and random coil, did not change during the whole process. And these results show that HSA-SeNPs self-assembly is intermolecular interaction between HSA under desolvation and reduction affection.
HSA-SeNPs Had Excellent Anti-Photoaging Ability
MTT method was used to explore the cytotoxicity on L929 cells (Figure 3A) and the protective effect of different concentrations of SeNPs and HSA-SeNPs on HaCaT cells (Figure 3B-E). In general, SeNPs and HSA-SeNPs had no obvious cytotoxicity within the concentration range of 100–500 ng/mL. However, when the concentration reached 1000 or 2000 ng/mL, SeNPs and HSA-SeNPs gradually showed obvious cytotoxicity. On the other hand, HSA-SeNPs exhibited reduced toxicity relative to SeNPs. Specifically, the cytotoxicity of HSA-SeNPs was approximately 92% that of the SeNPs group at a concentration of 1000 ng/mL and 81% at 2000 ng/mL. We selected SeNPs and HSA-SeNPs with 200 or 300 ng/mL in the following experiments.
After pretreated with PBS solution, SeNPs, or HSA-SeNPs, HaCaT cells were exposed to UVB light (60 J/cm2) for 18s and then cultured in the incubator. MTT assay was used to measure the cell survival rate after 24 h or 48 h. Microscopically, the number of cells in all groups decreased after UVB exposure, and even gradually reduced with the culture time extended from 24 h to 48 h after UVB irradiation (Figure 3B). The number of remaining living cells in the SeNPs group and the HSA-SeNPs group was more than that in the control group, and the cell structure was more stable in the SeNPs group and the HSA-SeNPs group. Cell survival rates measured in the SeNPs group and the HSA-SeNPs group were higher than those of control group. After UVB irradiation and a 24 h-culture, the cell survival rate in the 200 ng/mL HSA-SeNPs group was about 182% of that in the control group, while the cell survival rate was 137% of the control group in the 200 ng/mL SeNPs group. After UVB irradiation and a 48h-culture, the cell survival rate in the 200 ng/mL HSA-SeNPs group reduced to 132% of that in the control group, while the cell survival rate was 116% of the control group in the 200 ng/mL SeNPs group. When the concentration of SeNPs and HSA-SeNPs was added to 300 ng/mL, the survival rate in the HSA-SeNPs and the SeNPs group was surprisingly found to be 200% and 170% of that in the control group, respectively, 24 h after UVB exposure. The cell survival rate in the 300 ng/mL HSA-SeNPs and SeNPs group was 186% and 148% of the control group, respectively, 48 h after UVB exposure (Figure 3C). Overall, under the same concentration, after UVB irradiation, HSA-SeNPs showed a stronger protective effect on the keratinocyte cell survival than SeNPs, which indicated that HSA-SeNPs had better anti-photoaging ability and played a stronger role in maintaining the stability of keratinocytes under photoaging conditions.
HSA-SeNPs Had Superior Effect to SeNPs on Keratinocyte Cell Proliferation Under Photoaging Conditions
After UVB irradiation, the number of cells in the control group was significantly decreased, and there was a conspicuous increase in cell debris microscopically. In the HSA-SeNPs group, cells are arranged tightly and there is no significant reduction in cell number (Figure 3D). Following 24 hours of UV irradiation, the relative cell number for HSA-SeNPs at a concentration of 200 ng/mL stood at approximately 121% of that observed for SeNPs at the equivalent concentration, demonstrating a notable increase to 131% at 300 ng/mL. Similarly, after 48 hours of UV irradiation, the relative cell number for HSA-SeNPs at 200 ng/mL was observed to be 102% compared to SeNPs at the same concentration, with the figure rising to 115% at 300 ng/mL (Figure 3E). Consequently, HSA-SeNPs exhibit superior efficacy compared to SeNPs in enhancing keratinocyte proliferation under photoaging conditions.
HSA-SeNPs Protect the Skin from Photoaging Symptoms Such as Wrinkles and Sunburn
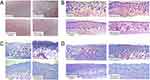
The back skin of ICR mice was randomly divided into four areas, denoted as an untreated group, UVB exposure group, SeNPs group, and HSA-SeNPs group. Macroscopically, the skin of mice in the untreated group was kept in the best condition, without any wrinkles or sunburn, appearing healthily ruddy (Figure 4A). After being exposed to UVB, the skin of mice in the UVB exposure control group was most seriously hurt, with numerous wrinkles, severe sunburn, multiple dry peeling, and pigmentation. In the SeNPs group, there is a small amount of massive sunburn, with partial pigmentation and dry peeling, but no obvious wrinkles were observed. Surprisingly, in the HSA-SeNPs group, the skin was almost the same as that of the untreated group. There were no skin photoaging symptoms such as wrinkles and sunburn, and the skin appearance was generally in normal condition.
