Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
IL-20RA is Associated with the Risk of Diabetic Microangiopathy: A Bidirectional Mendelian Randomization Analysis and Clinical Validation
Authors Li J , Yang H, Wang T, Ruan N, Lin Y, Fang Z
Received 29 May 2024
Accepted for publication 28 October 2024
Published 18 December 2024 Volume 2024:17 Pages 4803—4816
DOI https://doi.org/10.2147/DMSO.S480366
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Jinju Li,1 Hao Yang,2 Tingting Wang,1 Nuobing Ruan,1 Yixuan Lin,3 Zhaohui Fang3,4
1The First Clinical Medical College of Anhui University of Traditional Chinese Medicine, Hefei, Anhui, People’s Republic of China; 2Department of Geriatrics, The Second Affiliated Hospital of Anhui University of Traditional Chinese Medicine, Hefei, Anhui, People’s Republic of China; 3Department of Endocrinology, The First Affiliated Hospital of Anhui University of Traditional Chinese Medicine, Hefei, Anhui, People’s Republic of China; 4Centre for Xin’an Medicine and Modernization of Traditional Chinese Medicine of IHM, Hefei, Anhui, People’s Republic of China
Correspondence: Zhaohui Fang, Department of Endocrinology, The First Affiliated Hospital of Anhui University of Traditional Chinese Medicine, Hefei, Anhui, People’s Republic of China, Email [email protected]
Objective: Studies have demonstrated a link between chronic inflammatory responses and diabetic microangiopathy, which include diabetic nephropathy, diabetic retinopathy, and diabetic neuropathy. However, it remains unclear whether there is a causal association between circulating inflammatory cytokines and the development of diabetic microvascular complications. This study aimed to investigate whether altered genetically predicted concentrations of circulating inflammatory cytokines were associated with the development of diabetic microvascular complications using two-sample Mendelian randomization (MR) analysis and clinical validation.
Methods: Pooled data on diabetic nephropathy, diabetic retinopathy, diabetic neuropathy, and 91 circulating inflammatory cytokines were obtained from publicly available databases. The analysis was conducted mainly using the inverse variance weighting (IVW) method and the results were assessed based on the odds ratio (OR) and 95% confidence interval (CI). In addition, the stability and reliability of the results were verified using the leave-one-out method, heterogeneity tests, and horizontal pleiotropy. Finally, ELISA and RT-qPCR were utilized to assess the expression of relevant inflammatory cytokines associated with diabetic microvascular complications.
Results: Mendelian randomization analysis identified a total of 9 circulating inflammatory cytokines that exhibit causal associations with the diabetic microangiopathy, with IL-20RA being a common risk factor for all three conditions. Clinical studies have found elevated plasma IL-20RA concentrations in patients with diabetic peripheral neuropathy, and RT-qPCR testing of peripheral blood mononuclear cells revealed significantly higher IL-20RA mRNA expression in patients with diabetic peripheral neuropathy as compared to normal individuals.
Conclusion: This study highlights the potential role of specific inflammatory cytokines in the development of diabetic microangiopathy (diabetic nephropathy, diabetic retinopathy and diabetic neuropathy). Additionally, IL-20RA emerges as a potential common risk factor for three diabetic microvascular complications. These findings may provide novel insights into early prevention and new therapeutic strategies for diabetic microvascular complications.
Keywords: Mendelian randomisation, circulating inflammatory cytokines, diabetic microangiopathy, IL-20RA
Introduction
Diabetes is a chronic, non-communicable, and multi-system disease. According to data reported by the International Diabetes Federation (IDF), China has the largest number of people with diabetes in the world, with approximately 141 million adults suffering from this disease in 2021, and this number is projected to reach 174.4 million by 2045.1 With the increase of diabetes prevalence and the aging of the population, the occurrence of diabetic microvascular complications (such as diabetic retinopathy, diabetic nephropathy, and diabetic neuropathy) may also increase simultaneously.2 It has been found that although hyperglycemia-induced damage to the cardiovascular, cerebrovascular, and other macrovascular systems is the main cause of death in patients, microvascular hazards such as renal, ophthalmic, and neurological effects caused by abnormal blood glucose are more prevalent.3 Diabetic nephropathy is a chronic hyperglycemia-caused abnormality of renal structure and function, with the manifestations of glomerulosclerosis, arteriolar sclerosis, and renal papillary necrosis. Diabetic nephropathy has now surpassed glomerulonephritis as the most common cause of chronic kidney disease and end-stage renal disease worldwide.4 Early manifestations of diabetic retinopathy mainly include endothelial damage, microaneurysm formation, and punctate intraretinal hemorrhages, and as the disease progresses, fibrous tissue proliferation, intravitreal neovascularization, and retinal detachment may occur, which may ultimately result in blindness.5 Research studies have shown that the prevalence of type 2 diabetic neuropathy in China ranges from 8.4% to 61.8%, and is a major risk factor for the development of lower limb ulcers, amputations, and disability, imposing a heavy financial burden on the healthcare system and society.6,7
