Back to Journals » Journal of Pain Research » Volume 18
Hypervigilance to Pain May Predict the Transition from Subacute to Chronic Back Pain: A Longitudinal Observational Study
Authors Zhang W, Löffler M , Usai K , Mišić M, Nees F, Flor H
Received 17 December 2024
Accepted for publication 24 May 2025
Published 25 June 2025 Volume 2025:18 Pages 3141—3158
DOI https://doi.org/10.2147/JPR.S512911
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor King Hei Stanley Lam
Wen Zhang,1 Martin Löffler,1,2 Katrin Usai,1 Mina Mišić,1 Frauke Nees,1,3,* Herta Flor1,*
1Department of Neuropsychology and Psychological Resilience Research, Research Group Learning and Brain Plasticity in Mental Disorders, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany; 2Clinical Psychology, Department of Experimental Psychology, Heinrich Heine University Düsseldorf, Düsseldorf, Germany; 3Institute of Medical Psychology and Medical Sociology, University Medical Centre Schleswig-Holstein, Kiel University, Kiel, Germany
*These authors contributed equally to this work
Correspondence: Wen Zhang, Department of Neuropsychology and Psychological Resilience Research, Research Group Learning and Brain Plasticity in Mental Disorders, Central Institute of Mental Health, Square J5, Mannheim, 68159, Germany, Tel +49 621 1703-6302, Fax +49 621 1703-6305, Email [email protected]
Purpose: This study aimed to evaluate whether incorporating additional psychological and social risk factors, beyond those captured by the Örebro Musculoskeletal Pain Questionnaire (ÖMPQ), could enhance the prediction of the transition from subacute to chronic back pain.
Patients and Methods: Data of 75 patients with subacute back pain (SABP, 7– 12 weeks) from a longitudinal observational study were analyzed. The ÖMPQ and additional emotional, cognitive, behavioural, and social factors were assessed at baseline. Pain severity and pain-related interference were assessed at baseline and six months later to evaluate chronicity. Principal component analysis reduced psychological variables into interpretable components. Pearson’s correlation examined relationships between psychological factors, pain severity, and pain interference at baseline and 6-month follow-up. Factors linked to pain persistence were identified using best subsets regression and tested in multistage linear regression: (1) without baseline adjustment, (2) with baseline adjustment, and (3) assessing the effect of predictors from the previous steps on 6-month outcomes.
Results: The ÖMPQ and the Pain Vigilance and Awareness Questionnaire (PVAQ) scores significantly predicted pain severity, and the ÖMPQ, the Pain Behaviour Checklist (PBC), and PVAQ scores significantly predicted pain-related interference after six months. Multistage linear regression showed that PVAQ scores best predicted both pain severity (ß = 0.25, p = 0.017) and pain-related interference (ß = 0.33, p = 0.002) six months later, even after adjusting baseline pain levels.
Conclusion: The ÖMPQ score initially predicted pain persistence at six months, but its effect diminished after adjusting for baseline pain levels. In contrast, psychological risk factors such as pain hypervigilance and pain behaviors emerged as predictors of the pain severity or pain-related interference at six months. Pain hypervigilance became the strongest predictor of both pain severity and pain-related interference, regardless of initial pain, suggesting it as a target for early intervention.
Keywords: back pain, longitudinal studies, surveys and questionnaires, risk factors, anxiety, cognition
Introduction
Chronic back pain (CBP), particularly low back pain (LBP), is the leading cause of disability worldwide, with cases projected to rise from 619 million in 2020 to 843 million by 2050.1,2 While most patients recover after acute pain onset, 26–32% transition to chronic back pain, resulting in long-term disability.3,4 Identifying risk factors for this transition is important for reducing chronicity rates.
Over the past decades, extensive research has explored predictors of chronic pain. The biopsychosocial model emphasizes the role of psychological and social factors alongside biological ones in the development and persistence of chronic pain.5–8 Neuroimaging studies revealed changes in brain areas involved in emotional processing and cognitive control, which can predict the transition to chronic back pain.9–14 However, neuroimaging is costly and not routinely available in clinical settings, making psychological risk factor screening through questionnaires a practical and recommended alternative.15–18
Psychological risk factors in pain research cover a wide range of emotional, cognitive, and behavioural factors. Negative emotions such as anxiety, depression, stress are the earliest and most widely studied predictors of the chronicity in LBP.19 Later studies found cognitive factors, including pain catastrophizing, attention, self-efficacy, beliefs and expectations, to be associated with the outcome of LBP.20–24 Hypervigilance, characterized by heightened attention towards pain, may play a particularly significant role in the progression toward chronicity.25–28 Closely tied to emotion and cognition, pain-related behaviour—especially fear-avoidance behaviour—is related to pain severity and disability in patients with chronic pain and may also drive the transition from acute to chronic pain.29–31 Social factors—such as supportive and non-supportive responses from significant others (eg, spouses, caregivers), workplace conditions, cultural background, and economic status—further shape pain perception, coping with pain and disability, as well as overall quality of life in patients with back pain.32–34
Multidimensional questionnaires have been developed to improve screening and predict chronic pain by assessing multiple risk factors simultaneously. The Örebro Musculoskeletal Pain Questionnaire (ÖMPQ) is one of the most widely used instruments. It has been shown to be effective and reliable in predicting chronic pain, disability and absenteeism.35 However it does not capture all psychological risk factors, particularly certain cognitive and social risk factors.36 Identifying the key factors involved during the subacute stage of back pain (7 to 12 weeks post-onset) is important, as this period represents a critical window for the transition to chronicity, with pain characteristics differing significantly from those in the chronic stage.37–39 Despite its importance, only a limited number of studies have investigated psychological and social risk factors of chronicity during this period.40
This longitudinal observational study investigates the transition from subacute to chronic back pain, using pain severity and interference at six months post-initial evaluation as indicators of chronicity. It aimed to cluster psychological and social risk factors based on their intercorrelations and to assess whether incorporating these factors improves prediction beyond those already captured by the ÖMPQ. We hypothesize that the inclusion of additional psychological and social risk factors would enhance the prediction of the transition from subacute to chronic back pain.
Materials and Methods
Design and Setting
The data analyzed in this study were collected from an ongoing longitudinal observational study within the Heidelberg Pain Consortium. Neuroimaging data from this study have been reported in prior publications.33,41,42 Patients with SABP were followed longitudinally with a 6-month follow-up. Figure 1 outlines the study flowchart.
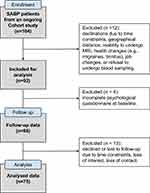 |
Figure 1 Flowchart of participant enrollment and exclusion in the study. |
Ethical approval was obtained from the Ethics Committee of the Medical Faculty Mannheim of Heidelberg University (2014–584N-MA). The study was registered in the German Clinical Trials Register under ID DRKS00008835, and the registered information is available online (https://www.bfarm.de/). It was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).43 All participants provided written informed consent.