H&E and Masson’s Staining Results Revealed a Regular Structure of the Skin in the HSA-SeNPs Group
As seen in sections of mouse skin stained with H&E assay, in the untreated group, collagen fibers in the dermis were normal-appearing and arranged in order (Figure 4B). After being exposed to UVB, the epidermis of the skin was thickened and wrinkled. Meanwhile, keratinocytes arranged disorderly, and collagen fibers lost normal connection and interaction. In the SeNPs group, the epidermis appeared smooth, but the collagen fibers were still broken and distributed unevenly. However, in the HSA-SeNPs group, the morphology and number of skin cells were normal, leading to a regular structure of the epidermis and dermis. The collagen fibers are arranged orderly as well, interweaving into a neat network.
Masson’s trichromatic staining method was used to observe collagen fibers and the muscular tissue. Compared with the untreated group, the epidermis in the UVB exposure group was thickened, and collagen fibers were significantly reduced with a broken and disorganized structure (Figure 4C and D). Dead cells with deep staining and pyknosis were occasionally observed in the UVB exposure group. In the SeNPs group, the degree of cell necrosis and collagen fiber degeneration was lighter than that in the UVB exposure group, but the epidermis remained thick and the number of collagen fibers was reduced significantly compared with the untreated group. However, in the HSA-SeNPs group, no significant thickening was observed in the epidermis, and the structure of skin cells and collagen was normal.
HSA-SeNPs balanced the mRNA expression of collagen metabolism-related factors, inflammatory factors, oxidative stress regulators, and senescence markers after UVB irradiation
To test the level of collagen content, inflammation, oxidative stress, and senescence in skin samples, col1a1, col3a3, MMP1, TNF-α, IL-1β, Nrf2, Gpx1, p16, and p21 mRNA expression was measured. Quantitative RT-PCR results showed that after UVB exposure, the mRNA expression of Col1a1 in all groups decreased. The col3a3 and MMP1 mRNA expression in the UVB exposure group and SeNPs group was significantly increased, whereas the col3a3 and MMP1 mRNA expression in the HSA-SeNPs group was similar to that in the untreated group, without significant differences (Figure 5A). The mRNA expression of TNF-α in all groups with UVB irradiation elevated dramatically. As for IL-1β mRNA expression, it significantly rose in the UVB exposure group, but there was no significant difference between the SeNPs group and the untreated group or between the HSA-SeNPs group and the untreated group (Figure 5B).
After UVB treatment, Nrf2 and Gpx1 mRNA expression in the UVB exposure group and the SeNPs group decreased significantly, with Nrf2 and Gpx1 mRNA expression in the UVB exposure group decreasing most severely, nearly only half of that in the untreated group (Figure 5C). However, there was no significant difference in Nrf2 and Gpx1 mRNA expression levels between the HSA-SeNPs group and the untreated group.
There was no significant difference in the p16 mRNA expression between 4 groups (Figure 5D). Notably, the mRNA expression level of p21 in the UVB exposure group was significantly higher than that in the untreated group, but there was no significant difference in p21 mRNA expression between the SeNPs group and the untreated group or between the HSA-SeNPs group and the untreated group.
In conclusion, UVB-induced photoaging leads to disrupted expression of collagen type I and III, increased the expression of MMP1, TNF-α and IL-1β, reduced the expression of Nrf2 and Gpx1, as well as elevated the expression of p16 and p21 in the skin. Notably, HSA-SeNPs exhibit significant protective effects against UVB-induced skin photoaging based on the mRNA expression profiles of multiple biological factors.
HSA-SeNPs Avoided UVB-Induced Abnormal Antioxidant Activity
The level of SOD in the skin was significantly decreased in the UVB exposure group and the SeNPs group compared with the untreated group (Figure 5E). But no significant difference was tested in SOD expression between the HSA-SeNPs group and the untreated group. At the same time, the MDA level of mice skin was significantly increased in the UVB exposure group (Figure 5F). Nonetheless, there was no significant difference in MDA content between the SeNPs group and the untreated group or between the HSA-SeNPs group and the untreated group.
Discussion
The histological and morphological injury of skin photoaging mainly occurs in the collagen fibers of dermis, related to the expression change of inflammatory factors and senescence markers. The present study revealed that in the skin dermis devoid of UVB irradiation, collagen fiber bundles exhibited a slender, uniform distribution, and orderly arrangement, reflecting a tense structure and stable performance. Conversely, in UVB-exposed skin, the collagen fiber bundles appeared thick, loose, and unevenly distributed, consistent with findings from previous investigations.33,34 These observed changes collectively suggest that UVB irradiation expedites the process of skin photoaging. At present, it is generally recognized that the main reason for collagen abnormalities in photoaged skin is the up-regulation of MMP1 expression induced by irradiation, contributing to the imbalance of collagen synthesis and degradation.35,36 Ying Rui et al found that the skin of nude mice subjected to UV irradiation for 30 days continuously contained more MMP-1 and less collagen type I.37 Some studies have also found that the content of collagen in the skin after UVB irradiation significantly reduced.38 Furthermore, collagen type I has stable structure and composition, while collagen type III is relatively immature and unstable with low elastic tension. When the skin is damaged under UVB light, the level of collagen type I decreases while the level of collagen type III increases, destroying the homeostatic collagen component and the structural disorder.39 Our results also confirmed that the mRNA expression of the collagen type I gene, Col1a1, was decreased and the mRNA expression of the collagen type III gene, col3a3, was increased in the UVB exposure group. Fortunately, HSA-SeNPs could efficaciously protect collagen type I and type III from a disturbing expression by down-regulating the level of MMP1, which was instrumental in maintaining the stability of the skin structure.