It has been evidenced that the inflammatory response is strongly associated with the development of diabetes and its complications. Immune cell activation and cytokine interactions are important in the development of the inflammatory response in diabetes, immune cells are activated to trigger a series of responses, releasing various inflammatory cytokines such as TNF-α, IL-1β, and IL-6, thereby activating diverse inflammatory signaling pathwayssuch as nuclear factor κB (NF-κB) and signal transducer-activated activator of transcription factor 3 (STAT3), which can lead to target organ damage.8 Hyperglycemia is the main driving force of diabetic nephropathy, and microinflammation and extracellular matrix proliferation are the main causes of progression. Activated inflammatory signaling pathways and the increased expression of cell adhesion molecules, chemokines, and pro-inflammatory cytokines in the kidney tissues of diabetic nephropathy lead to glomerular inflammation and sclerosis and renal interstitial fibrosis, ultimately affecting kidney function.9,10 The retinal endothelial cells, microglia, astrocytes, Müller cells, and neurons interact to maintain retinal homeostasis. Under the conditions of chronic low-grade inflammation, the activation of microglia leads to an increase in the expression of inflammatory cytokines, such as IL-1β. This increase induces pericyte apoptosis through the NF-κB signaling pathway, increases vascular permeability, and stimulates the Toll-like receptor 4 (TLR4) pathway through advanced glycation end products (AGEs), enhancing the expression of the angiogenic factor Galectin-1 in macrophages and microglia, thereby contributing to the development of diabetic retinopathy.11–13 The pathogenesis of diabetic neuropathy is complex, involving the interaction of metabolic, vascular, and neurotrophic factors. Studies have shown that pro-inflammatory cytokines, induced by hyperglycaemia-associated metabolic changes, were facilitators of neurotoxicity and were associated with the severity of the condition.14,15 For instance, it has been evidenced that excessive generation of reactive oxygen species (ROS) and NOD-like receptor protein 3 (NLRP3) inflammasomes induce the release of inflammatory cytokines (IL-1β and IL-18), triggering inflammatory responses and pyroptosis in Schwann cells, thereby promoting the development of diabetic peripheral neuropathy.16 However, the dynamic nature of the inflammatory response suggests that measurements at a particular point in time, whether high or low, may not accurately represent the overall changes in inflammatory factors. Furthermore, the results of these studies can be affected by unmeasured confounding, reverse causation, and a variety of biases that can complicate the establishment of a clear causal relationship.17 Limited by current clinical studies and animal experiments, the action mechanism of relevant inflammatory factors in specific diseases has not been fully elucidated. Therefore, further research and clarification are still needed to investigate the specific role of inflammatory factors in diabetic microvascular complications.
Mendelian randomization analysis (MR) is a method that leverages single nucleotide polymorphisms (SNPs), which are strongly associated with exposure factors, as instrumental variables (IVs) to investigate the causal relationship between exposure factors and outcomes. Compared to observational epidemiological studies, MR is based on Mendelian laws of inheritance, in which genetic variation follows the random assignment of alleles to offspring. MR can be regarded as a naturally randomized controlled trial, less susceptible to confounding factors and with a higher level of evidence.18 In the present study, in order to investigate the potential causal relationship between inflammatory factors and diabetic microangiopathy and to identify specific subtypes, we performed MR studies based on genome-wide association studies (GWAS) summary data. Additionally, the expression of specific inflammatory factors in diabetic microvascular complications was validated with a case-control study.
Materials and Methods
MR Studies
Research Design
Two-sample MR analysis was performed using publicly available GWAS statistics. SNPs were selected as IVs for MR analysis. Three key assumptions were used to guide this study: (a) relevance restriction: SNPs selected as IVs must show a strong correlation with exposure; (b) independence restriction: these SNPs should be independent of confounding factors associated with both exposure and outcomes; (c) exclusion restriction: instrumental variables should not affect the outcomes except through their effect on the exposure. The specific flow chart is shown in Figure 1.
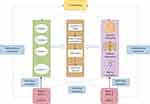 |
Figure 1 Flow chart of Mendelian randomization study. |
Data Sources for 91 Circulating Inflammatory Cytokines
Summary statistics for the 91 circulating inflammatory cytokines used in this study were obtained from a comprehensive large-scale GWAS meta-analysis. In EBIGWAS directory https://ftp.ebi.ac.uk/pub/databases/gwas/summary_statistics/ (GCST90274758 to GCST90274848) Download.19
Data Sources of Diabetic Microangiopathy
Aggregated statistics on diabetic nephropathy, diabetic retinopathy, and diabetic neuropathy were obtained from the Finngen dataset GWAS project (https://r9.finngen.fi). The diabetic nephropathy GWAS dataset covered 312,650 individuals, including 308,539 controls and 4,111 cases (1,434 females and 2,677 males, a mean age at first event of 49.43 years for females and 58.48 years for males), for a total of 20,168,510 SNPs. The diabetic retinopathy GWAS dataset covered 319,046 individuals, including 308,633 controls and 10,413 cases (4,568 females and 5,845 males, a mean age at first event of 55.95 years for females and 57.86 years for males), for a total of 20,168,725 SNPs. The diabetic neuropathy GWAS dataset covered 274,660 individuals, including 271,817 controls and 2843 cases (1,003 females and 1,840 males, a mean age at first event of 54.03 years for females and 58.86 years for males), for a total of 20,167,090 SNPs.
