Back to Journals » Drug Design, Development and Therapy » Volume 19
Impact of Food Physical Properties on Oral Drug Absorption: A Comprehensive Review
Authors Wang Z, Xu W, Liu D, Li X, Liu S, Wu X, Wang H
Received 23 September 2024
Accepted for publication 28 December 2024
Published 16 January 2025 Volume 2025:19 Pages 267—280
DOI https://doi.org/10.2147/DDDT.S497515
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Georgios Panos
Ziyang Wang,1 Wen Xu,2 Dan Liu,3 Xiuqi Li,1 Shupeng Liu,1 Xiaofei Wu,1 Hongyun Wang1
1Clinical Pharmacology Research Center, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 2CSPC Zhongqi Pharmaceutical Technology (Shijiazhuang) Co., Ltd, Shijiazhuang, People’s Republic of China; 3College of Pharmacy, Shenyang Pharmaceutical University, Shenyang, People’s Republic of China
Correspondence: Hongyun Wang, Clinical Pharmacology Research Center, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China, Tel +8610-69154637, Email [email protected]
Abstract: Food-Drug Interaction (FDI) refers to the phenomenon where food affects the pharmacokinetic or pharmacodynamic characteristics of a drug, significantly altering the drug’s absorption rate or absorption extent. These Interactions are considered as a primary determinant in influencing the bioavailability of orally administered drugs within the gastrointestinal tract. The impact of food on drug absorption is complex and multifaceted, potentially involving alterations in gastrointestinal physiology, increases in splanchnic blood flow rates, and shifts in the gut microbiota’s composition. Up to now, extensive research has focused on the interactions between food composition (such as proteins, fats, and vitamins) and drug absorption. In contrast, the impact of food physical properties (such as viscosity, volume, and pH) has received less attention in drug development. This article reviewed the impact of food properties on oral drug absorption based on a comprehensive literature search, focusing on the influence of food volume and food viscosity. From the perspective of pharmacokinetics, we examined interaction trends between food properties and drugs across different classification based on the Biopharmaceutics Classification System (BCS). In addition, we introduced the practical application of physiologically based pharmacokinetic (PBPK) modeling in predicting oral drug absorption under the influence of food Properties.
Plain Language Summary: In the development of new drugs, understanding how food affects drug absorption is crucial. Although scientists have deeply studied the role of food components–such as proteins, fats, and carbohydrates–in drug absorption, the impact of food’s physical properties, like viscosity, volume, and temperature, on drug safety is often overlooked. Dietary differences across cultures, such as the sticky porridge and steamed buns in China versus bread and milk in the West, suggest that food properties may differentially affect drug absorption. We emphasize the key role of food properties in drug absorption and review how these properties influence gastrointestinal function and drug bioavailability. Additionally, we discuss the specific interactions between different types of drugs and food properties, emphasizing the importance of incorporating food properties into the construction of drug absorption models.
Keywords: food properties, food-drug interactions, PBPK models, biopharmaceutics classification system
Introduction
Food-drug interactions can pose significant threats to the safety and efficacy of oral drug therapy. A thorough understanding of the potential mechanisms underlying these interactions is essential for informing clinical decisions and devising optimal treatment protocols.1 The currently known mechanisms of food effects primarily include chemical-physical interactions (a specific interaction between oral drugs and food components that elicit particular pharmacological responses) and alterations in the physiological environment of the gastrointestinal tract (such as gastric emptying, gut microbiota composition, and bile secretion etc).2
Over the past few decades, researchers have developed a clear understanding of how food composition (such as proteins, carbohydrates, lipids etc) affects oral drug absorption. However, the role of specific food properties (such as food volume, food viscosity etc) in mediating food-drug interactions remains unclear and has received comparatively less attention.
