Back to Journals » Drug Design, Development and Therapy » Volume 19
Identification of Lauric Acid as a Potent Sodium Channel NaV1.5 Blocker from Compound Chinese Medicine Wenxin Keli
Authors Xie W, Gao J , Liang Y, Huang C, Zhang B, Chen X, Yao X , Nan G, Wu H, Wang Y, Wu L, Wang T, Zhu Y
Received 5 July 2024
Accepted for publication 1 January 2025
Published 9 January 2025 Volume 2025:19 Pages 141—157
DOI https://doi.org/10.2147/DDDT.S485723
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Weiwei Xie,1,* Jiaming Gao,2,* Yingran Liang,1,* Chenxing Huang,3 Boyong Zhang,1 Xiaonan Chen,1 Xi Yao,1 Guo Nan,1 Honghua Wu,1 Yuefei Wang,1 Lin Wu,4 Taiyi Wang,3,5 Yan Zhu1
1State Key Laboratory of Component-Based Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, 301617, People’s Republic of China; 2Institute of Basic Medical Sciences of Xiyuan Hospital, Beijing Key Laboratory of Pharmacology of Chinese Materia, Beijing, 100091, People’s Republic of China; 3Institute of Acupuncture and Moxibustion, Shandong University of Traditional Chinese Medicine, Jinan, 250355, People’s Republic of China; 4Department of Cardiology, Peking University First Hospital, Beijing, 100034 People’s Republic of China; 5Shandong Key Laboratory of Innovation and Application Research in Basic Theory of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, 250355, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yan Zhu, State Key Laboratory of Component-based Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Beihua South Road, JingHai District, Tianjin, 301617, People’s Republic of China, Tel +86 22 59596168, Email [email protected] Taiyi Wang, Shandong Key Laboratory of Innovation and Application Research in Basic Theory of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, 250355, People’s Republic of China, Email [email protected]
Purpose: The major cardiac voltage-gated sodium channel NaV 1.5 (INa) is essential for cardiac action potential initiation and subsequent propagation. Compound Chinese medicine Wenxin Keli (WXKL) has been shown to suppress arrhythmias and heart failure. However, its active components have not been fully elucidated. This study focused on identifying the active inhibitor of INa in WXKL and exploring their mode of action in electrophysiological conduction.
Methods: A chemical fraction library was constructed from an aqueous extract of WXKL and screened using an automated patch-clamping system in cells stably expressing the NaV 1.5 gene SCN5A. Candidate fractions with INa-inhibition activity were analyzed by HPLC-ESI-IT-TOF-MS and GC-MS to identify the ingredients. NaV 1.5 blocker molecules identified by single-cell electrocardiogram were tested in hiPSC-derived cardiomyocytes. We evaluated the SCN5A inhibitory potential of Wenxin Keli effective monomer employing molecular docking and molecular dynamics simulation approaches.
Results: A primary screen of the WXKL chemical library identified five fractions that significantly inhibited the NaV 1.5 channel, with one of them rich in poly-saturated fatty acids. Molecular structural characterization revealed the presence of lauric acid, myristic acid, palmitic acid, and stearic acid in the active subfraction. Electrophysiological characterization demonstrated lauric acid (LA) as the most effective monomer for INa-inhibition with an IC50 at 27.40 ± 12.78 μM. LA shifted the steady-state inactivation of INa to more negative potentials and decreased the amplitude of extracellular field potential in hiPSC-derived cardiomyocytes. We demonstrate for the first time that naturally poly-saturated fatty acid, lauric acid, as a potential novel INa blocker. Molecular docking and molecular dynamics simulation suggested that LA binds to the NaV 1.5 protein, with a significant binding affinity forming interactions with functionally essential residues and blocks the inward flow of Na+. Mechanistically, lauric acid acts on the fast inactivation of NaV 1.5 alter electrophysiology conduction of hiPSC-derived cardiomyocytes and contribute to the antiarrhythmic effect of WXKL.
Conclusion: Lauric acid is a potent blocker for sodium channel NaV 1.5 and alleviates arrhythmia via inhibiting INa.
Keywords: lauric acid, Wenxin Keli, cardiac arrythmia, NaV 1.5 sodium channel, patch clamp recording, high-throughput electrophysiology
Graphical Abstract:

Introduction
Patients with heart failure and left ventricular dysfunction frequently present with arrhythmias, which can precipitate a reversible dilated cardiomyopathy. Arrhythmia-induced cardiomyopathy is a complex condition characterized by a decline in cardiac function resulting from irregular heart rhythms. These disorders include tachycardia, atrial fibrillation, and premature ventricular contractions, which illustrate the critical association between arrhythmias and heart failure. This highlights the necessity for timely intervention.1 Although there has been the application of non-pharmacological therapies to treat a vast variety of arrhythmias, in consideration of specific pathological conditions, more effective antiarrhythmic drugs are still in urgent need.2 Three main types of pharmacologic strategies are currently available for treating atrial fibrillation. The first type is sodium channel blockers like propafenone and flecainide, the second type is potassium channel blockers (largely IKr) such as sotalol and dofetilide, and the third type is mixed ion channel blockers such as dronedarone and amiodarone.3 Novel sodium ion channel INa remains an important target for antiarrhythmic drug discovery. However, the narrow therapeutic window of antiarrhythmic drugs represents a significant clinical challenge and has limited clinical application.