TNF-α can promote the early inflammatory response and increase the number of local fibroblasts, thus promoting skin wound healing by inducing the mitosis of fibroblasts and the synthesis of extracellular matrix.40,41 With the proinflammatory mediators, the body produces IL-1, IL-8, and other inflammatory factors to stimulate the defense behavior, as well as promote fibroblast proliferation and collagen synthesis with the help of growth factors to complete the process of skin tissue repair.42 Our experiment confirmed that the expression of inflammatory factors in the UVB exposure group was up-regulated. HSA-SeNPs could significantly promote the expression of TNF-α, being conducive to skin tissue repair, and could significantly inhibit the over-expression of inflammatory factors such as IL-1β on the other hand, to prevent excessive inflammatory reaction and avoid the aggravation of skin damage.
The P16 gene, also known as MTS1/CDKN2, directly participates in the regulation of the cell cycle and inhibits cell proliferation and division.43 P21 gene is an important member of cyclin-dependent kinase inhibitor family, which inhibits the activity of cyclin-dependent kinases, and suppresses DNA replication and DNA repair, thus inhibiting cell proliferation and division as well.44 P16 and p21 genes are considered as senescence markers. Their activation is regarded as cellular senescence.45,46 Under UVB irradiation, both SeNPs and HSA-SeNPs inhibited the activation of the p21 gene, and HSA-SeNPs showed stronger and better effects, which further reflected the value of HSA-SeNPs in resisting skin cell senescence caused by UVB irradiation.
SOD has distinct antioxidant effect. The decrease of SOD activity in the body indicates the declining ability to scavenge oxygen free radicals, therefore, the SOD activity reflects the degree of aging.47,48 MDA is the end product of lipid peroxidation caused by the accumulation of free radicals, which is cytotoxic.49 The amount of MDA often directly illustrates the degree of lipid peroxidation in the body and indirectly reflects the degree of cell lesion. In addition, MDA can promote the cross-link of collagen fibers. As report goes, excessive collagen fiber cross-linking leads to the hardening and shrinkage of the skin, contributing to skin aging.50,51 Therefore, SOD and MDA activities are important indicators of body aging, as well as skin aging. Our study found that, 1) the SOD activity in the skin of mice in the UVB exposure group was significantly decreased, which illustrated that the ability of skin cells in photoaged tissues to scavenge oxygen free radicals was decreased. 2) The level of MDA in the skin of mice in the UVB exposure group was significantly elevated, suggesting that numerous free radicals reacted with lipid and a large amount of MDA was produced with the oxidation reaction. So skin cells were seriously damaged in the UVB exposure group. However, comfortingly, in the HSA-SeNPs group, SOD levels were significantly higher than that in the UVB exposure group, while MDA levels were lower than that in the UVB exposure group, which revealed the ability of HSA-SeNPs to help scavenge oxygen free radicals. An it is seen that the antioxidant effect of HSA-SeNPs was better than SeNPs.
The abnormal activation of growth factor receptors and cytokine receptors can also lead to skin photoaging. Studies have shown that UVB radiation activates growth factor receptors and cytokine receptors on the cell surface, and then results in the activation of downstream signal transduction pathways,52 contributing to skin photoaging symptoms. EGF can activate JNK in combination with IL-1 or TNF. UVB is also a strong activator of JNK and, at the same time, can induce the production of IL-1 and TNF in keratinocytes. Thus, both UVB and EGF have similar effects on JNK activation.53 Meanwhile, Under UVB radiation, EGF-R, IL-1R, and TNF-R will specifically aggregate, activating the JNK pathway.54 EGF-R inhibitors can restrain the tyrosine phosphorylation of EGF-R induced by UV radiation, EGF, or IL-1β. Moreover, EGF-R inhibitors can also inhibit the activation of IL receptor-related kinase and suppress UV or IL-1β induced c-Jun kinase activation.55 In addition, IL-1β-induced activation of EGF-R and ERK pathways lead to MMP-1 overexpression, promoting the photoaging process.56 Therefore, EGF-R is a key mediator in multiple signal transduction pathways, which suggests that the suppression of EGF-R tyrosine kinase activation is also an important way to prevent skin photoaging induced by UV radiation.