With the continuous development of physiologically based pharmacokinetic (PBPK) modeling, a growing body of research indicates that food properties, especially food volume and food viscosity, significantly influence the prediction of oral drug absorption performance. This article examines the interplay among food properties, the Biopharmaceutics Classification System (BCS), Physiologically Based Pharmacokinetic (PBPK) modeling, and the pharmacokinetic and pharmacodynamic profiles of oral administered drugs, aiming to providing references for the precise prediction of oral drug absorption in the gastrointestinal tract.
Generally, food effect is investigated with a simple single-dose pharmacokinetic (PK) study and usually reflected in alterations of drug absorption rate and absorption extent, which can be quantified by measuring the rate and extent to which the drug is absorbed into systemic circulation.3 Common parameters used to measure the extent of absorption are area under the concentration-time curve (AUC) or oral bioavailability (F). For absorption rate, the maximum plasma or serum concentration(Cmax) and the time to reach Cmax(Tmax) are used as indicators.
Impact of Food Properties on Oral Drug Absorption
The physical and chemical properties of food, such as food viscosity and volume, can significantly affect the disintegration and dissolution of oral drugs by altering the physiological environment of the gastrointestinal tract, such as gastric pH and bile secretion, as well as gastrointestinal motility, including the changes in gastric emptying rate. Consequently, these alterations can lead to highly unpredictable changes in drug absorption and drug dosages.
Food Viscosity
Viscosity is generally categorized into microviscosity and macroviscosity, with macroviscosity referring to the macroscopic flow properties of the system. Intake of high-viscosity foods, particularly those rich in proteins, is a primary contributor to the increase in lumen macroviscosity. This increased macroviscosity can affect the disintegration process of pharmaceutical formulations by influencing water penetration.4 Postprandially, the shear rates within the gastrointestinal tract and the rapid intragastric dilution are two major factors affecting the viscosity of the lumen.5,6
Food Viscosity Can Affect Gastric Emptying and Bile Secretion
The rheological properties and physical state/structure of food can significantly affect the rate of gastric emptying, with viscosity being a crucial rheological property for liquid foods.7 Elevated food viscosity has been widely reported to decelerate gastric emptying, potentially due to complex interactions involving genetic and hormonal factors.8 High viscosity downregulates the expression level of gastrointestinal motility-related genes such as HCN1 and CX43, while inhibiting the increase in excitatory neurotransmitter 5-hydroxytryptamine (5-HT) and the decrease in inhibitory neurotransmitter vasoactive intestinal peptide (VIP)—thus increasing flow resistance and potentially countering gastrointestinal propulsion.7 Furthermore, the dense structure of high-viscosity foods can be more resistant to enzymatic hydrolysis and dilution, often resulting in uneven distribution in the stomach, restricting interactions with the gastric mucosal surface and nutrient diffusion, ultimately resulting in delayed gastric emptying.9
Notably, the rapid intragastric dilution and enhanced gastrointestinal motility can significantly mitigate the impact of food viscosity. Marciani et al summarized the effect of initial meal viscosity in human volunteers on half gastric emptying time and area under the curve(AUC) for gastric emptying and demonstrated that despite the initial meal viscosity varied by up to 1000-fold, there was only a 1.3-fold difference in gastric emptying rate (Table 1).10 This has led some researchers to conclude that the impact of food viscosity on gastric emptying may be relatively modest.11
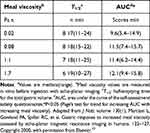 |
Table 1 Effect of Initial Meal Viscosity in Human Volunteers on Half Gastric Emptying Time and Area Under the Curve for Gastric Emptying (AUC) for the Four Locust Bean Gum Liquida |
Beyond these characteristics, high-viscosity meals can also inhibit gastric acid secretion and the mixing rate of food and gastric fluids. This results in an enhanced buffering capacity and a slower decline in pH, subsequently delaying both gastric emptying and intestinal propulsion.
