Back to Journals » Cancer Management and Research » Volume 17
IL-38 as a Novel Biomarker in Multiple Myeloma Patients: A Prospective Clinical Evaluation
Authors Shen H , Zhao P, Cao J
Received 12 February 2025
Accepted for publication 2 May 2025
Published 10 May 2025 Volume 2025:17 Pages 955—964
DOI https://doi.org/10.2147/CMAR.S520722
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Hailan Shen, Ping Zhao, Ju Cao
Department of Laboratory Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
Correspondence: Ju Cao, Department of Laboratory Medicine, The First Affiliated Hospital of Chongqing Medical University, No. 1 You-Yi Road, Yu-zhong District, Chongqing, 400016, People’s Republic of China, Tel +86-15213151261, Fax +86-023-89012513, Email [email protected]
Introduction: Multiple myeloma (MM) is a refractory haematological malignancy. Interleukin 38 (IL-38) is a novel cytokine that has attracted significant research in recently years. However, no study has investigated IL-38 expression in MM. This study aims to investigate the expression of IL-38 in MM and to provide valuable insights for clinical treatment and efficacy evaluation.
Methods: A total of 241 patients with MM (146 males, 95 females; R-ISS stage I: 111 cases, stage II: 74 cases, stage III: 56 cases) and 50 healthy individuals were included in this study. Medical records were reviewed for staging. Interleukin-1 (IL-1), interleukin-2 receptor (IL-2R), interleukin-8 (IL-8), and tumor necrosis factor-alpha (TNF-α) were detected by chemiluminescence method, IL-38 was detected by enzyme-linked immunosorbent assay (ELISA). Immunoglobulins, free light chains (FLC) and β 2-microglobulin (β 2-MG) were detected by immune nephelometry, and multiple biochemical indicators were detected by automatic biochemical analyzers.
Results: Compared with healthy control group, IL-1, IL-2R, IL-8, and TNF-α were elevated in all three stages of MM. In contrast, compared with normal control group, IL-38 was significantly decreased in patients with MM. When the cut-off value of IL-38 was 18.61 pg/mL, the diagnostic efficacy for MM had a sensitivity of 0.8176 and a specificity of 0.9000.
Discussion: IL-38 exhibited decrease in MM patients (p< 0.0001). It also showed a gradual increase with disease improvement after effective treatment, IL-38 may be a potential biomarker for the diagnosis and prognostic assessment of MM.
Keywords: interleukin 38, multiple myeloma, cytokine, blood disease, plasma cell disease
Introduction
Characterised by “CRAB” symptoms, elevated calcium levels, renal insufficiency, anemia, and bone disease, MM is a severe systemic disease. It manifests in diverse forms, including secondary amyloidosis.1 Despite the fact that chemotherapy and autologous transplantation currently prove effective in controlling MM and extending the patient’s life cycle, the unfortunate reality is that eventual relapse and irreversible disease progression create a despairing situation for individuals. The disease exhibits a high prevalence, yet its aetiology remains elusive, necessitating long-term treatment and monitoring. Timely diagnosis and intervention serve as effective means to impede disease progression.2
MM is a pathological condition characterised by the proliferation of abnormal haematopoietic stem cells that produce ineffective immunoglobulins. This results in the generation of non-functional immunoglobulins by many cloned plasma cells, ultimately leading to systemic harm.3 Due to the impaired function of immunoglobulins in MM, patients often exhibit severely diminished immune systems, rendering them highly susceptible to infections and subsequent inflammatory diseases.4,5
IL-38 has emerged as a prominent cytokine in recent years and has generated considerable interest in scientific community. Belonging to interleukin 1 family, IL-38 was initially identified in 2001. Notably, IL-38 is recognised for its anti-inflammatory properties, primarily achieved through the inhibition of pro-inflammatory cytokine secretion. The inhibitory effects of IL-38 extend to the reduction of T lymphocyte maturation, with a specific focus on targeting TH17 cells.6 Extensive research has been conducted on IL-38 in the context of chronic inflammatory conditions, encompassing such as rheumatoid arthritis, systemic lupus erythematosus (SLE), and multiple sclerosis. It has also demonstrated promise in treating diverse conditions, including colon tumours, allergic rhinitis and asthma, stroke, thyroid adenopathy, acute respiratory distress syndrome (ARDS), and sepsis.7
To our knowledge, IL-38 has not been extensively studied in the context of MM. Previous studies have demonstrated that IL-38 possesses potent anti-inflammatory properties with evidenced inhibitory effects on a range of diseases and a regulatory effect on autoimmune disorders.8 As Allegra et al stated, MM is associated with infectious diseases due to the dysfunction of immunoglobulins. Given that IL-38 has anti-inflammatory properties, there may be a potential link between IL-38 and MM.