Instrumental Variable Selection
Firstly, SNPs were selected from the exposure data as IVs for MR analysis. Initially, SNPs that were strongly associated with genome-wide significance threshold (P<5×10−8) exposure were selected. However, for most circulating inflammatory cytokines, there was not a sufficient number of SNPs to meet this threshold. Therefore, the threshold (P<5×10−6) was applied to identify SNPs associated with circulating inflammatory cytokines. To remove linkage disequilibrium, R2<0.001 and K=10000kb were set to ensure independence of each IV. Then, palindromic SNPs as well as outcome-associated SNPs were removed from the IVs. Finally, 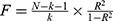 was used to calculate F values for individual IVs, and only IVs with F > 10 were retained to avoid bias caused by weak IVs. In the reverse MR analysis, the procedure for selecting IVs from pooled data on diabetic nephropathy, diabetic retinopathy, and diabetic neuropathy was consistent with the selection procedure for circulating inflammatory cytokines, noteworthly, SNPs associated with the three diabetic complications at the genome-wide significance threshold (P<5×10−8) were selected as potential IVs to obtain more comprehensive results. Finally, SNPs associated with potential confounders (such as age, fasting glucose, glycated haemoglobin, diabetes and insulin resistance) were examined and eliminated using the PhenoScanner online platform (http://www.phenoscanner.medschl.cam.ac.uk).20
was used to calculate F values for individual IVs, and only IVs with F > 10 were retained to avoid bias caused by weak IVs. In the reverse MR analysis, the procedure for selecting IVs from pooled data on diabetic nephropathy, diabetic retinopathy, and diabetic neuropathy was consistent with the selection procedure for circulating inflammatory cytokines, noteworthly, SNPs associated with the three diabetic complications at the genome-wide significance threshold (P<5×10−8) were selected as potential IVs to obtain more comprehensive results. Finally, SNPs associated with potential confounders (such as age, fasting glucose, glycated haemoglobin, diabetes and insulin resistance) were examined and eliminated using the PhenoScanner online platform (http://www.phenoscanner.medschl.cam.ac.uk).20
Statistical Analysis
MR Analysis
All analyses in this study were performed using R software (version 4.3.2). Four methods were mainly employed in this study to explore the causal relationship between 91 circulating inflammatory cytokines and diabetic microangiopathy. Among them, the inverse variance weighting (IVW) method was used as the main analytical method to evaluate the causal relationship,21 which assumed that all IVs were valid and combined the effects together to produce a weighted total effect. The significance threshold was set to P<0.05, and the outcome of causality is expressed as an odd ratio (OR) and a 95% confidence interval (95% CI). Additionally, other three MR analysis methods were also carried out to further prove the stability and directionality of the results and assess the causality, including the maximum likelihood (ML) method, MR-Egger regression method, and Weighted Median (WM) method. The ML method is a traditional method that introduces the most probable parameter values in the data by maximising a likelihood function with a low standard error.22 The WM is a method of combining the results of multiple MRs to reduce genetic variation-caused bias in the estimates.23 The MR-Egger method gives accurate estimates of causal effects in the presence of pleiotropic bias.24 In the reverse analysis, using the same criteria, SNPs associated with diabetic microangiopathy were used as IVs for the reverse MR analysis, and circulating inflammatory cytokines from the forward MR analysis were used as endpoints in order to explore whether diabetic microangiopathy has a causal effect on the above-identified cytokines.
Sensitivity Analysis
MR analysis results may be affected by heterogeneity or pleiotropy. Therefore, the validity and robustness of the MR analysis results were assessed by sensitivity analysis. Specifically, Cochran’s Q test was utilized to assess heterogeneity and considered IVs with P<0.05 to be heterogeneous. Mendelian randomisation polytropic residuals sum and outliers (MR PRESSO) was performed to detect the presence of outliers, and MR-Egger intercept was performed to detect horizontal pleiotropy, with P>0.05 indicating the absence of horizontal pleiotropy.25 Finally, leave-one-out sensitivity analysis was performed to assess the robustness of the results.26
Clinical Validation Study
Clinical Sample Collection
A total of 20 patients with diabetic peripheral neuropathy (DPN) aged 20–75 years who attended the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine from September 2023 to February 2024 were recruited in this study. The inclusion criteria of patients were: ① diagnosed as type 2 diabetes mellitus; ② neuropathy appeared at the time of diagnosis of type 2 diabetes mellitus or after; ③ symptoms and signs were consistent with the manifestation of DPN; ④ agreed to participate in the study and signed the informed consent form. The following conditions were excluded: the patients showed rapid disease progression, asymmetry of the lesion site, and more severe motor impairment than sensory impairment, as well as neuropathy caused by, for example, cervical and lumbar spine lesions, Guillain-Barré syndrome, ischemic stroke, severe vascular lesions, vitamin B12 deficiency, infections, and medication damage. Additionally, contemporaneous healthy individuals were recruited, propensity-matched for age and gender, and 20 cases were included as a control group. This study was approved by the Ethics Committee of the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine (2021AH-77).
Enzyme-Linked Immunosorbent Assay (ELISA) for Plasma Interleukin-20 Receptor Alpha (IL-20RA) Levels
Fasting venous blood (5mL) was collected from patients and healthy control individuals into EDTA tubes, and the supernatant was centrifuged. Each well was spiked with 100μL antibody and incubated at 37 °C for 60 min. The expression level of IL-20RA (pg/mL) was detected according to the instruction of the kit (Keshun Science, Shanghai, China; KS19571 &202403).
Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Detection of IL-20RA mRNA Expression
Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll density gradient centrifugation, and total RNA from PBMCs was extracted using the EZ-10 Total RNA Mini-Preps Kit (Sangon Biotech, Shanghai, China; B618583). The mRNA was reverse transcribed into cDNA as per the instructions of the Reverse Transcription Kit (ABclonal, Wuhan, China; RK20403). qPCR was performed using the CFX96 Real-Time PCR Detection System (Bio-Rad, China) according to the following steps: pre-denaturation at 95 °C for 3 min, followed by 40 cycles of denaturation at 95 °C for 5s, annealing at 60 °C for 30s, and extension at 72 °C for 30s. Beta-Actin served as an internal reference gene, and the relative gene expression was calculated using the 2−ΔΔCt method. The primer sequences are listed in Table 1.
 |
Table 1 Sequences of IL-20RA mRNA Primers in PBMCs |
Data Analysis
GraphPad Prism 10.1.2 software and IBM SPSS statistical package were employed for statistical analysis. Measurement data were expressed as mean ± standard deviation (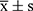 ). The independent samples t-test was used for comparisons between two groups. The difference was considered statistically significant at P < 0.05.
). The independent samples t-test was used for comparisons between two groups. The difference was considered statistically significant at P < 0.05.
