Back to Journals » Drug Design, Development and Therapy » Volume 19
Huyang Yangkun Formula Enhances Ovarian Function and Delays Reproductive Aging by Influencing Hypothalamic GnRH/LH Pulse Release Through GAT-1/GABA/GABAAR2
Authors Li Y, Peng Y, Nie G, Cheng F, Zhou Y, Liu J, Yang H
Received 29 November 2024
Accepted for publication 31 March 2025
Published 9 April 2025 Volume 2025:19 Pages 2677—2691
DOI https://doi.org/10.2147/DDDT.S504610
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianbo Sun
Yang Li,1 Yin Peng,1 Guangning Nie,1 Fangping Cheng,1 Yuling Zhou,1 Jian Liu,1 Hongyan Yang1,2
1Department of Gynaecology, The Second Clinical Medical College, Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, 510120, People’s Republic of China; 2Guangdong Provincial Key Laboratory of Clinical Research on Traditional Chinese Medicine Syndrome, Guangzhou, Guangdong, 510120, People’s Republic of China
Correspondence: Hongyan Yang; Jian Liu, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, No. 111 Dade Road, Yuexiu District, Guangzhou, 510120, People’s Republic of China, Tel +86-20-81887233, Email [email protected]; [email protected]
Background: Huyang Yangkun Formula (HYF), based on Yin and Yang principles, is effective in treating Premature Ovarian Insufficiency (POI) and enhancing reproduction, but its mechanism needs to be clarified.
Aim: This study investigates HYF’s impact on enhancing ovarian follicles development via GABA/GnRH neuron regulation.
Materials and Methods: The chemical components of water extract of HYF were analyzed by combining the ultra-high performance liquid chromatography coupled with Q-Exactive mass spectrum analysis (UHPLC-QE-MS). Female SD rats received HYF for 90 days to assess changes in the ovarian function. In addition, female SD rats underwent ovariectomy to evaluated the impact of HYF or GABA treatments on LH release induced by E2 to address age-related neuroendocrine damage. Reproductive neuroendocrine markers were analyzed through immunohistochemistry and immunofluorescence.
Results: HYF treatment improved the disrupted estrous cycle in aged female rats, breaking the continuous keratinized epithelial cell state and alternating between estrous periods. Aging reduces ovarian volume and follicle numbers, but after 3 months of HYF treatment, ovarian follicle development increased, and mature follicle and luteum numbers changed significantly. HYF improved sex hormone levels, advancing and enhancing the LH peak in older rats, thus boosting total 24-hour LH release. GABA injection into the lateral ventricle increased GnRH release in aging rats, highlighting GABA’s excitatory role. HYF also increased GnRH secretion by reducing GAT-1, leading to more GABA release, which activates GABAAR2 receptors in GnRH neurons, enhancing their function in the hypothalamic arcuate nucleus of elderly rats.
Conclusion: HYF shows promise as a treatment for improving ovarian and reproductive neuroendocrine function in early reproductive aging, providing insights into its effects on hypothalamic GnRH/LH pulse release via the GAT-1/GABA/GABAAR2/GnRH pathway.
Keywords: traditional Chinese medicine, Huyang yangkun formula, delays reproductive aging, GnRH/LH pulse, GABA neurons
Introduction
Female reproductive aging is distinctively characterized by a markedly reduced reproductive function due to a remarkable decline in quality and quantity of follicles acnd oocytes, recent evidence suggests that brain changes play a crucial role in early reproductive aging, even before ovarian and E2 changes occur.1,2 Before age-related loss of the estrous cycle, female rats exhibit a reduced LH surge, indicating impending reproductive decline. Aging disrupts GnRH neuron communication in the hypothalamus, leading to weakened LH peaks and altered GnRH pulse-release before ovulation.3 Thus, inhibited and delayed LH surges driven by GnRH are early markers of decreased reproductive capacity.4,5
GnRH neurons are the key link between the CNS and fertility regulation,6 expressing both GABAA and GABAB receptors and receiving GABA input with estrogen receptors.7 GABA’s role in GnRH activity and secretion is debated, with studies showing it as both a stimulant and suppressor of GnRH release. It is increasingly agreed that GABA can both depolarize and hyperpolarize GnRH neurons via GABAARs, with excitation being the primary effect, while GABAB receptor activation is consistently inhibitory.8,9 Furthermore, GABA transmits metabolic signals to GnRH neurons, and changes in GABAergic transmission are associated with hypothalamic problems in fertility disorders.10 In mouse models of polycystic ovary syndrome exposed to prenatal androgens, GABAergic postsynaptic currents increased, likely due to heightened GABAergic influence on GnRH neurons.11 However, no studies have examined GABA’s role in Premature Ovarian Insufficiency or its impact on disease progression via GnRH neurons, highlighting the need to explore GABA’s neuroendocrine role in reproductive aging.12
Currently, effective treatments for declining reproductive function are lacking, and there is growing interest in traditional Chinese medicine for enhancing reproductive health. For two thousand years, Chinese medicine has been used to prevent and treat diseases, including enhancing reproductive function with various formulas.13,14 One such formula, Huyang Yangkun (HYF), is derived from Danggui Buxue Tang and is used to treat ovarian aging symptoms in perimenopausal women.15 HYF consists of seven herbs, including Astragali Radix (Astragalus didymophysus Bunge), Herba Epimedii (Epimedium brevicornu Maxim), Dioscoreae Rhizoma (Dioscorea oppositifolia L)., Semen Cuscutae (Cuscuta chinensis Lam), Rehmanniae Radix (rehmannia Glutinosa Gaertn), Angelicae Sinensis Radix (Angelica sinensis (Oliv). Diels), Glehniae Radix (Glehnia littoralis F. Schmidt ex Miq, the plant name has been checked with http://www.worldfloraonline.org. accessed on: 07 Sep 2024) at a ratio of 5:1:1:1:1:1:1. Our research shows that HYF helps menstrual recovery, improves endocrine levels, and delays early ovarian insufficiency in patients with reproductive aging and amenorrhea. A retrospective study on IVF/ICIS outcomes revealed that low ovarian response patients treated with HYF had better results, including more eggs, embryos, and blastocysts, compared to a control group. Clinical studies suggest that HYF supplementation enhances ovulation, endocrine function, and pregnancy rates (under publication).