The study adheres to the STROBE guidelines for reporting observational longitudinal research (see Supplementary Material).44
Participants
Participants were recruited through the outpatient clinic of the Central Institute of Mental Health and collaborating pain clinics, flyers and newspaper announcements, online fora, and cooperation with general practitioners. All participants underwent comprehensive medical screening to exclude back pain due to specific underlying factors such as major injury, infection, systemic disease, or any known pathoanatomical conditions. The Structured Clinical Interview for DSM-IV (SCID-I) was used to assess comorbid mental disorders.45
Inclusion criteria for the SABP group included primary back pain—defined as back pain without a clear underlying cause, or pain (or its impact) disproportionate to any observable injury or disease.46 The pain had to be located in the upper or lower back (or both) and had to last 7 to 12 weeks, as previously suggested in the literature.39,47 Individuals with multiple short pain episodes lasting no more than three months were also included. Since this study also involved the examination of functional and structural magnetic resonance imaging, exclusion criteria were aligned with the overall cohort, excluding participants under 18 or over 70 years old, those with neurological complications, psychotic episodes, left-handedness, major illnesses, pregnancy, pacemakers, or metal implants.
The original sample size for patients with SABP for the ongoing prospective study was calculated based on an assumed mean conversion rate of 20%,48 as observed in previous studies.4,10 To ensure a minimum of 40 chronic pain cases after one year and thus adequate power, the study planned a sample size of N = 200 patients with SABP (power analysis based on G*Power 3.1.9.7, Heinrich-Heine-Universität Düsseldorf, Germany).49,50 Despite significantly increasing recruitment efforts, only 92 patients with SABP were enrolled, likely due to the strict inclusion criteria and lower motivation among patients with subacute compared to patients with chronic pain to participate in research.
Participants first underwent a telephone screening to gather demographic information and assess pain characteristics. They were then evaluated to identify factors that might influence pain or its chronicity using questionnaires, interviews, and clinical examinations. After six months, a follow-up telephone screening was conducted to reassess participants’ pain status.
To evaluate the adequacy of the sample size for a linear multiple regression analysis, a post hoc power analysis was conducted using G*Power 3.1.9.7.49,50 The analysis assessed the ability to detect a medium effect size (f2 = 0.15) at a significance level of α = 0.05, assuming 10 predictors and a total sample size of 92. The results indicated an achieved power of 0.98 (97.93%), exceeding the conventional threshold of 0.80 (80%) for adequate statistical power. Of the 92 enrolled patients with SABP, 88 completed baseline assessments, with follow-up data available for 75. Using the sample size of 75 in a recalculated post hoc power analysis, with other parameters unchanged, the actual achieved power was 0.95 (95.28%), confirming sufficient power to detect a medium effect size.
Measures
Assessment of Pain and Psychological Variables
The Örebro Musculoskeletal Pain Questionnaire (ÖMPQ) evaluates psychological risk factors for developing long-term pain-related disability in patients with musculoskeletal pain.35,51 It comprises subscales on function, pain, psychological factors, fear avoidance, and miscellaneous items. The function subscale includes five items describing daily life activities and pain-related disability, while the pain subscale assesses current pain intensity, pain intensity during the past three months, pain sites, pain duration, and frequency. The sub-scale of psychological reactions evaluates anxiety, depression, perceived ability to cope with pain, perceived chance of the pain becoming persistent, and the chance of being able to work in six months. The fear-avoidance subscale assesses beliefs that pain increases with physical activity, that pain is a signal to stop activities, and that one should not work while in pain. The miscellaneous category includes items that cannot be grouped into one factor such as age, gender, working status, previous sick leave, heavy or monotonous work, and job satisfaction. The ÖMPQ has demonstrated moderate to good predictive value in numerous studies on chronic back pain with good validity and reliability.52,53 Previously, a cutoff score of 105 and below indicated recovery with 95% accuracy, while a score of 130 and above indicated an 86% risk of patients developing a persistent back problem.54 For this study, we used the German version of the original (long-form) ÖMPQ, which includes 25 questions, 21 of which are scored on a 10-point scale. It has demonstrated good reliability and validity.35
The West Haven-Yale Multidimensional Pain Inventory (MPI) was used in this study to evaluate pain severity and pain-related interference as outcome variables and responses of significant others to pain as psychological predictors.55,56 It consists of three sections. The first section of the MPI encompasses five subscales: pain severity, pain-related interference, affective distress, social support, and life control. The second section includes responses of significant others to the patient’s pain, with subscales for punishing responses, solicitous responses, and distracting responses. The third section assesses the patient’s activity level with subscales for social and leisure activities, household activities, and outdoor activities that can be combined into a general activity level score. All MPI items are scored on a 6-point Likert Scale. The MPI has demonstrated high internal consistency and validity.55
The Hospital Anxiety and Depression Scale (HADS) measures anxiety and depression. It comprises 7 items in each subscale, with a 4-point scoring range.57,58 It has demonstrated good reliability and validity.57
The German version of the Perceived Stress Scale (PSS) evaluates perceived stress.59,60 The scale comprises 10 items, each rated on a 5-point Likert scale, with higher scores indicating higher levels of stress. It has been shown to be reliable and valid in measuring the level of stress.60
The German version of the Pain Behaviour Checklist (PBC) evaluates 11 pain-related behaviors, such as limping, grimacing, moaning, rigid posture, frequent changes in posture, verbal pain complaints, touching of painful areas, slowed movements, and refusal to perform activities due to pain, crying, and inactivity.55,56 The item scores range from 0 to 2 (never, sometimes, and always). The German version of the PBC has demonstrated high reliability and validity.61
The Pain Vigilance and Awareness Questionnaire (PVAQ) is a 16-item measure assessing attention bias to pain in a questionnaire format, with each item scores ranging from 0 to 5 (never to always).28,62 It has demonstrated good validity and reliability.62
The Fear of Pain Questionnaire III (FPQ III) evaluates fear of pain. It consists of three subscales with 30 items that assess severe, minor, and medical pain, respectively.63,64 Each item is scored on a 5-point Likert Scale, with higher scores indicating greater fear. It FPQ III used in this study has demonstrated good validity and reliability.64
The Pain-Related Self-Statement Scale (PRSS) is an 18-item scale designed to assess catastrophizing and active coping in two subscales, each with 9 items.65,66 The item scales range from 0 to 5 (nearly never to nearly always). The PRSS is a German equivalent to the Coping Strategy Questionnaire66 and has shown good reliability and validity.65
All questionnaires were either originally developed in a German language version or were translated and validated for the German version.
Outcome Parameters
Pain severity and pain-related interference as assessed in the MPI were used as primary and secondary outcomes to assess back pain persistence over six months. These data were collected at baseline and at the 6-month follow-up.
Statistical Analysis
Statistical analyses were performed using RStudio Version 2024.04.2+764 (RStudio, PBC, Boston, MA, USA) with R 4.4.0 (© R Foundation for Statistical Computing, Vienna, Austria). The normality of all the data was assessed using the Shapiro–Wilk test. Normally distributed data are reported as means and standard deviations (SD), while non-normally distributed data are presented as medians with interquartile ranges (IQR).