Food Viscosity Can Affect the Disintegration and Dissolution of Drugs
Lumen macroviscosity can significantly affect tablet disintegration and dissolution by modulating liquid permeability.12–15 The penetration rate of liquid into tablets is inversely related to viscosity, potentially explaining the reduced water absorption observed in tablets within highly viscous media.12 Drugs spanning BCS classes I to IV can exhibit altered pharmacokinetics due to food viscosity, while BCS class III drugs (characterized by high solubility and low permeability) with site-specific absorption in the proximal intestine being particularly susceptible to changes in luminal viscosity.13
Radwan et al reported that the BCS class III anticholinergic drug, trospium chloride, exhibited different dissolution patterns in equiviscous solutions of different viscosity enhancing agents (Figure 1).12 As the medium viscosity increased, the water uptake of all trospium chloride dosage forms (both film-coated and uncoated tablets) decreased. The overall effect was more pronounced for film-coated tablets, potentially due to the swelling of the hydroxypropyl methylcellulose(HPMC) coat layer surrounding the tablet, acting as a barrier for the diffusion of the water and drug molecules into and out of the tablet, as well as due to special interactions with excipients. Moreover, there may be more complex interactions, such as pH-medium-formulation interactions and charge interactions, between viscous media and drugs that could influence dissolution and absorption profiles.13
 |
Figure 1 Disintegration times of different trospium chloride products in various bio-relevant dissolution media. In all cases, increased medium viscosity significantly affected disintegration times. Spasmex, Spasmolyt and Trospi represents three commercially available trospium chloride tablet formulations. Reprinted from Radwan A, Amidon GL, Langguth P. Mechanistic investigation of food effect on disintegration and dissolution of BCS class III compound solid formulations: The importance of viscosity. Biopharm Drug Dispos. 2012;33(7):403–416. Copyright © 2012 John Wiley & Sons, Ltd.12 *pH=6.8; **pH=4.6. Abbreviations: SIF, simulated intestinal fluid; HPMC, hydroxypropyl methylcellulose. |
The practice of administering drugs with viscous foods or liquids is common, in order to enhance the safety of medication administration for patients with dysphagia, which can be caused by conditions such as stroke, dementia, Parkinson’s disease, or frailty.16 Healthcare providers often mix medication fragments or powders with viscous foods like yogurt, honey or jam to facilitate swallowing.17 However, this practice undoubtedly poses some hidden challenges for changes in pharmacokinetics and pharmacodynamics of oral drugs. Manrique et al found that the dissolution of four drugs, including warfarin and carbamazepine, was significantly delayed in the thickest consistency prescribed for individuals with dysphagia in Australia.14 Additionally, Almagate and ibuprofen formed precipitates when combined with a thickening agent.18 In countries like the USA, where even thicker consistencies may be used, it is conceivable that drug release and disintegration could be further impeded. For viscosity-mediated food effects, optimizing tablet formulations by using disintegrants that acts without gelling or which can counteract the effect of gelling (croscarmellose sodium or cross-linked polyvinylpolypyrrolidone) can achieve optimal results when taken with food.19
Food Viscosity Can Affect the Diffusion of Drug Molecules
Jaffe et al demonstrated that dietary pectin may affect drug diffusion and absorption through complexation, adsorption, or increase in the viscosity of gastrointestinal contents in a drug-food interaction study.20 In another in vitro study, natural gums hydrated rapidly and formed a gel network when added to water, which hindered the mixing of drugs with gastrointestinal fluids, with notable influence for the absorption of BCS class III drugs characterized by the limited permeability.14
Additionally, studies have reported that food viscosity is associated with postprandial glucose-insulin metabolism.8,21 Juvonen et al found that low-viscosity oat bran beverages elicited significant postprandial glucose-insulin responses, along with significantly elevated secretion of cholecystokinin (CCK) and glucagon-like peptide-1 (GLP-1), potentially due to an enhanced interaction between nutrients and the gastrointestinal mucosa.21 In contrast, consumption of a high-viscosity diet can slow gastric emptying, resulting in a slower postprandial blood glucose response and a reduced concentration of plasma glucose-dependent insulinotropic polypeptide(GIP).8,9
Food Size
The size of food intake is directly linked to the rate of energy delivery and the subsequent emptying from the stomach. Large sized and high-density foods can stimulate gastric antrum peristalsis by fully stretching the gastric wall or activating volume receptor.22 However, these foods require a more extended period to mechanical grind into 1–2mm particles within the gastric antrum before they can be emptied from the stomach. This results in a prolonged lag phase and extended gastric emptying time.