In previous studies with VCD-induced rat and mouse POI models, we investigated HYF’s effects on follicle development and ovarian function. Initial results indicate that the HYF may protect ovaries by decreasing granulosa cell apoptosis through the MAPK/JAK/P53 pathway.15,16 However, it’s unclear if HYF affects follicular development via the HPO axis, especially since age-related reproductive decline impacts the entire HPG axis and hypothalamic neurons. Considering that age-related decline in female reproductive function impacts all tiers of the hypothalamic-pituitary-gonadal (HPG) axis, and acknowledging the role of hypothalamic neurons in regulating gonadotropin-releasing hormone (GnRH) pulse secretion, it is essential to explore the reproductive neuroendocrine mechanisms of HYF at the levels of the hypothalamus and pituitary for its advancement and implementation.
This study utilized an integrated experimental approach, employing both an ovariectomy animal model and an early reproductive aging animal model, to examine two specific objectives. First, HYF improves ovarian function, follicle development, and reproductive neuroendocrine changes during early reproductive decline. Second, as female rats age, their sensitivity to estradiol (E2) feedback decreases, causing a weaker and delayed luteinizing hormone (LH) peak. HYF affects GnRH and LH pulse release by altering GABA expression and its receptors.
Materials and Methods
Herbal Materials and HYF Extract Preparation
The HYF consists of seven component herbs, sourced from Guangdong Kangmei Pharmaceutical Company, the herbs were authenticated by pharmacists Xiong Li and Wenliang Chen (The Second Affiliated Hospital of Guangzhou University of Chinese Medicine). All the preparation procedures conformed to the standards of the Chinese Pharmacopoeia, the composition of each dose is shown in Supplementary Material Table S1. The composition and preparation method are detailed in previous studies.15 Briefly, A reflux extraction device was used to decoct HYF after it had been soaked for half an hour. The decoction process was repeated once, then the liquid is concentrated to 1.1 g/mL using a rotary evaporator and then administered directly to animals.
Analysis of HYF by UPLC-MS/MS
UPLC-MS/MS analysis of HYF was performed using a Dionex Ultimate 3000 RSLC and Thermo Scientific Q Exactive Focus system with a Hypersil GOLD column (100*2.1 mm, 1.9 μm) at 35°C. The mobile phase consisted of 0.1% formic acid (A) and MeOH (B) with a flow rate of 200 μL/min and an injection volume of 5 μL. The gradient elution was as follows: 85–80% A (0–7 min), 80–70% A (7–15 min), 70–35% A (15–40 min), 35–30% A (40–43 min), 30–10% A (43–45 min), 10–85% A (45–46 min), and 85% A (46–50 min).
The MS analysis used negative electrospray mode with a scan range starting at m/z 120 and a speed of 35,000 Da/s. The HESI-II ion source was employed (Thermo Fisher Scientific, USA). Data-dependent MS/MS targeted the most intense ions from the full scan, using 40% normalized collision energy. Nitrogen was the sheath gas and helium the collision gas. Data was collected and analyzed with Xcalibur. The Q Exactive Focus mass analyzer was calibrated per the manufacturer’s instructions using a commercial calibration fluid.
Animal
The animals utilized in this study were SPF-grade Sprague-Dawley (SD) female rats (Certificate No. NO.44008500027961), supplied by the Guangdong Medical Laboratory Animal Center (License No. SCXK [Guangdong] 2021–0029). Rats were raised in a standard SPF barrier environment, including a temperature of 22±2°C, humidity levels between 50–60%, and a 12-hour light/dark cycle, with unlimited access to food and water. The entirety of the experimental procedures was conducted at the Laboratory Animal Center of Guangdong Hospital of Traditional Chinese Medicine (Experimental animal license: SYXK [Guangdong] 2018–0094). The animal experimentation protocol received ethical approval from the Animal Ethics Committee of the Guangdong Experimental Animal Center of Traditional Chinese Medicine (Ethics batch number: 2019032), and strictly follow the mandatory provisions on animal welfare in the Regulations on the Administration of Experimental Animals of China.