In ion channel pharmacology, prioritizing the mitigation of proarrhythmic risk is deemed more critical than elucidating the fundamental cellular mechanisms involved. Arrhythmia is classified as a type of palpitation within the framework of traditional Chinese medicine, and numerous traditional Chinese medicinal formulations have been documented for their efficacy in regulating heart rate. Interestingly, natural products, such as barberry, motherwort, cinchona, saponins, alkaloids, flavonoids, terpenes, and quinones, together with traditional acupuncture and yoga treatment, have been suggested to have certain effects on inhibition of various ion channel currents to exert antiarrhythmic effects, encompassing: Ito, INa, IKs, IKr, If, ICa.L.4–6 The cardiac sodium channel has two basic parts: peak INa and late INa, the peak INa reflects Phase 0 of the action potential.7 Because Peak INa contributes more to Na influx, inhibition of peak INa will cause a greater reduction of Na influx.8 The SCN5A gene encodes NaV1.5 channel protein in humans, and NaV1.5 current is tetrodotoxin-resistant.9 The NaV1.5 channel is a voltage-gated sodium channel playing an essential role in the peak INa of the heart and heart excitability and conduction.10
Wenxin Keli (WXKL) is a traditional Chinese medicine derived from Zhi Gan Cao Tang (recorded in Treatise on Cold Pathogenic and Miscellaneous Diseases) and approved by the China Food and Drug Administration, known for its effects in tonifying Qi and raising Yin. It is reported to be of benefit in the common treatment of cardiac arrhythmias by invigorating blood and eliminating blood stasis.11,12 WXKL, a herbal medicine consisting of Codonopsis Radix (Codonopsis pilosula (Franch.) Nannf.) (Dangshen), Polygonati Rhizoma (Polygonatumkingianum Coll. Et Hemsl.) (Huangjing), Notoginseng Radix Et Rhizoma (Panax notoginseng (Burk). F. H. Chen) (Sanqi), Ambrum (Resin of Pinaceae) (Hupo) and Nardostachyos Radix Et Rhizoma (Nardostachys jatamansi DC.) (Gansong), is the best-investigated Chinese medicine compound with confirmed effects in treating cardiac arrhythmias and heart failure.13,14 Clinical studies have reported the safety and effectiveness of WXKL as an alternative medicine for treating a variety of illnesses, such as ventricular premature complexes, angina pectoris, ventricular remodeling, histopathological damage, cardiac dysfunction, myocardial apoptosis, and angiotensin II concentrations in rats with myocardial infarction.15–17 It can also regulate cardiac rhythm and arrhythmic manifestations of Brugada syndrome in an experimental model.18,19 WXKL was reported to be effective in improving the left ventricular ejection fraction in patients with heart failure and its complications.20–22 WXKL shows protection of the ultrastructural gap junctions and Cx43 by regulating miR-1 and protein kinase C-mediated signal transduction and significantly increases the ventricular fibrillation threshold.23 Some previous studies indicate that the antiarrhythmic mechanism of WXKL is neither significantly bonded to closed nor open states of the sodium channel but binds very rapidly to the inactivated state of the channel and then dissociates rapidly from the closed state.24,25 Studies also show that WXKL attenuates intracellular Ca2+ overload induced by hypoxia-reoxygenation in ventricular myocytes by inhibiting INaL and ICaL and preventing arrhythmia in cardiac Purkinje cells.26 In addition, WXKL shows antiarrhythmic properties in the selective inhibition of INaL and regulates the CaMKII signal transduction pathway.27–29 WXKL inhibits transient outward current (Ito) to therapy Brugada syndrome,30 and it could also attenuate ischemia-induced ventricular arrhythmias in rats by regulating ICaL and Ito in a concentration-dependent manner.31 In addition, treatment with WXKL demonstrated superior performance compared to those of placebo for premature ventricular contractions-related symptoms, and no severe adverse effects were reported.32
Our previous network pharmacology prediction and validation study have identified an anti-arrhythmic component of WXKL as calaxin, which inhibited the CaV1.2 calcium channel.33 Natural fatty acids generally exist in traditional Chinese medicines. It has been reported that the ω-3 long-chain polyunsaturated fatty acids (PUFAs) have a protective effect against arrhythmia.6 Up till now, the WXKL fatty acids effect on sodium channels is unclear. Automated electrophysiology systems have been developed in recent years to enhance the capacity and lower the expenses of INa testing compared to conventional patch-clamp electrophysiology methods, which are regarded as the gold standard technique for studying ion channel function.34 IonWorks Barracuda, a high-throughput electrophysiology assay, is an automated patch-clamp system that uses a planar, multi-well substrate.35 By applying Head Space Solid Phase Micro Extraction (HS-SPME) and GC-MS techniques, our team has extracted and analyzed the chemical fractions of the volatile oil from WXKL, including the main constituents and their content percentages.36 In this study, we characterized WXKL and its monomers on the NaV1.5 channel by IonWorks Barracuda. Furthermore, we used the manual patch clamp to verify the active fatty acids and finally analyzed LA by UPLC-MS and GS-MS, which has the effect of suppressing NaV1.5.
Methods
Preparation of WXKL Fraction Library
WXKL (Buchang Pharmaceutical Co., Ltd, Lot No. Z10950026) was dissolved in 50% methanol aqueous solution at a concentration of 300 mg/mL. One hundred and thirty fractions of WXKL were separated on a preparative HPLC system as described in detail in the online Supplementary material 2.
High-Throughput Screen of the NaV1.5 Activities
WXKL fractions were screened electro-physiologically by an automated patch-clamping system (IonWorks Barracuda) in CHL cells stably expressing SCN5A. A detailed protocol, including cell culture conditions, solutions preparations, the NaV1.5 setup protocol, and editing of the recording protocol, was as previously described37 and provided in online Supplementary material 2.
Identification of Active Monomers
Positive fractions of WXKL were analyzed by high-performance liquid chromatography with electrospray ionization ion trap time-of-flight multistage mass spectrometry (HPLC-ESI-IT-TOF-MS) and gas chromatography-tandem mass spectrometric (GC-MS). We used the conventional patch clamp to identify the effective monomers. For conventional patch clamp and IonWork Barracuda automated recordings, the external buffer supplement with (in mM) NaCl, 140; KCL, 4; MgCl2, 1; CaCl2, 2; HEPES, 10 and D-(+)-Glucose, 10 with PH adjusted to 7.4 using NaOH. The internal solution was composed of (in mM) Potassium gluconate, 100; MgCl2, 3; EGTA, 5; and HEPES, 10, with PH adjusted to 7.2 using KOH. The resistance of each well was measured in a “hole test” by applying a short pulse to +10 mV from a potential of 0 mV. Cells were then mixed and dispensed, and 5 μL of the cell suspension was added to each of the 384 wells to the patch plate at a speed of 0.5 μL/s. Cells could allowed to seal on the patch plate substrate for 1 min before perfusion of the amphotericin B containing internal buffer. During that time, seal resistances were monitored by applying 100 ms pulses to −80 mV from a holding potential of −120 mV. After perfusing the amphotericin B (10 mg per 100 mL internal solution) for 115 s, cell perforation could take place for another 5 mins. See online Supplementary material 2.
Mechanism of Lauric Acid on NaV1.5 Ion Channel in CHL Cells
The Chinese Hamster Hung (CHL) cells stably expressing SCN5A were used to study the mechanism of LA on INa characteristics. The CHL cell line stably expressing the INa was a kind gift from Professor Zhaobing Gao of the Shanghai Institute of Materia Medica-Chinese Academy of Sciences. The experimental protocol for use of the donated cells was approved by the Laboratory Ethics Committee of Tianjin University of Traditional Chinese medicine. Protocol for conventional whole-cell current recordings was as depicted previously. Cells were placed in a glass-bottom cell culture dish and perfused with a standard external solution. Pipettes were pulled from borosilicate filament (BFI150-86-10, Sutter, USA). Glass microelectrodes (2–5 MΩ resistance between the pipette and extracellular solutions) were used to form tight seals (1 GΩ) on the cell surface. Whole-cell currents were recorded with an Axon 200B amplifier, filtered at 2 kHz, and current recordings were digitized with a DigiData 1550 interface under the control of pClamp10.4 software (Molecular Devices, Sunnyvale, CA). Data acquisition of whole-cell currents was done using Clampex 10.4 (Molecular Devices), and the results were plotted using Origin 10.0 (origin software). Experiments were conducted at room temperature of 22–25 °C. See the online Supplementary material 2 for a detailed protocol.