Results
Causal Effect of Circulating Inflammatory Cytokine Levels on Diabetic Microangiopathy
The causal relationship between 91 circulating inflammatory cytokines and diabetic nephropathy, retinopathy and neuropathy is shown in Figures 2 and 3. It was found that for diabetic nephropathy, C-C motif chemokine ligand 7 (CCL7) (OR: 1.14, 95% CI: 1.00–1.30, P=0.049), interleukin 24 (IL-24) (OR: 1.30, 95% CI: 1.00–1.68, P=0.048), interferon gamma (IFNG, IFN-γ) (OR: 1.278, 95% CI: 1.03–1.57, P=0.022), IL-20RA (OR: 1.28, 95% CI: 1.09–1.51, P=0.002) were potentially associated with an increased risk of diabetic nephropathy, whereas interleukin IL-1A (IL-1A, IL-1α) (OR: 0.80, 95% CI: 0.68–0.94, and P=0.008), and colony-stimulating factor 1 (CSF1) (OR: 0.86, 95% CI: 0.77–0.96, P=0.008) were related to a reduced risk of diabetic nephropathy. For diabetic retinopathy, we found that signal transduction interface molecule binding protein (STAMBP) (OR: 1.20, 95% CI: 1.01–1.43, P=0.037), IL-20RA (OR: 1.16, 95% CI: 1.01–1.33, P=0.033), and signaling lymphocytic activation molecule (SLAM) (OR: 1.9, 95% CI: 1.03–1.39, P=0.02) were potentially associated with an increased risk of diabetic retinopathy, while interleukin IL-1A (OR: 0.82, 95% CI: 0.72–0.94, P=0.004) was associated with a decreased risk of diabetic retinopathy. Regarding diabetic neuropathy lesions, IL-24 (OR: 1.39, 95% CI: 1.31–1.70, P=0.002), IL-20RA (OR: 1.53, 95% CI: 1.22–1.94, P=0.0003), and SLAM (OR: 1.20, 95% CI: 1.01–1.43, P=0.04) were potentially associated with an increased risk of diabetic neuropathy, while tumor necrosis factor superfamily member 14 (TNFSF14) (OR: 0.81, 95% CI: 0.72–0.92, P=0.001) was associated with a decreased risk of diabetic neuropathy. Among these relevant inflammatory cytokines, IL-20RA was found to be potentially associated with an increased risk of all these diabetic microvascular complications.
Causal Effect of Diabetic Microangiopathy on Circulating Inflammatory Cytokine Levels
The reverse MR analysis results revealed no causal effect of diabetic nephropathy, diabetic retinopathy, and diabetic neuropathy on circulating inflammatory cytokines in the forward MR analysis.
Results of Sensitivity Analyses
In this study, the MR-Egger regression, the WM method, and the ML method all supported the results of IVW, which enhanced the reliability of the analysis results. In sensitivity analyses, the results of the MR-Egger intercept test showed that MR analyses were not affected by any potential effect of horizontal pleiotropy (P>0.05). In causality analyses of circulating inflammatory cytokines and diabetic retinopathy, although the MR-PRESSO test and Cochran’s Q test for SLAM showed the presence of heterogeneity (P<0.05), the results of the MR-Egger intercept test for SLAM showed that the MR analysis was not potentially affected by horizontal pleiotropy (P>0.05) and that outliers were not detected by the MR-PRESSO test, therefore would not affect the MR results. Finally, the leave-one-out test results showed that the exclusion of any individual SNP would not affect these causal associations, which further confirmed the stability and reliability of our results, as shown in Table 2 and Figure 4.
 |
Table 2 Results of Sensitivity Analysis of the Causal Relationship Between 91 circulating Inflammatory Cytokines and Diabetic Microangiopathy |
Comparison of Baseline Levels and IL-20RA Concentration and Relative Expression Levels in Two Groups
As shown in Table 3 and Figure 5, there was no significant difference of gender in the baseline data between the two groups; patients in the DPN group showed a significantly higher plasma IL-20RA level than normal population (P=0.0007). Additionally, RT-qPCR results also demonstrated that the relative expression of IL-20RA mRNA in the PBMCs of DPN patients was remarkably higher than that in the normal population (P<0.0001).
 |
Table 3 Comparison of Baseline Information, IL-20RA Levels and Relative Expression Between the Two Groups |
Discussion
Current researches suggest a relationship between diabetic microangiopathy and inflammatory proteins; however, the exact causal relationship remains uncertain at the genetic level due to research limitations. In this comprehensive two-sample MR study, the causal relationship between 91 circulating inflammatory cytokines and common diabetic microvascular complications was explored. As genetically predicted by MR analysis, the levels of 9 different circulating inflammatory cytokines may be causally associated with the risk of these diabetic microvascular complications. These findings suggest that specific circulating inflammatory cytokines may play a key role in triggering microvascular complications in diabetes, and furthermore, IL-20RA was identified as a common risk factor for these three common microvascular conditions. In the present study, MR analysis was employed to mitigate the impact of confounding factors, which substantially enhanced the precision of research findings. Moreover, the genetic variants used -were derived from the largest available GWAS meta-analyses, and the advantage of this large sample size ensures the strength of the instrumental variables in MR analyses, as well as the robustness of the study results.