This study aims to investigate the expression of IL-38 in MM, preliminarily explore whether IL-38 holds potential utility in the diagnosis and prognosis of MM, and lay the groundwork for further mechanistic studies. We also detected some cytokines that have not been studied in MM, such as IL-1, IL-2R, IL-8, and TNF-α, hoping to provide some clues for clinicians.
Materials and Methods
Patient Characteristics
MM patients admitted to the First Affiliated Hospital of Chongqing Medical University from March 2022 and February 2024 were recruited in this study, according to the latest Chinese Guidelines for the Diagnosis and Treatment of Multiple Myeloma (2022 Revision). Exclusion criteria for case enrollment: patients who are using immunosuppressive medications, organ transplant recipients, diagnosed with malignancy, diabetic, kidney dialysis, pulmonary infection and with HIV infection or autoimmune diseases. For control comparisons, healthy individuals, relatively matched by age and gender were included in this study. The latest revised international staging standard (R-ISS, 2015) was consulted through the medical record system to determine the disease stage.9 MM patients were typed based on serum and urine immunofixation electrophoresis. Subsequently, serum examinations were conducted before and after treatment to identify any variance in the corresponding parameters. In this study, a total of 241 patients were included in the analysis, comprising 111 patients in Stage I, 74 patients in Stage II, and 56 patients in Stage III. The characteristics of the patients are described in Table 1. The treatment effects were graded following the guidelines of the International Myeloma Working Group (IMWG). The grading system comprised six categories: strictly complete remission (sCR), complete remission (CR), very good partial response (VGPR), partial remission (PR), stable disease (SD), and disease progression (PD).10
 |
Table 1 Chracteristics of MM Patients and Health Control |
Serum Collection and Sample Processing
Serum specimen was collected without anticoagulant and then centrifuged at 3000×g for 10 minutes at 4°C. The resulting serum was stored at −80°C for subsequent analysis. Samples with lipidaemia, haemolysis, or those stored in vitro for more than 24 h at room temperature were not included. Additionally, cases involving patients with cardiac disease, hypertension, hyperlipidaemia, pregnancy, and non-multiple myeloma diseases leading to increased plasma cell levels were excluded during case collection.
Instruments and Reagents
IL-38 was detected using the ELISA method (R&D Systems, USA, Lot #: DY9110-05), and the matching coating kit was purchased from R&D Systems (Lot #: DY008). The experiments were conducted strictly according to the manufacturer’s instructions, and a two-well calibration curve was established. An ELISA microplate reader was used to measure the absorbance and quantify IL-38 (TECAN, GENios Plus, Switzerland). IL-1, IL-2R, IL-8, and TNF-a were detected using the chemiluminescence method (Siemens immulite1000, Germany). FLC, β2-MG were detected using the immunoscatter turbidimetric (Siemens BNII, Germany). The immunoscatter turbidimetric (Beckman Immage800, USA) was used to detect immunoglobulin. Serum levels of albumin (ALB), lactate dehydrogenase (LDH), urea (BUN), creatinine (Cr), uric acid (UA), cystatin C (Cys C), total calcium (Ca), and magnesium (Mg) were quantified using the Cobas c701 automated biochemical analyzer (Roche Diagnostics, Switzerland) with the corresponding reagents provided by the manufacturer. Hemoglobin was measured using the automated hematology analyzer line system (Sysmex XN-20, Japan). The details of the manufacturers, batch numbers, and instrument models used for various laboratory indicators are presented in Supplementary Table 1.