Conclusions
In this study, to make selenium nanoparticles have better stability and water solubility, a form of human serum albumin/selenium nanoparticles (HSA-SeNPs) were prepared by self-assembly of denatured human serum albumin and inorganic selenite. Compared with SeNPs, HSA-SeNPs act as a more suitable protector for skin cells against UVB-induced skin photoaging with no obvious cytotoxicity, which prevents keratinocytes from cell death and effectively promotes cell proliferation under the UVB exposure condition. HSA-SeNPs mainly work on the skin epidermis and dermis, resisting wrinkles, pigmentation, and other damages caused by UVB exposure. MMP1 mRNA expression was inhibited by HSA-SeNPs under UVB irradiation, thus up-regulating the expression of collagen type I and reducing the expression of collagen type III, maintaining the stability of dermal collagen composition. The mRNA expression of TNF-α was increased by HSA-SeNPs under UVB irradiation, whereas IL-1β mRNA expression was inhibited, for the sake of skin tissue repair and the control of inflammation. Even more important, HSA-SeNPs can suppress the expression of p21 and prevent cellular senescence. The results showed that HSA-SeNPs could improve the activity of SOD, resisting the production of oxygen free radicals, and inhibit the level of MDA, restraining the happening of collagen cross-linking and wrinkling. In conclusion, this study verified the anti-photoaging effect of HSA-SeNPs on the skin (Figure 1). Our research provided innovative ideas for the development of new anti-aging products.
Experimental Methods
Preparation and Characterization of SeNPs and HSA-SeNPs
Three mL of 60% ethanol buffer including 100 mg HSA was first stirred at 40 °C for about 60 min. 600 μL of 25 mM sodium selenite was subsequently added into the HSA solution for 60 min under stirring, and then 0.4 mL of 60 mM Vitamin C was added into the mix buffer to react and induce self-assembly of HSA/SeNPs. After 3 h, the resulting HSA-SeNPs were dialyzed (MWCO 8–14 KD) in PBS at room temperature for 48 h to remove excess unreacted reagents, including Na2SeO3 and Vitamin C byproduct, and then were kept at 4 °C for use. And following the reported method,28 1 mL of 25 mM sodium selenite was mixed with 4 mL 25 mM glutathione (GSH) containing 20 mg HSA for the preparations of the middle sizes SeNPs. The pH of the mixture was adjusted to 7.2 with 1.0 M sodium hydroxide when the red elemental Se and oxidized glutathione (GSSG) formed. The red solution was dialyzed (MWCO 8–14 KD) against PBS for 48 h with the water changing every 12 h to separate GSSG from SeNPs. After centrifugation with 15,000 g for 20 min, while SeNPs and HSA-SeNPs were digested with 10% nitric acid in a 50 °C water bath overnight, the content of Se and HSA components was to be determined with ICP-OES. All measurements were performed in triplicate. Freshly prepared SeNPs and HSA-SeNPs were transferred onto a 200-mesh copper grid coated with carbon, dried at room temperature, and then analyzed by TEM. Circular dichroism (CD) spectra were recorded on an Applied Photophysics Chirascan instrument at 25 °C. The size of the freshly prepared sample (about 1 mg/mL) was also measured by dynamic light scattering (DLS), using a Malvern Zetasizer Nano ZS. Given the sensitivity of the instrument, multiple runs (>3) were performed to avoid erroneous results.
Cytotoxicity of SeNPs and HSA-SeNPs
L929 cells (Zhong Qiao Xin Zhou Biotechnology Co., Ltd, Shanghai, China) were cultured with Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Rockville, Md, USA) which contained 10% fetal bovine serum (Biological industries, Haemek, Israel) in a CO2 incubator at 37 °C. To test the toxicity of SeNPs and HSA-SeNPs, L929 cells were seeded in a 96-well plate at a density of 1.0×105 cells/well. SeNPs or HSA-SeNPs with a concentration range of 100–500 ng/mL were added respectively. MTT assay was used to measure the cell viability after 48h as we previously described elsewhere.57,58 The cell viability of the control group was considered 100%.
Trypan Blue Staining
HaCaT cells (Changsha bochu biotech co., ltd, Changsha, China) were cultured with DMEM which contained 10% fetal bovine serum in a CO2 incubator at 37 °C. With a density of 1.0×105 cell/well, HaCaT cells were divided randomly into 6 groups, a control group without UVB illumination (control group), a control group with UVB illumination (UVB exposure group), 200 or 300 ng/mL SeNPs group with UVB illumination (200 or 300 ng/mL SeNPs group), and 200 or 300 ng/mL HSA-SeNPs group with UVB illumination (200 or 300 ng/mL HSA-SeNPs group). After pretreatment, HaCaT cells were exposed to UVB light for 18 hours except for the untreated group. After that, cells were stained with trypan blue and the cell survival rate was calculated. The cell survival of the control group was considered 100%.
Cell Proliferation Under UVB Condition
With a density of 1.0×103 cell/well, HaCaT cells were seeded in the 96-well plate for the proliferation test. 200 and 300 ng/mL SeNPs or HSA-SeNPs were used for the following experiments. After pretreated with PBS solution, SeNPs or HSA-SeNPs for 16 h, HaCaT cells were exposed to UVB light (312nm, 60J/cm2; SIGMA High-tech Co., Ltd, Shanghai, China) for 18s and then cultured in the incubator for 24 or 48h. Photos of cell morphology were taken before and after UVB exposure. MTT assay was used as we previously described elsewhere57,58 to measure the relative cell number 24 or 48h after UVB exposure.