Treatment of Outliers and Missing Values
In this longitudinal study, 4 out of 92 participants were excluded due to incomplete baseline assessments. An additional 13 participants (drop-out ratio 14.77%) declined or were lost to follow-up due to time constraints, loss of interest, and loss of contact. The final dataset included 75 patients with SABP. Outliers were identified as values with a |z| ≥ 3.29 and were treated as missing values. The proportion of missing values across the 8 questionnaires and scales ranged from 9.3% to 22.7%. Little’s MCAR test indicated that the missing data were completely at random (MCAR) with Chi-Square = 184.92, df = 175, p =0.289. We employed scale-level multidimensional imputations using the method of linear regression model.
Dimensionality Reduction in Psychological Factors
Principal component analysis (PCA) was employed to extract psychological components and reduce the 13 psychological variables in the SABP sample. We used the varimax rotation method and accepted factors with Cronbach’s α above 0.5. Factor component scores were computed using Bartlett’s approach.67
Correlation Analysis
Pearson correlation coefficients were used to examine the bivariate relationships between psychological risk factors—assessed through questionnaire, subscale, and factor score—as well as pain severity and pain-related interference at baseline and 6-month follow-up. The “pheatmap” package was employed to generate correlation heatmaps combined with hierarchical clustering.
Prediction Analysis
First, best subset regression identified the optimal model by selecting psychological factors validated through prior correlation analysis, along with age and gender. Variables from the model with the lowest Mallow’s Cp and highest adjusted R² were chosen for further analysis.
Second, a multistage linear regression modeling procedure was used to predict the outcome variables and identify the best predictor. In the first step, predictors were tested without adjusting for baseline outcome values. In the second step, models were adjusted for these baseline values. Finally, significant predictors from the previous steps were evaluated for their effect on outcomes at the 6-month follow-up. A significance level of α = 0.05 was used for all analyses.
Results
Participant Characteristics
Table 1 presents the demographic and clinical characteristics of the sample. The cohort was 65.33% female and 34.67% male, with a mean age of 36.00 years (SD = 13.24, range = 19–69). Twenty-eight cases (37%) reported a history of recurrent back pain. Additionally, six participants were taking ibuprofen, one was taking diclofenac, two were using paracetamol, one was using acetylsalicylic acid, and one was using a combination of acetylsalicylic acid, paracetamol, and caffeine. Table 2 shows the baseline scores of the psychological (sub)scales for all patients with SABP.
 |
Table 1 Characteristics of the Sample with Subacute Back Pain (N=75) |
 |
Table 2 Descriptive Statistics of Baseline Psychological (Sub)scales |
 |
Table 3 Rotated Component Matrix of Principal Component Analysis (PCA) |
Dimensionality Reduction of Psychological Risk Factors
Table 3 presents the results of the principal component analysis (PCA) with a varimax rotation method and four fixed factors. The Kaiser–Meyer–Olkin’s measure of sampling adequacy was 0.723, and Bartlett’s Test of Sphericity was significant (chi-square (91) = 392.953, p <0.001), confirming the suitability for factor analysis. The four-factor solution explains 64.60% of the variance.
In the PCA analysis, the first factor (FAC1: negative emotion/stress) included MPI-Affective Distress, HADS, PSS, and MPI-Life Control (reversed). The second factor (FAC2: supportive social responses) comprised MPI-Solicitous Reactions, MPI-Distracting Reactions, and MPI-Support. PRSS-Active Coping (Coef. = 0.542), PVAQ (Coef. = 0.303), and MPI-General Activities (Coef. = 0.512) were kept as separate variables due to coefficients below the 0.6 threshold. Due to Cronbach’s α of <0.5, the third (PBC and PRSS-Catastrophizing) and the fourth factors (MPI-Punishing Responses and FPQIII) were also independently analyzed in subsequent correlation and prediction analyses.
Correlation Analysis
Figure 2 presents a correlation matrix of risk factors with hierarchical clustering. We followed widely cited guidelines for interpreting Pearson correlation coefficients, which suggest that r ≈ 0.1 indicates a small association, r ≈ 0.3 represents a moderate association, and r ≥ 0.5 reflects a large association between variables.68,69
The ÖMPQ score and FAC1(negative emotion/stress) showed a moderate-to-large positive correlation (r =0.47, p <0.001) and were clustered together, suggesting they might share the aspect of emotional distress. Similarly, the PBS and PRSS-catastrophizing showed a moderate positive correlation (r =0.41, p <0.001) and clustered together, indicating a potential association between maladaptive cognitions and pain-related behaviors. These four variables—ÖMPQ score, FAC1, PBS, and PRSS-catastrophizing—were further intercorrelated: The ÖMPQ score and FAC1 showed moderate positive correlation with PBC (r =0.37, p =0.001 and r =0.27, p =0.019, respectively) and small-to-moderate positive correlations with PRSS-catastrophizing (r =0.28, p =0.013 and r =0.25, p =0.033, respectively). In addition, the ÖMPQ score had a moderate positive correlation with FPQIII (r =0.34, p =0.003), suggesting a meaningful relationship between emotional distress and fear of pain.
MPI-General Activities, FAC2 (Supportive Social Responses), PRSS-Active Coping formed a separate cluster, suggesting a potential link to the aspect of adaptive resilience. FAC2 demonstrated strong positive significant correlations with PRSS-Active Coping (r =0.54, p <0.001) and MPI-General Activity (r =0.51, p <0.001), while the direct correlation between PRSS-Active Coping and MPI-General Activity was not significant. In addition, MPI-Punishing Responses were separated from other clusters and showed no significant correlations with other risk factors.
Notably, PVAQ showed small-to-moderate positive correlations with FAC2 (Supportive Social Responses, r = 0.30, p = 0.008), PBS (r =0.25, p =0.032), and a moderate positive correlation with PRSS-catastrophizing (r =0.40, p <0.001). While FAC2 strongly positively associated with MPI-General Activities (r =0.51, p <0.001), PBS and PRSS-catastrophizing were moderately negatively associated with MPI-General Activities, with r = −0.33, p =0.004, and r = −0.31, p =0.007, respectively.
