Back to Journals » International Journal of Nanomedicine » Volume 19
Hypoxia-Driven Changes in Tumor Microenvironment: Insights into Exosome-Mediated Cell Interactions
Received 23 May 2024
Accepted for publication 6 August 2024
Published 12 August 2024 Volume 2024:19 Pages 8211—8236
DOI https://doi.org/10.2147/IJN.S479533
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kamakhya Misra
Churan Wang,1 Shun Xu,2 Xiao Yang2
1Dalian Medical University, Dalian, 116000, People’s Republic of China; 2Department of Thoracic Surgery, The First Hospital of China Medical University, Shenyang, 110002, People’s Republic of China
Correspondence: Xiao Yang, Email [email protected]
Abstract: Hypoxia, as a prominent feature of the tumor microenvironment, has a profound impact on the multicomponent changes within this environment. Under hypoxic conditions, the malignant phenotype of tumor cells, the variety of cell types within the tumor microenvironment, as well as intercellular communication and material exchange, undergo complex alterations. These changes provide significant prospects for exploring the mechanisms of tumor development under different microenvironmental conditions and for devising therapeutic strategies. Exosomes secreted by tumor cells and stromal cells are integral components of the tumor microenvironment, serving as crucial mediators of intercellular communication and material exchange, and have consequently garnered increasing attention from researchers. This review focuses on the mechanisms by which hypoxic conditions promote the release of exosomes by tumor cells and alter their encapsulated contents. It also examines the effects of exosomes derived from tumor cells, immune cells, and other cell types under hypoxic conditions on the tumor microenvironment. Additionally, we summarize current research progress on the potential clinical applications of exosomes under hypoxic conditions and propose future research directions in this field.
Keywords: hypoxia, exosomes, tumor microenvironment, vesicles
Introduction
As research on the tumor microenvironment advances, the dynamic release and consumption of oxygen, as one of its characteristics, is gradually being recognized by researchers.1 In solid tumors, hypoxia is often considered a hallmark of the tumor microenvironment and is believed to be one of the reasons for poor patient prognosis and treatment outcomes.2,3 The essence of tumor hypoxia lies in the imbalance between the oxygen demand of cancer cells and the inadequate oxygen supply caused by dysfunctional tumor blood vessels, leading to a hypoxic state in virtually all areas of the tumor microenvironment.4,5 This not only induces proliferation, invasion, or resistance characteristics in tumor cells but also causes immune cell dysfunction or stimulates the stromal environment to further promote tumor development.4,5 There is no confirmed threshold for hypoxia standards in solid tumors, but they can be roughly classified into normoxic areas with functional blood vessels, hypoxic areas with oxygen partial pressures less than 10 mmHg, and necrotic zones with very low oxygen concentrations.6–8 Under such conditions, the communication and changes among various components of the tumor microenvironment generate complex mechanisms that regulate tumor occurrence and development. For example, tumor-associated macrophages (TAMs) tend to appear in hypoxic areas.9,10 Controlled by the mode of communication between cells in the tumor microenvironment, macrophages gradually polarize into the M2 subtype and exert functions that promote tumor progression.11 In addition, under the influence of hypoxia, M2 TAMs can drive tumor invasiveness, severely limiting the effectiveness of immunotherapy and other treatments.12 Hypoxia has been proven to be the most important influencing factor in such cell-to-cell communication, involving factors like chemokines and extracellular vesicles.13 Similarly, other components in the tumor microenvironment, especially tumor cells themselves, undergo changes or progression due to chemokines and extracellular vesicles in the hypoxic environment. In-depth research on cell-to-cell communication in hypoxic environments can provide promising strategies for understanding the mechanisms of tumor occurrence and development, as well as potential targets for future therapies. Increasing evidence suggests that extracellular vesicles, including exosomes, play a crucial role in complex hypoxic processes by acting as signaling transport proteins.14
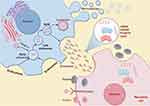 |
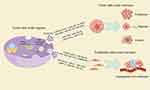
Figure 1 Diagram of the exosome formation process. Note: By Figdraw. |
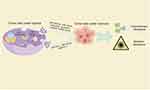 |
Figure 4 Provides an overview of the impact of exosomes derived from tumor cells in a hypoxic microenvironment and their cargo on tumor radiotherapy and chemotherapy resistance. Note: By Figdraw. |
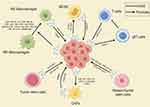 |
Figure 5 Summarizes the interaction between tumor cells in a hypoxic microenvironment with cancer-associated fibroblasts (CAFs), immune cells, and stem cells through exosomes. Note: By Figdraw. |
Extracellular vesicles, a diverse family of membrane-bound structures originating from cells, encompass both exosomes and microvesicles.15 These vesicles arise either from the endocytic pathway or through budding from the plasma membrane. Far from being mere cellular debris, extracellular vesicles have garnered significant attention in recent years for their remarkable capacity to facilitate intercellular communication. By trafficking various molecular cargo, including nucleic acids, lipids, and proteins, these vesicles play a pivotal role in maintaining normal cellular homeostasis and can also influence pathological processes.16,17 Among the different types of extracellular vesicles, exosomes stand out with their distinct size range of approximately 30–150 nm in diameter.18 In the past decade, research on exosomes has rapidly advanced, especially in their involvement in promoting various tumor activities through the exchange of functional contents between cells, such as anti-apoptosis, metastasis, angiogenesis, immune evasion, and chemotherapy resistance.19,20 Many studies have shown that hypoxic environments promote the biogenesis of exosomes and increase both local and distant cell-to-cell communication based on exosomes.19,20 The generation of exosomes is a complex, multistep process that commences with the invagination of the plasma membrane, progresses through the development of multivesicular bodies (MVBs), and culminates in the release of exosomes into the extracellular space. MVBs are specialized organelles that originate from the inward budding of endosomal membranes, resulting in the formation of intraluminal vesicles (ILVs) within their lumen. These ILVs, which are the precursors of exosomes, accumulate within the MVBs until they are secreted via the fusion of MVBs with the plasma membrane. This fusion event allows the ILVs to be released as exosomes, enabling them to engage in various signaling cascades and facilitate the intercellular transport of diverse molecular cargo.20,21 Exosomes have emerged as a promising drug delivery vehicle in clinical therapy due to their low toxicity, low immunogenicity, and high biocompatibility. However, their biological toxicity is a critical factor ensuring their safety in therapeutic applications. For instance, although stem cell-derived exosomes can minimize the inherent drawbacks of their parent stem cells (such as tumorigenic complications and fusion toxicity), the potential pro-tumorigenic activity of these exosomes (eg, promoting angiogenesis) still warrants careful consideration. Excluding the carcinogenic effects of tumor-derived exosomes (or the pathogenic functions of bacteria-derived exosomes) is essential for their use as therapeutic vehicles.22–24
This review aims to further summarize the mechanisms underlying the increased production and release of exosomes in hypoxic conditions and their impact on the malignant biological behavior of tumors, as well as the effects of exosomes on tumor therapy. To address the biological characteristics of tumor-derived exosomes under hypoxic conditions, communication with other cells, clearance from circulation, and cargo loading capabilities, future research directions should be proposed.
Hypoxia Promotes Exosomes Release
The biogenesis of exosomes encompasses multiple steps and intricate cellular mechanisms. Initially, the endoplasmic reticulum and Golgi apparatus are responsible for synthesizing and modifying proteins and lipids, which serve as the cargo for future exosomes. The cell membrane forms endosomes through endocytosis, whereby various molecules and substances are encapsulated within vesicles and transported into the cell. These vesicles are known as early endosomes. Early endosomes undergo a series of transformations and maturation processes within the cell, gradually transitioning into late endosomes. During this process, inward budding of the endosomal membrane occurs, resulting in vesicles rich in specific proteins, lipids, and nucleic acids. Late endosomes gradually accumulate numerous inward-budding vesicles, forming multivesicular bodies (MVBs). These vesicles encapsulate substances destined for extracellular release. Upon maturation, MVBs migrate towards the cell membrane. Under the regulation of various proteins and signaling molecules, the MVBs fuse with the cell membrane. As the membranes of the MVBs and the cell fuse, the internal vesicles are released into the extracellular environment. These extracellular vesicles are exosomes, which contain a plethora of bioactive molecules including proteins, lipids, and nucleic acids such as mRNA and miRNA15,25,26 (Figure 1).
Tumor-related cells, due to their abnormal proliferation and increased intercellular communication behaviors, secrete more exosomes compared to normal cells.27,28 The hypoxic tumor microenvironment promotes the synthesis and release of tumor cell-derived exosomes. In common prostate cancer, ovarian cancer, and breast cancer cell lines, the concentration of exosomes in hypoxic conditions is significantly increased compared to normoxic conditions.20,29,30 Research also suggests that high oxygen levels can reduce the number of exosomes in colon cancer.31 Furthermore, exosomes derived from non-tumor cells in the tumor microenvironment can also increase in response to hypoxia.32 Studies in these two directions demonstrate the important role of hypoxia in increasing the number of exosomes in the tumor microenvironment due to intercellular information exchange and nutrient exchange. Mechanistically, research has further explored the molecular mechanisms underlying the increased synthesis of exosomes under hypoxia and the sorting, transport, and plasma membrane fusion of exosomal contents.
Hypoxia-inducible factors (HIFs) are a group of cell transcription factors induced by hypoxia. At the transcriptional level, HIFs mediate the reprogramming of cell transcriptomes to cope with decreased O2 availability. HIF1, the pivotal component of the HIF family, is a heterodimeric protein consisting of HIF1α and HIF1β subunits. In normoxic environments, HIF1α is rapidly degraded. Conversely, under hypoxic conditions, HIF1α evades degradation, translocates to the nucleus, and dimerizes with HIF1β, thereby activating the transcription of target genes involved in the cellular response to low oxygen levels.33 HIFs are directly and indirectly involved in the release of exosomes, with the increase in exosomes in various tumors, including breast cancer, colon cancer, and pancreatic cancer, under hypoxic conditions being linked to HIF-1α-mediated mechanisms.34–37 Additionally, under hypoxic conditions, the activation of HIF1 and pyruvate kinase M2 (PKM2) promotes glucose uptake and lactate production. HIF1 also enhances the expression of PKM2 mRNA, and PKM2, when upregulated and phosphorylated in tumors, can control the release of tumor-derived exosomes. In the process of exosome release, PKM2 in its phosphorylated state functions as a protein kinase, targeting synaptosomal-associated protein 23 (SNAP-23) for phosphorylation. This modification of SNAP-23 contributes to the assembly of the synaptosome/SNARE complex, which plays a crucial role in promoting the secretion of exosomes from the cell.38,39 HIF-1α can also interact with miR-210, affecting exosome release, demonstrated by the high levels of exosomes and miR-210 released by breast cancer cells in a HIF-1α-dependent manner under hypoxic conditions.30,40 SHP2, a widely expressed non-receptor protein tyrosine phosphatase in the cytoplasm, has been shown to interact with HIF.41 SHP2 negatively controls exosome synthesis by dephosphorylating tyrosine 46 of synthesized proteins.42 The protein phosphatase has important research prospects in regulating exosome release under hypoxic conditions through its interaction with HIF. Head and neck squamous cell carcinoma (HNSCC) is one of the most hypoxic tumor types.43 Research by Wang et al confirmed an increase in exosomes quantity in HNSCC cells under hypoxic conditions, attributed to the acidic environment induced by hypoxia impairing lysosomal function in HNSCC cells, leading to reduced EV degradation. Mechanistic investigations have uncovered that HIF-1α interacts with the promoter region of ATP6V1A, a subunit of the lysosomal v-ATPase complex. This interaction leads to an imbalance in lysosomal pH, causing impaired lysosomal function and a decrease in the fusion of lysosomes with multivesicular bodies (MVBs). As a consequence, the release of extracellular vesicles (EVs) is significantly enhanced.44
Rab proteins, a subset of the small GTPase Ras family, are positioned at distinct locations on the cytoplasmic face of the plasma membrane. These proteins play a crucial role in orchestrating various membrane trafficking routes within the cell.45 Under hypoxic conditions, tumor cells upregulate Rab27a, downregulate Rab7, LAMP1/2, NEU-1, and increase the release of exosomes significantly by promoting a more secretory lysosomal phenotype. Mechanistically, reducing the expression of STAT3 in tumor cells decreases exosome release by altering Rab family proteins Rab7 and Rab27a.20 The gene Rab22a can also have increased expression in a HIF-dependent manner, leading to an increase in microvesicle (MV) production. Wang et al confirmed through dual immunofluorescence localization that Rab22a protein is directly associated with budding MV in hypoxic cancer cells, further validating this process.35
GABARAPL1, belonging to the GABARAP family, participates in autophagy, receptor trafficking, and other cellular processes. It maintains cellular homeostasis and is crucial for releasing angiogenic exosomes under hypoxic conditions.46 Keulers et al found that GABARAPL1 is a key factor required for the secretion of exosomes and growth factors during hypoxia, with increased expression under severe hypoxia. Additionally, GABARAPL1 is necessary for sorting cargo into endosomes and facilitating endosome maturation. Upon silencing GABARAPL1, early endosomes enlarge, Rab7 activity decreases, endosomal pathway function is impaired, and cargo sorting into endosomes is reduced. Correspondingly, cells with silenced GABARAPL1 gene sort fewer protein cargoes into secretory exosomes.47 In a separate investigation, a lack of GABARAPL1 offset the impact of hypoxia on exosomal miRNA loading, resulting in reduced levels of miR-148b-3p, miR-24-3p, miR-130b-3p, miR-345-5p, and miR-101-3p within exosomes48 These miRNAs are known to promote cancer cell migration, invasion, and metastasis. The findings imply that exosomes derived from cells deficient in GABARAPL1 possess properties that hinder the metastatic potential of tumors.