Animal Skin Photoaging Model
6-week-old ICR mice (n=4) with SPF grade, purchased from the SLAC Laboratory Animal Co., Ltd. (Shanghai, China), were fed in the animal experimental center of Xiangya Medical School, Central South University, under a standard breeding environment. The experimental protocol was approved by the Animal Care and Use Committee of Central South University, and all animal experiments followed the 3Rs’ principles and the Five Freedoms paradigm. The mouse UVB-induced photoaging model was made as previously described.59,60 In brief, after anesthesia and hair removal, the mice were placed on the platform 20 cm away from the lamp tube (8W, wavelength: 340nm, Yichen household products Co., Ltd, Shanghai, China) to receive UVB radiation on the dorsal skin for the 50s, 3 times a week for 10 weeks. As for the untreated group, the back skin is covered with tinfoil to avoid UVB radiation. After UVB exposure, 0.1 mL of 300 ng/mL SeNPs or HSA-SeNPs were injected subcutaneously in the SeNPs group or the HSA-SeNPs group. A saline solution was used as a control. Three days after the final irradiation, mice were sacrificed with 1% sodium pentobarbital (intraperitoneal injection), and the skin was taken for further investigation.
Hematoxylin and Eosin (H&E) and Masson Trichrome Staining
10% formalin was used to fix the skin samples for 48h, before dehydration and paraffin embedding. Then samples were sliced with a thickness of 4 μm, mounted onto slides, and stained with hematoxylin and eosin solution. For Masson trichrome staining, slices were stained with a Masson-Goldner staining kit (Sigma-Aldrich, St. Louis, MO, USA) following the instructions. Images were taken with a light microscope (Leica, Buffalo, NY, USA).
qRT-PCR
Total RNA was extracted from skin tissues by TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). After that, cDNA was synthesized using PrimeScript™ RT reagent Kit (TaKaRa Bio, Dalian, China), as templates for the following qRT-PCR experiments using HiScript II One Step qRT-PCR SYBR Green Kit (Vazyme Biotech Co.,Ltd, Nanjing, China). Primers selected were listed: Col1a1-F, 5’-CGCCATCAAGGTCTACTGC-3’; Col1a1-R, 5’-ACGGGAATCCATCGGTCA-3’; Col3a3-F, 5’-ACTGGTGCTCCTGGATTAAAG-3’; Col3a3-R, 5’-CTTCCTCGAGCTCCATCATTAC-3’; Mmp1-F, 5’-ATGAGACGTGGACCGACAAC-3’; Mmp1-R, 5’-TGAGTGAGTCCAAGGGAGTG-3’; TNF-α-F, 5’-CAGCGCTGAGGTAAGGTGGTGCAATCTGCC-3’; TNF-α-R, 5’-TGCCCGGACTCCGCAA-3’; IL-1β-F, 5’-GGCTTCCTTGTGCAAGTGTC-3’; IL-1β-R, 5’-TGTCGAGATGCTGCTGTGAG-3’; Nrf2-F, 5’-TCACACGAGATGAGCTTAGGGCAA-3’; Nrf2-R, 5’-TACAGTTCTGGGCGGCGACTTTAT-3’; Gpx1-F, 5’-CCAACACCCAGTGACGACC-3’; Gpx1-R, 5’-CTCAAAGTTCCAGGCAATGTC-3’; p16-F, 5’-CGTACCCCGATACAGGTGATG-3’; p16-R, 5’-ATACCGCAAATACCGCACGA-3’; p21-F, 5’-AGCAGTTGAGCCGCGATTG-3’; p21-R, 5’-ACCCAGGGCTCAGGTAGATCTTG-3’; Gapdh-F, 5’-CATACCAGGAAATGAGCTTG-3’; Gapdh-R, 5’-ATGACATCAAG-3’. The mRNA expression was measured from the threshold cycle value based on a standard curve and was normalized against Gapdh. The experiment was repeated 3 times at least.
SOD and Malondial Dehyde (MDA) Measurement
Levels of SOD and MDA were measured using the SOD assay kit (WST-1 method; Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and MDA assay kit (TBA method; Nanjing Jiancheng Bioengineering Institute, Nanjing, China), respectively. All procedures were conducted following the instructions. In brief, skin tissues were lysed by RIPA and centrifuged at 10,000 g for 10 min. The supernatant liquor was used for the SOD or MDA measurement by spectrophotometry with a microplate Reader (PerkinElmer, Waltham, MA, USA).
Statistical Analysis
Data were analyzed using unpaired, two-tailed Student’s t tests for comparisons between two groups and one-way analysis of variance for multiple comparisons. Results are expressed as mean ± SD. A p value less than 0.05 was considered statistically significant.
Abbreviations
SeNPs, Selenium nanoparticles; HSA-SeNPs, human serum albumin/selenium nanoparticles; UV, ultraviolet; MMPs, matrix metalloproteinases; IL-1, interleukin-1; AP-1, activating protein-1; ROS, reactive oxygen species; NF-κB, nuclear factor-kappa B; SOD, superoxide dismutase; JNK, Jun N-terminal kinase; TNF-α, tumor necrosis factor-α; Nrf2, nuclear factor erythroid 2-related factor 2; Gpx1, glutathione peroxidase 1; CD, circular dichroism; MDA, malondial dehyde.