Experimental Design
We previously used a POI rat model to compare the efficacy and toxicity of HYF multi-dose with estradiol valerate (EVT, positive controls). Results indicated that the clinical equivalent dose of HYF was more effective than EVT and showed no hepatorenal toxicity after 3 months.15,16 Thus, in line with the 3R (replacement, reduction and refinement) principle, we used only the optimal HYF dose in this experiment to explore its mechanism without affecting result interpretation.
Drug Effect Experiment on Early Reproductive Senescence in Rats
Female SD rats aged 8 weeks (Young) and 14 months (Aged) were divided into Young, Aged, and HYF groups. HYF was administered at 11g/kg for 90 days. The estrous cycle was monitored, and after the final dose, serum, ovary, hypothalamus, intestine, and pituitary tissues were collected for analysis.
LH Peak Induction Experiment
To study neuropeptide expression in the hypothalamus during reproductive aging, ensure OVX rats of different ages are exposed to the same hormone levels. Female SD rats aged 8 weeks and 14 months were anesthetized with 20% urethane (0.07 mL/10 g). A 0.5–1 cm incision was made along the dorsal midline under sterile conditions, starting from the intersection of the hind leg root line and the median line. After bluntly separating the muscle layer into the abdominal cavity, both ovaries were located and found tightly connected to the uterus with surgical thread to prevent bleeding. The ovaries were then removed using the same method for each side. The wound was sutured, disinfected with 57% alcohol, and treated with penicillin for antibacterial purposes. Group: Young-OVX, Young-OVX-HYF, Old-OVX, Old-OVX-HYF;The HYF group received an intragastric dose of 11g/kg for 90 days. After the final dose, rats were given estradiol benzoate (EB) via subcutaneous injection (0.1mL, 2 μg/day) for two days. After 48 hours, they were injected with progesterone (0.1mL, 500 μg). Blood samples were collected every 30 minutes after the progesterone injection to evaluate the LH and FSH level.
Intraventricular Injection
All laboratory instruments and work surfaces are disinfected. Rats were anesthetized with 2% pentobarbital sodium (2 mL/kg) and fixed to a stereotaxic apparatus. After the cranial roof was clipped and disinfected with alcohol, the skin and periosteum were cut open to expose the fonticulum site. According to the Paxinos&Wat map, Drill holes in the skull (Bregma:R:1.2mm, AP:1.0mm, H:3mm) with a cranial drill, be careful not to Pierce, insert a microinjector into the skull at a 90°Angle, and pump with a microinjector GABA (AS,56-12-2, NO. A2129. Sigma,0.75μg/g) was slowly injected into the lateral ventricle at a rate of 0.15 µL/min. The needle was kept for 5 minutes and the microsyringe was removed. Finally, the wound was sutured and penicillin was injected.
Estrous Cycle
The normal estrous cycle of rats is segmented into four distinct stages: interestrus, preestrus, estrus, and postestrus. These stages can be identified based on the type and proportion of vaginal exfoliated cells. During interestrus, the vaginal exfoliated cells predominantly consist of a large number of white blood cells and a small number of irregular epithelial cells. In the preestrus stage, the vaginal exfoliated cells are primarily nucleated epithelial cells, with occasional keratinized epithelium. The estrus stage is characterized by a substantial presence of keratinized epithelial cells. In the late estrus stage, the vaginal exfoliated cells include white blood cells (Leagene, DH0006, China), HE was observed under a microscope to record the stage of estrous cycle on that day. The results of continuous observation were recorded, and the disturbance of estrous cycle in each group was recorded.
HE Staining and Follicular Count
To continuously assess follicle numbers and observe pathological alterations in follicles, the ovaries were fixed, embedded, and sectioned according to previously described protocols. The sections were cut at a thickness of 5 μm, with every tenth slice being selected for hematoxylin and eosin (HE) staining. The HE-stained ovarian tissue sections were examined microscopically, and follicles were graded and counted based on a standardized criterion previously reported. Two pathologists, blinded to the experimental groups, independently performed the follicle counts, and the mean of their readings was recorded for analysis. The follicles were categorized into five distinct stages: primordial follicle, primary follicle, secondary follicle, antral follicle, and mature follicle. To prevent counting errors such as loss and duplication, only follicles with observable oocytes were included in the counts for small antral follicles, antral follicles, and mature follicles with larger diameters. This precaution was deemed unnecessary for the enumeration of small primary, primary, and secondary follicles.
Serum Hormone Tests
Anti-Müllerian hormone (AMH), follicle-stimulating hormone (FSH), luteinizing hormone (LH) and estradiol (E2) levels were detected referring to the instructions of the ELISA kits (CSB-E11162r, CSB-E06869r, CSB-E12654r, CSB-E07282r) which were provided by Wuhan Huamei Biotechnology Company.