Table 4 shows the correlations between psychological risk factors and pain outcomes at baseline and follow-up. The results revealed strong associations between pain severity and pain-related interference (baseline: r =0.53, p <0.001; follow-up: r =0.61, p <0.001), indicating a slightly increasing link over time. Baseline pain severity moderately positively correlated with the ÖMPQ score (r =0.38, p =0.001) and FAC2 (r =0.27, p =0.018), while pain-related interference showed moderate positive correlation with the ÖMPQ score (r =0.34, p =0.003) and small-to-moderate positive correlation with PBS (r =0.24, p =0.039). At 6-month follow-up, pain severity showed small but statistically significant positive correlation with the baseline ÖMPQ score (r =0.24, p =0.034) and a moderate correlation with the PVAQ (r =0.34, p =0.003). Pain-related interference showed moderate positive correlation with the ÖMPQ score (r =0.34, p =0.003), PBS (r =0.34, p =0.003), PVAQ (r =0.37, p =0.001), and small-to moderate correlations with PRSS-catastrophizing (r =0.30, p =0.010) and FAC1 (r =0.27, p =0.019). These factors associated with pain persistence were subsequently analyzed in the best subsets regression.
 |
Table 4 Correlations Between Psychological Risk Factors and Pain Outcomes at Baseline and Follow-up |
 |
Table 5 Summary of Best Subsets Regression |
Regression Analysis
Table 5 summarizes the results of the best subset regression. For predicting pain severity after 6 months, Model 2 (comprising the ÖMPQ score and PVAQ) and Model 3 (comprising Age, the ÖMPQ score and PVAQ) exhibited the highest adjusted R² value (0.13). However, Model 2 demonstrated the lowest Mallows’ Cp value (1.52), indicating a better model fit than Model 3. Regarding the prediction of pain-related interference after 6 months, Model 3 (comprising the ÖMPQ score, PBS, and PVAQ) exhibited the highest adjusted R² value (0.22) and lowest Mallows’ Cp value (1.48). Hence, the ÖMPQ score and PVAQ from Model 2, as well as the ÖMPQ score, PBS, and PVAQ from Model 3, were employed in the subsequent multiple linear regression analysis.
To predict follow-up pain severity after 6 months, we first included the ÖMPQ score and PVAQ in model 1 and then conducted a multiple regression analysis that controlled for baseline pain severity in addition to these variables. Results are presented in Table 6. Model 1 explained 13% of the variance in follow-up pain severity (R2 =0.16, R2adjusted =0.13, F (2, 72) = 6.64, p =0.002), with the PVAQ (ß =0.31, t (72) =2.86, p =0.006), but not the ÖMPQ score (ß =0.21, t (72) =1.88, p =0.064), which was only marginally significant, being a significant predictor. An increase of one point in the PVAQ score was associated with an average increase of 0.06 points in the pain severity score (B =0.06, 95% CI [0.02, 0.10]) after 6 months. Model 2, adjusting for baseline pain severity, accounted for 24% of the variance in follow-up pain severity (R2 =0.27, R2adjusted=0.24, F (3, 71) = 8.83, p <0.001). The PVAQ remained a significant predictor while the ÖMPQ score no longer approached significance (ß=0.07, t (71) =0.63, p=0.53). The final Model 3, which included baseline pain severity and the PVAQ, explained 25% of the variance in follow-up pain severity (R2 =0.27, R2adjusted=0.25, F (2, 72) = 13.16, p<0.0001). The PVAQ continued to significantly predict follow-up pain severity (ß=0.25, t (72) =2.43, p=0.017), with each unit increase in the score corresponding to a 0.05-point increase in follow-up pain severity (B =0.05, 95% CI [0.01, 0.08]), while each unit increase in the score of baseline pain severity corresponding to a 0.41-point increase in follow-up pain severity score (B =0.41, 95% CI [0.20, 0.62]).
 |
Table 6 Regression Coefficients for Predicting Follow-up Pain Severity |
The same multistage linear regression analysis was used to predict pain-related interference at the 6-month follow-up, with the ÖMPQ score, PBS and the PVAQ included as predictors. Results are presented in Table 7. Model 1 explained 22% of the variance of pain-related interference at the follow-up measurement (R2 =0.25, R2adjusted=0.22, F (3, 71) = 8.03, p =0.0001). Both the ÖMPQ score (ß=0.23, t (73) = 2.10, p=0.039) and PVAQ (ß=0.30, t (73) = 2.83, p=0.006) were significant predictors, while the PBS failed to significantly predict pain-related interference at the follow-up measurement (ß=0.18, t (71) =1.631 p=0.11). One unit increase in the ÖMPQ score corresponded to a 0.03 rise in pain-related interference (B=0.03, 95% CI [0.001, 0.05]), while one unit increase in the PVAQ score resulted in a 0.07-point increase (B=0.07, 95% CI [0.02, 0.11]). Model 2, after controlling for baseline pain-related interference, explained 26% of the variance of pain-related interference at the follow-up measurement (R2 =0.29, R2adjusted=0.26, F (3, 71) = 9.55, p <0.001). In this model, only PVAQ remained a significant predictor (ß=0.32, t (71) = 3.12, p=0.003), while the ÖMPQ score was only marginally significant in predicting pain-related interference at the follow-up measurement after controlling for pain-related interference at baseline (ß=0.21, t (71) = 1.96, p=0.054). Model 3 included both baseline pain-related interference and PVAQ. It explained 23% of the variance in follow-up pain-related interference (R2 =0.25, R2adjusted=0.23, F (2, 72) = 11.94, p <0.001). The PVAQ was a significant predictor of pain-related interference at the follow-up measurement in Model 3 (ß=0.33, t (72) = 3.24, p =0.002), with each unit of increase in the PVAQ score leading to a 0.07-point increase in follow-up pain-related interference (B =0.07, 95% CI [0.03, 0.12]), while each unit increase in the score of baseline pain-related interference to a 0.27-point increase in follow-up pain-related interference (B =0.27, 95% CI [0.10, 0.44]).
 |
Table 7 Regression Coefficients for Predicting Follow-up Pain-Related Interference |
Discussion
We evaluated whether incorporating additional psychological and social risk factors—beyond those captured by the ÖMPQ—could improve the accuracy of predicting the transition from subacute to chronic back pain. Our findings extend the results of previous studies by showing that additional factors such as emotional distress, hypervigilance and pain-related behavior, are associated with the development of chronic pain.
Correlation Patterns of Psychological Risk Factors and the Dual Role of Pain Hypervigilance
Correlation analysis and hierarchical clustering identified two distinct patterns of psychological and social risk factors included in this study. The first pattern, representing maladaptation, included negative emotions, cognitions, and behaviors, which clustered together (ÖMPQ, FAC1, PRSS-catastrophizing, and PBS) as mutually reinforcing factors. The second pattern, reflecting adaptive resilience, included social support, positive coping, and engagement in activities (FAC2, PRSS-Active Coping, and MPI-General Activity). Interestingly, pain hypervigilance was correlated with both patterns and could influence general activity levels in opposite ways: it can encourage activity through the positive correlation with social supports (FAC2), yet discourage it through its association with negative cognitive (PRSS-catastrophizing) and behavioral (PBS) factors. Furthermore, the association with supportive social responses (FAC2) suggests that pain hypervigilance may also serve a communicative function.
Pain hypervigilance, which can either promote or reduce activity levels at the subacute stage, has been identified as a possible predictor of chronic back pain in subsequent regression analysis. Initially, hypervigilance may be a modifiable factor; however, its association with catastrophizing and pain-related behaviors suggests that heightened pain awareness, combined with negative cognition-behavior pattern, might lead to activity avoidance, ultimately as pain persists, it may shift into dominant maladaptive patterns that intensify pain severity, interference, disability and finally lead to pain chronicity.