Molecular Docking and Molecular Dynamics
Molecular docking was conducted using Sybyl X-2.1.1 software, employing the Surflex-Dock Geom method for docking simulations. The three-dimensional structure of the target active compound was obtained from the NCBI database. The crystal structure of the NaV1.5 protein was retrieved from the RCSB Protein Data Bank (PDB ID: 6LQA). This PDB structure was imported into Sybyl to create an active site pocket based on the Ligand mode. Subsequently, the target protein was optimized and saved in SFXC format. All parameters were set to the default values within the Sybyl software. The preparation of the NaV1.5 protein included the addition of hydrogen atoms.38
Molecular Dynamics Simulations were conducted using Schrodinger/2023-4 (academic version), with initial conformations derived from the best-docked pose by Sybyl molecular docking. The OPLS_2005 force field was employed for the protein, while the protein structure was placed at the center of a cubic box filled with SPC water molecules. The study was carried out under isothermal-isobaric (NPT) conditions, maintaining a temperature of 310K (37 °C), with a total of 160069 atoms. The boundaries of the box were strategically set to ensure a minimum distance of 10Å between the farthest point of the protein and the box edge, establishing an adequate water buffer. To balance the overall charge, a small amount of Na+ and Cl− ions were introduced, resulting in a solution containing 0.15 M NaCl.
Prior to initiating the simulations, energy minimization was performed to correct for any possible steric overlaps. This was achieved using the steepest descent method and conjugate gradient method for energy optimization. To maintain consistency in the simulations, the Berendsen thermostat and barostat were utilized, keeping the temperature at 310 K and the pressure at 1 atm (101.325 kPa). Following the initialization and energy optimization steps, the system entered the molecular dynamics simulation phase, which spanned 100 ns, with trajectory data captured every 1 ns. The simulation results were saved in the data file.
hiPSC-Derived Cardiomyocytes Culture
hiPSC-derived cardiomyocytes (Cat log no. CA2201106) were purchased from Saibei Biological Company (Beijing, China). Cells were cultured on a CardioExcyte 96 sensor plate (Nanion Technologies GmbH, Germany) at 37°C and 5% CO2 for 4 days to allow the formation of a stable synchronous beating pattern. The whole medium (Cat. no. CA203100, Saibei Biological company) was changed every 48 h, and online parameters were monitored daily with the CardioExcyte Control software (Nanion Technologies GmbH, Germany).
CardioExcyte 96 Recordings
Cells were seeded in 96-well plates at a density of 4.0 × 104 cells/well of a CardioExcyte 96 system. Before compound addition, 200 μL fresh media were changed to ensure the exact volume per well. Online parameters were monitored for 2 h to ensure a stable baseline. Information about compound plates and reference solutions was inserted before recording. Culture media was used for LA stock preparation at 10x concentration and serial dilution. The final DMSO concentration was less than 0.1%, which did not affect sodium currents. After the application of the compound, impedance and EFP measurements were taken every 5 mins for 30 s for 2 h and then changed to every 60 mins for 30 s for 10 h.39
Immunofluorescence Assay (IF)
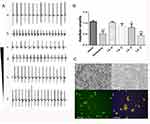
Cardiomyocytes induced by human stem cells not only maintain the pluripotency of stem cells that was verified by pluripotent marker OCT4 which should be expressed in greater than 95% of the cells,40 but also express cardiomyocyte-specific proteins such as α-actinin. Cardiomyocytes derived from hiPSC were seeded in 96-well plates at a density of 1.0 × 104 cells/well and kept at 37°C with 5% CO2 for 24 h. The attached cells were fixed with 4% paraformaldehyde, perforated with 10% Trizon for 20 mins, and then blocked for 2 h. The cells were incubated at 4°C overnight with OCT4 (1:500 dilution) and α-actinin (1:200 dilution) antibodies for 24 h, rinsed with PBS 3 times, incubated with Donkey Anti-Rabbit IgG H&L (Alexa Fluor® 555, 1:500 dilution) and Donkey Anti-Rabbit IgG H&L (Alexa Fluor® 488, 1:500 dilution) for 2 h and washed with PBS 3 times. Subsequently, Hoechst was added to identify the nuclei. After 30 mins incubation, the cells were washed with PBS 3 times and then subjected to immunofluorescence imaging analysis using a PE Operetta instrument in the dark with a ×200 objective.
Statistics Analysis
The data were subjected to statistical analysis, which involved the utilization of paired t-tests, unpaired t-tests, and one-way repeated measures or multiple comparison analysis of ANOVA followed by Bonferroni’s test, as appropriate. The mean ± SD was used to express all data. A p-value of less than 0.05 was deemed significant.
Results
Validation of the High-Throughput NaV1.5 Ion Channel Recordings by IonWorks Barracuda
The activation properties of INa current in CHL cells stably expressing SCN5A in an automated patch clamping system (IonWorks Barracuda) vs in a conventional manual recording system were compared. Since the PPC mode, which featured 64 holes per well and an average current from 64 cells, was successful in recording 375 of the 384 wells tested, it was used to screen the WXKL library. During the optimization process, the mean currents were approximately −1.5 nA, and the mean seal resistances were >20 MΩ. Additionally, 98.53% of the baseline3/baseline1 current was over 0.9, indicating a minimal run down.
The INa currents recorded by the conventional patch clamp electrophysiology or by IonWorks Barracuda are shown in Figure 1A and B. To determine the voltage dependence of INa activation (Figure 1C and D), current–voltage curves were generated based on peak currents. The voltage at which the channels were half-activated was −39.9 ± 1.0 mV by conventional electrophysiology and −39.9 ± 0.5 mV by automated patch clamp. The slope of Boltzmann fits of INa activation was 2.31 ± 0.28 on the conventional patch clamp and 4.9 ± 0.5 on the automated patch clamp, indicating a significant difference (P < 0.01).