Acknowledgments
The authors acknowledge the support from the National Natural Science Foundation of China (82272280) and the Wisdom Accumulation and Talent Cultivation Project of The Third Xiangya Hospital of Central South University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fang JY, Wang PW, Huang CH, Chen MH, Wu YR, Pan TL. Skin aging caused by intrinsic or extrinsic processes characterized with functional proteomics. Proteomics. 2016;16(20):2718–2731. doi:10.1002/pmic.201600141
2. McDaniel D, Farris P, Valacchi G. Atmospheric skin aging-Contributors and inhibitors. J Cosmet Dermatol. 2018;17(2):124–137. doi:10.1111/jocd.12518
3. Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Res Rev. 2015;21:16–29. doi:10.1016/j.arr.2015.01.001
4. Awad F, Assrawi E, Louvrier C, et al. Photoaging and skin cancer: is the inflammasome the missing link? Mech Ageing Dev. 2018;172:131–137. doi:10.1016/j.mad.2018.03.003
5. Bae YC, Bae EJ, Wang JH, Gilchrest BA. Changes in self-perceptions of photoaging severity and skin cancer risk after objective facial skin quality analysis. J Drugs Dermatol. 2017;16(5):453–459.
6. Naylor EC, Watson RE, Sherratt MJ. Molecular aspects of skin ageing. Maturitas. 2011;69(3):249–256. doi:10.1016/j.maturitas.2011.04.011
7. Fisher GJ, Talwar HS, Lin J, Voorhees JJ. Molecular mechanisms of photoaging in human skin in vivo and their prevention by all-trans retinoic acid. Photochemistry and photobiology. 1999;69(2):154–157. doi:10.1562/0031-8655(1999)069<0154:mmopih>2.3.co;2
8. Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. New Engl J Med. 1997;337(20):1419–1428. doi:10.1056/NEJM199711133372003
9. Inui M, Ooe M, Fujii K, Matsunaka H, Yoshida M, Ichihashi M. Mechanisms of inhibitory effects of CoQ10 on UVB-induced wrinkle formation in vitro and in vivo. BioFactors. 2008;32(1–4):237–243. doi:10.1002/biof.5520320128
10. Chaiprasongsuk A, Lohakul J, Soontrapa K, Sampattavanich S, Akarasereenont P, Panich U. Activation of Nrf2 reduces UVA-mediated MMP-1 upregulation via MAPK/AP-1 signaling cascades: the photoprotective effects of sulforaphane and hispidulin. J Pharmacol Exp Ther. 2017;360(3):388–398. doi:10.1124/jpet.116.238048
11. Oh JH, Karadeniz F, Lee JI, Park SY, Seo Y, Kong CS. Anticatabolic and anti-inflammatory effects of myricetin 3-O-β-d-galactopyranoside in UVA-irradiated dermal cells via repression of MAPK/AP-1 and activation of TGFβ/Smad. Molecules. 2020;25(6):6. doi:10.3390/molecules25061331
12. Oh Y, Lim HW, Park KH, et al. Ginsenoside Rc protects against UVB‑induced photooxidative damage in epidermal keratinocytes. Mol Med Rep. 2017;16(3):2907–2914. doi:10.3892/mmr.2017.6943
13. Watson RE, Gibbs NK, Griffiths CE, Sherratt MJ. Damage to skin extracellular matrix induced by UV exposure. Antioxid. Redox Signaling. 2014;21(7):1063–1077. doi:10.1089/ars.2013.5653
14. Amaro-Ortiz A, Yan B, D’Orazio JA. Ultraviolet radiation, aging and the skin: prevention of damage by topical cAMP manipulation. Molecules. 2014;19(5):6202–6219. doi:10.3390/molecules19056202
15. Meinke MC, Müller R, Bechtel A, et al. Evaluation of carotenoids and reactive oxygen species in human skin after UV irradiation: a critical comparison between in vivo and ex vivo investigations. Exp Dermatol. 2015;24(3):194–197. doi:10.1111/exd.12605
16. Peres PS, Terra VA, Guarnier FA, Cecchini R, Cecchini AL. Photoaging and chronological aging profile: understanding oxidation of the skin. J Photochem Photobiol B Biol. 2011;103(2):93–97. doi:10.1016/j.jphotobiol.2011.01.019
17. Sullivan NJ, Tober KL, Burns EM, et al. UV light B-mediated inhibition of skin catalase activity promotes Gr-1+ CD11b+ myeloid cell expansion. J Investig Dermatol. 2012;132(3 Pt 1):695–702. doi:10.1038/jid.2011.329
18. Chung JH, Kang S, Varani J, Lin J, Fisher GJ, Voorhees JJ. Decreased extracellular-signal-regulated kinase and increased stress-activated MAP kinase activities in aged human skin in vivo. J Investig Dermatol. 2000;115(2):177–182. doi:10.1046/j.1523-1747.2000.00009.x
19. Huang CY, Lin YT, Kuo HC, Chiou WF, Lee MH. Compounds isolated from Eriobotrya deflexa leaves protect against ultraviolet radiation B-induced photoaging in human fibroblasts. J Photochem Photobiol B Biol. 2017;175:244–253. doi:10.1016/j.jphotobiol.2017.08.042