This dual role of pain hypervigilance underscores the potential of interventions, such as attention management and cognitive-behavioral therapy (CBT),70 to enhance the adaptive aspects of hypervigilance—such as fostering social support and active coping—while reducing maladaptive patterns linked to emotional distress and catastrophizing. Recognizing hypervigilance as a risk factor is important for clinicians and researchers, as inadequate awareness or miscommunication may contribute to nocebo effects, ultimately reducing treatment efficacy for musculoskeletal pain.71,72
Prediction of Pain Progression
The ÖMPQ is a multidimensional questionnaire that focuses on screening psychological risk factors for chronic pain development, particularly emotional distress components like depression, anxiety, and fear avoidance. In this study, we intended to test if the use of additional psychological variables such as pain hypervigilance, catastrophizing or pain behaviors beyond the ÖMPQ could provide predictive accuracy for pain progression at the subacute stage. The ÖMPQ score alone as well as with the scores of PBC and PVAQ at this stage predicted pain persistence after 6 months. However, the effect of these predictors diminished after baseline adjustment, and only the PVAQ remained predictive of chronicity. One possible explanation is the moderate positive correlation between the ÖMPQ and baseline pain severity, as 11 of its 21 items assess pain characteristics, suggesting that its predictive effects may partly reflect autoregressive influences.
Alternatively, the psychological risk factors identified by the ÖMPQ may only impact pain progression within certain time frames rather than during the entire subacute stage. Correlation analysis revealed the shifts in psychological risk factors over time. At baseline, pain severity was associated with the ÖMPQ score and social support (FAC2), suggesting a link to increased emotional distress and social support needs at the subacute stage. By follow-up, the ÖMPQ score remained consistently correlated with pain severity, while pain hypervigilance, as measured by PVAQ, emerged as a relevant factor and the impact of social support diminished, suggesting the intensified influence of cognitive factors. Pain-related interference showed consistent correlations with the ÖMPQ score and pain-related behaviors (PBS) from baseline to follow-up, indicating stable contributions of these factors. At follow-up, additional correlations emerged with catastrophizing (PRSS), negative emotions and stress (FAC1), and pain hypervigilance (PVAQ), suggesting full involvement of negative emotions and an increased role of maladaptive cognitive factors as back pain became chronic.
These findings suggest that psychological factors may exert their effects within specific time frames or shift in influence as pain progresses. While the impact of maladaptive cognitions intensifies as SABP progresses, the ÖMPQ’s primary focus on emotional distress associates it more closely with pain severity in the subacute stages.
Hypervigilance to Pain Emerged as a Potential Predictor at the Subacute Stage for Pain Chronicity
Hypervigilance to pain emerged as a strongest predictor at the subacute stage for the chronicity of back pain. This aligns with evidence linking pain hypervigilance to higher pain severity.73–76
Attention plays a direct role in modulating sensation. Animal and human neuroimaging studies have shown that neural activity of somatosensory neurons could be modulated by attention, with focused attention amplifying signals in pain perception and distraction reducing it.77,78 Clinical observations align with these findings: concentrating on pain tends to amplify the perception of its intensity, whereas distracting or redirecting attention can alleviate it.27,79,80 However, clinical studies also found that individual responses to attention manipulation vary, with some participants experiencing no or paradoxical pain reductions when using the same attention manipulation strategies.75,81,82 These differences may stem from individual variations in attention, where some individuals are naturally more vigilant to pain-related stimuli.83 This hypervigilance is also associated with an increased risk of developing chronic pain.84
In patients with chronic pain, attention bias to pain has been confirmed by a meta-analysis study.85 Attention bias is generally divided into avoidance and hypervigilance from the direction of the bias of attention.86 Hypervigilance to pain correlates with higher pain intensity, emotional distress, and psychological disability, even when controlling for baseline pain intensity and demographic factors.28 In previous studies, vigilance exacerbated pain by intensifying pain sensations and reducing engagement in productive activities.28,84
Attention Bias Modification (ABM), an intervention aimed at altering attentional deployment to symptom-relevant emotionally salient stimuli, has shown potential in improving pain outcomes by raising experimental pain thresholds in healthy participants.87 Clinically, ABM has demonstrated effectiveness in reducing pain intensity, disability and unfavorable psychological consequences in patients with chronic pain.88 This suggests that heightened vigilance to pain may contribute to chronicity by influencing an individual’s pain perception.
Our study highlights the potential predictive role of hypervigilance to pain in the transition from subacute to chronic back pain, suggesting that hypervigilance during this period may significantly influence pain progression. Therefore, attention management at the subacute stage could be an effective strategy for preventing the development of chronic back pain.
Strengths and Limitations
This study has several strengths. First, it addresses a critical gap in the literature by focusing on the subacute stage of back pain, a period that represents a key window for intervention to prevent chronicity. Second, the assessment of psychological and social risk factors is extensive. Third, the use of robust statistical methods ensures methodological rigor. Especially, the use of correlations and hierarchical clustering revealed two distinct resilience patterns—maladaptive and adaptive—and their correlations with pain hypervigilance, offering a novel perspective on the predictive value of hypervigilance. Fourth, the longitudinal design provides valuable insights into the dynamic nature of risk factors over time. Finally, the study’s findings have significant clinical relevance, offering actionable insights for developing targeted early interventions to reduce the burden of chronic back pain.
This study also has several limitations. First, our cohort consisted of a carefully selected sample of SABP participants with primary pain. This, along with the relatively small sample size, may limit the generalizability of our findings and reduce the feasibility of more detailed analyses. Second, the strict inclusion criteria may have introduced selection bias. Third, there was an imbalance in the gender distribution of SABP participants in our study, with approximately two times more women than men. This ratio is slightly higher than 1.4, which was reported in a large-scale randomized cohort study in patients with acute low back pain,4 but lower than 2.6 observed in a cross-sectional study on chronic pain.89 This discrepancy may stem from the higher prevalence of pain in women compared to men and potential gender-based differences in clinical trial participation.90–92 Due to the limited sample size in our study, gender matching was not feasible. Although the best subset regression analysis indicated that gender had no significant effect on the outcome variables, this imbalance may still limit the applicability of our results to male participants. Fourth, baseline data indicated that participants experienced mild to moderate pain severity. Since pain and psychological risk factors are interrelated, different risk factors may emerge in individuals with more severe pain during the subacute phase. Fifth, our analysis lacks post-hoc correction. Recommendations for post-hoc correction are inconsistent, and using different correction methods can yield conflicting p-value results; additionally, post-hoc correction itself may increase Type II errors.93 Given our study’s exploratory nature and focus on managing Type I errors, we choose not to apply post-hoc correction and have interpreted the findings with caution. Lastly, in this study, we have selectively focused on psychological and social variables related to chronicity, without addressing other potentially relevant predictors such as personality traits, central sensitization, brain imaging characteristics, or other biological factors.94 Future studies should evaluate the relative contribution of these determinants to chronicity.
Conclusion
At the subacute stage, the ÖMPQ score, alone or with PBC and PVAQ, predicted pain persistence at six months but lost significance after the adjustment of baseline pain, likely due to overlap with pain severity. Our data suggest that maladaptive cognitions (eg, pain hypervigilance, catastrophizing) gain influence as pain transitions to chronicity.