RT-qPCR
The frozen hypothalamic or ovarian tissue extracted from the brain was pulverized in a mortar with liquid nitrogen. Subsequently, Trizol reagent (Invitrogen, 15596026, USA) was added to the tissue powder at a ratio of 100 mg/mL. RNA extraction from the tissue was performed using the chloroform-isopropyl alcohol method. The concentration and purity of the extracted RNA were measured, and cDNA synthesis was carried out according to the instructions provided with the reverse transcription kit (AG11728, AG11718, AG/Ecore Bio, China). Following the amplification reaction (ABI, Quant Studio 7 Flex, Singapore), the relative expression levels of mRNA were determined by 2-ΔΔCT. Primer sequence can be found in Supplementary Materials Table S2.
IF
Following dewaxing of the paraffin sections, antigen retrieval was performed. The sections were then incubated in 3% H2O2 for blocking. Subsequently, the sections were placed in TBST after delineating the areas of interest, followed by the addition of 10% goat serum for blocking at room temperature for 30 minutes. The primary antibody solution, diluted at a ratio of 1:100 to 1:500, was then applied, and the sections were incubated at 4°C overnight. On the following day, the sections were washed three times with TBST. The corresponding secondary antibody solution, 50 µL at a dilution of 1:1000 to 1:2000, was added and incubated at 37°C for 45 minutes. Finally, the sections were washed three times with TBST, each wash lasting 5 minutes. Each section was treated with 50 μL of DAPI working solution (diluted from DAPI stock solution at a ratio of 1:500), stained for 5 minutes to achieve a dark core, rinsed with TBST, mounted with fluorescent mounting medium, and subsequently examined under a microscope. Fluorescence intensity was quantified using ImageJ software. The primary antibodies used were:GnRH (ab281844, Abcam), GAT-1 (ab259971, Abcam).
Immunohistochemical Staining
Paraffin sections were dewaxed and hydrated, sodium citrate was used for tissue antigen repair, 3% hydrogen peroxide was used to remove endogenous peroxides, and blocked with 5%BSA for 2 h. The primary antibody was incubated overnight at 4◦C, and the secondary antibody was incubated for 40 min at room temperature. The sections were stained with DAB mixed solution for 1 min. The sections were soaked in hematoxylin dye for 3 min and then dehydrated, transparent and sealed. The results of immunohistochemical staining were observed with optical microscope (OLYMPUS BX53, Japan). And Image Pro Plus 6.0 software was used to obtain the optical density(mean) for quantitative analysis. Primary antibodies:Anti-GABA B Receptor 1, 1:250, ab238130, abcam; Anti-GABRA2, 1:250, ab307359, abcam.
Statistical Analysis
Data analysis was processed by SPSS 21.0 software. Mean ± Standard was chose to describe the data, depending on whether the data meet the normal distribution. T-test or Mann–Whitney U-test was used to compare within groups. One-way ANOVA or Kruskal–Wallis test was used for comparison of groups. P < 0.05 was considered statistically significant. And graphs were drawn by GraphPad Prism 8 software.
Result
Characterization of Compounds in HYF
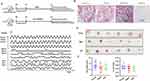
Batch-to-batch consistency was assessed by quantifying marker compounds using UPLC-MS/MS analysis. Figure 1 illustrate the quantification results obtained in negative and positive ion modes. UPLC-MS/MS facilitated the identification of the active constituents of HYF. Through comparison with standard compounds, ten major active components were identified, as detailed in Table 1.
 |
Table 1 The Information of Nine Compounds Identified in HYF by UPLC-MS/MS |
 |
Figure 1 Compounds in HYF. (1. chlorogenic acid, 2. coumaroylquinic, 3. calycosin, 4. ononin, 5. quercetin, 6. epimedoside A, 7. epimedin B, 8. icariin, 9. epemedin L, 10. palmitic acid). |
HYF Enhances Estrous Cycle and Ovarian Development in Aged Rats
Overall animal experimental design is depicted in Figure 2A, daily vaginal smears were taken to monitor the estrous cycle, which has four stages: interestrus, preestrus, estrus, the preestrus and estrus stages were denoted as 0, while the postestrus and interestrus stages were denoted as 1.17 Young female rats displayed regular estrous cycles, whereas 14-month-old female rats exhibited a transition from regular to irregular estrous cycles, the results are in line with what was previously reported.17,18 The disturbed estrous cycle of the aged female rats was improved after HYF treatment, which showed that the state of the continuous keratinized epithelial cells was disrupted, alternating between the estrous period and the late estrous period (Figure 2B and C).
The ovaries of the rats are depicted in Figure 2D. Both ovaries from each rat were weighed, and their long diameter (L, mm) and short diameter (S, mm) were measured. The ovarian volume was subsequently calculated using the formula V = L * S² / 2 (mm³). Compared to the Young group, the Aged group demonstrated a significant reduction in both ovarian volume and weight (p < 0.05). In contrast, the HYF group exhibited a significant increase in ovarian volume by 23.54% relative to the Aged group (p < 0.05, Figure 2E).