Using tetracaine (an INa inhibitor) as a positive control, the feasibility of utilizing the automated patch clamp for a concentration-response assay of INa blockers was explored. The concentration–response curve was first established on a manual patch clamp to examine the stability of INa and the seal resistances over external buffer additions. The tetracaine suppression of INa was concentration-depend with an IC50 of 0.28 ± 0.03 μM (Figure 1E). On IonWorks Barracuda, the IC50 for tetracaine was 3.44 ± 0.12 μM (Figure 1F). The sigmoidal curve was fit by 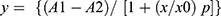 , using ORIGIN 9.0 Microcal Software. The INa recorded from the automated patch clamp was compared with the manual patch clamp current (Figure 1G), which also showed a good correlation between the manual patch clamp and the automated patch clamp (R2=0.9335).
, using ORIGIN 9.0 Microcal Software. The INa recorded from the automated patch clamp was compared with the manual patch clamp current (Figure 1G), which also showed a good correlation between the manual patch clamp and the automated patch clamp (R2=0.9335).
HTP Screen of the WXKL Fractions for INa Activity
Next, we examined the effects of 130 fractions prepared by the HPLC on the INa (Figure 2A). We used the automated patch clamp to validate the inhibitors and added the 130 fractions to the external bath. After a series of optimizations for several important screening parameters, we used a standard procedure for the automated patch clamp to screen an in-house collection of the 130 fractions. With an initial test concentration of 2 g/L, we selected extracts that inhibit INa for further investigation. Of all the extracts tested, five were found to be active, including 50% methanol aqueous solution extracts, which are No. 82, 97, 102, 105, and 121 (Figure 2B).
Identification of WXKL Active Monomers
HPLC-ESI-IT-TOF-MS analysis was performed to identify WXKL active compounds (Figure 3). The total ion chromatograms of the whole WXKL preparation (Figure 3B) showed major peaks resolved compared to the blank solution (Figure 3A). Secondary separation of one of the active fractions (#82 in Figure 2B) identified that the 3rd, 4th, 8th, and 11th peaks are lauric acid (LA), myristic acid, palmitic acid, and stearic acid, respectively (Figure 3C and Table 1).
 |
Table 1 Identification of Active Fractions of WXKL by HPLC-ESI-IT- TOF-MS |
 |
Figure 3 HPLC-MS characterization of the chemical constituents in the active fraction of WXKL. (A) HPLC-MS total ion chromatogram of blank solution. (B) Ion chromatogram (positive ion) of whole WXKL. (C) Ion chromatograms (positive ion) of fraction No.82. Peaks 3, 4, 8 and 11 were putatively identified as lauric acid, myristic acid, palmitic acid and stearic acid, respectively as shown in Table 1. |
Since the INa-blocking positive fractions tend to be of lower polarity, they were submitted to GC-MS for analysis to confirm the chemical identities. Compared to that of blank (Figure 4A) and LA standard (Figure 4C), the total ion chromatogram of WXKL (Figure 4B) positively identified LA in WXKL. A comparison of the qualitative LA fingerprint patterns retrieved from the NIST library (Figure 4D), and the one generated by MS fragmentation (Figure 4E) showed a perfect match. Quantitatively, the percentage of LA in WXKL is about 2%, which is relatively high, and the literature suggested the source could be from Codonopsis pilosula (Franch). Nannf. and Polygonatum sibiricum Red.
Effects of Lauric Acid on NaV1.5 Ion Channel Activation
The INa was elicited by a single-step pulse from −120 to −20 mV, sustained with 50 ms. As a result, whole-cell voltage-clamp traces from CHL cells expressing INa exert a concentration-dependent curve. When the time course of INa was examined, LA had the function of dose-dependent suppress INa (Figure 5). The inhibition of LA on NaV1.5 was reversible after the washout of the perfusate. The IC50 for LA was 27.40 ± 12.78 μM.
The activation curves in the absence and presence of 25 μM LA exhibited overlap (Figure 6A). The halfway channel activation was at −40.20 ± 3.7 mV and −42.80 ± 1.5 mV for the control and LA, respectively. At a membrane potential at −80 mV, currents were generated by double-pulse involving a test pulse of 25 ms at −30 mV after a pre-pulse of 500 ms that ranged from −160 to −30 mV in increments of 5 mV, at a frequency of 0.1 hz. Bath perfusion of 25 μM LA solution significantly suppressed INa and LA washout reversed the inhibition effect (Figure 6A). The normalized steady-state inactivation curve of INa was significantly shifted toward the hyperpolarizing direction, from −61.50±1.8 mV for the control to −82.90 ± 2.4 mV (P<0.001) in the presence of 25 μM LA. Therefore, the results in Figure 6 illustrate that 25 μM LA modified the steady-state inactivation.
The impact of LA on the kinetics of INa recovery from inactivation was further studied using a pulse protocol that involved a depolarizing pulse from −80 mV to −65 mV, followed by a hyperpolarizing pulse of −140 mV with gradually increasing durations, and a subsequent test pulse of −30 mV. The results indicated that the presence of 25 μM LA significantly prolonged the time taken for halfway recovery from inactivation of INa, from 2.10 ± 0.8 ms in the control group to 34.80 ± 2.1 ms (Figure 6B, P < 0.001).
A double exponential function was used to model the time course of recovery from inactivation of INa in the absence and presence of LA. The equation used was y = A0 + A1exp-t/τ1+ A2exp-t/τ2. In the absence of LA, the amplitudes and time constants for recovery from inactivation of INa were −30.70 ± 7.5% (A1), −3.50 ± 0.9% (A2), 1.10 ± 0.3ms (τ1), and 175.20 ± 168.5 ms (τ2). In the presence of LA, the amplitudes and time constants for recovery from inactivation of INa were −87.60 ± 10.00% (A1), −12.90 ± 2.1% (A2), 1.50 ± 0.3ms (τ1), and 150.00 ± 44.3 ms (τ2). Although there was a decrease in τ2 in the presence of 25 μM LA, this difference was not statistically significant due to measurement variability. Additionally, τ1 was not significantly increased in the presence of LA. These findings suggest that LA may affect both the fast and slow components of NaV1.5 inactivation.
LA May Block the Inward Flow of Na+ Through Binding to the Pore Region of NaV1.5 Channel
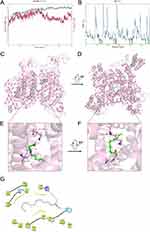
The structural stability of the protein and ligand during the molecular dynamics simulation is reflected in the RMSD curves shown in Figure 7A. The blue curve represents the RMSD of the protein, which reached an equilibrium state after approximately 60 ns of simulation, indicating that the protein structure remained relatively stable after this time point. In contrast, the red curve representing the ligand’s RMSD exhibited significant fluctuations, especially within the first 60 ns, which may be associated with the high flexibility of the ligand within the binding pocket. However, as time progressed, the fluctuations of the ligand gradually diminished and tended towards equilibrium in the last 1 ns, suggesting that the ligand’s binding became more stable.