Pain hypervigilance may have a dual role at the subacute stage, fostering social support through increased activity but also limiting it through its association with catastrophizing and pain-related behaviors. As pain advanced, individuals with SABP who exhibited hypervigilance were more likely to report greater pain severity and interference six months later.
These findings highlight the role and predictive value of pain hypervigilance in the transition from subacute to chronic back pain. Early interventions targeting hypervigilance may help to prevent chronicity by enhancing adaptive coping while reducing maladaptive patterns tied to negative emotions, cognitions and behaviors during the subacute stage. Future longitudinal studies should incorporate behavioral measures of attentional bias to confirm these findings and to clarify the mechanisms linking hypervigilance to pain chronicity.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (Collaborative Research Center) SFB 1158, project B03 to FN and HF within the Heidelberg Pain Consortium.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ferreira ML, de Luca K, Haile LM. GBD 2021 Low Back Pain Collaborators. Global, regional, and national burden of low back pain, 1990-2020, its attributable risk factors, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5(6):e316–e329. doi:10.1016/S2665-9913(23)00098-X
2. Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. Lancet Lond Engl. 2018;391(10137):2356–2367. doi:10.1016/S0140-6736(18)30480-X
3. Chou R. Will this patient develop persistent disabling low back pain? JAMA. 2010;303(13):1295. doi:10.1001/jama.2010.344
4. Stevans JM, Delitto A, Khoja SS, et al. Risk factors associated with transition from acute to chronic low back pain in us patients seeking primary care. JAMA NetwOpen. 2021;4(2):e2037371. doi:10.1001/jamanetworkopen.2020.37371
5. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133(4):581–624. doi:10.1037/0033-2909.133.4.581
6. Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119–130. doi:10.1037/a0035514
7. Raja SN, Carr DB, Cohen M, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–1982. doi:10.1097/j.pain.0000000000001939
8. Crombez G, Veirman E, Van Ryckeghem D, Scott W, De Paepe A. The effect of psychological factors on pain outcomes: lessons learned for the next generation of research. Pain Rep. 2023;8(6):e1112. doi:10.1097/PR9.0000000000001112
9. Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463–484. doi:10.1016/j.ejpain.2004.11.001
10. Apkarian AV, Baliki MN, Farmer MA. Predicting transition to chronic pain. Curr Opin Neurol. 2013;26(4):360–367. doi:10.1097/WCO.0b013e32836336ad
11. Baliki MN, Petre B, Torbey S, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15(8):1117–1119. doi:10.1038/nn.3153
12. Robertson JW, Aristi G, Hashmi JA. White matter microstructure predicts measures of clinical symptoms in chronic back pain patients. NeuroImage Clin. 2023;37:103309. doi:10.1016/j.nicl.2022.103309
13. Hashmi JA, Baliki MN, Huang L, et al. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain J Neurol. 2013;136(Pt 9):2751–2768. doi:10.1093/brain/awt211
14. Gustin SM, Peck CC, Cheney LB, Macey PM, Murray GM, Henderson LA. Pain and plasticity: is chronic pain always associated with somatosensory cortex activity and reorganization? J Neurosci off J Soc Neurosci. 2012;32(43):14874–14884. doi:10.1523/JNEUROSCI.1733-12.2012
15. Tracey I. Can neuroimaging studies identify pain endophenotypes in humans? Nat Rev Neurol. 2011;7(3):173–181. doi:10.1038/nrneurol.2011.4
16. Korownyk CS, Montgomery L, Young J, et al. PEER simplified chronic pain guideline: management of chronic low back, osteoarthritic, and neuropathic pain in primary care. Can Fam Physician Med Fam Can. 2022;68(3):179–190. doi:10.46747/CFP.6803179
17. Nicol V, Verdaguer C, Daste C, et al. Chronic low back pain: a narrative review of recent international guidelines for diagnosis and conservative treatment. J Clin Med. 2023;12(4):1685. doi:10.3390/JCM12041685
18. Oliveira CB, Maher CG, Pinto RZ, et al. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2018;27(11):2791–2803. doi:10.1007/s00586-018-5673-2
19. Otero-Ketterer E, Peñacoba-Puente C, Pinheiro-Araujo CF, Valera-Calero JA, Ortega-Santiago R. Biopsychosocial factors for chronicity in individuals with non-specific low back pain: an umbrella review. Int J Environ Res Public Health. 2022;19(16):10145. doi:10.3390/ijerph191610145
20. Petrini L, Arendt-Nielsen L, Pammi VSC, Dutt V. Understanding pain catastrophizing: putting pieces together. Front Psychol. 2020;11:11. doi:10.3389/fpsyg.2020.603420
21. Sullivan MJL. The communal coping model of pain catastrophising: clinical and research implications. Can Psychol Psychol Can. 2012;53(1):32–41. doi:10.1037/a0026726
22. Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull. 1999;125(3):356–366. doi:10.1037/0033-2909.125.3.356
23. Villemure C, Bushnell CM. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95(3):195–199. doi:10.1016/S0304-3959(02)00007-6
24. Myers SS, Phillips RS, Davis RB, et al. Patient expectations as predictors of outcome in patients with acute low back pain. J Gen Intern Med. 2008;23(2):148–153. doi:10.1007/s11606-007-0460-5
25. Diotaiuti P, Corrado S, Mancone S, et al. Influence of cognitive orientation and attentional focus on pain perception. Int J Environ Res Public Health. 2021;18(13):7176. doi:10.3390/ijerph18137176
26. Janvin A, Weltzien F. The relationship between chronic pain and attention: a clinical perspective [master’s thesis]. Oslo: University of Oslo. 2022 Available from: https://www.duo.uio.no/handle/10852/94673.