The Selection of Chemotherapy Regimens for Multiple Myeloma
The chemotherapy regimen for newly diagnosed multiple myeloma (NDMM) patients with MM needs to be selected based on whether the patient is eligible for autologous stem cell transplantation (ASCT). For NDMM patients who are eligible for transplantation, induction therapy regimens are typically based on combination therapies, most of which include proteasome inhibitors (such as bortezomib), immunomodulatory agents (such as lenalidomide), and dexamethasone. For NDMM patients who are not suitable for transplantation, the selection of chemotherapy regimens should take into account factors such as the patient’s age, physical condition, and disease characteristics to achieve the best therapeutic effect and quality of life. The specific treatment regimen can refer to the Diagnostic Guidelines for Multiple Myeloma (2022 Revision).
Statistical Analysis
All analyses were conducted using GraphPad Prism version 8.0.2 (GraphPad Software, San Diego, CA, USA). p value < 0.05 was considered statistically significant. Comparisons between groups were conducted using the Mann–Whitney U-test with non-parametric tests. The detection results of IL-1, IL-2R, IL-8, IL38, and TNF-a are presented in scatterplots, showing individual values for each parameter. Receiver operating characteristic (ROC) curves were generated using GraphPad Software, the sensitivity and specificity were imported into excel to calculate the Youden Index and obtain the cut-off values.
Results
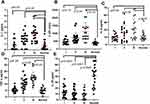
The Cytokine IL-38 Decreased in MM Patients
Compared to normal group, the level of IL-38 in MM patients was lower. On the contrary, patients with MM had elevated IL-1, IL-2R, IL-8, and TNF-a levels than normal control (Figure 1). The correlation between the four cytokines and IL-38 is not very good (Supplementary Figure 1). Table 1 shows the levels of IL-38 (pg/mL) in each stage (median, IQR): Health control 42.6 (22.0; 53.7) versus R-ISS I 12.0 (6.34; 20.46) versus R-ISS II 9.46 (2.59; 15.13) versus R-ISS III 9.01 (2.09; 15.15). The p-values obtained using the Mann–Whitney non-parametric test in the unpaired t-test were as follows: R-ISS I versus control p<0.0001, R-ISS II versus control p<0.0001, R-ISS III versus control p<0.0001 (Figure 1E).
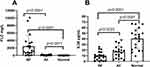
The Levels of IL-38 Increased in MM Patients After Effective Chemotherapy
Once diagnosed with MM, patients subsequently receive chemotherapy, with the efficacy evaluated according to the IMWG guidelines. Effective treatment generally correlates with improvements in disease progression, with grading progressing from PD to SD, PR, VGPR, and sCR. In this section, we observed the clinical data of 20 patients with effective treatment. As the patient’s condition improved, the levels of IL-38 gradually increased (Figure 2). However, both before and after treatment improved, the patients consistently exhibited lower IL-38 levels than healthy individuals.
IL-38 as An Independent Diagnostic Predictor for MM
To better demonstrate the diagnostic efficacy of IL-38 for MM, we plotted the ROC curve for other 13 laboratory items, as shown in Figure 3. When the cut-off value of IL-38 is 18.61 pg/mL, the diagnostic efficacy for MM has a sensitivity of 0.8176 and a specificity of 0.9000, with an area under the curve(AUC) of 0.9288. This indicator can be comparable to β2MG, LDH, and Ca, that currently included in the diagnostic guidelines for multiple myeloma (2022 Revision). Among these 14 indicators, IL-8 and Cr have the lowest diagnostic efficacy for MM (IL-8 AUC=0.5808, Cr AUC=0.5229). It is worth noting that Hb and ALB have higher diagnostic efficacy for MM (Hb AUC=0.9610, ALB AUC=0.9568).