Figure 7B displays the RMSF curves, which illustrate the changes in the flexibility of different residues during the molecular dynamics simulation. The RMSF values of the residues varied between 1 and 10 Å, with peaks corresponding to regions of higher flexibility, typically located on the protein surface or near the active pocket. In comparison, residues with lower RMSF values were more stable, possibly situated in the core of the protein or regions involved in strong interactions with other molecules.
Analysis of the trajectory in the last 1 ns of the dynamics, as shown in Figure 7C to E, reveals that lauric acid forms ionic interactions with amino acid LYS 1419. Additionally, lauric acid establishes hydrogen bond interactions with ASN 927. The formation of these ionic and hydrogen bonds is likely crucial for the stability of the ligand’s binding. These interactions suggest that lauric acid binds to the NaV1.5 channel through a combination of ionic and hydrogen bonding interactions, which may play a significant role in modulating the channel’s activity.
LA Lowered the Amplitude of EFP in Stem Cell-Derived Cardiomyocytes
To evaluate the possible impact of LA on hiPSC-derived cardiomyocytes (hiPSC-CMs), the extracellular field potential (EFP) generated by the propagation of action potentials was monitored from hiPSC-MC along the electrode array. Cardiomyocytes induced by human stem cells maintained the pluripotency of stem cells and expressed the specific cardiomyocyte markers (Figure 8C). Since the EFP signal is similar to the electrocardiogram (ECG) of the heart, the recorded impedance provides an indirect assessment of the cell’s contractions. In the absence of any experimental intervention, the cardiomyocytes exhibited spontaneous and consistent EFP and impedance signals, with a rate of 54 ± 6 beats per minute. The presence of LA from 1 mM to 100 nM inhibited EFP amplitude in a dose-dependent manner. The amplitude variability of 10−3M LA was 0.43 ± 0.06, and 10−4M LA was 0.80 ± 0.20. As a control, the amplitude variability of INa current inhibitor tetracaine (10 nM) was 0.52 ± 0.12 (Figure 8A and B). A lower concentration of LA (<100 nM) did not affect the peak sodium current in hiPSC-derived cardiomyocytes. Our results demonstrated that LA could reduce NaV1.5 availability during the cardiac action potential.
Discussion
In the current study, we screened 130 fractions of WXKL and revealed that lauric acid, a 12-carbon atom medium chain fatty acid (MCFA) from Rhizoma Polygonati,41 which can block the NaV1.5 channel. Research findings indicate that common dietary PUFAs of both the ω-3 and ω-6 fatty acids can modulate the contraction rate of spontaneously beating neonatal cardiac myocytes.42 The normalized steady-state inactivation curve of INa was significantly shifted to unsteady-state activation and recovery, with the same result compared to lauric acid. Screened fatty acids almost all are with two or more unsaturated carbon bonds (C=C), but lauric acid is a saturated fatty acid. Myristic acid, palmitic acid, and stearic acid are the same as lauric acid; we have identified that the solubility of these acids is low negatively to block the NaV1.5 channel.
Dietary and policy recommendations have traditionally emphasized the importance of cutting down on saturated fatty acid intake to enhance cardiometabolic health. These recommendations have mainly been drawn from ecological and animal studies. However, with the latest breakthroughs in nutritional science, it has become possible to examine crucial issues relating to the impact of saturated fatty acids on health.43 Free fatty acids are crucial constituents of cells, serving pivotal functions in lipid and carbohydrate metabolism and fundamental in configuring the physicochemical qualities of the lipid bilayer and the cell’s response to its surroundings. While the human body produces some fatty acids, certain essential fatty acids, primarily PUFAs, must be obtained through dietary intake. Consuming α-linolenic and linoleic acid is imperative for acquiring the necessary PUFAs. Fatty acids have important roles as cellular messengers in signaling and as essential components of plasma membrane phospholipids. Most naturally occurring fatty acids possess a carboxylic acid structure combined with an unbranched aliphatic hydrocarbon tail. PUFAs are capable of modulating ion channels, consequently exerting substantial physiological and pharmacological influences. Studies have suggested that various types of PUFAs affect certain voltage-sensor domains in ion channels, including but not limited to voltage-gated sodium, potassium, calcium, and proton channels, as well as calcium-activated potassium and transient receptor potential channels. While some effects of fatty acids appear to be specific to certain channels, others exhibit more generalized characteristics.44
Although there were no previous studies on ion channel regulation by lauric acid, other pharmacological activities have been reported. For instance, lauric acid alleviates insulin resistance,45 improves hormonal profiles, antioxidant properties, sperm quality in diabetic infertility rats,46 provides neuroprotection against oxidative stress in hyperglycemic stroke,47 inhibits Escherichia coli persistence and biofilm formation,48 alleviates deoxynivalenol-induced intestinal stem cell damage,49 accelerates glycolytic muscle fiber formation,50 attenuates hepato-metabolic complications in high-fat diet-induced nonalcoholic fatty liver disease and ameliorates lipopolysaccharide-induced liver inflammation rats.51,52 Most interestingly, LA modulates cancer-associated microRNA expression, inhibits cancer cell growth,53 and overcomes hypoxia-induced gemcitabine chemoresistance in pancreatic ductal adenocarcinoma.54 Whether these anticancer effects are due to the sodium channel-blocking activity remains to be investigated.