The Role of Tumor Cell-Derived Exosomes Under Hypoxic Conditions
Every decade, top oncology scholars Hanahan D and Weinberg RA summarize the hallmarks of cancer, laying the foundation for future generations to study cancer. As we enter 2022, the hallmarks of cancer have been updated to include 14 categories. These include the ability to maintain continuous cell division, circumvent growth-limiting factors, and resist apoptosis. Additionally, cancer cells acquire the capacity for unlimited replication, stimulate the formation of new blood vessels, and initiate the processes of invasion and metastasis. Furthermore, they reprogram their energy metabolism, escape immune system surveillance, exhibit genomic instability and increased mutation rates, and foster inflammation that supports tumor growth. Moreover, cancer cells unlock phenotypic plasticity, undergo epigenetic changes independent of mutations, harbor a diverse microbiome, and evade immune-mediated destruction.49–51 In a hypoxic microenvironment, different tumor cells can secrete exosomes containing proteins, RNA, and lipids, which are taken up by various cells, triggering different cancer hallmarks.51 When tumor cell-derived exosomes in hypoxic microenvironment are taken up by other tumor cells in the microenvironment, they can promote malignant biological behaviors such as proliferation, migration, invasion, and metastasis.52 When taken up by endothelial cells, they can enhance angiogenesis, and when taken up by immune cells, they can promote macrophage polarization and create an immune-suppressive microenvironment.52 This section will detail how hypoxia-induced exosomes act on other cells to promote tumor progression (Figure 2).
Proliferation, Migration, and Invasion
Non-coding RNAs are a class of RNA molecules that do not have the ability to be translated into proteins, playing important regulatory roles within cells. Non-coding RNAs can regulate gene expression, cell signaling, and metabolic pathways through various mechanisms, participating in cellular differentiation, proliferation, and apoptosis. Among them, microRNAs, long non-coding RNAs, and circular RNAs are important representatives of non-coding RNAs.53–55 Numerous studies have shown that non-coding RNAs can enter exosomes and exert functional effects on target cells through the exosome pathway.21,56 In bladder cancer, hypoxia-derived exosomes containing lncRNA-UCA1 modulate the expression of EMT markers. These exosomes upregulate E-cadherin while downregulating vimentin and MMP9 in tumor cells. This alteration in gene expression induces EMT, leading to enhanced tumor cell proliferation, invasiveness, and migratory potential.57 In renal cell carcinoma (RCC), exosomes derived from hypoxic cancer cells contain lncHILAR, which can be internalized by normoxic RCC cells. Acting as a ceRNA, lncHILAR sponges miR-613/206/1-1-3p, resulting in the increased expression of Jagged-1 and CXCR4. The upregulation of the Jagged-1/Notch/CXCR4 signaling pathway promotes tumor cell invasion and metastasis under normoxic conditions.58 In pancreatic cancer, hypoxia-induced HIF1A activation upregulates circPDK1 expression in both cancer cells and their secreted exosomes. Exosomal circPDK1 functions as a miR-628-3p sponge, activating the BPTF/c-myc pathway and promoting aerobic glycolysis. Moreover, circPDK1 facilitates the interaction between UBE2O and BIN1, leading to UBE2O-mediated BIN1 ubiquitination and degradation, thus enhancing tumor cell proliferation.59 Pancreatic stellate cells (PSCs), a crucial component of the pancreatic cancer microenvironment, play a significant role in cancer progression60 PSC-derived exosomes containing miR-4465 and miR-616-3p target PTEN in pancreatic cancer cells, leading to the activation of the AKT pathway. This, in turn, enhances tumor cell proliferation, migration, and invasive potential.61
Moreover, in colorectal cancer (CRC), hypoxia-derived exosomes enhance tumor growth, motility, and invasiveness via multiple pathways. One mechanism involves hypoxic CRC cell-derived exosomes reducing mitotic duration, thereby accelerating cancer cell proliferation.62 Additionally, the activation of intracellular STAT3, which is associated with proliferation, is upregulated by hypoxia-induced exosomes in colon cancer cells.62 Furthermore, two different miRNAs in exosomes exert their effects through different pathways. In cells, miR-361-3p is significantly upregulated under the influence of HIF1α, and exosomal miR-361-3p enters tumor cells targeting TRAF3 protein to activate the non-canonical NF-κB2 signaling pathway, promoting tumor proliferation and reducing apoptosis.63 HIF1α also regulates the expression of miR-4299, and exosomes carrying miR-4299 target the tumor suppressor gene ZBTB4 in normoxic cells, thereby promoting colorectal cancer proliferation and metastasis.36 Exosomes released by glioma cells under hypoxic conditions carry miR-1246 and miR-10b-5p, targeting two tumor suppressor genes, FRK and FAP2A, to enhance neuroglial tumor migration and invasion.64 Neuroblastoma, a prevalent neuroectodermal malignancy in young children, can be influenced by hypoxic conditions. Exosomes secreted by hypoxic neuroblastoma cells contain miR-210-3p, which promotes tumor cell migration and invasion in vitro. Furthermore, these exosomes can induce the migratory and invasive potential of xenografted tumor cells in zebrafish embryo models.65 However, the specific mechanism by which miR-210-3p functions remains to be explored. In lung adenocarcinoma, hypoxic tumor cells secrete exosomes enriched with miR-31-5p, which targets SATB2 and activates the MEK/ERK pathway. This promotes cancer cell invasion and migration in vitro and enhances lung metastasis in vivo. Notably, exosomal miR-31-5p levels are significantly elevated in the blood of lung adenocarcinoma patients compared to healthy individuals, suggesting its potential as a diagnostic biomarker for this malignancy.66 Proteomic analysis of exosomes derived from hypoxic triple-negative breast cancer reveals increased protein abundance and diversity compared to exosomes from normoxic conditions, with a high enrichment of components involved in promoting invasion signaling pathways. Phenotypic analysis shows that exosomes from hypoxic conditions induce MDA-MB-231 cells under normoxic conditions to adopt a migratory phenotype, develop invasive pseudopods, and exhibit behaviors such as ECM degradation and matrix metalloproteinase (MMP) secretion to promote invasion.67 Unfortunately, this study did not analyze the key proteins within exosomes that mediate invasion, and the authors acknowledge certain limitations regarding the use of a single cell line in this research (Table 1) (Figure 3).
 |
Table 1 The Effect of Exosomes Under Hypoxic Conditions on Tumor Cell Proliferation, Invasion, and Migration |
Angiogenesis and Metastasis
Metastasis, the most lethal aspect of cancer, occurs when cancer cells proliferate in organs far from the primary tumor site. It is the leading cause of cancer-related deaths, rather than the primary tumor itself. The metastatic process involves a sequence of biological steps, during which primary tumor cells progressively gain the capacity to invade deeper tissues through the mucosa, disseminate via the bloodstream, lymphatic system, or direct infiltration of nearby structures, and finally colonize distant organs by proliferating anew. Each step is orchestrated by tumor cells adopting distinct phenotypic states and recruiting surrounding immune and stromal cells within the tumor microenvironment to support their growth and immune evasion.69–71
Judah Folkman’s seminal paper in 1971 revolutionized cancer research by suggesting that angiogenesis is essential for tumors to fulfill their growing oxygen and nutrient requirements.72 Tumor angiogenesis is a complex process involving the cooperation of multiple cell types within the tumor microenvironment (TME). These include vascular endothelial cells, which form tight junctions to maintain vascular integrity; perivascular cells, which cover the vessels and regulate vascular maturity; and bone marrow-derived progenitors, whose recruitment is influenced by hypoxic conditions.73,74 The hypoxic tumor microenvironment has been shown to promote angiogenesis, leading to tumor metastasis.75,76 Therefore, exosomes derived from hypoxic tumor cells also play a role in angiogenesis and metastasis. As early as 2010, Siu Kwan Sze et al discovered that under hypoxic conditions, tumor cells exhibit various phenotypes that promote metastasis, such as reduced adhesion, increased invasiveness, and enhanced angiogenic activity.77 Moreover, proteomic studies reveal that hypoxic tumor cells release proteins that participate in angiogenesis, focal adhesion formation, interactions between the extracellular matrix and cell surface receptors, and the recruitment of immune cells to the tumor site. Moreover, protein enrichment analysis of exosome-related proteins, including CD81, CD9, and Alix, extracted from the supernatant of cells centrifuged at high speeds, suggested for the first time that exosomes derived from hypoxic tumor cells may be involved in angiogenesis and metastatic potential, laying the foundation for subsequent comprehensive research.77 Furthermore, Mattias Belting et al revealed the presence of hypoxia-regulated mRNA and proteins (such as matrix metalloproteinases, IL-8, PDGF, caveolin 1, and lysyl oxidase) in exosomes derived from hypoxic glioblastoma multiforme (GBM) cells, with these proteins being associated with tumor angiogenesis and poor prognosis. Furthermore, exosomes secreted by hypoxic glioblastoma multiforme (GBM) cells can reprogram endothelial cells, inducing them to release a variety of growth factors and cytokines. This, in turn, activates the PI3K/AKT signaling pathway and promotes migration in nearby cells, ultimately increasing their vascular coverage.78
In addition to the mRNA and proteins mentioned in the above studies, miRNAs in exosomes derived from hypoxic glioblastoma cells also play a role in promoting angiogenesis. Under hypoxic conditions, the upregulation of transcription factor SOX9 in glioblastoma cells promotes the transcription of miR-204-3p. Concurrently, hypoxic conditions lead to increased SUMOylation of hnRNP A2/B1, facilitating its translocation from the nucleus to the cytoplasm. As a crucial factor in loading miR-204-3p into exosomes, hnRNP A2/B1 enables the transfer of this microRNA to endothelial cells. Once internalized, miR-204-3p promotes endothelial tube formation via the ATXN1/STAT3 signaling pathway.79 These findings shed light on the potential mechanisms underlying glioblastoma angiogenesis and suggest that the SUMOylation inhibitor TAK-981 may serve as a promising therapeutic option for this aggressive brain tumor. In oral squamous cell carcinoma, exosomes rich in miR-21 derived from low oxygen conditions significantly promote EMT, and the level of circulating exosomal miR-21 is closely associated with lymph node metastasis in OSCC patients, serving as a marker of poor prognosis.80 Additionally, exosomal miR-1825 from hypoxic conditions can promote angiogenesis by targeting TSC2 in endothelial cells to activate the mTOR pathway.81 Esophageal cancer, also a squamous cell carcinoma, shows enhanced effects on proliferation, migration, invasion, and tube formation of endothelial cells when exposed to exosomes derived from low oxygen conditions. Furthermore, exosomes from low oxygen conditions significantly enhance tumor growth and lung metastasis in a nude mouse model, although the specific mechanisms require further exploration.82 In nasopharyngeal carcinoma, exosomal MMP13 levels from hypoxic NPC cells increase in a HIF-1α-dependent manner, and exosomal MMP-13 enhances EMT levels in tumor cells, promoting tumor metastasis.83 Furthermore, exosomal miR-455 from hypoxic nasopharyngeal carcinoma cells can promote vascular permeability and angiogenesis by targeting the tight junction component ZO-1, contributing to nasopharyngeal carcinoma metastasis.84 Likewise, lung cancer-derived exosomes containing miR-23a can modulate vascular permeability and endothelial cell migration by targeting ZO-1. Additionally, exosomal miR-23a directly suppresses prolyl hydroxylase 1 and 2 (PHD1 and PHD2), resulting in the accumulation of HIF-1α in endothelial cells, which further promotes angiogenesis.85 This mechanism has been validated in multiple myeloma (MM), where exosomal miR-135b from hypoxic sources directly targets endothelial cells to inhibit factor inhibiting HIF-1 (FIH-1), enhancing HIF-1α expression and promoting angiogenesis.86 In pancreatic cancer, exosomes derived from hypoxic cells are enriched with the long non-coding RNA UCA1. Upon transfer to human umbilical vein endothelial cells (HUVECs), UCA1 sponges miR-96-5p, thereby derepressing its target gene AMOTL2 and activating the ERK1/2 pathway, ultimately promoting angiogenesis.87 Furthermore, hypoxic exosomes can directly deliver miR-30b-5p to endothelial cells, downregulating gap junction protein (GJA1) and enhancing tube formation and migration.88 Notably, serum levels of exosomal lncRNA UCA1 and miR-30b-5p are significantly elevated in pancreatic cancer patients compared to healthy individuals, suggesting their potential as biomarkers for early detection and diagnosis. Similarly, in hypoxic thyroid cancer, exosomal miR-181a promotes HUVEC proliferation and capillary-like network formation through a dual mechanism: downregulating DACT2 by targeting MLL3, and upregulating YAP and vascular endothelial growth factor (VEGF).89 In terms of promoting angiogenesis, there is a certain crosstalk between exosomes and autophagy. Increased expression of the LC3/GABARAP protein family member GABARAPL1 is essential for endosome maturation, cargo sorting, exosome secretion, and GABARAPL1-positive exosomes exhibit angiogenic properties. Targeting antibodies against GABARAPL1 may serve as a key to blocking exosome-mediated angiogenesis and inhibiting tumor metastasis47 (Table 2) and (Figure 3).
 |
Table 2 The Effect of Exosomes Under Hypoxic Conditions on Tumor Cell Metastasis |
Therapeutic Resistance
In the clinical treatment of tumors, besides surgical intervention, chemotherapy and radiotherapy are commonly used methods for the treatment of malignant tumors. They can be used individually or in combination to treat cancer. Chemotherapy involves the use of chemical drugs to kill tumor cells or prevent their growth. Chemotherapeutic drugs enter the patient’s bloodstream, reaching throughout the body to kill disseminated tumor cells and prevent or delay tumor recurrence and metastasis. On the other hand, radiotherapy uses high-energy radiation to irradiate the tumor area, causing destruction of tumor cells to achieve therapeutic goals. Radiotherapy is primarily used for localized treatment, aiming to reduce tumor volume, alleviate symptoms, and control tumor growth.90–93 Resistance to radiotherapy and chemotherapy is a major contributor to the poor prognosis observed in cancer patients. This section will focus on the role of hypoxia-derived exosomes in promoting tumor resistance to these therapeutic modalities (Figure 4).