Diabetic nephropathy has been shown as one of the major causes of end-stage renal disease,27 and chronic inflammation has been considered as an underlying mechanism of diabetic nephropathy.28–30 The occurrence of inflammatory response is tightly associated with the damage of glomerular vascular endothelial cells, disruption of glomerular filtration barrier, and leakage of macromolecular proteins.31 As suggested by our research findings, CCL7, IL-24, and IL-20RA were potentially associated with an increased risk of diabetic nephropathy, while IL-1A and CSF-1 appeared to be associated with a reduced risk of diabetic nephropathy. A previous study has revealed that renal glomerular macrophage infiltration plays a major role in diabetes nephropathy-induced lipotoxicity, in which CSF-1 acts as a preventive factor; treatment with CSF-1 can inhibit the infiltration of glomerular macrophages and urinary albumin excretion in hypercholesterolemic diabetic rats.32 IL-1α is a regulator of the immune system and cell differentiation of the interleukin 1 family, The present study identified IL-1α as a protective factor against diabetic nephropathy and diabetic retinopathy. Previous studies have found reduced serum IL-1α concentrations in patients with type 1 diabetes compared to subjects with normal glucose tolerance.33 Additionally, a related study found that in diabetic retinopathy rats have demonstrated significant increases in IL-1β, IL-1RI, IL-RII, and IL-1Ra protein expression in the retinal layer but IL-1α was not significantly increased.34 Based on the above findings, it was speculated that elevated levels of IL-1α may represent a compensatory response aimed at maintaining body homeostasis in the presence of systemic inflammation. IFN-γ is produced by T cells, NK cells and other cells of the immune system and is a classical cytokine of the pro-inflammatory immune response of type 1 helper T cells (Th1).35 It has been evidenced that the serum IFN-γ level of diabetic nephropathy patients is significantly higher than that of control subjects.36 Transcriptome analysis of the kidneys of uninephrectomized db/db mice showed that CXCL16-mediated signal transduction induced by IFN-γ and TNF-α between 26 and 35 weeks of age may lead to renal fibrosis, ultimately resulting in severe disease.37 IL-24 is a part of the IL-10 family and shares the IL-20RA/IL-20RB and IL-22RA/IL-20RB receptor heterodimers with IL-20 to exert its biological activity. IL-24 expression was found to be increased in STZ-induced diabetic nephropathy rats and LPS-induced acute kidney injury mice. The in-vitro studies have demonstrated that IL-24 was able to induce transforming growth factor β (TGF-β), platelet-derived growth factor-B (PDGF-B), and connective tissue growth factor (CTGF) synthesis in HK-2 renal tubular epithelial cells, which suggests that IL-24 may indirectly influence renal myofibroblasts by increasing epithelial production of pro-fibrotic growth factors.38 CCL7, also known as monocyte chemotactic protein 3 (MCP3), is a major cytokine involved in macrophage chemotaxis and activation, CCL7 expression has been found to be increased in the kidneys of mice with diabetic nephropathy, this increase may be associated with the progression of islet damage and renal fibrosis.39–41
Diabetic retinopathy is a highly tissue-specific neurovascular complication of diabetes mellitus and one of the leading causes of blindness worldwide, which affects approximately 103.12 million global individuals.42 Inflammatory responses represent one of the core pathogenic mechanisms of diabetic retinopathy. Research has revealed that the retina is in a systemic pro-inflammatory environment long before the onset of clinical symptoms, due to enhanced expression of various inflammatory chemicals (including cytokines, chemokines, and growth factors) in the retina during the course of diabetes.43 According to MR analysis results, STAMBP, SLAM, and IL-20RA were potentially associated with an increased risk of diabetic retinopathy, while interleukin IL-1A was associated with a reduced risk of diabetic retinopathy. SLAM, also known as CD150, is a glycoprotein expressed on the surface of T cells, B cells, natural killer cells and dendritic cells. Currently, there is no study proving the correlation between CD150 and the development of diabetic retinopathy as well as diabetic neuropathy. However, a previous study has indicated that the CD150 expression is increased in retinal adipose tissue of subjects with severe obesity and insulin resistance.44 Hence, it is hypothesized that the potential relationship between CD150 and diabetic retinopathy may be related to insulin resistance. STAMBP is a JAMM family deubiquitinating enzyme that regulates the stability of substrate proteins in cells by cleaving the ubiquitin portion of the molecule; proteomics and other research techniques have shown that STAMBP appears to exhibit high expression in various diseases as an inflammatory biomarker, including breast cancer, ischemic stroke, and autism.45–47 However, the expression of STAMBP in diabetic retinopathy has not yet been reported.
Long-term mild inflammation has been found to have a significant negative impact on the pathogenesis of diabetic neuropathy.48 In the present study, MR analysis results indicated a potentially positive association between IL-24, IL-20RA, and SLAM and the risk of diabetic neuropathy, while TNFSF14 is negatively related to the risk of diabetic neuropathy. TNFSF14, also known as LIGHT or CD258, is a type II transmembrane protein expressed mainly on activated T lymphocytes and other immune cells, which has been widely demonstrated to be an important regulator of immune and fibrotic diseases.49 Previous studies have identified that TNFSF14 prevents high-fat diet-caused obesity and prediabetes; treatment of human primary adipocytes with TNFSF14 effectively inhibits adipocyte differentiation and accumulation.50,51 However, in vivo ablation of TNFSF14 can promote high-fat diet-induced obesity, glucose intolerance, insulin resistance, hyperinsulinemia, hepatic steatosis, and adipocyte hypertrophy and inflammation.52 These results further support our findings that TNFSF14 may be a protective factor against diabetic neuropathy. IL-24 proteins were found to widely expressed in the brain and peripheral nervous tissues of mammals, and it can enhance T currents by stimulating the coupling of IL-22R1 with tyrosine protein kinase Lyn-dependent protein kinase A (PKA) signaling transduction, leading to excessive excitation of trigeminal ganglion TG neurons and pain hyperalgesia.53
MR analysis in this study identified IL-20RA as a common risk factor for these three types of diabetic microvascular complications. IL-20RA, located in the chromosomal region 6q23, is a subunit of the IL-10 (including IL-19, IL-20, IL-22, IL-24, and IL-26) family member IL-20RA/IL-20RB receptor dimer.54 It can activate the JAK/STAT signaling pathway, leading to downstream cascade reactions and regulating the expression of various genes involved in immune responses, cell growth, and angiogenesis, which are closely related to the occurrence of autoimmune diseases and tumors.55 Studies have demonstrated the upregulation of IL-20 expression is observed in the serum of patients with diabetic nephropathy and in the kidneys of streptozotocin-induced diabetic mice. IL-20 may cause podocyte apoptosis directly or indirectly by triggering mesangial cells to produce ROS in the microenvironment of the glomerulus. IL-20RA is also highly expressed in the kidneys of diabetic mice. IL-20RA-deficient diabetic mice show improved renal function, reduced blood glucose levels, and higher survival rates relative to wild-type STZ-induced early diabetic nephropathy mice. This indicates that the IL-20/IL-20RA axis may play a crucial role in mediating inflammatory responses during the progression of diabetic nephropathy.56,57 This study is the first to discover a causal relationship between IL-20RA and diabetic retinopathy. In a previous study, immunofluorescence staining identified the expression of IL-20 and its receptor IL-20RA in the basal epithelial cells of the injured corneal limbus, as well as in the retina and optic nerve head in the DBA/2J mouse model of glaucoma.58 Currently, there is limited documentation on the expression of IL-20RA in neural tissues. Research has indicated that the IL-20RA protein is highly expressed in human brain microvascular endothelial cells and may signal through IL-20RB at the blood-brain barrier, thereby triggering neuroinflammation.59 The increase of IL-20 in the skin can act on the upregulated IL-20RA in sensory neurons, leading to neuronal hyperalgesia.60 According to ELISA and RT-qPCR experiment results, the present study found that IL-20RA expression in the blood of patients with diabetic peripheral neuropathy was remarkably higher than that in the normal subjects. However, the specific action mechanism of IL-20RA still requires further research.