Furthermore, the physical size of food particles significantly affects the rate of gastric emptying. For instance, the T1/2 of 10-mm chicken liver particles labeled with radionuclide is significantly longer than that of 0.25-mm chicken liver particles.23 Christian et al also reported that meals consisting of small portions of vegetables, meat, and beverages were associated with more rapid gastric emptying, which could potentially affect the pharmacokinetics of oral drugs.24
Consuming large portions of food can delay gastric emptying, which prolongs the dissolution time for drugs like nitrofurantoin that have poor dissolution characteristics, consequently leading to a significant increase in their bioavailability.25 Conversely, for the antiparkinsonism drug levodopa, such delayed gastric emptying will prolong the reaction time between drug and gastric mucosal dopa decarboxylase (DDC), resulting in increased systemic metabolism of the drug.26
Moreover, the size of food particles can directly affect drug bioavailability. Shinkuma D et al found that both the type and size of the food directly affected the intestinal transport of drug formulations.27 Large portions of food reduced the diffusion path of drug molecules to the gastrointestinal mucosa, leading to a decrease in the bioavailability of drugs like sulpiride. This highlights the importance of considering food size and type when assessing the pharmacokinetics of orally administered medications.28
Solid Food and Liquid Food
Food rheology significantly influences gastrointestinal physiology and drug bioavailability. Solid foods, compared to liquid foods, induce more frequent and forceful contractions in the gastric antrum contractions and require a more extended period to be ground into 1–2 millimeter particles. This extended mechanical processing delays food transport and gastric emptying, decelerates the pace of consumption, and enhances the secretion of pancreatic and bile fluids, all of which can subsequently affect the bioavailability of orally administrated drugs.29–32
Solid Food and Liquid Food Can Affect Gastric Emptying
Noncaloric liquids, when consumed postprandially, can quickly empty within few minutes via the “Magenstrasse” shortcut, bypassing the bulk stomach contents.33–35 When noncaloric liquids are taken with medication, their rapid emptying can take away some dissolved drugs, potentially affecting the absorption of oral drug.36 However, the gastric emptying rate of noncaloric liquids will significantly decrease when they are homogenized with food.
In contrast, caloric liquids empty from the stomach more slowly and follow first-order kinetics, where the rate of emptying proportional to the volume of liquid present.37 Conversely, solid particles exhibit a biphasic emptying pattern, beginning with an initial lag phase during which minimal emptying occurs, followed by a linear emptying phase characterized by zero-order kinetics (Figure 2).5,38
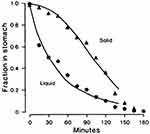 |
Figure 2 The gastric emptying curves of a healthy volunteer after ingesting solid and liquid meals. Liquid emptying begins immediately in an exponential manner, whereas the linear solid emptying begins after the lag phase. ▲ represents the fraction of residual food in the stomach after ingesting solid meals. ● represents the fraction of residual food in the stomach after ingesting liquid meals. Reprinted with permission from Camilleri M, Malagelada JR, Brown ML, Becker G, Zinsmeister AR. Relation between antral motility and gastric emptying of solids and liquids in humans. Am J Physiol. 1985;249(5 Pt 1): G580-585. Copyright © 1985 the American Physiological Society.38 |
The presence of solid foods during a meal significantly impedes the gastric emptying of liquid components.39 Increasing the volume of solid components in a mixed meal can further delay the distribution of liquid in the stomach. Moreover, the liquid components can also prolong both the lag phase and the emptying phases of the solid meal.40,41 After majority of the liquid components have been emptied from the stomach, the emptying of solid components begins.42,43
Food-induced delays in gastric emptying can result in prolonged drug transit times and increased net efficiency of apical efflux transport.43,44 Notably, substrates of efflux transporters, including drugs like indinavir and omadacycline, are subject to negative food effects, with this phenomenon being particularly pronounced in drugs of high permeability and those in immediate release preparations.44–46
Therefore, accurately simulating the impact of solid/liquid meals on gastric emptying is crucial for predicting the effects of food. Winter et al proposed a model that predicts gastric emptying parameters for solid foods based on specific food characteristics, while also considering the effects of gastric secretion and the Magenstrasse shortcut.35,46 This model ultimately applies a physiologically-based pharmacokinetic (PBPK) approach to predict the effects of food under real-world conditions.