Chemotherapy Resistance
Exosomes derived from hypoxic glioblastoma cells can enhance temozolomide resistance in glioblastoma by carrying miR-106a-5p. Mechanistically, temozolomide increases the levels of PTEN, Bax, and p53 while decreasing p-Akt levels in glioblastoma cells to inhibit tumor growth and promote apoptosis. However, the drug effects of temozolomide can be reversed by exosomes from hypoxic glioblastoma cells.94 Proteomic analysis of exosomes derived from ovarian cancer under hypoxic and normoxic conditions revealed that hypoxic exosomes carry a variety of proteins potentially related to drug resistance. Co-culturing experiments showed that hypoxic exosomes significantly reduce dsDNA damage in ovarian cancer cells and increase cell survival rates after cisplatin treatment.20 Hypoxic conditions enhance the secretion of exosomes from bone marrow-derived mesenchymal stem cells (BMSCs). RNA sequencing reveals that these exosomes are enriched with miR-140-5p and miR-28-3p. Acting in concert, these miRNAs target SPRED1, leading to the activation of the MAPK pathway and diminishing the sensitivity of multiple myeloma cells to bortezomib treatment.95 In pancreatic cancer, exosomes derived from hypoxic conditions exhibit multiple mechanisms inducing resistance to gemcitabine. Firstly, hypoxic exosomes carry circZNF91 into normoxic pancreatic cancer cells, competitively binding to miR-23b-3p to relieve the inhibition of the deacetylase enzyme Sirtuin1 (SIRT1). Elevated SIRT1 levels increase the deacetylation-mediated stability of HIF-1α, promoting glycolysis and gemcitabine resistance in pancreatic cancer cells.37 Additionally, exosomal lncROR derived from hypoxic conditions suppresses the activation of the Hippo/YAP pathway in pancreatic cancer cells, further contributing to gemcitabine resistance.96 Finally, hypoxic pancreatic stellate cells secrete exosomes containing lncRNA UCA1, which are taken up by pancreatic cancer cells. Once internalized, lncRNA UCA1 recruits EZH2 to the SOCS3 promoter region, leading to increased histone methylation and suppressed SOCS3 transcription. This ultimately contributes to enhanced gemcitabine resistance in pancreatic cancer.97
Radiation Resistance
Under hypoxic conditions, the highly expressed miR-301a in glioblastoma tumor tissues is released via exosomes and taken up by normoxic glioblastoma cells. MiR-301a specifically targets TCEAL7, which has been identified as a tumor suppressor in GBM malignancies. TCEAL7 negatively regulates the Wnt/β-catenin pathway activation by blocking the translocation of β-catenin from the cytoplasm to the nucleus. Furthermore, activation of the Wnt/β-catenin pathway reduces the sensitivity of glioblastoma to radiation therapy.98 Similarly, exosomal miR-340-5p derived from hypoxic esophageal squamous cell carcinoma enhances radioresistance by targeting KLF10/UVRAG. Interestingly, metformin can increase the expression of KLF10, thereby counteracting the radioresistance induced by exosomal miR-340-5p. This provides a potential combination therapy option of metformin with radiotherapy for improving radioresistance in OSCC.99 ANGPTL4 protein, a secreted glycoprotein, is widely considered a crucial regulator of angiogenesis and an inflammatory carcinogenic mediator. ANGPTL4 protein is enriched in hypoxic lung cancer tissues and exosomes derived from lung cancer cells, leading to radiotherapy resistance in lung cancer through two parallel mechanisms. Firstly, ANGPTL4 protein in hypoxic cells induces radioresistance in a GPX4-dependent manner, with GPX4 being a key protein in ferroptosis, a type of iron-dependent cell death linked to radioresistance. Secondly, uptake of hypoxic exosomal ANGPTL4 by normoxic cells promotes resistance to radiation therapy by enhancing GPX expression and inhibiting cell ferroptosis, thereby causing radioresistance in adjacent normoxic cells100 (Table 3).
 |
Table 3 The Effect of Exosomes Under Hypoxic Conditions on Tumor Treatment Resistance |
Changes in Tumor Immune Microenvironment
The complex interplay between tumor cells and the surrounding tumor microenvironment (TME) plays a crucial role in cancer progression. The TME is a multifaceted system primarily consisting of tumor cells, infiltrating immune cells (eg, macrophages, dendritic cells, and lymphocytes), cancer-associated stromal cells (eg, cancer-associated fibroblasts or CAFs), endothelial cells, adipocytes, extracellular matrix (ECM), and a variety of signaling molecules.101,102 Exosomes, as mediators of intercellular communication, not only deliver substances and information to tumor cells but also to other stromal cells in the TME. This chapter will review the impact of hypoxia-derived exosomes from tumor cells on other immune cells in the microenvironment (Figure 5).
Macrophages
Macrophages in tumors are important immune cells that play a crucial role in the development and progression of tumors. Macrophages can be divided into two different subtypes: M1 and M2. M1 macrophages are pro-inflammatory cells whose main function is to clear foreign bodies, pathogens, and abnormal cells in the tumor. They can release various inflammatory mediators, activate other immune cells, and promote the occurrence of anti-tumor immune responses. M1 macrophages play a crucial role in the early stages of tumors, inhibiting tumor progression. In contrast, M2 macrophages are anti-inflammatory cells whose main function is to promote the resolution and repair of inflammation. They can secrete growth factors and cytokines, promoting the proliferation of tumor cells and angiogenesis, thereby facilitating tumor growth and metastasis. M2 macrophages play a major role in the late stages of tumors, promoting tumor growth and metastasis.103–105 Exosomes in the hypoxic microenvironment have a dual role in regulating macrophage polarization, closely related to the different contents in different types of tumors. In hypoxic glioblastoma, the high expression levels of NEDD4L can inhibit the activation of the PI3K/AKT pathway by increasing the ubiquitination degradation of PIK3CA. Exosomal miR-10b-5p can target NEDD4L, activating the PI3K/AKT pathway and leading to increased polarization of M2 macrophages.106 Furthermore, exosomal IL-6 and miR-155-3p carried in hypoxic exosomes induce macrophage autophagy by activating the STAT3 pathway, resulting in an increased polarization of M2 macrophages.107 Lastly, exosomal miR-1246 targets TERF2IP to activate the STAT3 signaling pathway and inhibit the NF-κB signaling pathway, mediating the polarization of M2 macrophages induced by hypoxic exosomes.108 In lung cancer, exosomes under hypoxic conditions can carry various miRNAs to induce macrophage polarization towards the M2 phenotype. For example, exosomal miR-103a targets PTEN to activate the classical AKT-STAT3 pathway, exosomal miR-1290 induces M2 macrophage polarization by activating the SOCS3/STAT3 pathway in macrophages, and exosomal miR-21 promotes M2 polarization in macrophages by targeting IRF1, thereby promoting tumor progression.109–111 Additionally, exosomes from hypoxic lung cancer cells can directly carry PKM2 to induce M2 polarization in macrophages by activating the AMPK pathway.108 Overexpression of miR-101 can polarize macrophages towards a pro-inflammatory state by targeting CDK8. However, under hypoxic conditions, the decreased levels of miR-101 in exosomes derived from lung cancer fail to suppress CDK8, preventing macrophages from polarizing towards a pro-inflammatory phenotype, thus leading to increased tumor immune evasion.112 Hypoxic conditions lead to a significant enrichment of miR-301a-3p in both pancreatic cancer cells and their secreted exosomes. The levels of circulating exosomal miR-301a-3p in patients strongly correlate with the extent of pancreatic cancer invasion, lymph node metastasis, TNM stage, and poor prognosis. Once internalized by macrophages, hypoxic exosomal miR-301a-3p promotes their polarization towards an M2 phenotype by activating the PTEN/PI3Kγ signaling pathway, ultimately facilitating pancreatic cancer metastasis.113 In epithelial ovarian cancer (EOC), hypoxia induces the exosomal transfer of three miRNAs (miR-21-3p, miR-125b-5p, and miR-181d-5p) to macrophages. These miRNAs target SOCS4/5, promoting STAT3 phosphorylation and driving macrophage polarization towards an M2 phenotype, which enhances EOC cell proliferation and migration.114 Similarly, in hepatocellular carcinoma (HCC), hypoxia-induced HIF-1α binds to the promoter of lncRNA HMMR-AS1, increasing its transcription and exosomal secretion. Once taken up by macrophages, exosomal HMMR-AS1 sponges miR-147a, preventing ARID3A degradation and promoting M2 polarization, thereby accelerating HCC progression.115
T Cells
In the tumor microenvironment, there are various types of T cells, including cytotoxic T cells (Tc cells) responsible for attacking and killing tumor cells, helper T cells (Th cells) that activate and regulate the activities of other immune cells to promote the generation of immune responses, and regulatory T cells (Treg cells) that suppress excessive immune responses to prevent the immune system from attacking normal tissues. In tumors, tumor cells can escape T cell attacks through various mechanisms, leading to immune evasion and tumor development, with exosomes playing a significant role in this process. For example, tumor-derived exosomes can promote CD8+ T cell exhaustion or induce Treg cell differentiation to advance tumor progression.116–118The impact of exosomes on T cells in hypoxic environments requires further investigation. Nasopharyngeal carcinoma-derived exosomal miR-24-3p directly targets FGF11, increasing the expression of P-ERK, P-STAT1, and P-STAT3 while decreasing P-STAT5 levels. This inhibits T cell proliferation, Th1 and Th17 differentiation, and Treg cell induction. Hypoxia further enhances the inhibitory effect of these exosomes on T cell proliferation and differentiation by increasing cellular and exosomal miR-24-3p levels.119 γδ T cells, a unique T cell subset with either antitumor or protumor functions depending on the cancer type, can also be influenced by exosomes. In hypopharyngeal squamous cell carcinoma, exosomal miR-21 targets PTEN in myeloid-derived suppressor cells (MDSCs), upregulating PD-L1 expression and strengthening the inhibitory effect on γδ T cells.120
Mdsc
MDSCs primarily impact tumor immune responses in two ways: first, by inhibiting the activity of T cells (as mentioned earlier); and second, by promoting tumor-related inflammation and angiogenesis. MDSCs can enhance the production of tumor-related inflammatory factors and angiogenic factors, creating a suitable microenvironment for tumor growth and spread.121 Exosomes released by hypoxic glioblastoma cells can carry miR-10a and miR-21 to target RORA and PTEN, respectively, activating the IκBα/NF-κB and PI3K/AKT pathways to induce the expansion and activation of MDSCs, thereby promoting tumor progression122(Table 4).
 |
Table 4 The Effect of Exosomes Under Hypoxic Conditions on Immune Cells |
Activate Cancer Associated Fibroblasts
Tumor-associated fibroblasts are a type of cell present in the tumor microenvironment that plays a crucial role. Compared to normal fibroblasts, tumor-associated fibroblasts exhibit differences in morphology, function, and expression characteristics. Tumor-associated fibroblasts are typically involved in regulating processes such as tumor growth, metastasis, angiogenesis, and drug resistance.124,125 Exosomes derived from hypoxic head and neck squamous cell carcinoma (HNSCC) cells are enriched with miR-192 and miR-215. These microRNAs target Caveolin-1, suppressing TGF-β/SMAD signaling and driving the differentiation of normal fibroblasts into cancer-associated fibroblasts (CAFs).126 Moreover, HIF1α enhances miR-21 transcription and facilitates the secretion of exosomes from HNSCC cells. These hypoxic tumor cell-derived exosomes, which are enriched with miR-21, promote the transformation of normal fibroblasts into CAFs by targeting YOD1.127 Proteomic analysis of exosomes derived from normoxic and hypoxic conditions revealed the highest increase in LOXL2 levels. Exosomes derived from hypoxic cells promote the formation of pre-metastatic niches through a dual mechanism. Firstly, hypoxia-derived exosomes carry a significant amount of LOXL2 to non-hypoxic HNSCC cells, inducing epithelial-mesenchymal transition and promoting cancer cell invasion.128 Additionally, these LOXL2-enriched exosomes are internalized by distant fibroblasts, activating the FAK/Src signaling pathway. The resulting increase in fibronectin production, mediated by the FAK/Src pathway, facilitates the recruitment of bone marrow-derived suppressor cells, contributing to the formation of pre-metastatic niches.128 In addition, exosomes derived from hypoxic liver cancer cells activate lung fibroblasts and promote the formation of pre-metastatic niches. Mechanistically, exosomal miR-4508 targets RFX1 to activate the p38 MAPK-NF-κB signaling pathway, inducing fibroblast activation and PMN formation.129
The Effect of Exosomes Derived from Other Stromal Cells on Tumors Under Hypoxic Conditions
In the hypoxic conditions of tumors, exosomes originating from other components of the TME play a crucial role in tumor progression. In this chapter, we will summarize the regulatory effects of exosomes derived from non-cancer cells within hypoxic conditions (Figure 5).