20. Dalle Carbonare M, Pathak MA. Skin photosensitizing agents and the role of reactive oxygen species in photoaging. J Photochem Photobiol B Biol. 1992;14(1–2):105–124. doi:10.1016/1011-1344(92)85086-A
21. Zhang J, Wang X, Xu T. Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with se-methylselenocysteine in mice. Toxicol Sci. 2008;101(1):22–31. doi:10.1093/toxsci/kfm221
22. Zhang J, Wang H, Yan X, Zhang L. Comparison of short-term toxicity between Nano-Se and selenite in mice. Life Sci. 2005;76(10):1099–1109. doi:10.1016/j.lfs.2004.08.015
23. Benko I, Nagy G, Tanczos B, et al. Subacute toxicity of nano-selenium compared to other selenium species in mice. Environ Toxicol Chem. 2012;31(12):2812–2820. doi:10.1002/etc.1995
24. Xu J, Spitale RC, Guan L, et al. Novel gene expression profile of women with intrinsic skin youthfulness by whole transcriptome sequencing. PLoS One. 2016;11(11):e0165913. doi:10.1371/journal.pone.0165913
25. Khera A, Dong LF, Holland O, et al. Selenium supplementation induces mitochondrial biogenesis in trophoblasts. Placenta. 2015;36(8):863–869. doi:10.1016/j.placenta.2015.06.010
26. Zhou YJ, Zhang SP, Liu CW, Cai YQ. The protection of selenium on ROS mediated-apoptosis by mitochondria dysfunction in cadmium-induced LLC-PK(1) cells. Toxicol in vitro. 2009;23(2):288–294. doi:10.1016/j.tiv.2008.12.009
27. Chang Y, He L, Li Z, et al. Designing core-shell gold and selenium nanocomposites for cancer radiochemotherapy. ACS nano. 2017;11(5):4848–4858. doi:10.1021/acsnano.7b01346
28. Zhang JS, Gao XY, Zhang LD, Bao YP. Biological effects of a nano red elemental selenium. BioFactors. 2001;15(1):27–38. doi:10.1002/biof.5520150103
29. Jaswal VS, Banipal PK, Kaura A, Bakshi MS. Bovine serum albumin driven interfacial growth of selenium-gold/silver hybrid nanomaterials. Journal of Nanoscience and Nanotechnology. 2011;11(5):3824–3833. doi:10.1166/jnn.2011.3874
30. Liu L, Bi Y, Zhou M, et al. Biomimetic human serum albumin nanoparticle for efficiently targeting therapy to metastatic breast cancers. ACS Appl Mater Interfaces. 2017;9(8):7424–7435. doi:10.1021/acsami.6b14390
31. Shan W, Zhu X, Liu M, et al. Overcoming the diffusion barrier of mucus and absorption barrier of epithelium by self-assembled nanoparticles for oral delivery of insulin. ACS nano. 2015;9(3):2345–2356. doi:10.1021/acsnano.5b00028
32. Zhang J, Wang H, Bao Y, Zhang L. Nano red elemental selenium has no size effect in the induction of seleno-enzymes in both cultured cells and mice. Life Sci. 2004;75(2):237–244. doi:10.1016/j.lfs.2004.02.004
33. Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol. 2006;55(1):1–19. doi:10.1016/j.jaad.2005.05.010
34. Moloney SJ, Edmonds SH, Giddens LD, Learn DB. The hairless mouse model of photoaging: evaluation of the relationship between dermal elastin, collagen, skin thickness and wrinkles. Photochemistry and Photobiology. 1992;56(4):505–511. doi:10.1111/j.1751-1097.1992.tb02194.x
35. Fisher GJ, Datta SC, Talwar HS, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379(6563):335–339. doi:10.1038/379335a0
36. Chung JH, Seo JY, Choi HR, et al. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J Investig Dermatol. 2001;117(5):1218–1224. doi:10.1046/j.0022-202x.2001.01544.x
37. Rui Y, Zhaohui Z, Wenshan S, Bafang L, Hu H. Protective effect of MAAs extracted from Porphyra tenera against UV irradiation-induced photoaging in mouse skin. J Photochem Photobiol B Biol. 2019;192:26–33. doi:10.1016/j.jphotobiol.2018.12.009
38. Inomata S, Matsunaga Y, Amano S, et al. Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J Investig Dermatol. 2003;120(1):128–134. doi:10.1046/j.1523-1747.2003.12021.x
39. Yi R, Zhang J, Sun P, Qian Y, Zhao X. Protective effects of kuding Tea (Ilex kudingcha C. J. Tseng) polyphenols on UVB-induced skin aging in SKH1 hairless mice. Molecules. 2019;24(6):6. doi:10.3390/molecules24061016
40. Yan C, Grimm WA, Garner WL, et al. Epithelial to mesenchymal transition in human skin wound healing is induced by tumor necrosis factor-alpha through bone morphogenic protein-2. Am J Pathol. 2010;176(5):2247–2258. doi:10.2353/ajpath.2010.090048