With the advancements in biological technology and the introduction of patch clamp techniques, the understanding of arrhythmia mechanisms has become more precise. Notably, natural medicines from various Chinese medicines have been acknowledged globally for their potent antiarrhythmic properties. The complex chemical structures of these medicines include alkaloids, cardiac glycosides, flavonoids, saponins, coumarins, and naphtha. This study exclusively focused on NaV1.5, and there is a need for further research to screen other cardiac channels. It should be noted that future studies should involve in vivo arrhythmic animal models, and drug candidates must be tested for activity at the INa cardiac sodium channel before progressing to clinical trials. It is also worth noticing that since many ion channels are found to be critical in cancers, one of the potential pharmacological effects of traditional toxin medicines is their regulation of ion channels in cancers.55 The regulation of ion channels in cancer therapy to exert local anesthetic effects provides a positive idea for the application and expansion of ion channel inhibitors, and the screening of small molecule compounds for voltage-gated sodium channels will provide a more comprehensive choice for clinical treatment.56,57 Interestingly, the latest research has found that voltage-gated sodium channels also play an important role in cancer.58 Identifying potential liabilities of a molecule early in a lead optimization can save drug developers significant time and resources required to advance it to first-time human studies. Interestingly, we found that LA does not have a typical structure for sodium channel modulators.59 However, since the active fraction of WXKL in our high-throughput screen contained a series of saturated fatty acids with different alkyl chain lengths, we also tested myristic acid (14-C alkane chain), palmitic acid (16-C alkane chain), and stearic acid (18-C alkane chain). Only the lauric acid (12-C alkane chain) had a significant inhibitory effect on NaV1.5. This suggests that the blocking effect of saturated fatty acids on sodium channels may be related to the length of the alkane chain (see Supplementary material 1 for details). We performed molecular docking and molecular dynamics simulations of lauric acid with the pore region of the NaV1.5 protein and found that the carboxyl group of LA primarily interacts through hydrogen bonding with the amino acid side chains in the cavity below the ion selectivity filter region, close to the intracellular side of the pore (Figure 7E and G). Its alkyl chain extends towards the intracellular side, with the terminal C atom located precisely at the exit of the cavity facing the intracellular side, and it is less than an atomic distance from the nearest S6 helix structure (Figure 7F). These results suggest that LA may reduce sodium currents by directly obstructing the sodium ion permeation path, and the length of the alkyl chain determines that an excessively long saturated alkyl chain structure cannot form a reasonable pose to block the permeation path in the pore region.
Finally, as reported in many studies, maximal peak INa amplitudes appear considerably larger in hiPSC-CMs than those reported for native human ventricular CMs.60 The change of field potential has a certain correspondence with the opening of EFP waveforms and action potentials. The highest peak of EFP represents the depolarization corresponding to the sodium channel. By this, we once again demonstrated the inhibitory effect of LA on sodium channel currents. In summary, the outcomes of our study propose that lauric acid found in Chinese medicine Wenxin Keli is a robust inhibitor of NaV1.5. The IonWorks Barracuda automated electrophysiology platform is adept at assessing the impact of natural products on ion channel regulation in a comprehensive manner. This enables high-throughput, cost-effective, and predictive testing of INa through the electrophysiology technique.
Voltage-gated Na+ channel blockers constitute a significant category of antiarrhythmic agents, as outlined in the latest classification of such medications.61 The results of this study suggest that lauric acid may have the potential to alleviate arrhythmia. However, this preliminary finding necessitates more vigorous validation. One limitation of the present investigation is that although we provided in vitro evidence that lauric acid is antiarrhythmic using hiPSC-derived cardiomyocytes (Figure 8), in vivo evidence is still needed. Subsequent research will aim to further substantiate the pharmacological effects of lauric acid in more intricate animal models before its clinical application as an antiarrhythmic agent.
Conclusion
In the current study, we established a large-scale, cell-based screen of HPLC-prepared fraction library of a compound Chinese medicine Wenxin Keli. A high-throughput automatic patch clamp INa assay followed by refined structural resolution using HPLC-ESI-IT-TOF-MS/GC-MS identified lauric acid as a potent NaV1.5 channel blocker. Molecular docking analysis suggested that LA binds to the pore region of NaV1.5 protein and blocks the inward flow of Na+. Manual patch-clamping and cell-based EFP assays in hiPSC-derived cardiomyocytes reveal that LA may affect both the fast and slow components of NaV1.5 inactivation and is antiarrhythmic in vitro.
Abbreviations
WXKL, Wenxin Keli; LA, lauric acid; CHL, Chinese Hamster Lung; GC-MS gas chromatography-tandem mass spectrometric; HPLC, High Performance Liquid Chromatography; NaV1.5, human SCN5A gene; INa, NaV1.5 sodium current; PUFA, Polyunsaturated fatty acids; EFP, extracellular field potential; HTP, High throughput.
Data Sharing Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding authors.
Acknowledgment
We thank our colleagues, Drs Peng Zhang, Meng Wang, Yuxin Feng, Jian Yang, Ying Cui, Pengzhi Dong, and John Orgah for stimulating discussions and their technical support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the National Natural Science Foundation [82474109 to Y Zhu] and [82374075 to T Wang], the International Cooperation Project of MOST [2013DFA31620 to Y Zhu], the National Key R&D Program Project (China) [2018YFC1704502 to Y Zhu], the Postdoctoral Fellowship Program of CPSF [GZC20242024 to J Gao] and Taishan Scholar Youth Project of Shandong Province [tsqn202306188 to T Wang].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Huizar JF, Ellenbogen KA, Tan AY, Kaszala K. Arrhythmia-induced cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(18):2328–2344. doi:10.1016/j.jacc.2019.02.045
2. Ruskin JN. The cardiac arrhythmia suppression trial (CAST) investigators. preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppress ion after myocardial in fraction. N Engl J Med. 1989;321:406–412.
3. Antzelevitch C, Burashnikov A. Atrial-selective sodium channel block as a novel strategy for the management of atrial fibrillation. J Eletrocardiol. 2009;42(6):543–548. doi:10.1016/j.jelectrocard.2009.07.007
4. Kanmanthareddy A, Reddy M, Ponnaganti G, et al. Alternative medicine in atrial fibrillation treatment-Yoga, acupuncture, biofeedback and more. J Thorac Dis. 2015;7(2):185–192. doi:10.3978/j.issn.2072-1439.2015.01.13
5. Brenyo A, Aktas MK. Review of complementary and alternative medical treatment of arrhythmias. Am J Cardiol. 2014;113(5):897–903. doi:10.1016/j.amjcard.2013.11.044
6. Xiao YF, Sterling NW, Ging KW, James PM, Alexander F. Fatty acid suppresses voltage-gated Na+ current in HEK293 cells transfected with the α-subunit of the human cardiac Na+ channel. Physiology. 1998;95(5):2680–2685.