Solid Food and Liquid Food Can Affect Postprandial pH
Reynaud et al found significant differences in the postprandial pH kinetics stimulated by solid versus liquid meals in pigs.47 After consuming solid meals, the gastric pH decreased rapidly in an exponential manner, with large food particles creating a more pronounced and persistent pH gradient in the stomach. Conversely, liquid meals induced an S-shaped pH decline following an initial plateau phase and eventually stabilized after more than 2 hours.
Malagelada et al investigated the potential relationship between dietary homogeneity and gastric pH in humans, noting a markedly lower gastric pH within the first hour after consuming solid/liquid meals compared to homogenized ones (Figure 3).48 Moreover, homogenized meals resulted in a substantially higher soluble buffering capacity in the stomach than solid/liquid meals.
 |
Figure 3 The gastric pH curves of six healthy volunteers after consuming solid/liquid meals and homogenized meals. The pH was significantly lower after solid/liquid meals compared to homogenized meals during the first hour. Thereafter the pH for both meal types remained similar until the end of the observation. (○ represents solid/liquid meal; ● represents homogenized meals. * represents the differences in pH after ingesting solid/liquid meal and homogenized meal. Reprinted from Malagelada JR, Go VL, Summerskill WH. Different gastric, pancreatic, and biliary responses to solid-liquid or homogenized meals. Dig Dis Sci. 1979;24(2):101–110.48 |
Compared to other types of diets, homogenized liquid diets are associated with a notable increase in gastric pH within 3 hours post-consumption.49 Semi-solid meals, on the other hand, produce a more pronounced pH response in the proximal stomach and have a strong correlation with the gastric emptying rate.50
These postprandial changes in pH gradients not only affect the dissolution and absorption of pH-sensitive drugs such as erythromycin, penicillin, and quinidine, but also significantly alter the bioavailability of pH-dependent release tablets.51
Liquid Food Can Affect the Buffering or Solubilizing Capacity of Intestinal Lumen Contents
Liquid diets (with similar composition, caloric content and volume to reference meals) are utilized to assess the effects of food consumption on the buffering or solubilizing capacity within the intestinal lumen.49
The ingestion of such diets leads to alterations in physiological parameters, including gastrointestinal pH, buffering capacity, and solubility change, which has a significant impact on drugs with poor dissolution characteristic (BCS class II). Brouwers et al reported that liquid diets can delay the absorption of poorly soluble drugs like fosamprenavir, by influencing tablet disintegration.52 This effect may be attributed to the formation of a hydrophobic protein barrier around the tablets in the liquid diet, which hindered water permeation.53
Furthermore, beverages such as sports drinks and fruit juices, rich in sugars and salts, can interfere with the hydration and gel layer of polymeric matrices, leading to a hastened release of medications from HPMC matrix sustained-release formulations.54
Acidic Food and Alkaline Food
In the fasting state, the human stomach’s pH typically ranges from 1.4 to 2.1. Consuming acidic foods (such as high protein diets) or alkaline foods (such as vegetables, fresh fruits, and high carbohydrate diets) can affect the pH of the gastrointestinal tract and urine, thereby influencing the dissolution and release of weakly acidic or weakly alkaline drugs such as memantine and flecainide, especially those that are low in solubility and high in permeability with a low pKa value (BCS class II).55–60