 |
Figure 3 The ROC curve demonstrates the diagnostic efficacy of IL-38. |
IL-38 in Three Stages of R-ISS Demonstrated Significant Diagnostic Value
We plotted ROC curves for MM patients in the three stages of R-ISS. In R-ISS I MM patients, when the cut-off value is 17.86, it has a sensitivity of 0.85 and a specificity of 0.9, with AUC=0.9400; in R-ISS II patients, when the cut-off value is 18.61, it has a sensitivity of 0.85 and a specificity of 0.90, with AUC=0.9350; in R-ISS III patients, when the cut-off value is 15.75, it has a sensitivity of 0.70 and a specificity of 0.95, with AUC=0.9113 (Figure 4). Although some differences can be seen from the figures, there were no significant differences in IL-38 among the three stages: R-ISS I versus R-ISS II, p=0.199; R-ISS I versus R-ISS III, p=0.126; R-ISS II versus R-ISS III, p=0.597 (Figure 5).
 |
Figure 4 The ROC curves of MM patients in three different R-ISS stages. |
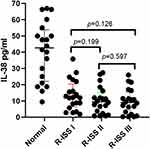 |
Figure 5 The outcomes of MM patients in three different R-ISS stages. |
Discussion
IL-38 is one member of interleukin-1 family, which was newly discovered in the 21st century. It plays an important role as an anti-inflammatory factor in tumours,11,12 inflammation,13–15 and autoimmune diseases.16–20 Some studies focused on to discover whether IL38 increases or decreases in one disease.21 This also sparked our interest in investigating the expression profile of IL-38 in MM.
In our study, we included MM patients across the three R-ISS stages and examined the changes in IL-38 levels before and after treatment in patients who responded well to chemotherapy. We ultimately found that, compared with the healthy control group, untreated patients had significantly lower IL-38 levels. In contrast, patients whose conditions improved with treatment experienced a rebound in IL-38 values.
In terms of MM disease, the most interleukins studied are IL-6,22–24 IL-10,25,26 IL-34,27 IL-17,28 and IL-16.29,30 Few studies have been conducted on IL-37,31 IL-32,32 and IL-9.33 Most of the interleukins were elevated in MM, including cytokines IL-1, IL-2R, IL-8, TNF-a in this study, and the increase in cytokines may be associated with the disease state to some extent. The cytokine IL-38 investigated in our study is consistent with the findings of Zun et al, who studied IL-37, which is also found to be downregulated in MM.31 It is worth noting that both IL-37 and IL-38 are expressed at lower levels in MM. They are both members of the cytokine family, and there may be some correlation between the two in the pathogenic mechanisms of MM, which warrants further investigation. Specifically, whether a hierarchical relationship exists and how it might influence the growth of MM cells are questions that require additional study. Further research is required to verify this hypothesis.
To demonstrate the diagnostic efficacy of IL-38 in MM, we analyzed the ROC curves of various serological markers. Surprisingly, we found that the diagnostic efficacy of IL-38 for MM had an AUC of 0.9288, which is second only to the currently listed markers in the MM guidelines: β2MG, Ca, and LDH. Its diagnostic efficacy is significantly higher than that of the renal function indicators BUN and Cr, indicating that IL-38 has considerable diagnostic value for MM.
It is evident that, from the ROC curve, we can also observe that ALB and Hb demonstrate the highest diagnostic value in MM (ALB, AUC = 0.9568; Hb, AUC = 0.9610). The results of these two indicators are consistent with the findings of Wan et al (ALB, AUC = 0.910; Hb, AUC = 0.952).34
IL-38 decreased significantly in each stage compared with the healthy control group (p<0.0001), but the difference between the three groups of R-ISS was not significant (Figure 5), and there may be many factors involved, such as the disease course of each patient between different stages, different treatment regime (chemotherapy cycle, chemotherapy drugs used, interactions between drugs), and so on. It also involves the correlation between the staging indexes (β2MG, ALB, LDH) and IL-38, which may not be closely correlated with the staging indexes. In the diagnostic indicators of a disease, if the correlation between multiple indicators is not good, there may be the following reasons. The relationship between these markers may not be a simple linear relationship, but is influenced by a variety of factors. For example, in some diseases, multiple indicators may influence the disease process through different mechanisms, resulting in insignificant correlations between them. Different diagnostic indicators may reflect different aspects of the disease. For example, some indicators may primarily reflect inflammatory responses, while others may be related to tissue damage or metabolic status. This difference in biological characteristics can lead to poor correlation between them. The disease itself may be highly heterogeneous, and manifestations may vary greatly between different patients or within the same patient at different stages. This heterogeneity may lead to inconsistent correlations between indicators. By increasing the sample size, we can improve the effectiveness of statistical analysis and reflect the relationship between indicators more accurately.