7. Burashnikov A. Late INa inhibition as an antiarrhythmic strategy. J Cardiovasc Pharmacol. 2017;70(3):159–167. doi:10.1097/FJC.0000000000000510
8. Burashnikov A, Antzelevitch C. Effectiveness of late INa versus Peak INa block in the setting of ventricular fibrillation. Circ Arrhythm Electrophysiol. 2017;10(3):e005111. doi:10.1161/CIRCEP.117.005111
9. Wang Q, Shen J, Splawski I, et al. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80(5):805–811. doi:10.1016/0092-8674(95)90359-3
10. Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The cardiac arrhythmia suppression trial. N Engl J Med. 1991;324(12):781–788. doi:10.1056/NEJM199103213241201
11. Huang P, Luo Y, Chen J, et al. Efficacy and safety of Wenxin Keli combined with metoprolol tartrate in the treatment of premature ventricular contractions: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;29(9):952657. doi:10.3389/fcvm.2022.952657
12. Shi Q, Chen J, Yu Y. Efficacy analysis of Wenxin granule combined with metoprolol tartrate in the treatment of elderly patients with arrhythmia. Minerva Med. 2024;115(4):516–518. doi:10.23736/S0026-4806.22.08479-8
13. Xue X, Guo D, Sun H, et al. Wenxin Keli suppresses ventricular triggered arrhythmias via selective inhibition of late sodium current. Pacing Clin Electrophysiol. 2013;36(6):732–740. doi:10.1111/pace.12109
14. Chen Y, Li Y, Guo L, et al. Effects of Wenxinkeli on the action potential and L-type calcium current in rats with transverse aortic constriction-induced heart failure. Evid Based Complement Alternat Med. 2013;2013:572078. doi:10.1155/2013/572078
15. Wang X, Wang Y, Feng X, et al. Systematic review and meta-analysis of randomized controlled trials on Wenxin keli. Drug Des Devel Ther. 2016;10:3725–3736. doi:10.2147/DDDT.S112333
16. Li M, Qiu R, Tian G, et al. Wenxin Keli for ventricular premature complexes with heart failure: a systematic review and meta-analysis of randomized clinical trials. Complement Ther Med. 2017;33:85–93. doi:10.1016/j.ctim.2017.06.006
17. He M, Lv Z, Yang ZW, Huang JL, Liu F. Efficacy and safety of Chinese herbal medicine Wenxin Keli for ventricular premature be ats: a systematic review. Complement Ther Med. 2016;29:181–189. doi:10.1016/j.ctim.2016.10.007
18. Minoura Y, Panama BK, Nesterenko VV, et al. Effect of Wenxin Keli and Quinidine to suppress arrhythmogenesis in an experimental model of Brugada syndrome. Heart Rhythm. 2013;10(7):1054–1062. doi:10.1016/j.hrthm.2013.03.011
19. Antzelevitch C, Patocskai B. Brugada syndrome: clinical, genetic, molecular, cellular, and ionic aspects. Curr Probl Cardiol. 2016;41(1):7–57. doi:10.1016/j.cpcardiol.2015.06.002
20. Chen Y, Xiong X, Wang C, et al. The effects of wenxin keli on left ventricular ejection fraction and brain natriuretic peptide in patients with heart failure: a meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2014;2014:242589. doi:10.1155/2014/242589
21. Chen Y, Nie S, Gao H, et al. The effects of wenxin keli on p-wave dispersion and maintenance of sinus rhythm in patients with paroxysmal atrial fibrillation: a meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2013;2013:245958. doi:10.1155/2013/245958
22. Kalifa J, Avula UM. The Chinese herb extract Wenxin Keli: atrial selectivity from the Far East. Heart Rhythm. 2012;9(1):132–133. doi:10.1016/j.hrthm.2011.11.030
23. Wu A, Zhao M, Lou L, et al. Effect of Wenxin granules on gap junction and MiR-1 in rats with myocardial infarction. Biomed Res Int. 2017;2017:3495021. doi:10.1155/2017/3495021
24. Burashnikov A, Petroski A, Hu D, Barajas-Martinez H, Antzelevitch C. Atrial-selective inhibition of sodium-channel current by WenxinKeli is effective in suppressing atrial fibrillation. Heart Rhythm. 2012;9(1):125–131. doi:10.1016/j.hrthm.2011.08.027
25. Hua W, Gao RL, Zhao BC, et al. The efficacy and safety of Wenxin Keli in patients with frequent premature ventricular contractions: a randomized, double-blind, placebo- controlled, parallel-group, multicenter trial. Chin Med J. 2015;128(19):2557–2564. doi:10.4103/0366-6999.166026
26. Luo A, Liu Z, Cao Z, et al. Wenxin keli diminishes Ca2+overload induced by hypoxia/reoxygenation in cardiomyocytes through inhibiting INaL and ICaL. Pacing Clin Electrophysiol. 2017;40(12):1412–1425. doi:10.1111/pace.13206
27. Hou JW, Li W, Guo K, et al. Antiarrhythmic effects and potential mechanism of WenXin KeLi in cardiac Purkinje cells. Heart Rhythm. 2016;13(4):973–982. doi:10.1016/j.hrthm.2015.12.023
28. Yang X, Chen Y, Li Y, Ren X, Xing Y, Shang H. Effects of Wenxin Keli on cardiac hypertrophy and arrhythmia via regulation of the calcium/calmodulin dependent kinase II signaling pathway. Biomed Res Int. 2017;2017:1569235. doi:10.1155/2017/1569235
29. Xing Y, Gao Y, Chen J, et al. Wenxin-Keli regulates the calcium/calmodulin-dependent protein kinase II signal transduction pathway and inhibits cardiac arrhythmia in rats with myocardial infarction. Evid Based Complement Alternat Med. 2013;2013:464508. doi:10.1155/2013/464508
30. Patocskai B, Antzelevitch C. Novel therapeutic strategies for the management of ventricular arrhythmias associated with the Brugada syndrome. Expert Opin Orphan Drugs. 2015;3(6):633–651. doi:10.1517/21678707.2015.1037280
31. Wang X, Wang X, Gu Y, Wang T, Huang C. Wenxin Keli attenuates ischemia-induced ventricular arrhythmias in rats: involvement of L type calcium and transient outward potassium currents. Mol Med Rep. 2013;7(2):519–524. doi:10.3892/mmr.2012.1195
32. Liu Y, Zhang Z, Yang Y, Zhang N, Li G, Liu T. The Chinese herb extract Wenxin Keli: a promising agent for the management of atrial fibrillation. Int J Cardiol. 2016;203:614–615. doi:10.1016/j.ijcard.2015.10.211
33. Wang T, Lu M, Du Q, et al. An integrated anti-arrhythmic target network of a compound Chinese medicine, Wenxin Keli, revealed by combined machine learning and molecular pathway analysis. Mol Biosyst. 2017;13(5):1018–1030. doi:10.1039/C7MB00003K
34. Gillie DJ, Novick SJ, Donovan BT, Payne LA, Townsend C. Development of a high-throughput electrophysiological assay for the human ether-à-go-go related potassium channel hERG. Pharmaco Toxicol Meth. 2013;67(1):33–44. doi:10.1016/j.vascn.2012.10.002
35. Schroeder K, Neagle B, Trezise DJ, Worley J. Ionworks HT: a new high-throughput electrophysiology measurement platform. J Biomol Screen. 2003;8(1):50–64. doi:10.1177/1087057102239667
36. Wu HH, Wang ZP, Nan G, Chen YP, Lu CS, Zhu Y. Analysis of volatile components of Wenxin granule by HS-SPME-GC-MS technique. Chinese J Exp Trad Med Form. 2015;21(11):73–76.