Inevitably, there are still some certain limitations in this study. Firstly, inthis research, the use of GWAS summary data instead of raw data precludes the exploration of potential stratification effects or nonlinear relationships that may arise from differences in specific subtypes, age, disease duration, and gender of diabetic nephropathy, diabetic retinopathy, and diabetic neuropathy, which could lead to heterogeneity. Secondly, the two-sample MR analysis method is a theoretical approach for causal relationship analysis, and the biological pathways and functional roles of circulating inflammatory cytokines in diabetic microvascular complications have not yet been fully elucidated. Further validation using advanced techniques (such as single-cell RNA sequencing, proteomics, and gene editing in cellular and animal models) is needed. Additionally, considering that diabetic neuropathy is the most prevalent chronic complication of diabetes,61 only the expression of IL-20RA in patients with diabetic peripheral neuropathy was examined. In future studies, the population should be expanded to include patients with other diabetic microvascular complications to enhance the generalizability of the research findings.
Conclusion
In summary, the present study investigated the causal relationships between circulating inflammatory cytokines and three common microvascular complications of diabetes utilizing large GWAS datasets and MR studies, thereby minimizing the impact of confounding factors and biases due to reverse causality. A total of 6, 4, and 4 circulating inflammatory cytokines associated with diabetic nephropathy, diabetic retinopathy, and diabetic neuropathy were identified, separately. It was found that IL-20RA was a common risk factor for all three diabetic microangiopathies. Relevant research findings have confirmed our discoveries, while some results still require further investigation. In the future, more research is necessary to verify the exact role of specific circulating inflammatory cytokines in the pathogenesis of these diabetic microvascular complications, and to better understand the underlying disease mechanisms.
Data Sharing Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Ethical Statement
This MR study utilized summary statistics from public databases and all original studies have been approved by their Institutional Review Boards or local ethics committees. Studies using publicly available databases are exempt from ethical review and approval according to item 1 and 2 of Article 32 of the Measures for Ethical Review of Life Science and Medical Research Involving Human Subjects dated February 18, 2023, China. We express our gratitude to all contributors who have made efforts to provide and share the data publicly. Clinical Study was approved by the Ethics Committee of the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine (2021AH-77).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (82174153), the Collaborative Innovation Project of Universities in Anhui Province (GXXT-2020-025), and Center for Xin’an Medicine and Modernization of Traditional Chinese Medicine of IHM (2023CXMMTCM003).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045 [published correction appears in Diabetes Res Clin Pract2023 Oct;204:110945]. Diabetes Res Clin Pract. 2022;183:109119. doi:10.1016/j.diabres.2021.109119
2. Sabanayagam C, Chee ML, Banu R, et al. Association of diabetic retinopathy and diabetic kidney disease with all-cause and cardiovascular mortality in a multiethnic Asian population. JAMA Network Open. 2019;2(3):e191540. doi:10.1001/jamanetworkopen.2019.1540
3. An J, Nichols GA, Qian L, et al. Prevalence and incidence of microvascular and macrovascular complications over 15 years among patients with incident type 2 diabetes. BMJ Open Diabetes Res Care. 2021;9(1):e001847. doi:10.1136/bmjdrc-2020-001847
4. Fu H, Liu S, Bastacky SI, et al. Diabetic kidney diseases revisited: a new perspective for a new era. Mol Metab. 2019;30:250–263. doi:10.1016/j.molmet.2019.10.005
5. Lin KY, Hsih WH, Lin YB, et al. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J Diabetes Investig. 2021;12(8):1322–1325. doi:10.1111/jdi.13480
6. Jadhao P, Swain J, Das S, et al. Prevalence and predictors of diabetic peripheral neuropathy in newly diagnosed type 2 diabetes mellitus patients. Curr Diabetes Rev. 2024;12. doi:10.2174/0115733998282818240125110248
7. Jende JME, Groener JB, Oikonomou D, et al. Diabetic neuropathy differs between type 1 and type 2 diabetes: insights from magnetic resonance neurography. Ann Neurol. 2018;83(3):588–598. doi:10.1002/ana.25182
8. Zhao L, Hu H, Zhang L, et al. Inflammation in diabetes complications: molecular mechanisms and therapeutic interventions. MedComm. 2024;5(4):e516. doi:10.1002/mco2.516
9. Ke D, Zhang Z, Liu J, et al. RIPK1 and RIPK3 inhibitors: potential weapons against inflammation to treat diabetic complications. Front Immunol. 2023;14:1274654. doi:10.3389/fimmu.2023.1274654