CAFs
CAFs are the most prevalent stromal cells within the TME and significantly contribute to tumor initiation, progression, and metastasis. The dysregulation of various genes in CAFs, such as the Ataxia Telangiectasia Mutated (ATM) gene, can influence cancer progression. Under hypoxic conditions, oxidized ATM is activated in breast cancer CAFs, promoting abnormal proliferation of breast CAFs. Oxidized ATM can phosphorylate BNIP3 and ATP6V1G1 in hypoxic breast CAFs, inducing autophagosome accumulation and leading to lysosomal dysfunction, as well as fusion of autophagosomes with multivesicular bodies (MVBs) instead of lysosomes to facilitate exosome release. Released exosomes from hypoxic CAFs are enriched with autophagy-related GPR64, which activates the atypical NF-κB signaling pathway, upregulating MMP9 and IL-8 expression in recipient breast cancer cells, enhancing their invasive capabilities.130 In colorectal cancer, hypoxia-treated CAFs secrete more exosomes and circEIF3K. In vitro and in vivo experiments showed that knockdown of circEIF3K in exosomes derived from CAFs led to reduced proliferation of CRC cells compared to CRC cells treated with exosomes from control group. Mechanistically, the phenomenon of CAF-promoted malignant biological behavior of tumor cells under hypoxic conditions mediated by circEIF3K can be rescued by miR-214, and PD-L1 is a potential target of the circEIF3K/miR-214 axis in exerting these effects.131
Stem Cells
Stem cells are a type of cells with self-renewal and differentiation capabilities, continuously producing new cells and possessing potential pluripotency, meaning they can differentiate into various cell types. In the TME, two types of stem cells cannot be overlooked: tumor stem cells and mesenchymal stem cells. You et al found that under hypoxic conditions, glioma stem cells upregulate the transcription of miR-30b-3p via HIF1α and STAT3, which can bind to hnRNPA2B1 and promote its transfer to exosomes. Exosomal miR-30b-3p directly targets RHOB in glioma cells, leading to reduced apoptosis and increased proliferation in vitro and in vivo, as well as increased chemoresistance in gliomas.132 Xiong et al found that under hypoxic conditions, glioma stem cells transfer Linc01060 to glioma cells via exosomes. Linc01060 directly interacts with and stabilizes MZF1, promoting its ability to enhance c-Myc transcription. In turn, c-Myc increases HIF1α accumulation post-transcriptionally. HIF1α binds to the hormone response element in the Linc01060 promoter, upregulating its transcription and forming a positive feedback loop. Clinically, Linc01060 is overexpressed in gliomas and strongly correlates with tumor grade and poor prognosis. Serum levels of exosomal Linc01060 are also associated with tumor progression.133 Exosomes from hypoxia-treated mesenchymal stem cells significantly increase the proliferation, survival, invasiveness, and EMT of non-small cell lung cancer cells, as well as M2 polarization of macrophages, with exosomal miR-21-5p mainly acting by downregulating PTEN, PDCD4, and RECK gene expression.134 Likewise, the exosomal transfer of miR-193a-3p, miR-210-3p, and miR-5100 from mesenchymal stem cells to lung cancer cells can induce epithelial-mesenchymal transition (EMT) by activating the STAT3 signaling pathway, thereby promoting lung cancer invasion. These findings suggest that the aforementioned exosomal miRNAs may serve as potential non-invasive biomarkers for metastatic lung cancer.135 Furthermore, exosomes from mesenchymal stem cells also exhibit anti-cancer effects, as hypoxia-induced HIF1α in mesenchymal stem cells increases the level of microRNA let-7f and promotes cell autophagy, leading to increased motility and invasion abilities of mesenchymal stem cells. Notably, hypoxic conditions lead to an increase in let-7f levels within exosomes derived from mesenchymal stem cells. When these exosomes are internalized by recipient 4T1 tumor cells, the mesenchymal stem cell-derived exosomal let-7f significantly suppresses tumor cell proliferation and invasion.136 However, the precise mechanisms underlying this effect require further investigation.
Immune Cells
Contrary to the tumor-derived exosomes promoting M2 polarization of macrophages and expansion of MDSCs mentioned in the previous chapter, MDSCs and M2-polarized macrophages also release exosomes to promote tumor progression. Under hypoxic conditions, MDSCs promote exosome release in a HIF1α-dependent manner. Exosomal S100A9 enhances the stemness and growth of colorectal cancer cells, and the plasma levels of exosomal S100A9 in colorectal cancer patients are significantly higher than in healthy individuals, making exosomal S100A9 a potential biomarker for diagnosing colorectal cancer.31 Excessive expression of Hif-1α in macrophages under low oxygen conditions leads to its direct binding to different regions of the Hsp90 promoter, resulting in elevated levels of Hsp90 protein both intracellularly and in exosomes. The crucial interaction between exosomal Hsp90 and colorectal cancer cell Lats1 leads to Lats1 inactivation, inhibiting Yap phosphorylation and ultimately inactivating the Hippo signaling pathway to promote colorectal cancer progression.137 Furthermore, under low oxygen conditions, exosomal miR-155-5p from macrophages can directly interact with the HuR protein in renal cell carcinoma cells, increasing the stability of IGF1R mRNA and activating the PI3K/AKT pathway to promote the progression of renal cell carcinoma.138 With a variety of immune cell types, the direct or indirect effects of exosomes from other immune cells on tumors remain an important direction for further exploration.
Clinical Application
Liquid biopsy has emerged as a groundbreaking approach in cancer diagnosis and prognosis prediction, offering a minimally invasive and more easily implemented alternative to traditional tissue biopsies. In recent years, the use of liquid biopsy in cancer treatment has garnered significant attention. This technique can be applied to various body fluids, including blood, urine, saliva, and cerebrospinal fluid. Among these, biomarkers isolated from plasma and serum in peripheral blood have shown promising potential for cancer detection due to their ease of acquisition.139 For blood samples, common biomarkers include circulating tumor cells (CTCs) and CTC clusters, cell-free DNA (cfDNA) or circulating tumor DNA (ctDNA), extracellular vesicles (EVs), and circulating non-coding RNAs. Among them, the detection of blood exosomes as a liquid biopsy approach has its special advantages. Firstly, exosomes not only contain DNA but also a variety of proteins, RNAs, carbohydrates, and lipids. Additionally, exosomes are detectable in almost all body fluids, making them easily accessible. This makes exosomes an ideal biomarker for monitoring the dynamic occurrence and progression of tumors, providing valuable clinical information for diagnosis and prognosis prediction. Many studies have shown exosomes and their contents to be highly effective biomarkers for cancer diagnosis and treatment. For example, in 2016, ExoDx™ Lung (ALK) was proposed for the first time. This method isolates and analyzes exosomal RNA from blood samples, enabling the detection of EML4-ALK mutations in NSCLC patients with a sensitivity of 88% and specificity of 100%. It is a more direct and sensitive method for gene fusion detection compared to cfDNA. The heterogeneity of exosomes, including their size, origin, and the diversity of their cargo, poses challenges for their use in liquid biopsy for cancer diagnosis and prognosis. However, exosome barcoding and single exosome profiling aid in overcoming these limitations and play a significant role in precision oncology. Exosome barcoding technology attaches unique molecular labels (such as DNA barcodes, RNA barcodes, or protein tags) to the surface of each exosome, giving them a distinct “identity marker”. These labels can be detected and analyzed using high-throughput sequencing or other molecular biology techniques. Zhang et al developed a method utilizing surface-enhanced Raman spectroscopy (SERS) nanotags, enabling high-sensitivity, multiplexed analysis of multiple protein biomarkers on cancer-derived small extracellular vesicles (EVs) in a single test through a sandwich immunoassay without complex separation steps. This allows for non-invasive cancer diagnosis and monitoring of therapeutic responses.140 Similarly, Zhuang et al fabricated an innovative sandwich-type biosensor utilizing amino-functionalized Fe3O4 nanoparticles and two-dimensional MXene nanosheets, achieving highly sensitive detection of exosomes.141 SORTER, a dual-surface protein-guided orthogonal recognition and microRNA in situ analysis technology for tumor-derived exosomes, enhances the diagnostic accuracy of tumors by detecting exosomal miRNA. SORTER employs two aptamers targeting exosomal marker CD63 and tumor marker EpCAM to create orthogonal barcode tags, enabling selective classification of tumor-specific exosome subtypes.142 Additionally, the nucleic acid lateral flow assay (NALFA) of DNA barcodes, combined with stem-loop primer reverse transcription, generates a colorimetric signal only in the presence of target exosomal miRNA. This system successfully detected overexpressed miR-92a and miR-141 in CRC exosomes, with sensitivity and specificity reaching 95.24% and 100.0%, respectively.143 Single exosome analysis is an avant-garde technique aimed at providing detailed molecular and functional analysis of individual exosomes. This method overcomes the limitations of traditional bulk exosome analysis, offering more precise and profound biological insights. Proximity Barcode Analysis (PBA) is used for the protein analysis of single exosome surfaces. This technique combines antibody-DNA conjugates with next-generation sequencing technology, enabling the identification and quantification of surface protein compositions from exosomes of different origins, thereby distinguishing and quantifying specific tissue-derived exosomes in mixed samples.144 Song et al developed an integrated microfluidic platform consisting of a single-cell capturing chip and a spatially encoded antibody barcode chip for multiplex profiling of single-cell exosomes. Using this platform, they simultaneously analyzed five phenotypic exosomes from over 1000 single cells and inflammatory factor secretion from the same single cells, identifying a special functional subpopulation of ovarian tumor cells with unique phenotypic exosomes (HSP70+, EPCAM+).145 Furthermore, through single exosome analysis, Guo et al discovered two differentially abundant exosome subsets in colorectal cancer: ITGB3+ and ITGAM+ exosome subpopulations. In vitro and in vivo experiments demonstrated that ITGB3+ exosomes promote CRC proliferation, migration, and invasion, whereas ITGAM+ exosomes inhibit CRC development.146 While multicenter, large-scale trials focusing on hypoxia-derived exosomes are currently lacking, there is still great clinical potential for hypoxia-derived exosomes.
Kucharzewska et al discovered that hypoxia-derived exosomes can reflect the tumor status of glioblastoma (GBM). In hypoxic experiments with glioma cells in vitro and patient samples, exosomes were found to be rich in hypoxia-regulated matrix metalloproteases, IL-8, PDGFs, caveolin-1, and lysyl oxidase. Furthermore, hypoxia-derived exosomes from GBM cells can modulate endothelial cells to secrete various potent growth factors and cytokines, stimulating the activation and migration of pericytes through PI3K/AKT signaling, enhancing the induction of tumor vascularization, pericyte coverage, and GBM cell proliferation. By monitoring exosomes, the hypoxic state of GBM, as well as the tumor’s invasiveness and prognosis, can be closely observed.78 The potential of hypoxia-derived exosomes in cancer diagnosis and treatment can be further enhanced by combining them with other materials engineering approaches. One such approach is magnetic particle imaging (MPI), an emerging tracking imaging technology that utilizes superparamagnetic iron oxide (SPIO) nanoparticles as tracers. This method enables close monitoring of in vivo tumor hypoxia models.147 Jung et al developed an exosome platform for targeted therapy of tumor hypoxic regions. They modified exosomes derived from hypoxic breast cancer cells to carry superparamagnetic iron oxide (SPIO) nanoparticles and Olaparib (a PARP inhibitor). By labeling the exosomes with a lipophilic fluorescent tracer DiO and monitoring in vivo using MPI, they found that hypoxic cells preferentially took up exosomes released by hypoxic cells. Furthermore, Olaparib-loaded exosomes increased tumor cell apoptosis and slowed tumor growth in vivo, demonstrating their therapeutic efficacy. This method demonstrates the possibility of monitoring hypoxia-derived exosomes and delivering therapeutic drugs through them.148 Detecting hypoxia-derived exosomes to reflect tumor progression, improve the tumor microenvironment’s hypoxic state, and achieve antitumor effects is a highly promising research direction in this field. Additionally, as previously mentioned, hypoxia-derived exosomes from non-tumor cells, such as immune cell-derived exosomes, are also worth investigating. Combining engineering methods with hypoxia-derived exosomes to improve the tumor immune microenvironment and exert antitumor effects is another promising direction. There is great potential for research on how to effectively load drugs into exosomes, enhance the stability of exosomes, ensure a longer half-life, and improve specificity and targeting.