Two limitations should be considered. First, after assessing the treatment outcomes of the patients included in the experiment, we only tested the levels of IL-38 before and after treatment in those who responded effectively to the therapy. We did not conduct tests on patients who did not respond to the treatment or whose conditions worsened during treatment. We hope to further improve this part of the experimental data in subsequent experiments. Second, in the detection of IL-38, we selected 20 patients for analysis in each R-ISS stage. The limited sample size in this study, primarily due to financial constraints, may affect the generalizability of our findings. However, the significant results obtained suggest that the observed effects are robust and warrant further investigation with larger cohorts. Future studies should aim to validate these findings in larger cohorts to confirm the observed effects and to explore the underlying mechanisms in greater detail. Our findings provide a novel perspective and lay the groundwork for future research.
Conclusion
In conclusion, this study elucidates the value of IL-38 in the diagnosis and therapeutic efficacy assessment of MM. IL-38 levels were significantly reduced in MM patients across different stages and gradually rebounded following treatment improvement, suggesting that IL-38 may serve as a biomarker for the diagnosis and prognosis of MM. This study may provide some scientific evidence for the early diagnosis and therapeutic efficacy evaluation in patients with multiple myeloma.
Abbreviations
MM, multiple myeloma; IL-38, Interleukin 38; IL-1, Interleukin-1; IL-2R, interleukin-2 receptor; IL-8, interleukin-8; TNF-α, tumor necrosis factor-alpha; ELISA, enzyme-linked immunosorbent assay; FLC, free light chains; β2-MG, β2-microglobulin; IMWG, International Myeloma Working Group; sCR, strictly complete remission; CR, complete remission; VGPR, very good partial response; PR, partial remission; SD, stable disease; PD, disease progression; ALB, albumin; LDH, lactate dehydrogenase; BUN, urea; Cr, creatinine; UA, uric acid; Cys C, cystatin C; Ca, total calcium; Mg, magnesium; Hb, Hemoglobin; ROC, Receiver operating characteristic.
Data Sharing Statement
All data are included in this article.
Ethical Declaration
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University under Ethical Approval No. 2021 Research Ethics (2021-361). Prior to participation, each participant was provided with detailed information about the research and gave their informed consent to participate as a research subject.
Acknowledgments
We wish to thank all the patients for collecting their serum after needed medical serum tests.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Chongqing medical scientific research project (Joint project of Chongqing Health Commission and Science and Technology Bureau, number: 2024ZDXM002).
Disclosure
All authors declare that they have no potential conflicts of interest. There is no commercial conflict of interest in this article. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Anderson KC. Vision statement for multiple myeloma: future directions. Cancer Treat Res. 2016;169:15–22. PMID: 27696255. doi:10.1007/978-3-319-40320-5_2
2. Yan W, Shi H, He T, et al. Employment of artificial intelligence based on routine laboratory results for the early diagnosis of multiple myeloma. Front Oncol. 2021;11:608191. PMID: 33854961. doi:10.3389/fonc.2021.608191