10. Oda Y, Nishi H, Nangaku M. Role of inflammation in progression of chronic kidney disease in type 2 diabetes mellitus. Clinical Implications Semin Nephrol. 2023;43(3):151431. doi:10.1016/j.semnephrol.2023.151431
11. Sorrentino FS, Allkabes M, Salsini G, et al. The importance of glial cells in the homeostasis of the retinal microenvironment and their pivotal role in the course of diabetic retinopathy. Life Sci. 2016;162:54–59. doi:10.1016/j.lfs.2016.08.001
12. Kaštelan S, Orešković I, Bišćan F, et al. Inflammatory and angiogenic biomarkers in diabetic retinopathy. Biochem Med. 2020;30(3):030502. doi:10.11613/BM.2020.030502
13. Kanda A, Dong Y, Noda K, Saito W, Ishida S. Advanced glycation endproducts link inflammatory cues to upregulation of galectin-1 in diabetic retinopathy. Sci Rep. 2017;7(1):16168. doi:10.1038/s41598-017-16499-8
14. Jin HY, Park TS. Role of inflammatory biomarkers in diabetic peripheral neuropathy. J Diabetes Investig. 2018;9(5):1016–1018. doi:10.1111/jdi.12794
15. Okdahl T, Brock C, Fløyel T, et al. Increased levels of inflammatory factors are associated with severity of polyneuropathy in type 1 diabetes. Clin Endocrinol. 2020;93(4):419–428. doi:10.1111/cen.14261
16. Cheng YC, Chu LW, Chen JY, et al. Loganin attenuates high glucose-induced Schwann cells pyroptosis by inhibiting ROS generation and NLRP3 inflammasome activation. Cells. 2020;9(9):1948. doi:10.3390/cells9091948
17. Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22. doi:10.1093/ije/dyg070
18. Hemani G, Zheng J, Elsworth B, et al. The MR-base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. doi:10.7554/eLife.34408
19. Zhao JH, Stacey D, Eriksson N, et al. Author correction: genetics of circulating inflammatory proteins identifies drivers of immune-mediated disease risk and therapeutic targets. Nat Immunol. 2023;24(11):1960. doi:10.1038/s41590-023-01635-6
20. Kamat MA, Blackshaw JA, Young R, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35(22):4851–4853. doi:10.1093/bioinformatics/btz469
21. Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42(5):1497–1501. doi:10.1093/ije/dyt179
22. Xue H, Shen X, Pan W. Constrained maximum likelihood-based Mendelian randomization robust to both correlated and uncorrelated pleiotropic effects. Am J Hum Genet. 2021;108(7):1251–1269. doi:10.1016/j.ajhg.2021.05.014
23. Bowden J, Davey Smith G, Haycock PC, et al. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–314. doi:10.1002/gepi.21965
24. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–525. doi:10.1093/ije/dyv080
25. Xiang K, Wang P, Xu Z, et al. Causal effects of gut microbiome on systemic lupus erythematosus: a two-sample Mendelian randomization study. Front Immunol. 2021;12:667097. doi:10.3389/fimmu.2021.667097
26. Xue Y, Zhang L, Chen Y, et al. Gut microbiota and atopic dermatitis: a two-sample Mendelian randomization study. Front Med. 2023;10:1174331. doi:10.3389/fmed.2023.1174331
27. Qiu Y, Tang J, Zhao Q, et al. From diabetic nephropathy to end-stage renal disease: the effect of chemokines on the immune system. J Diabetes Res. 2023;2023:3931043. doi:10.1155/2023/3931043
28. Cebeci E, Cakan C, Gursu M, et al. The main determinants of serum resistin level in type 2 diabetic patients are renal function and inflammation not presence of microvascular complication obesity and insulin resistance. Exp Clin Endocrinol Diabetes. 2019;127(4):189–194. doi:10.1055/s-0043-121262
29. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease and frailty. Nat Rev Cardiol. 2018;15(9):505–522. doi:10.1038/s41569-018-0064-2
30. Li X, Wang L, Liu M, et al. Association between neutrophil-to-lymphocyte ratio and diabetic kidney disease in type 2 diabetes mellitus patients: a cross-sectional study. Front Endocrinol. 2024;14:1285509. doi:10.3389/fendo.2023.1285509
31. Xue R, Xiao H, Kumar V, et al. The molecular mechanism of renal tubulointerstitial inflammation promoting diabetic nephropathy. Int J Nephrol Renovasc Dis. 2023;16:241–252. doi:10.2147/IJNRD.S436791
32. Utsunomiya K, Ohta H, Kurata H, Tajima N, Isogai Y. The effect of macrophage colony-stimulating factor (M-CSF) on the progression of lipid-induced nephrotoxicity in diabetic nephropathy. J Diabetes Complicat. 1995;9(4):292–295. doi:10.1016/1056-8727(95)80025-A
33. Klimontov VV, Mavlianova KR, Orlov NB, et al. Serum cytokines and growth factors in subjects with type 1 diabetes: associations with time in ranges and glucose variability. Biomedicines. 2023;11(10):2843. doi:10.3390/biomedicines11102843
34. Scuderi S, D’amico AG, Federico C, et al. Different retinal expression patterns of IL-1α, IL-1β, and their receptors in a rat model of type 1 STZ-induced diabetes. J Mol Neurosci. 2015;56(2):431–439. doi:10.1007/s12031-015-0505-x
35. De George DJ, Ge T, Krishnamurthy B, et al. Inflammation versus regulation: how interferon-gamma contributes to type 1 diabetes pathogenesis. Front Cell Dev Biol. 2023;11:1205590. doi:10.3389/fcell.2023.1205590
36. Aly RH, Ahmed AE, Hozayen WG, et al. Patterns of toll-like receptor expressions and inflammatory cytokine levels and their implications in the progress of insulin resistance and diabetic nephropathy in type 2 diabetic patients. Front Physiol. 2020;11:609223. doi:10.3389/fphys.2020.609223