Exosomal DNA, as a novel intercellular communication molecule, has been extensively studied and has demonstrated significant clinical therapeutic and diagnostic potential. Exosomal DNA primarily comprises linear double-stranded DNA (dsDNA), mitochondrial DNA (mtDNA), and circular DNA. These DNA molecules can span the entire genome, covering all chromosomes and even including mutated genes. As a non-invasive biomarker, exosomal DNA holds potential clinical diagnostic value. Through liquid biopsy to detect exosomal DNA in the blood, it is possible to achieve early detection and monitoring of cancer and other diseases.149,150 Franz L. Ricklefs et al, utilizing super-resolution single EV microscopy, have demonstrated that DNA is encapsulated within EVs secreted by glioblastoma cells. Methylation array analysis and targeted next-generation sequencing (NGS) revealed that EV-associated DNA mirrors the comprehensive mutation landscape and copy number variations (CNV) present in the parental glioblastoma cells as well as the primary tumor. By examining EV-DNA derived from glioblastoma cells, it is possible to accurately determine the tumor’s methylation classification and the methylation status of the MGMT promoter.151 This renders exosomal DNA a potent tool for classifying brain tumors. Functionalized DNA engineered into hinges enables the anchoring of quantum dots (QDs) onto the exosomal surface, thereby achieving a moderately bio-compatible labeling strategy. Exosomes labeled with QDs (exosome-DNA-QD complexes) can be rapidly internalized by tumor cells, indicating their potential use as a specific reagent for tumor marking.152 Moreover, exosome-packaged DNA has exhibited advantages in tumor therapy. Bovine colostrum exosomes and polyethyleneimine matrix (EPM) can deliver small interfering RNA (siRNA) or plasmid DNA (pDNA) to achieve effective gene therapy.153 The advent of nano-flow cytometry (nFCM) allows for the detection of single EVs as small as 40 nm in diameter and individual DNA fragments of 200 bp after SYTO 16 staining, facilitating the study of EV-DNA within individual vesicles.154 In the realm of hypoxia, research related to exosomal DNA remains in its nascent stages, paving the way for future advancements in understanding tumorigenesis mechanisms and developing diagnostic and therapeutic strategies under hypoxic conditions.
Common Exosome Isolation Methods
There is an increasing focus on the role of exosomes in tumor diagnosis and therapy, and their isolation and purification methods are being progressively proposed by numerous researchers. The bioactivity of exosomes largely depends on the method of isolation. Ultracentrifugation is the most commonly used method for exosome extraction, considered the “gold standard” for exosome isolation.155 By adjusting centrifugation parameters, it effectively removes dead cells, cellular debris, and apoptotic bodies, allowing for the acquisition of large-scale samples without the need for complex sample preparation.156 Furthermore, using density gradient media (such as sucrose or iodixanol) in conjunction with ultracentrifugation can enhance the purity of exosome isolation.157 Ultrafiltration is another method that separates exosomes by size, utilizing extremely small pores (diameter≈100 nm).158 This method increases the efficiency of exosome separation compared to ultracentrifugation, but it may damage exosomes due to shear stress or cause exosome loss due to membrane clogging from membrane adhesion and particle aggregation.159 The precipitation method, using polymers like polyethylene glycol to bind water molecules and achieve precipitation, is also employed for exosome isolation. Numerous commercial kits based on this principle are widely used.160 Currently, various engineering approaches such as microfluidics and chromatography are also extensively applied for exosome isolation.161 The advantages and disadvantages of these methods are briefly summarized in the Table 5 within this paper. Many studies have compared these methods for exosome extraction and isolation, yet optimal protocols may vary depending on specific research requirements. Through technological advancements, the challenges in exosome extraction and isolation are gradually being addressed, necessitating further attention from researchers.162
 |
Table 5 Common Exosome Isolation Methods and Their Advantages and Disadvantages |
Summary and Outlooks
The tumor microenvironment, as a complete system, consists of various components that interact with each other to collectively drive tumor adaptive changes. Hypoxia, as a characteristic of the tumor microenvironment, leads to epigenetic, immunometabolic, and other changes in multiple components of the tumor microenvironment, thereby promoting tumor initiation and progression. Under hypoxic conditions, various signaling transduction factors and pathways, including HIF-1, PI3K-mTOR, and NF-κB, undergo sequential alterations, influencing processes such as cell metabolism, cell growth/death, cell proliferation, glycolipid metabolism, immune responses, and more. With increased intercellular communication and material exchange, exosomes play a crucial role as key mediators in the communication of multiple components in the tumor microenvironment under hypoxic conditions. Numerous studies have shown that hypoxia promotes the synthesis and release of exosomes from tumor cells, stromal cells, and immune cells, with their contents significantly contributing to intercellular communication, exacerbating the overall malignant behaviors of the tumor microenvironment. This is manifested by increased tumor proliferation, migration, invasion abilities, angiogenesis, and metastatic capabilities, as well as resistance to systemic anti-tumor therapies such as chemotherapy and radiotherapy. As research in this field progresses, utilizing exosomes and engineered exosomes as targets for tumor therapy in hypoxic environments shows promising research prospects and has the potential for clinical translation.
However, there are currently many limitations in the research in this field. Firstly, the investigation of the role of hypoxia and HIF-1, HIF-2 in the tumor microenvironment has not received enough attention from researchers. Most studies explore tumor cells, immune cells, stromal cells, etc. in the tumor microenvironment under normoxic conditions in in vivo and in vitro models, neglecting the crucial influence of hypoxia. In particular, the impact of HIF factors on various signaling pathways should not be overlooked as a factor in studying the mechanisms of tumor progression.171 Artificial inhibitors or natural inhibitors of HIF factors have great potential as therapeutic agents for improving malignant tumors, and there are many clinical trials in this field that can be found on the ClinicalTrials website. Furthermore, exosomes, with their ability to deliver cargo to target cells, can produce therapeutic effects by blocking or inhibiting them, thereby improving the hypoxic characteristics of the tumor microenvironment and exerting anti-tumor effects.27 This aspect also deserves further attention from researchers. Additionally, improving exosome-based therapeutic approaches through materials engineering can enhance the clinical translation of this treatment strategy, addressing issues such as drug loading, therapeutic efficacy, and treatment plan stability. For example, Creeden et al developed “ExoSmart”, engineered exosomes displaying both RGD and CD47p110-130. ExoSmart binds to αvβ3 on pancreatic ductal adenocarcinoma (PDAC) cells, increasing cellular uptake. CD47p110-130 interacts with signal-regulatory protein alpha (SIRPα) on macrophages, inhibiting phagocytosis and significantly reducing the clearance rate of exosomes by the liver and spleen. In therapy, this approach significantly improves the effectiveness of chemotherapy for PDAC.172
Overall, exosomes derived from hypoxic conditions exhibit numerous advantages and disadvantages. 1. Under hypoxic conditions, the expression patterns of biomolecules such as proteins, mRNA, and miRNA within exosomes undergo significant changes. These alterations can serve as specific biomarkers for disease diagnosis and prognosis assessment. Given that many tumor environments are hypoxic, exosomes originating from such conditions can provide valuable insights into the tumor microenvironment, aiding in the identification and progression of tumor types. 2. Owing to their inherent cellular membrane structure, exosomes can protect encapsulated drugs and nucleic acids from degradation within the body. Exosomes derived from hypoxic conditions can serve as drug delivery vehicles, targeting and delivering specific therapeutic substances to diseased tissues. 3. Exosomes under hypoxic conditions contain immunomodulatory molecules that can regulate immune responses. For instance, within the tumor microenvironment, these exosomes can influence the immune escape mechanisms of tumors by modulating immune cell activity, potentially becoming a target for combinatorial immunotherapy. Conversely, the drawbacks include: 1. The molecular composition and function of hypoxia-derived exosomes are highly heterogeneous, posing challenges for standardization and consistency in research and clinical applications. This heterogeneity may lead to poor reproducibility of experimental results. 2. The separation and purification of hypoxia-derived exosomes are complex and time-consuming since they are often mixed with other types of exosomes and cellular debris. Current isolation techniques, such as ultracentrifugation and immunocapture, have limited efficiency, making it difficult to obtain highly pure exosomes. 3. Although hypoxia-derived exosomes exhibit numerous potential biological functions and applications, standardized methods to validate these functions have yet to be established, limiting their clinical application. 4. Culturing cells under hypoxic conditions and isolating exosomes is costly. Moreover, the storage and transportation of exosomes require special conditions, increasing their economic burden. 5. While exosomes are considered to possess good biocompatibility as natural nanocarriers, their immunogenicity and potential toxicity need further investigation, particularly concerning long-term application and high-dose usage.
In conclusion, research in this field is fascinating and holds great potential to advance the current landscape of studies on the tumor microenvironment, providing potential strategies for clinical treatment. Incorporating hypoxic conditions and the downstream pathway changes into the research vision can deepen researchers’ understanding of the mechanisms of tumor development. Additionally, combining the hot research topic of exosomes to specifically study intercellular communication and material transfer in the hypoxic tumor microenvironment interactions can help researchers explore the mechanisms in this field and provide more powerful solutions for clinical treatment translation.
Acknowledgments
Thank you very much Figraw for providing beautiful illustrations in this review.
Author Contributions
Each contributor played a pivotal role in the research presented herein, be it through conceptualization, experimental design, implementation, data collection, analysis, interpretation, or a combination thereof. All authors participated in the drafting process, either by composing the initial manuscript, revising its content, or providing critical feedback. Moreover, they have unanimously approved the final version intended for publication and concur with the choice of journal to which the article has been submitted. Furthermore, the authors collectively accept responsibility for all facets of the work. Churan Wang was primarily responsible for the written content and visual elements of the manuscript, while Shun Xu and Xiao Yang contributed conceptually and conducted thorough reviews and revisions of the text.
Disclosure
These authors declare that they have no conflicts of interest in this work.
References
1. L HARRISA. Hypoxia--a key regulatory factor in tumour growth [J]. Na Revi Can. 2002;2(1):38–47. doi:10.1038/nrc704
2. L CODONYV, Tavassoli M. Hypoxia-induced therapy resistance: available hypoxia-targeting strategies and current advances in head and neck cancer [J]. Transl Oncol. 2021;14(3):101017. doi:10.1016/j.tranon.2021.101017
3. WOUTERS A, PAUWELS B, LARDON F, et al. Review: implications of in vitro research on the effect of radiotherapy and chemotherapy under hypoxic conditions [J]. Oncologist. 2007;12(6):690–712. doi:10.1634/theoncologist.12-6-690
4. WU Q, L YOU, NEPOVIMOVA E, et al. Hypoxia-inducible factors: master regulators of hypoxic tumor immune escape [J]. J Hemat Oncology. 2022;15(1):77.
5. ABOU KHOUZAM R, JANJI B, THIERY J, et al. Hypoxia as a potential inducer of immune tolerance, tumor plasticity and a driver of tumor mutational burden: impact on cancer immunotherapy [J]. Semi Can Bio. 2023;97:104–123. doi:10.1016/j.semcancer.2023.11.008
6. WIGERUP C, Påhlman S, BEXELL D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer [J]. Pharm Therape. 2016;164:152–169.