The figure displays HE images of ovaries from three groups. The young control group shows follicles at various development stages and corpora lutea (CL). In contrast, the Aged group has very few follicles, with occasional developing ones. The HYF group has fewer follicles than the young control group but more than the Aged group, with a higher number at different developmental stages (Figure 3A and B). A histological descriptive analysis of ovarian tissue was conducted, involving the quantification of various follicle grades within each section. When compared to the young group, the old group exhibited a significant reduction in the number of follicles across all grades (P < 0.01, P < 0.001). In contrast, the HYF group demonstrated a significant increase in the number of primary follicles, secondary follicles, mature follicles, and corpora lutea (Figure 3C), findings that are largely consistent with data obtained from our previous VCD model.
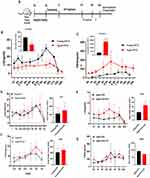
The Effect of HYF on Serum AMH, E2, FSH and LH
Monitoring follicle development and the estrous cycle revealed a notable rise in mature follicles and corpus luteum, prompting us to measure luteinizing hormone (LH) levels in orbital blood from day-old rats over five days. The control group showed significant periodic fluctuations and distinct pre-ovulation LH peaks. The aged group’s LH profile showed a disordered pattern with no wave peaks, delayed pulses, or multiple peaks, while the HYF group’s LH peaks were slightly more regular.(Figure 4A). The 5-day LH mean significantly decreased in the Aged group (p < 0.01), while the HYF group showed a 112% increase compared to the Aged group, consistent with estrous cycle findings and more mature follicles. Serum AMH and E2 levels, crucial for ovarian reserve, declined in aged rats (p < 0.01, p < 0.001), whereas FSH levels rose significantly (p < 0.01, Figure 4B). HYF treatment significantly increased serum AMH and E2 levels and decreased (p < 0.01, Figure 4B). FSH levels in aged rats, aligning partially with previous POI animal model findings.15 These results suggest HYF may enhance the estrous cycle, ovarian development, and neuroendocrine functions in older rats.
HYF Enhances Estrogen-Stimulated LH Release in Older Ovarian-Castrated Rats
Since GnRH and LH regulate gonadotropin release, assessing their pulsatility offers insights into hypothalamic function during reproductive aging. Our study revealed that castrated young rats exhibited a strong LH surge, whereas aged rats showed a significantly weaker surge and reduced total LH secretion over 24 hours compared to young rats. In the aged group, FSH peak amplitude and total secretion were significantly higher (Figure 5A–C). GnRH secretion frequency affects FSH and LH differently: fast pulses increase LH, while slow pulses raise FSH.19 Aging reduces E2’s ability to trigger GnRH/LH hypersecretion, delaying and diminishing the LH surge, which affects ovarian maturation. Abnormal FSH release can alter follicle recruitment and development.
In young ovariectomized rats, the HYF intervention adjusted the timing of LH and FSH peaks and slightly increased their total concentrations, but not significantly compared to controls (Figure 5D–G). In aged rats, HYF advanced the LH peak, increased its value, and significantly raised the total 24-hour LH release (p < 0.01), while reducing total FSH release by 22.8% (Figure 5D–G). Overall, HYF appears to enhance the hypothalamus’s sensitivity to estrogen feedback, increasing LH pulse release.
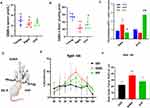
HYF Boosts GnRH/LH Release by Increasing GABA Levels
To investigate GABA changes during reproductive aging and HYF regulation, we used an enzyme-linked immunosorbent assay on serum and brain tissue from three groups of rats. Results indicated that GABA levels in the serum and brain of the elderly group were significantly lower than those in the young group (p < 0.05, p < 0.01, respectively), the concentration of GABA in the brain of the aged rats increased after HYF intervention (p < 0.05, Figure 6A and B). The mRNA expression levels of Gaba and Gnrh in the hypothalamus were measured. Unlike the GABA content results, Gaba mRNA levels were significantly higher in the aging group (p < 0.01) but lower in the HYF group (p < 0.05, Figure 6C). To further assess the impact of GABA and HYF on GnRH/LH release in aging rats, we induced an LH peak using an estrogen-progesterone method 30 days after administering GABA via lateral ventricle injection or HYF orally to ovariectomized rats, and then measured LH peak release in each group (Figure 6D). Our results showed that GABA injection into the lateral ventricle significantly increased LH release in early aging rats, surpassing the HYF group (Figure 6E). Compared with OVX group, total LH secretion (AUC) in GABA group was also significantly increased (p < 0.001, Figure 6F).
HYF Regulates GnRH/LH Release Through GAT-1/GABA/GABAAR2 Pathway
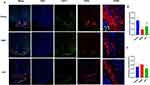
GABA Transporter GAT-1 (GABA Transporter 1), a protein crucial for recycling GABA (gamma-aminobutyric acid) from the synaptic gap into presynaptic membrane neurons and is essential for maintaining homeostasis of the neurotransmitter GABA.20 Immunofluorescence staining in rats’ hypothalamus shows co-expression of GAT-1 and GnRH. GnRH-positive products were primarily found in the lateral arcuate nucleus and median uplift. The elderly group showed significantly lower GnRH fluorescence intensity compared to the youth group, while GAT-1 expression increased but not significantly (Figure 7A). In the HYF group, GnRH fluorescence intensity was significantly higher, and GAT-1 was significantly lower than in the aging group (p<0.05, Figure 7B and C). The findings of this section suggest a decline in the neuronal expression and function of GABA and GnRH associated with reproductive aging. Based on the conclusions drawn from prior literature, we tentatively infer that the enhancement of GnRH/LH release pulsatility by HYF may be attributed to an increase in GABA release, potentially mediated by GAT-1.