27. Lautenbacher S, Pauli P, Zaudig M, Birbaumer N. Attentional control of pain perception: the role of hypochondriasis. J Psychosom Res. 1998;44(2):251–259. doi:10.1016/S0022-3999(97)00214-6
28. McCracken LM. “Attention” to pain in persons with chronic pain: a behavioral approach. Behav Ther. 1997;28(2):271–284. doi:10.1016/S0005-7894(97)80047-0
29. Turk DC, Wilson HD. Fear of pain as a prognostic factor in chronic pain: conceptual models, assessment, and treatment implications. Curr Pain Headache Rep. 2010;14(2):88–95. doi:10.1007/s11916-010-0094-x
30. Vlaeyen JWS, Crombez G, Linton SJ. The fear-avoidance model of pain. PAIN. 2016;157(8):1588. doi:10.1097/j.pain.0000000000000574
31. Vlaeyen JWS, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85(3):317–332. doi:10.1016/S0304-3959(99)00242-0
32. Flor H, Breitenstein C, Birbaumer N, Fürst M. A psychophysiological analysis of spouse solicitousness towards pain behaviors, spouse interaction, and pain perception. Behav Ther. 1995;26(2):255–272. doi:10.1016/S0005-7894(05)80105-4
33. Nees F, Usai K, Kandić M, et al. The association of spouse interactions and emotional learning in interference related to chronic back pain. Neurobiol Pain. 2023;13:100122. doi:10.1016/J.YNPAI.2023.100122
34. Wilson JM, Colebaugh CA, Flowers KM, Meints SM, Edwards RR, Schreiber KL. Social support and psychological distress among chronic pain patients: the mediating role of mindfulness. Personal Individ Differ. 2022;190:111551. doi:10.1016/J.PAID.2022.111551
35. Langenfeld A, Bastiaenen C, Brunner F, Swanenburg J. Validation of the Orebro musculoskeletal pain screening questionnaire in patients with chronic neck pain. BMC Res Notes. 2018;11(1):161. doi:10.1186/s13104-018-3269-x
36. Karran EL, McAuley JH, Traeger AC, et al. Can screening instruments accurately determine poor outcome risk in adults with recent onset low back pain? A systematic review and meta-analysis. BMC Med. 2017;15(1):13. doi:10.1186/s12916-016-0774-4
37. Heneweer H, Aufdemkampe G, van Tulder MW, Kiers H, Stappaerts KH, Vanhees L. Psychosocial variables in patients with (sub)acute low back pain: an inception cohort in primary care physical therapy in the Netherlands. Spine. 2007;32(5):586–592. doi:10.1097/01.brs.0000256447.72623.56
38. Reckziegel D, Vachon-Presseau E, Petre B, Schnitzer TJ, Baliki MN, Apkarian AV. Deconstructing biomarkers for chronic pain: context and hypothesis dependent biomarker types in relation to chronic pain. Pain. 2019;160(Suppl 1):S37. doi:10.1097/J.PAIN.0000000000001529
39. Chanda ML, Alvin MD, Schnitzer TJ, Apkarian AV. Pain characteristic differences between subacute and chronic back pain. J Pain. 2011;12(7):792–800. doi:10.1016/j.jpain.2011.01.008
40. Hruschak V, Cochran G. Psychosocial predictors in the transition from acute to chronic pain: a systematic review. Psychol Health Med. 2018;23(10):1151–1167. doi:10.1080/13548506.2018.1446097
41. Kandić M, Moliadze V, Andoh J, Flor H, Nees F. Brain circuits involved in the development of chronic musculoskeletal pain: evidence from non-invasive brain stimulation. Front Neurol. 2021;12:732034. doi:10.3389/fneur.2021.732034
42. Löffler M, Levine SM, Usai K, et al. Corticostriatal circuits in the transition to chronic back pain: the predictive role of reward learning. Cell Rep Med. 2022;3(7). doi:10.1016/J.XCRM.2022.100677
43. World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human participants. JAMA. 2024. doi:10.1001/jama.2024.21972
44. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi:10.1016/j.jclinepi.2007.11.008
45. Wittchen HU, Wunderlich U, Gruschwitz S, Zaudig M. Strukturiertes Klinisches Interview Für DSM-IV, Achse I-SKID-IV [Structured Clinical Interview for DSM-IV. Axis I: Mental Disorders]. Göttingen. Hogrefe; 1997.
46. Nicholas M, Vlaeyen JWS, Rief W, et al. The IASP classification of chronic pain for ICD-11: chronic primary pain. PAIN. 2019;160(1):28–37. doi:10.1097/j.pain.0000000000001390
47. Dionne CE, Dunn KM, Croft PR, et al. A consensus approach toward the standardization of back pain definitions for use in prevalence studies. Spine. 2008;33(1):95–103. doi:10.1097/BRS.0b013e31815e7f94
48. Nees F, Löffler M, Usai K, Flor H. Hypothalamic-pituitary-adrenal axis feedback sensitivity in different states of back pain. Psychoneuroendocrinology. 2019;101:60–66. doi:10.1016/j.psyneuen.2018.10.026
49. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi:10.3758/BF03193146
50. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–1160. doi:10.3758/BRM.41.4.1149
51. Linton SJ, Halldén K. Can we screen for problematic back pain? A screening questionnaire for predicting outcome in acute and subacute back pain. Clin J Pain. 1998;14(3):209–215. doi:10.1097/00002508-199809000-00007
52. Sattelmayer M, Lorenz T, Röder C, Hilfiker R. Predictive value of the acute low back pain screening questionnaire and the örebro musculoskeletal pain screening questionnaire for persisting problems. Eur Spine J. 2012;21(S6):773–784. doi:10.1007/s00586-011-1910-7
53. Brown G. The Orebro musculoskeletal pain questionnaire. Occup Med. 2008;58(6):447–448. doi:10.1093/occmed/kqn077
54. Linton SJ, Boersma K. Early identification of patients at risk of developing a persistent back problem: the predictive validity of the Orebro musculoskeletal pain questionnaire. Clin J Pain. 2003;19(2):80–86. doi:10.1097/00002508-200303000-00002
55. Flor H, Rudy TE, Birbaumer N, Streit B, Schugens MM. Zur Anwendbarkeit des West Haven-Yale Multidimensional Pain Inventory im deutschen Sprachraum [The applicability of the West Haven-Yale multidimensional pain inventory in German-speaking countries]. Schmerz Pain. 1990;4(2):82–87. doi:10.1007/BF02527839
56. Kerns RD, Turk DC, Rudy TE. The west haven-yale multidimensional pain inventory (WHYMPI). Pain. 1985;23(4):345–356. doi:10.1016/0304-3959(85)90004-1
57. Petermann F. Hospital anxiety and depression scale, deutsche version (Hads-D) [HADS-D hospital and anxiety depression scale – German version]. Zeitschrift für Psych Psychol Psychother. 2015;59(3):251–253. Httpdxdoiorg1010241661-4747a000077. doi:10.1024/1661-4747/A000077
58. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/J.1600-0447.1983.TB09716.X
59. Cohen S. Perceived stress in a probability sample of the United States. In: The Social Psychology of Health. The Claremont Symposium on Applied Social Psychology. Sage Publications, Inc; 1988:31–67.
60. Klein EM, Brähler E, Dreier M, et al. The German version of the Perceived Stress Scale – psychometric characteristics in a representative German community sample. BMC Psychiatry. 2016;16(1):159. doi:10.1186/s12888-016-0875-9
61. Flor H, Heimerdinger K. Effectiveness of attention bias modification with and without trans cranial direct current stimulation in. In: Geissner E, Jungnitsch G, editors. Psychologie Des Schmerzes.Diagnose Und Therapie. Psychologie Verlags Union [Psychology of Pain.Diagnose and Therapy. Psychology Publishing Union]. BeltzPVU; 1992:99–105.