7. LEE P, S CHANDELN, C SIMONM. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond [J]. Nat Revi Mole Cell Biol. 2020;21(5):268–283. doi:10.1038/s41580-020-0227-y
8. W ALTAMEEMI, P DALET, K AL-JUMAILYRM, et al. Hypoxia-Modified Cancer Cell Metabolism [J]. Front Cell Devel Bio. 2019;7:4. doi:10.3389/fcell.2019.00004
9. ZHENG X, Weigert A, S REU, et al. Spatial Density and Distribution of Tumor-Associated Macrophages Predict Survival in Non-Small Cell Lung Carcinoma [J]. Cancer Rese. 2020;80(20):4414–4425. doi:10.1158/0008-5472.CAN-20-0069
10. YANG M, MCKAY D, W POLLARDJ, et al. Diverse Functions of Macrophages in Different Tumor Microenvironments [J]. Cancer Rese. 2018;78(19):5492–5503. doi:10.1158/0008-5472.CAN-18-1367
11. J BOUTILIERA, F ELSAWAS. Macrophage Polarization States in the Tumor Microenvironment [J]. Inter J Mole Scien. 2021;22(13). doi:10.3390/ijms22136995
12. J GAO, LIANG Y, WANG L. Shaping Polarization Of Tumor-Associated Macrophages In Cancer Immunotherapy [J]. Front Immuno. 2022;13:888713. doi:10.3389/fimmu.2022.888713
13. J WEI, CHEN Z, HU M, et al. Characterizing Intercellular Communication of Pan-Cancer Reveals SPP1+ Tumor-Associated Macrophage Expanded in Hypoxia and Promoting Cancer Malignancy Through Single-Cell RNA-Seq Data [J]. Front Cell Devel Bio. 2021;9:749210. doi:10.3389/fcell.2021.749210
14. KUMAR A, DEEP G. Hypoxia in tumor microenvironment regulates exosome biogenesis: molecular mechanisms and translational opportunities [J]. Cancer Letters. 2020;479:23–30. doi:10.1016/j.canlet.2020.03.017
15. KALLURI R, S LEBLEUV. The biology, function, and biomedical applications of exosomes [J]. Science. 2020;367(6478). doi:10.1126/science.aau6977
16. COLOMBO M, RAPOSO G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles [J]. Annual Rev Cell Devel Biology. 2014;30:255–289. doi:10.1146/annurev-cellbio-101512-122326
17. BUSCHMANN D, KIRCHNER B, HERMANN S, et al. Evaluation of serum extracellular vesicle isolation methods for profiling miRNAs by next-generation sequencing [J]. J Extracel Vesicl. 2018;7(1):1481321. doi:10.1080/20013078.2018.1481321
18. CRESCITELLI R, Lässer C, Lötvall J. Isolation and characterization of extracellular vesicle subpopulations from tissues [J]. Nature Protocols. 2021;16(3):1548–1580. doi:10.1038/s41596-020-00466-1
19. RAMTEKE A, TING H, AGARWAL C, et al. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules [J]. MolecCarcinogen. 2015;54(7):554–565. doi:10.1002/mc.22124
20. P DORAYAPPANKD, WANNER R, J WALLBILLICHJ, et al. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: a novel mechanism linking STAT3/Rab proteins [J]. Oncogene. 2018;37(28):3806–3821. doi:10.1038/s41388-018-0189-0
21. XU Z, CHEN Y, MA L, et al. Role of exosomal non-coding RNAs from tumor cells and tumor-associated macrophages in the tumor microenvironment [J]. Molec Ther. 2022;30(10):3133–3154. doi:10.1016/j.ymthe.2022.01.046
22. HE G, J LIU, YU Y, et al. Revisiting the advances and challenges in the clinical applications of extracellular vesicles in cancer [J]. Cancer Lett. 2024;593:216960. doi:10.1016/j.canlet.2024.216960
23. K HERRMANNI, A WOODMJ, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform [J]. Nat Nanotechnol. 2021;16(7):748–759. doi:10.1038/s41565-021-00931-2
24. ZHANG K, Cheng K. Stem cell-derived exosome versus stem cell therapy [J]. Nat Rev Bioeng. 2023:1–2.
25. B ARYAS, P COLLIES, A PARENTC. The ins-and-outs of exosome biogenesis, secretion, and internalization [J]. Trends Cell Biol. 2024;34(2):90–108. doi:10.1016/j.tcb.2023.06.006
26. J LEEY, SHIN KJ, C CHAEY. Regulation of cargo selection in exosome biogenesis and its biomedical applications in cancer [J]. Exp Mol Med. 2024;56(4):877–889. doi:10.1038/s12276-024-01209-y
27. Möller A, J LOBBR. The evolving translational potential of small extracellular vesicles in cancer [J]. Nature Rev Ca. 2020;20(12):697–709. doi:10.1038/s41568-020-00299-w
28. KALLURI R. The biology and function of exosomes in cancer [J]. J Clin Invest. 2016;126(4):1208–1215. doi:10.1172/JCI81135
29. K PANIGRAHIG, P PRAHARAJP, C PEAKT, et al. Hypoxia-induced exosome secretion promotes survival of African-American and Caucasian prostate cancer cells [J]. Scientific Repo. 2018;8(1):3853. doi:10.1038/s41598-018-22068-4
30. W KINGH, Z MICHAELM, M GLEADLEJ. Hypoxic enhancement of exosome release by breast cancer cells [J]. BMC Cancer. 2012;12:421. doi:10.1186/1471-2407-12-421
31. WANG Y, K YIN, TIAN J, et al.. Granulocytic Myeloid-Derived Suppressor Cells Promote the Stemness of Colorectal Cancer Cells through Exosomal S100A9. Advanced Scien. Baden-Wurttemberg, Germany) 2019;618:1901278. doi:10.1002/advs.201901278
32. W LIU, LI L, RONG Y, et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126 [J]. Acta biomaterialia. 2020;103:196–212. doi:10.1016/j.actbio.2019.12.020
33. K KNUTSONA, L WILLIAMSA, A BOISVERTW, et al.. HIF in the heart: development, metabolism, ischemia, and atherosclerosis [J]. J Clin Invest. 2021;131(17). doi:10.1172/JCI137557
34. ZHANG W, ZHOU X, Q YAO, et al. HIF-1-mediated production of exosomes during hypoxia is protective in renal tubular cells [J]. American J Phy Renal Phys. 2017;313(4):F906–f13. doi:10.1152/ajprenal.00178.2017
35. WANG T, M GILKESD, TAKANO N, et al. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis [J].
36. WU L, M XUE, S LAI, et al. Hypoxia derived exosomes promote the proliferation and metastasis of colorectal cancer through the regulation of HIF-1α/miR-4299/ZBTB4 [J]. Life Sciences. 2023;329:121872. doi:10.1016/j.lfs.2023.121872
37. ZENG Z, ZHAO Y, CHEN Q, et al. Hypoxic exosomal HIF-1α-stabilizing circZNF91 promotes chemoresistance of normoxic pancreatic cancer cells via enhancing glycolysis [J]. Oncogene. 2021;40(36):5505–5517. doi:10.1038/s41388-021-01960-w
38. W LUO, HU H, CHANG R, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1 [J]. Cell. 2011;145(5):732–744. doi:10.1016/j.cell.2011.03.054
39. Y WEI, WANG D, F JIN, et al. Pyruvate kinase type M2 promotes tumour cell exosome release via phosphorylating synaptosome-associated protein 23 [J]. Nature Commu. 2017;8:14041. doi:10.1038/ncomms14041
40. DANG K, A MYERSK. The role of hypoxia-induced miR-210 in cancer progression [J]. Inter J Mole Scien. 2015;16(3):6353–6372. doi:10.3390/ijms16036353
41. HE Q, LI X, HE L, et al. Pericyte dysfunction due to Shb gene deficiency increases B16F10 melanoma lung metastasis. International J Ca. 2020;147(9):2634–2644. doi:10.1002/ijc.33110
42. ZHANG Y, LI Y, P LIU, et al. Phosphatase Shp2 regulates biogenesis of small extracellular vesicles by dephosphorylating Syntenin [J]. J Extracell Vesicl. 2021;10(5):e12078. doi:10.1002/jev2.12078
43. BHANDARI V, HOEY C, Y LIUL, et al. Molecular landmarks of tumor hypoxia across cancer types [J]. Nature Genetics. 2019;51(2):308–318. doi:10.1038/s41588-018-0318-2
44. WANG X, WU R, ZHAI P, et al. Hypoxia promotes EV secretion by impairing lysosomal homeostasis in HNSCC through negative regulation of ATP6V1A by HIF-1alpha [J]. J Extrace Vesicles. 2023;12(2):e12310. doi:10.1002/jev2.12310
45. STENMARK H. Rab GTPases as coordinators of vesicle traffic [J]. Nature Revi Molec Cell Biology. 2009;10(8):513–525. doi:10.1038/nrm2728
46. B SCHAAFM, G KEULERST, A VOOIJSM, et al. LC3/GABARAP family proteins: autophagy-(un)related functions [J]. FASEB J. 2016;30(12):3961–3978. doi:10.1096/fj.201600698R
47. G KEULERST, F LIBREGTSS, J BEAUMONTJE, et al. Secretion of pro-angiogenic extracellular vesicles during hypoxia is dependent on the autophagy-related protein GABARAPL1 [J]. J Extracell Vesicles. 2021;10(14):e12166. doi:10.1002/jev2.12166
48. J BEAUMONTJE, JU J, O BARBEAULM, et al. GABARAPL1 is essential in extracellular vesicle cargo loading and metastasis development [J]. Radio Oncol. 2024;190:109968. doi:10.1016/j.radonc.2023.109968
49. HANAHAN D, A WEINBERGR. The hallmarks of cancer [J]. Cell. 2000;100(1):57–70. doi:10.1016/S0092-8674(00)81683-9
50. HANAHAN D, A WEINBERGR. Hallmarks of cancer: the next generation [J]. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
51. HANAHAN D. Hallmarks of Cancer: new Dimensions. Cancer Discovery. 2022;12(1):31–46. doi:10.1158/2159-8290.CD-21-1059
52. HE G, PENG X, S WEI, et al. Exosomes in the hypoxic TME: from release, uptake and biofunctions to clinical applications [J]. Molecr Can. 2022;21(1):19. doi:10.1186/s12943-021-01440-5
53. ESTELLER M. Non-coding RNAs in human disease [J]. Nature Revi Genet. 2011;12(12):861–874. doi:10.1038/nrg3074
54. H YAN. Non-coding RNA in cancer [J]. Essays in biochemistry. 2021. 65. 4:625–639. doi:10.1042/EBC20200032
55. ANASTASIADOU E, S JACOBL, J SLACKF. Non-coding RNA networks in cancer [J]. Nature Reviews Cancer. 2018;18(1):5–18. doi:10.1038/nrc.2017.99
56. LI C, Q NIY, XU H, et al. Roles and mechanisms of exosomal non-coding RNAs in human health and diseases [J]. Signal Transduction and Targeted Therapy. 2021;6(1):383. doi:10.1038/s41392-021-00779-x
57. M XUE, CHEN W, XIANG A, et al. Hypoxic exosomes facilitate bladder tumor growth and development through transferring long non-coding RNA-UCA1. Molecular Cancer. 2017;16(1):143. doi:10.1186/s12943-017-0714-8
58. HU G, MA J, ZHANG J, et al. Hypoxia-induced lncHILAR promotes renal cancer metastasis via ceRNA for the miR-613/206/ 1-1-3p/Jagged-1/Notch/CXCR4 signaling pathway [J]. Molecular Therapy: the Journal of the American Society of Gene Therapy. 2021;29(10):2979–2994. doi:10.1016/j.ymthe.2021.05.020
59. J LIN, WANG X, ZHAI S, et al. Hypoxia-induced exosomal circPDK1 promotes pancreatic cancer glycolysis via c-myc activation by modulating miR-628-3p/BPTF axis and degrading BIN1 [J]. Journal of Hematology & Oncology. 2022;15(1):128. doi:10.1186/s13045-022-01348-7
60. SCHNITTERT J, BANSAL R, PRAKASH J. Targeting Pancreatic Stellate Cells in Cancer. Trends in Cancer. 2019;5(2):128–142. doi:10.1016/j.trecan.2019.01.001
61. Cao, W Zeng, Z He, Z et al. Hypoxic pancreatic stellate cell-derived exosomal mirnas promote proliferation and invasion of pancreatic cancer through the PTEN/AKT pathway. Aging. 2021;13(5):7120-32. doi:10.18632/aging.202569
62. R REN, H SUN, MA C, et al. Colon cancer cells secrete exosomes to promote self-proliferation by shortening mitosis duration and activation of STAT3 in a hypoxic environment [J]. Cell & bioscience. 2019;9:62. doi:10.1186/s13578-019-0325-8
63. LI J, YANG P, CHEN F, et al. Hypoxic colorectal cancer-derived extracellular vesicles deliver microRNA-361-3p to facilitate cell proliferation by targeting TRAF3 via the noncanonical NF-κB pathways [J]. Clinical and Translational Medicine. 2021;11(3):e349. doi:10.1002/ctm2.349
64. Qian M, CHEN Z, X GUO, et al. Exosomes derived from hypoxic glioma deliver miR-1246 and miR-10b-5p to normoxic glioma cells to promote migration and invasion [J]. Labo Investi J Tech Meth Pathology. 2021;101(5):612–624. doi:10.1038/s41374-020-00522-0
65. FUSCO P, FIETTA A, R ESPOSITOM, et al. miR-210-3p enriched extracellular vesicles from hypoxic neuroblastoma cells stimulate migration and invasion of target cells [J]. Cell & bioscience. 2023;13(1):89. doi:10.1186/s13578-023-01045-z
66. YU F, LIANG M, HUANG Y, et al. Hypoxic tumor-derived exosomal miR-31-5p promotes lung adenocarcinoma metastasis by negatively regulating SATB2-reversed EMT and activating MEK/ERK signaling [J]. J Exper Clini Cancer Rese. 2021;40(1):179. doi:10.1186/s13046-021-01979-7
67. C PACHANEB, NUNES ACC, R CATALDIT, et al.. Small Extracellular Vesicles from Hypoxic Triple-Negative Breast Cancer Cells Induce Oxygen-Dependent Cell Invasion [J]. Inter J Molec Scie. 2022;23(20). doi:10.3390/ijms232012646
68. W CAO, ZENG Z, HE Z, et al. Hypoxic pancreatic stellate cell-derived exosomal mirnas promote proliferation and invasion of pancreatic cancer through the PTEN/AKT pathway [J]. Aging. 2021;13(5):7120–7132. doi:10.18632/aging.202569
69. Massagué J, GANESH K. Metastasis-Initiating Cells and Ecosystems. Cancer Discovery. 2021;11(4):971–994. doi:10.1158/2159-8290.CD-21-0010
70. GERSTBERGER S, JIANG Q, GANESH K. Metastasis. Cell. 2023;186(8):1564–1579. doi:10.1016/j.cell.2023.03.003
71. E TALMADGEJ, J FIDLERI. AACR Centennial series: the biology of cancer metastasis: historical perspective [J]. Cancer Research. 2010;70(14):5649–5669. doi:10.1158/0008-5472.CAN-10-1040
72. FOLKMAN J, Parris EE, Folkman J. Tumor angiogenesis: therapeutic implications [J]. New England J Med. 1971;285(21):1182–1186. doi:10.1056/NEJM197111182852108
73. M WEISS, A CHERESHD. Tumor angiogenesis: molecular pathways and therapeutic targets [J]. Nature Medicine. 2011;17(11):1359–1370. doi:10.1038/nm.2537
74. F QUAILD, A JOYCEJ. Microenvironmental regulation of tumor progression and metastasis [J]. Nature Medicine. 2013;19(11):1423–1437. doi:10.1038/nm.3394
75. X WEI, CHEN Y, JIANG X, et al. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments [J]. Molecular Cancer. 2021;20(1):7. doi:10.1186/s12943-020-01288-1
76. L SEMENZAG. Cancer-stromal cell interactions mediated by hypoxia-inducible factors promote angiogenesis, lymphangiogenesis, and metastasis [J]. Oncogene. 2013;32(35):4057–4063. doi:10.1038/onc.2012.578
77. E PARKJ, S TANH, DATTA A, et al.. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. MCP. 2010;96:1085–1099. Mole Cell Proteomics. doi:10.1074/mcp.M900381-MCP200