Gamma-aminobutyric acid (GABA) exerts its effects in the brain predominantly through two receptor types: GABAA and GABAB receptors.21,22 Preliminary polymerase chain reaction (PCR) screening results indicate that aging is associated with differential expression patterns of mRNA for GABA-related receptors (Figure 8A). Specifically, the expression levels of gabra2 and gabbr1 were significantly upregulated (p < 0.05, p < 0.001, respectively), whereas gabrb2a and gabrg2 exhibited significant downregulation (p < 0.05). Further analysis of potential changes associated with HYF revealed a significant upregulation in the mRNA expression of gabra2, gabra5, and gabbr2, in contrast to a significant downregulation of gabbr1.
Following an extensive evaluation and analysis, we proceeded with additional immunohistochemical validation of GABRA2 and GABBR1, identified as the most significantly differentially expressed genes between the groups. The findings revealed divergent expression patterns of GABABR1 and GABAAR2 in aged rats. Specifically, the expression of GABABR1 was upregulated, aligning with the mRNA expression results (Figure 8B). Conversely, GABAAR2 expression was markedly downregulated, contradicting the mRNA expression findings for GABAAR2. The analysis of changes in the HYF and Aged groups revealed a significant increase in GABAAR2 expression exclusively in the HYF group, indicating its potential role in the regulation of GABA-GnRH/LH secretion by HYF. Immunofluorescence double staining for GABAAR2 and GnRH demonstrated their co-expression within the hypothalamic arcuate nucleus of rats (Figure 8C). Notably, the fluorescence intensity of GnRH and GABAAR2 neurons significantly declined with aging but showed a marked increase following HYF treatment.
Discussion
Female reproductive aging, a leading cause of infertility, is marked by reduced ovarian function and fewer high-quality follicles and oocytes.23 Recent research indicates that brain changes, particularly in the hypothalamus, play a crucial role in early reproductive aging, even before ovarian and estrogen changes occur. Aging disrupts GnRH neuron communication, leading to altered LH peaks and GnRH pulse release, early indicators of declining fertility.24–27 Thus, understanding how GnRH neurons are regulated in the hypothalamus is vital for grasping the neuroendocrine aspects of reproductive aging.
Our earlier study utilized a POI rat model and transcriptomics to examine HYF’s impact on primordial follicle activation, primary follicle development, and the prevention of ovarian granulosa cell apoptosis to maintain ovarian function.15 However, female infertility linked to stress, mood changes, and lifestyle shifts is not solely due to reproductive aging. Alterations in reproductive neuroendocrine function are crucial for assessing reproductive health.28,29 These alterations encompass changes in neurotransmitter secretion, pituitary hormone levels, and follicular development, ultimately leading to the cessation of reproductive cycles. This study examined the impact of HYF on reproductive aging in naturally aging female rats. HYF was found to significantly enhance the estrus cycle, ovarian volume, follicle count, sex hormone levels, and LH peaks. These improvements suggest that HYF may boost reproductive function by affecting hypothalamic GnRH/LH pulse release.
Our findings indicate that age-related declines in reproductive function are linked to decreased hypothalamic neuroendocrine activity, shown by a diminished capacity to release estrogen-induced luteinizing hormone pulses. This is associated with hypothalamus regulation of altered GnRH release, leading to reduced GnRH secretion and changes in ovarian structure and hormone levels. GABA is crucial for GnRH neuron activity and secretion, as these neurons have GABAA and GABAB receptors and receive GABA input. Depending on which receptor is activated, GABA can either stimulate or inhibit GnRH neuron activity.12,30 In addition, GABA regulates GnRH neurons via metabolic signals and is linked to hypothalamic issues in reproductive disorders.7,9 However, research on GABA’s regulatory role in GnRH during reproductive aging is limited. Consequently, we investigated the potential role of GABA in reproductive aging by examining several key aspects. This study found that older rats had reduced GABA levels and increased GAT-1 expression, indicating higher GABA reuptake. HYF treatment raised GABA levels and lowered GAT-1 expression, suggesting it enhances GnRH/LH pulse release by modulating GABA.