41. Ono M, Masaki A, Maeda A, et al. CCN4/WISP1 controls cutaneous wound healing by modulating proliferation, migration and ECM expression in dermal fibroblasts via α5β1 and TNFα. Matrix Biol. 2018;68-69:533–546. doi:10.1016/j.matbio.2018.01.004
42. Ahmed OM, Mohamed T, Moustafa H, Hamdy H, Ahmed RR, Aboud E. Quercetin and low level laser therapy promote wound healing process in diabetic rats via structural reorganization and modulatory effects on inflammation and oxidative stress. Biomed Pharmacothe. 2018;101:58–73. doi:10.1016/j.biopha.2018.02.040
43. Caldas C, Hahn SA, da Costa LT, et al. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nature Genet. 1994;8(1):27–32. doi:10.1038/ng0994-27
44. Jiang C, Liu G, Luckhardt T, et al. Serpine 1 induces alveolar type II cell senescence through activating p53-p21-Rb pathway in fibrotic lung disease. Aging Cell. 2017;16(5):1114–1124. doi:10.1111/acel.12643
45. Mosteiro L, Pantoja C, de Martino A, Serrano M. Senescence promotes in vivo reprogramming through p16INK 4a and IL −6. Aging Cell. 2018;17(2):2. doi:10.1111/acel.12711
46. Storer M, Mas A, Robert-Moreno A, et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155(5):1119–1130. doi:10.1016/j.cell.2013.10.041
47. Kim HY, Sah SK, Choi SS, Kim TY. Inhibitory effects of extracellular superoxide dismutase on ultraviolet B-induced melanogenesis in murine skin and melanocytes. Life Sci. 2018;210:201–208. doi:10.1016/j.lfs.2018.08.056
48. Barygina V, Becatti M, Lotti T, Moretti S, Taddei N, Fiorillo C. ROS-challenged keratinocytes as a new model for oxidative stress-mediated skin diseases. J Cell Biochem. 2019;120(1):28–36. doi:10.1002/jcb.27485
49. Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal. Biochem. 2017;524:13–30. doi:10.1016/j.ab.2016.10.021
50. Khan A, Bai H, Shu M, Chen M, Khan A, Bai Z. Antioxidative and antiphotoaging activities of neferine upon UV-A irradiation in human dermal fibroblasts. Biosci Rep. 2018;38(6):6. doi:10.1042/BSR20181414
51. Granger C, Aladren S, Delgado J, Garre A, Trullas C, Gilaberte Y. Prospective evaluation of the efficacy of a food supplement in increasing photoprotection and improving Selective markers related to skin photo-ageing. Dermatol. Ther. 2020;10(1):163–178. doi:10.1007/s13555-019-00345-y
52. Li Y, Bi Z, Yan B, Wan Y. UVB radiation induces expression of HIF-1alpha and VEGF through the EGFR/PI3K/DEC1 pathway. IntJ Mol Med. 2006;18(4):713–719.
53. Baek M, Kim M, Lim JS, et al. Epidermal-specific deletion of TC-PTP promotes UVB-induced epidermal cell survival through the regulation of Flk-1/JNK signaling. Cell Death Dis. 2018;9(7):730. doi:10.1038/s41419-018-0781-9
54. Zhai Y, Dang Y, Gao W, et al. P38 and JNK signal pathways are involved in the regulation of phlorizin against UVB-induced skin damage. Exp Dermatol. 2015;24(4):275–279. doi:10.1111/exd.12642
55. Peus D, Vasa RA, Meves A, Beyerle A, Pittelkow MR. UVB-induced epidermal growth factor receptor phosphorylation is critical for downstream signaling and keratinocyte survival. Photochemistry and photobiology. 2000;72(1):135–140. doi:10.1562/0031-8655(2000)072<0135:UIEGFR>2.0.CO;2
56. Wu TJ, Lin CY, Tsai CH, Huang YL, Tang CH. Glucose suppresses IL-1β-induced MMP-1 expression through the FAK, MEK, ERK, and AP-1 signaling pathways. Environ Toxicol. 2018;33(10):1061–1068. doi:10.1002/tox.22618
57. Peng Y, Wu S, Tang Q, Li S, Peng C. KGF-1 accelerates wound contraction through TGF-beta1/Smads signaling pathway in a double paracrine manner. J Biol Chem. 2019;294(21):8361–8370. doi:10.1074/jbc.RA118.006189
58. Pan A, Zhong M, Wu H, et al. Topical application of keratinocyte growth factor conjugated gold nanoparticles accelerate wound healing. Nanomedicine. 2018;14(5):1619–1628. doi:10.1016/j.nano.2018.04.007
59. Hwang E, Park SY, Lee HJ, Lee TY, Sun ZW, Yi TH. Gallic acid regulates skin photoaging in UVB-exposed fibroblast and hairless mice. Phytother Res. 2014;28(12):1778–1788. doi:10.1002/ptr.5198
60. Urikura I, Sugawara T, Hirata T. Protective effect of Fucoxanthin against UVB-induced skin photoaging in hairless mice. Biosci Biotechnol, Biochem. 2011;75(4):757–760. doi:10.1271/bbb.110040
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.