78. KUCHARZEWSKA P, C CHRISTIANSONH, E WELCHJ, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development [J].
79. Q GUO, Y FAN, WANG Q, et al. Glioblastoma upregulates SUMOylation of hnRNP A2/B1 to eliminate the tumor suppressor miR-204-3p, accelerating angiogenesis under hypoxia [J]. Cell Death & Disease. 2023;14(2):147. doi:10.1038/s41419-023-05663-w
80. LI L, LI C, WANG S, et al. Exosomes Derived from Hypoxic Oral Squamous Cell Carcinoma Cells Deliver miR-21 to Normoxic Cells to Elicit a Prometastatic Phenotype [J]. Cancer Research. 2016;76(7):1770–1780. doi:10.1158/0008-5472.CAN-15-1625
81. CAPIK O, GUMUS R, F KARATASO. Hypoxia-induced tumor exosomes promote angiogenesis through miR-1825/TSC2/mTOR axis in oral squamous cell carcinoma [J]. Head & Neck. 2023;45(9):2259–2273. doi:10.1002/hed.27460
82. Y MAO, WANG Y, DONG L, et al. Hypoxic exosomes facilitate angiogenesis and metastasis in esophageal squamous cell carcinoma through altering the phenotype and transcriptome of endothelial cells [J]. J Experim Clini Canc Res. 2019;38(1):389. doi:10.1186/s13046-019-1384-8
83. SHAN Y, B YOU, S SHI, et al. Hypoxia-Induced Matrix Metalloproteinase-13 Expression in Exosomes from Nasopharyngeal Carcinoma Enhances Metastases [J]. Cell Death & Disease. 2018;9(3):382. doi:10.1038/s41419-018-0425-0
84. L XIE, ZHANG K, B YOU, et al. Hypoxic nasopharyngeal carcinoma-derived exosomal miR-455 increases vascular permeability by targeting ZO-1 to promote metastasis [J]. Molecular Carcinogenesis. 2023;62(6):803–819. doi:10.1002/mc.23525
85. L HSUY, Y HUNGJ, A CHANGW, et al. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1 [J]. Oncogene. 2017;36(34):4929–4942. doi:10.1038/onc.2017.105
86. UMEZU T, TADOKORO H, AZUMA K, et al. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124(25):3748–3757. doi:10.1182/blood-2014-05-576116
87. Z GUO, WANG X, YANG Y, et al. Hypoxic Tumor-Derived Exosomal Long Noncoding RNA UCA1 Promotes Angiogenesis via miR-96-5p/AMOTL2 in Pancreatic Cancer [J]. Molecular Therapy Nucleic Acids. 2020;22:179–195. doi:10.1016/j.omtn.2020.08.021
88. CHEN K, WANG Q, X LIU, et al. Hypoxic pancreatic cancer derived exosomal miR-30b-5p promotes tumor angiogenesis by inhibiting GJA1 expression [J]. Int J Biolog Scie. 2022;18(3):1220–1237. doi:10.7150/ijbs.67675
89. WANG Y, A CEN, YANG Y, et al. miR-181a, delivered by hypoxic PTC-secreted exosomes, inhibits DACT2 by downregulating MLL3, leading to YAP-VEGF-mediated angiogenesis [J]. Molecular Therapy Nucleic Acids. 2021;24:610–621. doi:10.1016/j.omtn.2021.02.027
90. BASKAR R, A LEEK, R YEO, et al. Cancer and radiation therapy: current advances and future directions [J]. Inte J Medical Scie. 2012;9(3):193–199. doi:10.7150/ijms.3635
91. SCHAUE D, H MCBRIDEW. Opportunities and challenges of radiotherapy for treating cancer [J]. Nature Reviews Clinical Oncology. 2015;12(9):527–540. doi:10.1038/nrclinonc.2015.120
92. K BEUTELA, J HALBROOKC. Barriers and opportunities for gemcitabine in pancreatic cancer therapy [J]. Ame J Physio Cell Phys. 2023;324(2):C540–c52. doi:10.1152/ajpcell.00331.2022
93. KARACHI A, DASTMALCHI F, A MITCHELLD, et al. Temozolomide for immunomodulation in the treatment of glioblastoma [J]. Neuro-Oncology. 2018;20(12):1566–1572. doi:10.1093/neuonc/noy072
94. WU P, J GUO, YANG H, et al. Exosomes Derived from Hypoxic Glioma Cells Reduce the Sensitivity of Glioma Cells to Temozolomide Through Carrying miR-106a-5p [J]. Drug Desig Develop Therapy. 2022;16:3589–3598. doi:10.2147/DDDT.S382690
95. ZHANG H, DU Z, TU C, et al. Hypoxic Bone Marrow Stromal Cells Secrete miR-140-5p and miR-28-3p That Target SPRED1 to Confer Drug Resistance in Multiple Myeloma [J]. Cancer Research. 2024;84(1):39–55. doi:10.1158/0008-5472.CAN-23-0189
96. WANG H, J MIN, XU C, et al. Hypoxia-elicited Exosomes Promote the Chemoresistance of Pancreatic Cancer Cells by Transferring LncROR via Hippo Signaling [J]. J Cancer. 2023;14(6):1075–1087. doi:10.7150/jca.81320
97. Y CHI, H XIN, Z LIU. Exosomal lncRNA UCA1 Derived From Pancreatic Stellate Cells Promotes Gemcitabine Resistance in Pancreatic Cancer via the SOCS3/EZH2 Axis [J]. Frontiers in oncology. 2021;11:671082. doi:10.3389/fonc.2021.671082
98. X YUE, F LAN, T XIA. Hypoxic Glioma Cell-Secreted Exosomal miR-301a Activates Wnt/β-catenin Signaling and Promotes Radiation Resistance by Targeting TCEAL7 [J]. Molecul Ther. 2019;27(11):1939–1949. doi:10.1016/j.ymthe.2019.07.011
99. CHEN F, XU B, LI J, et al. Hypoxic tumour cell-derived exosomal miR-340-5p promotes radioresistance of oesophageal squamous cell carcinoma via KLF10 [J]. Journal of Experimental & clinical Cancer Research: CR. 2021;40(1):38. doi:10.1186/s13046-021-01834-9
100. ZHANG Y, X LIU, ZENG L, et al. Exosomal protein angiopoietin-like 4 mediated radioresistance of lung cancer by inhibiting ferroptosis under hypoxic microenvironment [J]. British Journal of Cancer. 2022;127(10):1760–1772. doi:10.1038/s41416-022-01956-7
101. BEJARANO L, C JORDĀOMJ, A JOYCEJ. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discovery. 2021;11(4):933–959. doi:10.1158/2159-8290.CD-20-1808
102. TIWARI A, TRIVEDI R, Y LINS. Tumor microenvironment: barrier or opportunity towards effective cancer therapy [J]. Journal of Biomedical Science. 2022;29(1):83. doi:10.1186/s12929-022-00866-3
103. Z QIANB, W POLLARDJ. Macrophage diversity enhances tumor progression and metastasis [J]. Cell. 2010;141(1):39–51. doi:10.1016/j.cell.2010.03.014
104. NGAMBENJAWONG C, H GUSTAFSONH, PUN SH. Progress in tumor-associated macrophage TAM-targeted therapeutics [J]. Advanced Drug Delivery Reviews. 2017;114:206–221. doi:10.1016/j.addr.2017.04.010
105. ZHENG H, PENG X, YANG S, et al. Targeting tumor-associated macrophages in hepatocellular carcinoma: biology, strategy, and immunotherapy [J]. Cell Death Discov. 2023;9(1):65. doi:10.1038/s41420-023-01356-7
106. LI B, YANG C, Z ZHU, et al. Hypoxic glioma-derived extracellular vesicles harboring MicroRNA-10b-5p enhance M2 polarization of macrophages to promote the development of glioma [J]. CNS Neuroscience & Therapeutics. 2022;28(11):1733–1747. doi:10.1111/cns.13905
107. XU J, ZHANG J, ZHANG Z, et al. Hypoxic glioma-derived exosomes promote M2-like macrophage polarization by enhancing autophagy induction [J]. Cell Death & Disease. 2021;12(4):373. doi:10.1038/s41419-021-03664-1
108. QIAN M, WANG S, X GUO, et al. Hypoxic glioma-derived exosomes deliver microRNA-1246 to induce M2 macrophage polarization by targeting TERF2IP via the STAT3 and NF-κB pathways [J]. Oncogene. 2020;39(2):428–442. doi:10.1038/s41388-019-0996-y
109. J JIN, YU G. Hypoxic lung cancer cell-derived exosomal miR-21 mediates macrophage M2 polarization and promotes cancer cell proliferation through targeting IRF1 [J]. World Journal of Surgical Oncology. 2022;20(1):241. doi:10.1186/s12957-022-02706-y
110. GU J, YANG S, WANG X, et al. Hypoxic lung adenocarcinoma-derived exosomal miR −1290 induces M2 macrophage polarization by targeting SOCS3. Cancer Medicine. 2023;12(11):12639–12652. doi:10.1002/cam4.5954
111. L HSUY, Y HUNGJ, A CHANGW, et al. Hypoxic Lung-Cancer-Derived Extracellular Vesicle MicroRNA-103a Increases the Oncogenic Effects of Macrophages by Targeting PTEN [J]. Mole Therapy. 2018;26(2):568–581. doi:10.1016/j.ymthe.2017.11.016
112. LI J, XU P, WU D, et al. Hypoxic stress suppresses lung tumor-secreted exosomal miR101 to activate macrophages and induce inflammation [J]. Cell Death & Disease. 2021;12(8):776. doi:10.1038/s41419-021-04030-x
113. WANG X, G LUO, ZHANG K, et al. Hypoxic Tumor-Derived Exosomal miR-301a Mediates M2 Macrophage Polarization via PTEN/PI3Kγ to Promote Pancreatic Cancer Metastasis [J]. Cancer Research. 2018;78(16):4586–4598. doi:10.1158/0008-5472.CAN-17-3841
114. CHEN X, ZHOU J, LI X, et al. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype [J]. Cancer Letters. 2018;435:80–91. doi:10.1016/j.canlet.2018.08.001
115. WANG X, ZHOU Y, DONG K, et al. Exosomal lncRNA HMMR-AS1 mediates macrophage polarization through miR −147a/ ARID3A axis under hypoxia and affects the progression of hepatocellular carcinoma. Environmental Toxicology. 2022;37(6):1357–1372. doi:10.1002/tox.23489
116. YANG C, WU S, Z MOU, et al. Exosome-derived circTRPS1 promotes malignant phenotype and CD8+ T cell exhaustion in bladder cancer microenvironments [J]. Molecular Ther. 2022;30(3):1054–1070. doi:10.1016/j.ymthe.2022.01.022
117. J LIU, PENG X, YANG S, et al. Extracellular vesicle PD-L1 in reshaping tumor immune microenvironment: biological function and potential therapy strategies [J]. Cell Communication and Signaling: CCS. 2022;20(1):14. doi:10.1186/s12964-021-00816-w
118. WANG M, Z QIN, J WAN, et al. Tumor-derived exosomes drive pre-metastatic niche formation in lung via modulating CCL1(+) fibroblast and CCR8(+) Treg cell interactions [J]. Cancer Immunology, Immunotherapy: CII. 2022;71(11):2717–2730. doi:10.1007/s00262-022-03196-3
119. Ye S-B, ZHANG H, Cai -T-T, et al. Exosomal miR −24-3p impedes T-cell function by targeting FGF11 and serves as a potential prognostic biomarker for nasopharyngeal carcinoma. The Journal of Pathology. 2016;240(3):329–340. doi:10.1002/path.4781
120. LI L, B CAO, LIANG X, et al. Microenvironmental oxygen pressure orchestrates an anti- and pro-tumoral γδ T cell equilibrium via tumor-derived exosomes [J]. Oncogene. 2019;38(15):2830–2843. doi:10.1038/s41388-018-0627-z
121. I GABRILOVICHD. Myeloid-Derived Suppressor Cells [J]. Cancer Immunology Research. 2017;5(1):3–8. doi:10.1158/2326-6066.CIR-16-0297
122. X GUO, W QIU, Q LIU, et al. Immunosuppressive effects of hypoxia-induced glioma exosomes through myeloid-derived suppressor cells via the miR-10a/Rora and miR-21/Pten Pathways [J]. Oncogene. 2018;37(31):4239–4259. doi:10.1038/s41388-018-0261-9
123. ZHOU S, Y LAN, LI Y, et al. Hypoxic Tumor-Derived Exosomes Induce M2 Macrophage Polarization via PKM2/AMPK to Promote Lung Cancer Progression [J]. Cell Transplantation. 2022;31:9636897221106998. doi:10.1177/09636897221106998