37. Maekawa M, Maekawa T, Sasase T, et al. Renal transcriptome analysis of uninephrectomized db/db mice identified a mechanism for the transition to severe diabetic nephropathy. Exp Anim. 2024;73(1):29–40. doi:10.1538/expanim.22-0168
38. Pap D, Veres-Székely A, Szebeni B, et al. Characterization of IL-19, −20, and −24 in acute and chronic kidney diseases reveals a pro-fibrotic role of IL-24. J Transl Med. 2020;18(1):172. doi:10.1186/s12967-020-02338-4
39. Har R, Scholey JW, Daneman D, et al. The effect of renal hyperfiltration on urinary inflammatory cytokines/chemokines in patients with uncomplicated type 1 diabetes mellitus. Diabetologia. 2013;56(5):1166–1173. doi:10.1007/s00125-013-2857-5
40. Kamijo-Ikemori A, Sugaya T, Sekizuka A, et al. Amelioration of diabetic tubulointerstitial damage in liver-type fatty acid-binding protein transgenic mice. Nephrol Dial Transplant. 2009;24(3):788–800. doi:10.1093/ndt/gfn573
41. Chang TT, Chen C, Chen JW. CCL7 as a novel inflammatory mediator in cardiovascular disease, diabetes mellitus, and kidney disease. Cardiovasc Diabetol. 2022;21(1):185. doi:10.1186/s12933-022-01626-1
42. Teo ZL, Tham YC, Yu M, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045 systematic review and meta-analysis. Ophthalmology. 2021;128(11):1580–1591. doi:10.1016/j.ophtha.2021.04.027
43. Yao Y, Li J, Zhou Y, et al. Macrophage/microglia polarization for the treatment of diabetic retinopathy. Front Endocrinol. 2023;14:1276225. doi:10.3389/fendo.2023.1276225
44. Guglielmi V, Cardellini M, Cinti F, et al. Omental adipose tissue fibrosis and insulin resistance in severe obesity. Nutr Diabetes. 2015;5(8):e175. doi:10.1038/nutd.2015.22
45. Li L, Yang X, He M, et al. The expression and clinical significance of STAMBP in breast cancer. Mol Biol Rep. 2023;50(1):899–906. doi:10.1007/s11033-022-07964-3
46. Angerfors A, Brännmark C, Lagging C, et al. Proteomic profiling identifies novel inflammation-related plasma proteins associated with ischemic stroke outcome. J Neuroinflammation. 2023;20(1):224. doi:10.1186/s12974-023-02912-9
47. Bao XH, Chen BF, Liu J, et al. Olink proteomics profiling platform reveals non-invasive inflammatory related protein biomarkers in autism spectrum disorder. Front Mol Neurosci. 2023;16:1185021. doi:10.3389/fnmol.2023.1185021
48. Cheng Y, Chen Y, Li K, et al. How inflammation dictates diabetic peripheral neuropathy: an enlightening review. CNS Neurosci Ther. 2024;(4):e14477. doi:10.1111/cns.14477
49. Mauri DN, Ebner R, Montgomery RI, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8(1):21–30. doi:10.1016/s1074-7613(00)80455-0
50. Tiller G, Laumen H, Fischer-Posovszky P, et al. LIGHT (TNFSF14) inhibits adipose differentiation without affecting adipocyte metabolism. Int J Obes. 2011;35(2):208–216. doi:10.1038/ijo.2010.126
51. Agostino M, Rooney J, Herat L, et al. TNFSF14-derived molecules as a novel treatment for obesity and type 2 diabetes. Int J Mol Sci. 2021;22(19):10647. doi:10.3390/ijms221910647
52. Saunders BM, Rudnicka C, Filipovska A, et al. Shining LIGHT on the metabolic role of the cytokine TNFSF14 and the implications on hepatic IL-6 production. Immunol Cell Biol. 2018;96(1):41–53. doi:10.1111/imcb.1002
53. Cai H, Chen S, Sun Y, et al. Interleukin-22 receptor 1-mediated stimulation of T-type Ca channels enhances sensory neuronal excitability through the tyrosine-protein kinase Lyn-dependent PKA pathway. Cell Commun Signal. 2024;22(1):307. doi:10.1186/s12964-024-01688-62
54. Wu J, Yang S, Yu D, et al. CRISPR/cas9 mediated knockout of an intergenic variant rs6927172 identified IL-20RA as a new risk gene for multiple autoimmune diseases. Genes Immun. 2019;20(2):103–111. doi:10.1038/s41435-018-0011-6
55. Yu D, Yang X, Lin J, et al. Super-enhancer induced IL-20RA promotes proliferation/metastasis and immune evasion in colorectal cancer. Front Oncol. 2021;11:724655. doi:10.3389/fonc.2021.724655
56. Hsu YH, Li HH, Sung JM, et al. Interleukin-20 targets podocytes and is upregulated in experimental murine diabetic nephropathy. Exp Mol Med. 2017;49(3):e310. doi:10.1038/emm.2016.169
57. Chang MS, Hsu YH. The role of IL-20 in chronic kidney disease and diabetic nephropathy pathogenic and therapeutic implications. J Leukoc Biol. 2018;104(5):919–923. doi:10.1002/JLB.MR1217-489R
58. Wirtz MK, Keller KE. The role of the IL-20 subfamily in glaucoma. Mediators Inflamm. 2016;2016:4083735. doi:10.1155/2016/4083735
59. Dayton JR, Yuan Y, Pacumio LP, et al. Expression of IL-20 receptor subunit β is linked to EAE neuropathology and CNS neuroinflammation. Front Cell Neurosci. 2021;15:683687. doi:10.3389/fncel.2021.683687
60. Lu Z, Xiao S, Chen W, et al. IL-20 promotes cutaneous inflammation and peripheral itch sensation in atopic dermatitis. FASEB J. 2022;36(6):e22334. doi:10.1096/fj.202101800R
61. Chinese Medical Association Diabetes Branch. Guidelines for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chinese J Diabetes. 2021;13(4):315–409.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