3. Dowling M. Multiple myeloma. Prof Nurse. 1997;12(5):354–357. PMID: 9128689.
4. Allegra A, Tonacci A, Musolino C, Pioggia G, Gangemi S. Secondary immunodeficiency in hematological malignancies: focus on multiple myeloma and chronic lymphocytic leukemia. Front Immunol. 2021;12:738915. doi:10.3389/fimmu.2021.738915
5. Li L, Wang L. Multiple myeloma: what do we do about immunodeficiency? J Cancer. 2019;10(7):1675–1684. doi:10.7150/jca.29993
6. Xu WD, Huang AF. Role of Interleukin-38 in chronic inflammatory diseases: a comprehensive review. Front Immunol. 2018;9:1462. PMID: 29988385. doi:10.3389/fimmu.2018.01462
7. Xia HS, Liu Y, Fu Y, Li M, Wu YQ. Biology of interleukin-38 and its role in chronic inflammatory diseases. Int Immunopharmacol. 2021;95:107528. PMID: 33725637. doi:10.1016/j.intimp.2021.107528
8. Ohno M, Imai T, Chatani M, et al. The anti-inflammatory and protective role of interleukin-38 in inflammatory bowel disease. J Clin Biochem Nutr. 2022;70(1):64–71. PMID: 35068683. doi:10.3164/jcbn.21-104
9. González-Calle V, Slack A, Keane N, et al. Evaluation of Revised International Staging System (R-ISS) for transplant-eligible multiple myeloma patients. Ann Hematol. 2018;97(8):1453–1462. PMID: 29623394. doi:10.1007/s00277-018-3316-7
10. Ludwig H, Miguel JS, Dimopoulos MA, et al. International Myeloma Working Group recommendations for global myeloma care. Leukemia. 2014;28(5):981–992. PMID: 24177258. doi:10.1038/leu.2013.293
11. Kinoshita F, Tagawa T, Akamine T, et al. Interleukin-38 promotes tumor growth through regulation of CD8+ tumor-infiltrating lymphocytes in lung cancer tumor microenvironment. Cancer Immunol Immun. 2021;70(1):123–135. PMID: 32653939. doi:10.1007/s00262-020-02659-9
12. Chen F, Zhang F, Tan Z, Hambly BD, Bao S, Tao K. Interleukin-38 in colorectal cancer: a potential role in precision medicine. Cancer Immunol Immun. 2020;69(1):69–79. PMID: 31786620. doi:10.1007/s00262-019-02440-7
13. de Graaf DM, Maas RJA, Smeekens SP, et al. Human recombinant interleukin-38 suppresses inflammation in mouse models of local and systemic disease. Cytokine. 2021;137:155334. PMID: 33128926. doi:10.1016/j.cyto.2020.155334
14. Xu F, Lin S, Yan X, et al. Interleukin 38 Protects Against Lethal Sepsis. J Infect Dis. 2018;218(7):1175–1184. PMID: 29762676. doi:10.1093/infdis/jiy289
15. Zhu J, Zhang J, Wang Y, et al. The effect of interleukin 38 on inflammation-induced corneal neovascularization. Curr Mol Med. 2019;19(8):589–596. PMID: 31244436. doi:10.2174/1566524019666190627122655
16. Rudloff I, Godsell J, Nold-Petry CA, et al. Brief report: interleukin-38 exerts anti-inflammatory functions and is associated with disease activity in systemic lupus erythematosus. Arthritis Rheumatol. 2015;67(12):3219–3225. PMID: 26314375. doi:10.1002/art.39328
17. Zhang J, Tabush N, Wei C, Luo L. Regulatory effect of IL-38 on NF-κB pathway in systemic lupus erythematosus. Immunobiology. 2022;228(2):152322. PMID: 36621308. doi:10.1016/j.imbio.2022.152322
18. Fazeli P, Saeidnia M, Erfani M, Kalani M. An overview of the biological and multifunctional roles of IL-38 in different infectious diseases and COVID-19. Immunol Res. 2022;70(3):316–324. PMID: 35260945. doi:10.1007/s12026-022-09275-y
19. Xu WD, Su LC, He CS, Huang AF. Plasma interleukin-38 in patients with rheumatoid arthritis. Int Immunopharmacol. 2018;65:1–7. PMID: 30268016. doi:10.1016/j.intimp.2018.09.028