124. X MAO, XU J, WANG W, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives [J]. Molecular Cancer. 2021;20(1):131. doi:10.1186/s12943-021-01428-1
125. LAVIE D, BEN-SHMUEL A, EREZ N, et al. Cancer-associated fibroblasts in the single-cell era [J]. Nature Cancer. 2022;3(7):793–807. doi:10.1038/s43018-022-00411-z
126. G ZHU, B CAO, LIANG X, et al. Small extracellular vesicles containing miR-192/215 mediate hypoxia-induced cancer-associated fibroblast development in head and neck squamous cell carcinoma [J]. Cancer Letters. 2021;506:11–22. doi:10.1016/j.canlet.2021.01.006
127. YE B, DUAN Y, ZHOU M, et al. Hypoxic tumor-derived exosomal miR-21 induces cancer-associated fibroblast activation to promote head and neck squamous cell carcinoma metastasis [J]. Cell Signall. 2023;108:110725. doi:10.1016/j.cellsig.2023.110725
128. G ZHU, WANG L, Meng W, et al. LOXL2-enriched small extracellular vesicles mediate hypoxia-induced premetastatic niche and indicates poor outcome of head and neck cancer [J]. Theranostics. 2021;11(19):9198–9216. doi:10.7150/thno.62455
129. W JIA, LIANG S, W LIN, et al. Hypoxia-induced exosomes facilitate lung pre-metastatic niche formation in hepatocellular carcinoma through the miR-4508-RFX1-IL17A-p38 MAPK-NF-κB pathway [J]. International Journal of Biological Sciences. 2023;19(15):4744–4762. doi:10.7150/ijbs.86767
130. PENG XIL, LIU S M. Hypoxia-stimulated ATM activation regulates autophagy-associated exosome release from cancer-associated fibroblasts to promote cancer cell invasion [J]. Journal of Extracellular Vesicles. 2021;10(11):e12146. doi:10.1002/jev2.12146
131. YANG K, ZHANG J, Bao C. Exosomal circEIF3K from cancer-associated fibroblast promotes colorectal cancer (CRC) progression via miR-214/PD-L1 axis [J]. BMC Cancer. 2021;21(1):933. doi:10.1186/s12885-021-08669-9
132. J YIN, GE X, Z SHI, et al. Extracellular vesicles derived from hypoxic glioma stem-like cells confer temozolomide resistance on glioblastoma by delivering miR-30b-3p [J]. Theranostics. 2021;11(4):1763–1779. doi:10.7150/thno.47057
133. LI J, Liao T, H LIU, et al. Hypoxic Glioma Stem Cell-Derived Exosomes Containing Linc01060 Promote Progression of Glioma by Regulating the MZF1/c-Myc/HIF1α Axis [J]. Cancer Research. 2021;81(1):114–128. doi:10.1158/0008-5472.CAN-20-2270
134. W REN, J HOU, YANG C, et al. Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via miR-21-5p delivery [J]. J Exper clinical CancRese. 2019;38(1):62. doi:10.1186/s13046-019-1027-0
135. ZHANG X, B SAI, WANG F, et al. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT [J]. Molecular Cancer. 2019;18(1):40. doi:10.1186/s12943-019-0959-5
136. EGEA V, KESSENBROCK K, LAWSON D, et al. Let-7f miRNA regulates SDF-1α- and hypoxia-promoted migration of mesenchymal stem cells and attenuates mammary tumor growth upon exosomal release [J]. Cell Death & Disease. 2021;12(6):516. doi:10.1038/s41419-021-03789-3
137. JIANG J, WANG W, L ZHU, et al. Unveiling the role of hypoxic macrophage-derived exosomes in driving colorectal cancer progression [J]. Frontiers in Immunology. 2023;14:1260638. doi:10.3389/fimmu.2023.1260638
138. GU W, GONG L, WU X, et al. Hypoxic TAM-derived exosomal miR-155-5p promotes RCC progression through HuR-dependent IGF1R/AKT/PI3K pathway [J]. Cell Death Discov. 2021;7(1):147. doi:10.1038/s41420-021-00525-w
139. LI Y, YE J, XU S, et al. Circulating noncoding RNAs: promising biomarkers in liquid biopsy for the diagnosis, prognosis, and therapy of NSCLC [J]. Discover Oncology. 2023;14(1):142. doi:10.1007/s12672-023-00686-3
140. ZHANG W, JIANG L, J DIEFENBACHR, et al. Enabling Sensitive Phenotypic Profiling of Cancer-Derived Small Extracellular Vesicles Using Surface-Enhanced Raman Spectroscopy Nanotags [J]. ACS Sens. 2020;5(3):764–771. doi:10.1021/acssensors.9b02377
141. Zhuang L, Q YOU, SU X, et al. High-Performance Detection of Exosomes Based on Synergistic Amplification of Amino-Functionalized Fe(3)O(4) Nanoparticles and Two-Dimensional MXene Nanosheets. Sensors. 2023;23(7):3508. doi:10.3390/s23073508
142. Y LEI, X FEI, DING Y, et al. Simultaneous subset tracing and miRNA profiling of tumor-derived exosomes via dual-surface-protein orthogonal barcoding [J]. Sci Adv. 2023;9(40):eadi1556. doi:10.1126/sciadv.adi1556
143. S KIM, J HAN, S PARKJ, et al. DNA barcode-based detection of exosomal microRNAs using nucleic acid lateral flow assays for the diagnosis of colorectal cancer [J]. Talanta. 2022;242:123306. doi:10.1016/j.talanta.2022.123306
144. WU D, J YAN, SHEN X, et al. Profiling surface proteins on individual exosomes using a proximity barcoding assay [J]. Nature Communications. 2019;10(1):3854. doi:10.1038/s41467-019-11486-1
145. SONG F, WANG C, WANG C, et al. Multi-Phenotypic Exosome Secretion Profiling Microfluidic Platform for Exploring Single-Cell Heterogeneity [J]. Small Methods. 2022;6(9):e2200717. doi:10.1002/smtd.202200717
146. W GUO, AI Y C, X LIU, et al. Single-Exosome Profiling Identifies ITGB3+ and ITGAM+ Exosome Subpopulations as Promising Early Diagnostic Biomarkers and Therapeutic Targets for Colorectal Cancer. Research. 2023;6:0041. doi:10.34133/research.0041
147. Y YUE, BISHOP M, ZHENG B, et al. Magnetic Particle Imaging: a Novel in Vivo Imaging Platform for Cancer Detection [J]. Nano Letters. 2017;17(3):1648–1654. doi:10.1021/acs.nanolett.6b04865
148. O JUNGK, H JO, YU JH, et al. Development and MPI tracking of novel hypoxia-targeted theranostic exosomes [J]. Biomaterials. 2018;177:139–148. doi:10.1016/j.biomaterials.2018.05.048
149. SHARMA A, JOHNSON A. Exosome DNA: critical regulator of tumor immunity and a diagnostic biomarker [J]. J Cell Physiol. 2020;235(3):1921–1932. doi:10.1002/jcp.29153
150. K THAKURB, ZHANG H, BECKER A, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection [J]. Cell Research. 2014;24(6):766–769. doi:10.1038/cr.2014.44
151. L MAIREC, M FUHM, Kaulich K, et al. Genome-wide methylation profiling of glioblastoma cell-derived extracellular vesicle DNA allows tumor classification [J]. Neuro-Oncology. 2021;23(7):1087–1099. doi:10.1093/neuonc/noab012
152. Z FAN, XIAO K, J LIN, et al. Functionalized DNA Enables Programming Exosomes/Vesicles for Tumor Imaging and Therapy [J]. Small. 2019;15(47):e1903761. doi:10.1002/smll.201903761
153. MUNAGALA R, Aqil F, JEYABALAN J, et al. Exosome-mediated delivery of RNA and DNA for gene therapy [J]. Cancer Letters. 2021;505:58–72. doi:10.1016/j.canlet.2021.02.011
154. H LIU, TIAN Y, C XUE, et al. Analysis of extracellular vesicle DNA at the single-vesicle level by nano-flow cytometry [J]. J Extracel Vesic. 2022;11(4):e12206. doi:10.1002/jev2.12206
155. K LUDWIGA, DE MIROSCHEDJI K, DOEPPNER TR, et al. Precipitation with polyethylene glycol followed by washing and pelleting by ultracentrifugation enriches extracellular vesicles from tissue culture supernatants in small and large scales [J]. J Extracel Vesic. 2018;7(1):1528109. doi:10.1080/20013078.2018.1528109
156. KAMERKAR S, LEBLEU VS, SUGIMOTO H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer [J]. Nature. 2017;546(7659):498–503. doi:10.1038/nature22341
157. IWAI K, MINAMISAWA T, SUGA K, et al. Isolation of human salivary extracellular vesicles by iodixanol density gradient ultracentrifugation and their characterizations [J]. J Extracel Vesicles. 2016;5:30829. doi:10.3402/jev.v5.30829
158. LI P, KASLAN M, H LEES, et al. Progress in Exosome Isolation Techniques [J]. Theranostics. 2017;7(3):789–804. doi:10.7150/thno.18133
159. CHERUVANKY A, ZHOU H, PISITKUN T, et al. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator [J]. Am J Physiology Renal Physiology. 2007;292(5):F1657–61. doi:10.1152/ajprenal.00434.2006
160. J VANDEUN, MESTDAGH P, SORMUNEN R, et al. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling [J]. J Extracell Vesicl. 2014;3.
161. LIGA A, D VLIEGENTHARTA, OOSTHUYZEN W, et al. Exosome isolation: a microfluidic road-map [J]. Lab Chip. 2015;15(11):2388–2394. doi:10.1039/C5LC00240K
162. J LOBBR, BECKER M, W WENS, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma [J]. J Extracell Vesicles. 2015;4:27031. doi:10.3402/jev.v4.27031
163. MOMEN-HERAVI F, BALAJ L, ALIAN S, et al. Current methods for the isolation of extracellular vesicles [J]. Biol Chem. 2013;394(10):1253–1262. doi:10.1515/hsz-2013-0141
164. SUNKARA V, PARK J, HAN J, et al. Exosome Precipitation by Ionic Strength Modulation: exoPRISM [J]. ACS Appl Mater Interfa. 2023;15(49):56807–56819. doi:10.1021/acsami.3c13527
165. K PATELG, A KHANM, ZUBAIR H, et al. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications [J]. Scientific Repo. 2019;9(1):5335. doi:10.1038/s41598-019-41800-2
166. SHARMA P, LUDWIG S, MULLER L, et al. Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma [J]. J Extracell Vesic. 2018;7(1):1435138. doi:10.1080/20013078.2018.1435138
167. S LIN, YU Z, CHEN D, et al. Progress in Microfluidics-Based Exosome Separation and Detection Technologies for Diagnostic Applications [J]. Small. 2020;16(9):e1903916. doi:10.1002/smll.201903916
168. AMONDARAIN M, GALLEGO I, PURAS G, et al. The role of microfluidics and 3D-bioprinting in the future of exosome therapy [J]. Trends Biotechno. 2023;41(11):1343–1359. doi:10.1016/j.tibtech.2023.05.006
169. Q KOHY, B ALMUGHLLIQF, VASWANI K, et al. Exosome enrichment by ultracentrifugation and size exclusion chromatography. Front Bioscienc. 2018;23(5):865–874. doi:10.2741/4621
170. SIDHOM K, O OBIP, SALEEM A. A Review of Exosomal Isolation Methods: is Size Exclusion Chromatography the Best Option? Inte J Mole Sciences. 2020;21(18).
171. L SEMENZAG. Hypoxia-inducible factors: coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype [J]. THE EMBO Journal. 2017;36(3):252–259. doi:10.15252/embj.201695204
172. F Creedenj, Sevier J, T Zhangj, et al. Smart exosomes enhance PDAC targeted therapy [J]. J Control Rel. 2024;368:413–429. doi:10.1016/j.jconrel.2024.02.037
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.