In the OVX E rat model, administering GABA into the lateral ventricle significantly increased the peak and total release of luteinizing hormone (LH). Changes in GABA delivery to GnRH neurons can be triggered by activating GABAA receptors. Although the direct effect of GABA on GnRH neurons is debated, this study adds evidence supporting GABA’s potential excitatory role in the adult brain, especially in the context of aging. In HYF-treated rats, the ovaries showed more follicles at various stages, an increase in mature follicles, and more luteal bodies. We suggest this might be due to higher systemic GnRH levels caused by HYF, as GABAergic neurons are known to transmit metabolic signals to GnRH neurons.31,32 We propose that GABA mediates HYF’s impact on GnRH regulation. While studying GABA’s role in reproductive aging, we found that HYF partially counteracts age-related changes in the expression of GABA, GAT-1, and related receptors. Further experiments showed HYF regulates GnRH neurons through GABAAR2 receptors, with increased expression after treatment, enhancing neuron excitability and promoting GnRH/LH release. In older rats, reduced GABAAR2 levels may result from lower GABA levels or decreased neuronal activity. HYF enhances GAT-1 expression and GABA release, boosting GABAAR2 levels. This mechanism offers new insights into HYF’s biological role. Previous research links GABAAR2 expression to neuronal excitability, showing that increasing its levels can improve neural function. Our findings align with these studies, highlighting GABAAR2’s role in reproductive function regulation. The dynamic balance of GABAAR2 levels reflects age-related changes and HYF intervention, providing a basis for new treatment strategies. We plan further studies, including GABAAR2 gene knockout and overexpression, to explore its role in reproductive aging.
Recent investigations reveal that KNDy neurons significantly influence the pulsatile release of GnRH and LH, raising questions about whether HYF acts directly on GnRH neurons via GABA or indirectly through neurons like KNDy, particularly those with kisspeptin. We noted mRNA expression differences, such as KNDy, between groups (see Supplementary Materials). HYF might also impact KNDy neurons by modulating GABAergic neurons, crucial for GnRH pulse release. While the exact mechanism of HYF remains unclear, these findings suggest its potential in addressing reproductive aging. Understanding this mechanism is a key research focus. Additionally, ongoing studies suggest HYF may affect GABA and its receptors in the brain via the vagus nerve in the gut (unpublished data), which is crucial for enhancing fertility outcomes.
Conclusion
Our study found that aging female rats experience reduced ovarian function and hormonal changes due to fewer GnRH neurons, influenced by GABAergic neurons, affecting LH pulse production. This underscores the GABA-GnRH pathway’s role in reproductive aging. We identified a Chinese herbal compound, HYF, that enhances the estrous cycle, ovarian development, and neuroendocrine functions in aged rats by modulating the hypothalamic GnRH/LH pulse through the GAT-1/GABA/GABAAR2 pathway (Figure 8D). This suggests HYF as a potential treatment for early reproductive aging. We plan to conduct clinical trials of HYF in humans to assess its impact on ovarian function and hormone levels. Further studies will explore HYF’s mechanism, particularly its effect on GABAergic neurons and GnRH release, to develop better treatment strategies.
Data Sharing Statement
All data used and analyzed to support the current study are available from the corresponding author upon request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was financially supported by the National Natural Science Foundation of China (NO. 82274568, 82004407, 81903903), Guangzhou Science and Technology Planning Project (2025A03J4132), the Clinical Research Special Project Fund from Guangdong Provincial Hospital of Chinese Medicine (NO. YN10101912). The funding sources had no involvement in the study design, the collection, analysis, and interpretation of data, in the writing of the report; or the decision to submit the article for publication.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Ishizuka B. Current understanding of the etiology, symptomatology, and treatment options in Premature Ovarian Insufficiency (POI). Front Endocrinol. 2021;12:626924. doi:10.3389/fendo.2021.626924
2. Sang Q, Ray PF, Wang L. Understanding the genetics of human infertility. Science. 2023;380(6641):158–163. doi:10.1126/science.adf7760
3. Brann DW, Mahesh VB. The aging reproductive neuroendocrine axis. Steroids. 2005;70(4):273–283. doi:10.1016/j.steroids.2004.12.008
4. Dai X, Hong L, Shen H, et al. Estradiol-induced senescence of hypothalamic astrocytes contributes to aging-related reproductive function declines in female mice. Aging. 2020;12(7):6089–6108. doi:10.18632/aging.103008
5. Finch CE. The menopause and aging, a comparative perspective. J Steroid Biochem mol Biol. 2014;142:132–141. doi:10.1016/j.jsbmb.2013.03.010
6. Stamatiades GA, Kaiser UB. Gonadotropin regulation by pulsatile GnRH: signaling and gene expression. Mol Cell Endocrinol. 2018;463:131–141. doi:10.1016/j.mce.2017.10.015
7. Christian CA, Moenter SM. Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci. 2007;27(8):1913–1921. doi:10.1523/JNEUROSCI.4738-06.2007
8. Temple JL, Wray S. Developmental changes in GABA receptor subunit composition within the gonadotrophin-releasing hormone-1 neuronal system. J Neuroendocrinol. 2005;17(9):591–599. doi:10.1111/j.1365-2826.2005.01348.x
9. Lee K, Porteous R, Campbell RE, Lüscher B, Herbison AE. Knockdown of GABA(A) receptor signaling in GnRH neurons has minimal effects upon fertility. Endocrinology. 2010;151(9):4428–4436. doi:10.1210/en.2010-0314
10. Sim JA, Skynner MJ, Pape JR, Herbison AE. Late postnatal reorganization of GABA(A) receptor signalling in native GnRH neurons. Eur J Neurosci. 2000;12(10):3497–3504. doi:10.1046/j.1460-9568.2000.00261.x
11. Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci USA. 2004;101(18):7129–7134. doi:10.1073/pnas.0308058101
12. Watanabe M, Fukuda A, Nabekura J. The role of GABA in the regulation of GnRH neurons. Front Neurosci. 2014;8:387. doi:10.3389/fnins.2014.00387
13. Zhang QL, Lei YL, Deng Y, et al. Treatment progress in diminished ovarian reserve: western and Chinese medicine. Chin J Integr Med. 2023;29(4):361–367. doi:10.1007/s11655-021-3353-2
14. Li M, Xiao YB, Wei L, Liu Q, Liu PY, Yao JF. Beneficial effects of traditional Chinese medicine in the treatment of premature ovarian failure. Evid Based Complementary Altern Med. 2022;2022:5413504.