62. Kunz M, Capito ES, Horn-Hofmann C, et al. Psychometric properties of the German version of the pain vigilance and awareness questionnaire (PVAQ) in pain-free samples and samples with acute and chronic pain. Int J Behav Med. 2017;24(2):260–271. doi:10.1007/s12529-016-9585-4
63. McNeil DW, Rainwater AJ. Development of the fear of pain questionnaire--III. J Behav Med. 1998;21(4):389–410. doi:10.1023/a:1018782831217
64. Osman A, Breitenstein JL, Barrios FX, Gutierrez PM, Kopper BA. The fear of pain questionnaire-III: further reliability and validity with nonclinical samples. J Behav Med. 2002;25(2):155–173. doi:10.1023/A:1014884704974
65. Flor H, Behle DJ, Birbaumer N. Assessment of pain-related cognitions in chronic pain patients. Behav Res Ther. 1993;31(1):63–73. doi:10.1016/0005-7967(93)90044-u
66. Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983;17(1):33–44. doi:10.1016/0304-3959(83)90125-2
67. Distefano C, Zhu M, Mîndrilã D. Understanding and Using Factor Scores: considerations for the applied researcher. Pract Assess Res Eval. 2009;14:20. doi:10.7275/da8t-4g52
68. Cohen J. Statistical Power Analysis for the Behavioral Sciences.
69. Mukaka M. A guide to appropriate use of Correlation coefficient in medical research. Malawi Med J J Med Assoc Malawi. 2012;24(3):69–71.
70. Lim JA, Choi SH, Lee WJ, et al. Cognitive-behavioral therapy for patients with chronic pain: implications of gender differences in empathy. Medicine. 2018;97(23):e10867. doi:10.1097/MD.0000000000010867
71. Hohenschurz-Schmidt D, Thomson OP, Rossettini G, et al. Avoiding nocebo and other undesirable effects in chiropractic, osteopathy and physiotherapy: an invitation to reflect. Musculoskelet Sci Pract. 2022;62:102677. doi:10.1016/j.msksp.2022.102677
72. Rossettini G, Campaci F, Bialosky J, Huysmans E, Vase L, Carlino E. The biology of placebo and nocebo effects on experimental and chronic pain: state of the art. J Clin Med. 2023;12(12):4113. doi:10.3390/jcm12124113
73. Baum C, Huber C, Schneider R, Lautenbacher S. Prediction of experimental pain sensitivity by attention to pain-related stimuli in healthy individuals. Percept Mot Skills. 2011;112(3):926–946. doi:10.2466/02.09.22.PMS.112.3.926-946
74. Crombez G, Damme SV, Eccleston C. Hypervigilance to pain: an experimental and clinical analysis. Pain. 2005;116(1):4–7. doi:10.1016/j.pain.2005.03.035
75. Goubert L, Crombez G, Eccleston C, Devulder J. Distraction from chronic pain during a pain-inducing activity is associated with greater post-activity pain. Pain. 2004;110(1):220–227. doi:10.1016/j.pain.2004.03.034
76. Huber C, Kunz M, Artelt C, Lautenbacher S. Attentional and emotional mechanisms of pain processing and their related factors: a structural equations approach. Pain Res Manag J Can Pain Soc. 2010;15(4):229–237. doi:10.1155/2010/516176
77. Gomez-Ramirez M, Hysaj K, Niebur E. Neural mechanisms of selective attention in the somatosensory system. J Neurophysiol. 2016;116(3):1218–1231. doi:10.1152/jn.00637.2015
78. Wiesman AI, Wilson TW. Attention modulates the gating of primary somatosensory oscillations. NeuroImage. 2020;211:116610. doi:10.1016/j.neuroimage.2020.116610
79. Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain J Neurol. 2002;125(Pt 2):310–319. doi:10.1093/BRAIN/AWF022
80. Chayadi E, McConnell BL. Gaining insights on the influence of attention, anxiety, and anticipation on pain perception. J Pain Res. 2019;12:851–864. doi:10.2147/JPR.S176889
81. Damme S, Crombez G, Wever KN, Goubert L. Is distraction less effective when pain is threatening? An experimental investigation with the cold pressor task. Eur J Pain. 2008;12(1):60–67. doi:10.1016/j.ejpain.2007.03.001
82. Prins B, Decuypere A, Damme SV. Effects of mindfulness and distraction on pain depend upon individual differences in pain catastrophizing: an experimental study. Eur J Pain. 2014;18(9):1307–1315. doi:10.1002/j.1532-2149.2014.491.x
83. Baum C, Kappesser J, Schneider R, Lautenbacher S. Does vigilance to pain make individuals experts in facial recognition of pain? Pain Res Manag. 2013;18(4):191–196. doi:10.1155/2013/371428
84. Herbert MS, Goodin BR, Pero ST, et al. Pain hypervigilance is associated with greater clinical pain severity and enhanced experimental pain sensitivity among adults with symptomatic knee osteoarthritis. Ann Behav Med Publ Soc Behav Med. 2014;48(1):50–60. doi:10.1007/s12160-013-9563-x
85. Todd J, van Ryckeghem DML, Sharpe L, Crombez G. Attentional bias to pain-related information: a meta-analysis of dot-probe studies. Health Psychol Rev. 2018;12(4):419–436. doi:10.1080/17437199.2018.1521729
86. Tabira T, Maruta M, Matsudaira K, et al. Relationship between attention bias and psychological index in individuals with chronic low back pain: a preliminary event-related potential study. Front Hum Neurosci. 2020;14:561726. doi:10.3389/fnhum.2020.561726
87. Sharpe L, Johnson A, Dear BF. Attention bias modification and its impact on experimental pain outcomes: comparison of training with words versus faces in pain. Eur J Pain. 2015;19(9):1248–1257. doi:10.1002/ejp.648
88. Shiasy Y, Shakiba S, Taremian F, Akhavan Hejazi SM, Abasi A. The effectiveness of attention bias modification with and without trans cranial direct current stimulation in chronic low back pain. Iran J Psychiatry. 2020;15(2):112–125.
89. Molander P, Dong HJ, Äng B, Enthoven P, Gerdle B. The role of pain in chronic pain patients’ perception of health-related quality of life: a cross-sectional SQRP study of 40,000 patients. Scand J Pain. 2018;18(3):417–429. doi:10.1515/sjpain-2018-0003
90. Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Sex RJL. Gender, and pain: a review of recent clinical and experimental findings. J Pain off J Am Pain Soc. 2009;10(5):447–485. doi:10.1016/j.jpain.2008.12.001
91. Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev. 2000;24(4):485–501. doi:10.1016/S0149-7634(00)00017-8
92. Lobato L, Bethony JM, Pereira FB, Grahek SL, Diemert D, Gazzinelli MF. Impact of gender on the decision to participate in a clinical trial: a cross-sectional study. BMC Public Health. 2014;14(1):1156. doi:10.1186/1471-2458-14-1156
93. García-Pérez MA. Use and misuse of corrections for multiple testing. Methods Psychol. 2023;8:100120. doi:10.1016/j.metip.2023.100120
94. Sariyildiz A, Coskun Benlidayi I, Olmez Engizek S, Deniz V. The relation of psychological status and type D personality with central sensitization in knee osteoarthritis: everything is in your mind! Rheumatol Int. 2023;43(12):2261–2269. doi:10.1007/s00296-023-05471-7
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