20. Boutet MA, Nerviani A, Pitzalis C. IL-36, IL-37, and IL-38 cytokines in skin and joint inflammation: a comprehensive review of their therapeutic potential. Int J Mol Sci. 2019;20(6):1257. PMID: 30871134. doi:10.3390/ijms20061257
21. Boutet MA, Bart G, Penhoat M, et al. Distinct expression of interleukin (IL)-36α, β and γ, their antagonist IL-36Ra and IL-38 in psoriasis, rheumatoid arthritis and Crohn’s disease. Clin Exp Immunol. 2016;184(2):159–173. PMID: 26701127. doi:10.1111/cei.12761
22. Li J, Luo S, Hong W, Zhou Z, Zou W. [Influence of thalidomide on interleukin-6 and its transmission in multiple myeloma patients]. Zhonghua Zhong Liu Za Zhi. 2002;24(3):254–256. PMID: 12515619. Polish
23. Podar K, Tai YT, Cole CE, et al. Essential role of caveolae in interleukin-6- and insulin-like growth factor I-triggered Akt-1-mediated survival of multiple myeloma cells. J Biol Chem. 2003;278(8):5794–5801. PMID: 12482878. doi:10.1074/jbc.M208636200
24. Nageshwari B, Merugu R. Effect of levamisole on expression of CD138 and interleukin-6 in human multiple myeloma cell lines. Indian J Cancer. 2017;54(3):566–571. PMID: 29798960. doi:10.4103/ijc.IJC_349_17
25. Alexandrakis MG, Goulidaki N, Pappa CA, et al. Interleukin-10 induces both plasma cell proliferation and angiogenesis in multiple myeloma. Pathol Oncol Res. 2015;21(4):929–934. PMID: 25743259. doi:10.1007/s12253-015-9921-z
26. Lu ZY, Gu ZJ, Zhang XG, et al. Interleukin-10 induces interleukin-11 responsiveness in human myeloma cell lines. FEBS Lett. 1995;377(3):515–518. PMID: 8549788. doi:10.1016/0014-5793(95)01322-9
27. Baghdadi M, Ishikawa K, Nakanishi S, et al. A role for IL-34 in osteolytic disease of multiple myeloma. Blood Adv. 2019;3(4):541–551. PMID: 30782613. doi:10.1182/bloodadvances.2018020008
28. Song XN, Yang JZ, Sun LX, et al. Expression levels of IL-27 and IL-17 in multiple myeloma patients: a higher ratio of IL-27:IL-17 in bone marrow was associated with a superior progression-free survival. Leukemia Res. 2013;37(9):1094–1099. PMID: 23849453. doi:10.1016/j.leukres.2013.06.022
29. Atanackovic D, Hildebrandt Y, Templin J, et al. Role of interleukin 16 in multiple myeloma. J Natl Cancer I. 2012;104(13):1005–1020. PMID: 22745469. doi:10.1093/jnci/djs257
30. Gu YL, Xiao XC, Yang S. Change of plasma interleukin-16 level in patients with multiple myeloma and its clinical significance. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2017;25(3):823–826. PMID: 28641643. doi:10.7534/j.issn.1009-2137.2017.03.034
31. Li ZC, Sun MD, Zheng YQ, Fu HJ. The low expression of IL-37 involved in multiple myeloma - associated angiogenesis. Med Sci Monit. 2016;22:4164–4168. PMID: 27807338. doi:10.12659/msm.897451
32. Yan H, He D, Qu J, et al. Interleukin-32γ promotes macrophage-mediated chemoresistance by inducing CSF1-dependent M2 macrophage polarization in multiple myeloma. Cancer Immunol Immun. 2023;72(2):327–338. PMID: 35881196. doi:10.1007/s00262-022-03241-1
33. Chang Y, Xing X, Jiang Y, et al. Serum interleukin-9 concentration is associated with the hemoglobin level and renal function in patients with multiple myeloma. Ann Clin Lab Sci. 2019;49(4):513–518. PMID: 31471342.
34. Wan Z, Zhao J, Chen W, et al. Establishment of a diagnostic model for multiple myeloma based on LASSO regression. Int J Lab Med. 2022;43(24):2987–2990, 2995. doi:10.3969/j.issn.16734130.2022.24.010
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.