37. Rotordam MG, Obergrussberger A, Brinkwirth N, et al. Reliable identification of cardiac conduction abnormalities in drug discovery using automated patch clamp II: best practices for NaV1.5 peak current in a high throughput screening environment. J Pharmacol Toxicol Methods. 2021;112:107125. doi:10.1016/j.vascn.2021.107125
38. Li Z, Jin X, Wu T, et al. Structural basis for pore blockade of the human cardiac sodium channel Nav 1.5 by the antiarrhythmic drug quinidine. Angew Chem Int Ed Engl. 2021;60(20):11474–11480. doi:10.1002/anie.202102196
39. Bot CT, Juhasz K, Haeusermann F, Polonchuk L, Traebert M, Stoelzle-Feix S. Cross - site comparison of excitation-contraction coupling using impedance and field potential recordings in hiPSC cardiomyocytes. J Pharmacol Toxicol Methods. 2018;93:46–58. doi:10.1016/j.vascn.2018.06.006
40. Lian X, Zhang J, Azarin SM, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nature Protocols. 2013;8(1):162–175. doi:10.1038/nprot.2012.150
41. Wang J, Yue Y, Tang F, Tao W. Comparative analysis of volatile fractions in Polygonati Rhizoma and its processed products by GC-MS. Chian J Chinese Materia Medica. 2011;36(16):2187–2191.
42. Jing XK, Alexander L. Effects of long-chain polyunsaturated fatty acids on the contraction of neonatal rat cardiac myocytes. Physiology. 1994;91(21):9886–9890.
43. Micha R, Mozaffarian D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: a fresh look at the evidence. Lipids. 2010;45(10):893–905. doi:10.1007/s11745-010-3393-4
44. Fredrik E, Sara I. Actions and mechanisms of polyunsaturated fatty acids on voltage-gated ion channels. Front Physiol. 2017;2(6):103389.
45. Tham YY, Choo QC, Muhammad TST, Chew CH. Lauric acid alleviates insulin resistance by improving mitochondrial biogenesis in THP-1 macrophages. Mol Biol Rep. 2020;47(12):9595–9607. doi:10.1007/s11033-020-06019-9
46. Anuar NS, Shafie SA, Maznan MAF, et al. Lauric acid improves hormonal profiles, antioxidant properties, sperm quality and histomorphometric changes in testis and epididymis of streptozotocin-induced diabetic infertility rats. Toxicol Appl Pharmacol. 2023;470:116558. doi:10.1016/j.taap.2023.116558
47. Shaheryar ZA, Khan MA, Hameed H, Zaidi SAA, Anjum I, Rahman MSU. Lauric acid provides neuroprotection against oxidative stress in mouse model of hyperglycaemic stroke. Eur J Pharmacol. 2023;956:175990. doi:10.1016/j.ejphar.2023.175990
48. Jin X, Zhou J, Richey G, Wang M, Hong SMC, Hong SH. Undecanoic acid, lauric acid, and N-tridecanoic acid inhibit Escherichia coli persistence and biofilm formation. J Microbiol Biotechnol. 2021;31(1):130–136. doi:10.4014/jmb.2008.08027
49. Liu ZH, Xie WW, Zan GX, et al. Lauric acid alleviates deoxynivalenol-induced intestinal stem cell damage by potentiating the Akt/mTORC1/S6K1 signaling axis. Chem Biol Interact. 2021;348:109640. doi:10.1016/j.cbi.2021.109640
50. Wang L, Luo L, Zhao W, et al. Lauric acid accelerates glycolytic muscle fiber formation through TLR4 signaling. J Agric Food Chem. 2018;66(25):6308–6316. doi:10.1021/acs.jafc.8b01753
51. Sedik AA, Elgohary R, Khalifa E, et al. Lauric acid attenuates hepato-metabolic complications and molecular alterations in high-fat diet-induced nonalcoholic fatty liver disease in rats. Toxicol Mech Methods. 2024;34(4):454–467. doi:10.1080/15376516.2023.2301344
52. Khan HU, Aamir K, Jusuf PR, et al. Lauric acid ameliorates lipopolysaccharide (LPS)-induced liver inflammation by mediating TLR4/MyD88 pathway in Sprague Dawley (SD) rats. Life Sci. 2021;265:118750. doi:10.1016/j.lfs.2020.118750
53. Verma P, Ghosh A, Ray M, Sarkar S. Lauric acid modulates cancer-associated microRNA expression and inhibits the growth of the cancer cell. Anticancer Agents Med Chem. 2020;20(7):834–844. doi:10.2174/1871520620666200310091719
54. Takagi T, Fujiwara-Tani R, Mori S, et al. Lauric acid overcomes hypoxia-induced gemcitabine chemoresistance in pancreatic ductal adenocarcinoma. Int J Mol Sci. 2023;24(8):7506. doi:10.3390/ijms24087506
55. Hengrui L. Toxic medicine used in Traditional Chinese Medicine for cancer treatment: are ion channels involved? J Tradit Chin Med. 2022;42(6):1019–1022. doi:10.19852/j.cnki.jtcm.20220815.005
56. Sonkin D, Thomas A, Teicher BA. Cancer treatments: past, present, and future. Cancer Genet. 2024;286–287:18–24. doi:10.1016/j.cancergen.2024.06.002
57. Liu H, Weng J, Huang CL, Jackson AP. Voltage-gated sodium channels in cancers. Biomark Res. 2024;12(1):70. doi:10.1186/s40364-024-00620-x
58. Liu H, Dilger JP, Lin J. Effects of local anesthetics on cancer cells. Pharmacol Ther. 2020;212:107558. doi:10.1016/j.pharmthera.2020.107558
59. Ahmed M, Jalily Hasani H, Ganesan A, Houghton M, Barakat K. Modeling the human Nav1.5 sodium channel: structural and mechanistic insights of ion permeation and drug blockade. Drug Des Devel Ther. 2017;4(11):2301–2324. doi:10.2147/DDDT.S133944
60. Casini S, Verkerk AO, Remme CA. Human iPSC-derived cardiomyocytes for investigation of disease mechanisms and therapeutic strategies in inherited arrhythmia syndromes: strengths and limitations. Cardiovasc Drugs Ther. 2017;31(3):325–344. doi:10.1007/s10557-017-6735-0
61. Lei M, Wu L, Terrar DA, Huang CL. Modernized classification of cardiac antiarrhythmic drugs. Circ. 2018;138(17):1879–1896. doi:10.1161/CIRCULATIONAHA.118.035455
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.