15. Wang L, Li M, Liu J, Nie G, Li Y, Yang H. Protective effect of Huyang Yangkun formula on ovarian function in premature ovarian insufficiency rats based on apoptotic mechanism. J Ethnopharmacol. 2021;280:114477.
16. Xie L, Wu S, Cao D, et al. Huyang yangkun formula protects against 4-vinylcyclohexene diepoxide-induced premature ovarian insufficiency in rats via the Hippo-JAK2/STAT3 signaling pathway. Biomed Pharmacothe. 2019;116:109008. doi:10.1016/j.biopha.2019.109008
17. Mora OA, Cabrera MM. Pheromonal male-induced diestrus and cyclicity in aging intact and young estrogenized female rats. Biol Reprod. 1994;50(3):603–606. doi:10.1095/biolreprod50.3.603
18. Rodríguez SS, Schwerdt JI, Barbeito CG, et al. Hypothalamic IGF-I gene therapy prolongs estrous cyclicity and protects ovarian structure in middle-aged female rats. Endocrinology. 2013;154(6):2166–2173. doi:10.1210/en.2013-1069
19. Gross KM, Matsumoto AM, Bremner WJ. Differential control of luteinizing hormone and follicle-stimulating hormone secretion by luteinizing hormone-releasing hormone pulse frequency in man. J Clin Endocrinol Metab. 1987;64(4):675–680. doi:10.1210/jcem-64-4-675
20. Soudijn W, van Wijngaarden I. The GABA transporter and its inhibitors. Curr Med Chem. 2000;7(10):1063–1079. doi:10.2174/0929867003374363
21. Nakahiro M, Nishi N, Fukuchi I, et al. Effects of pantoyl-GABA on GABAA and GABAB receptors in the rat brain. Jpn J Pharmacol. 1987;45(2):292–294. doi:10.1016/S0021-5198(19)44546-0
22. Negri S, Scolari F, Vismara M, et al. GABA(A) and GABA(B) receptors mediate GABA-induced intracellular Ca(2+) signals in human brain microvascular endothelial cells. Cells. 2022;11(23):3860. doi:10.3390/cells11233860
23. May-Panloup P, Boucret L, Chao de la Barca JM, et al. Ovarian ageing: the role of mitochondria in oocytes and follicles. Human Reproduction Update. 2016;22(6):725–743. doi:10.1093/humupd/dmw028
24. Artini PG, Obino ME, Vergine F, Sergiampietri C, Papini F, Cela V. Assisted reproductive technique in women of advanced fertility age. Minerva Ginecologica. 2018;70(6):738–749. doi:10.23736/S0026-4784.18.04247-8
25. Guarente L, Sinclair DA, Kroemer G. Human trials exploring anti-aging medicines. Cell Metab. 2024;36(2):354–376. doi:10.1016/j.cmet.2023.12.007
26. Liang J, Huang F, Song Z, Tang R, Zhang P, Chen R. Impact of NAD+ metabolism on ovarian aging. Immun Ageing I A. 2023;20(1):70. doi:10.1186/s12979-023-00398-w
27. Hornos Carneiro MF, Colaiácovo MP. Beneficial antioxidant effects of coenzyme Q10 on reproduction. Vitamin Hormon. 2023;121:143–167.
28. Bala R, Singh V, Rajender S, Singh K. Environment, lifestyle, and female infertility. Reprod Sci. 2021;28(3):617–638. doi:10.1007/s43032-020-00279-3
29. Lainez NM, Coss D. Obesity, neuroinflammation, and reproductive function. Endocrinology. 2019;160(11):2719–2736. doi:10.1210/en.2019-00487
30. Tomaszewska-Zaremba D, Przekop F. The role of GABA(A) and GABA(B) receptors in the control of GnRH release in anestrous ewes. Reprod Biol. 2006;6(Suppl 2):3–12.
31. Liu JH, Patel B, Collins G, et al. Endotext. In: Feingold KR, Anawalt B, Blackman MR, editors. Central Causes of Amenorrhea. South Dartmouth (MA): MDText.com, Inc.; 2000.
32. Ratra DV, Elias CF. Chemical identity of hypothalamic neurons engaged by leptin in reproductive control. J Chem Neuroanat. 2014;61-62:233–238. doi:10.1016/j.jchemneu.2014.05.005
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.