When administering medication to children, the impact of acidic and alkaline foods on drug absorption must be carefully considered, as it is quite common to combine medications with acidic foods such as fruit juice, applesauce, or yogurt to improve compliance in young children.15 These acidic liquid foods are known to have a slow gastric emptying rate, and research has shown that excessive intake of acidic foods is closely associated with disorders in calcium and phosphorus metabolism, hormone resistance, and accelerated skeletal muscle breakdown.61
However, for acquired immunodeficiency syndrome(AIDS) patients with achlorhydria, the bioavailability of itraconazole can be significantly improved by providing acidic beverages (such as cola).62 Appropriate intake of alkaline foods can enhance the body’s buffering capacity, ameliorate metabolic dysfunction and physical performance, stabilize the levels of metabolic enzymes and various hormones, and positively influence drug metabolism in the body.63
In addition, changes in gastric pH can affect drug release from pH-dependent sustained-release matrix tablets (such as alginate-based matrix tablets) and formulations containing pH-dependent excipients (such as calcium sulfate dehydrate).60 For instance, the enteric-coated tablets of chlorophenol and metronidazole exhibit pH-dependent disintegration.64 Elevated gastric pH may precipitate premature release or disintegration of these enteric-coated tablets in the stomach, diminishing their bioavailability.
Food Temperature
Food temperature can also affect gastrointestinal physiology (such as gastric emptying and gastric acid diffusion) and pharmacokinetics (such as drug disintegration and dissolution). However, current research has not yet definitively established the relationship between food temperature and gastric emptying.
Sun et al reported that both hot drinks (50°C) and cold drinks (4°C) can inhibit gastric emptying.65 Moreover, there was a significant correlation between the intragastric temperature after consuming cold drinks and the initial rate of gastric emptying. Verhagen et al also found that cold meals (4°C) reduced the frequency of gastric myoelectrical activity, subsequently leading to a decrease in the frequency of gastric antrum contractions.66 However, other studies have suggested that different food temperatures may result in similar patterns of gastric activity.67
Mishima et al conducted a more detailed study on the effect of dietary temperature and found that various food temperatures predominantly influenced the initial stage of gastric emptying, without affecting the physiological function of the small intestine (duodenum).68 They observed that the gastric emptying rate was significantly higher after consuming a meal at 60°C compared to other temperatures (Figures 4 and 5), possibly due to the thermal stimulation enhancing the release of cholecystokinin (CCK) and glucagon-like peptide-1 (GLP-1).69
 |
Figure 4 The time-course thermal changes of liquid and solid meals initially at 4°C or 6°C when placed in a 37°C water bath. Reprinted from Mishima Y, Amano Y, Takahashi Y et al. Gastric emptying of liquid and solid meals at various temperatures: Effect of meal temperature for gastric emptying. J Gastroenterol. 2009;44(5):412–418.68 |
 |
Figure 5 The [13C]-labeled acetate breath test for liquid test meals at three different temperatures showed the percentage of 13CO2 excretion in 1 h(% dose/h). At the early stages of the study (5,10,20, and 30 minutes of the study), the excretion values of 60°C liquid meals were significantly higher than those for the 4°C and 37°C liquid meals (*P<0.05). Reprinted from Mishima Y, Amano Y, Takahashi Y et al. Gastric emptying of liquid and solid meals at various temperatures: Effect of meal temperature for gastric emptying. J Gastroenterol. 2009;44(5):412–418.68 |
Furthermore, many medications and excipients (such as taste masking agent) degrade at higher temperatures, so it is advisable to avoid mixing medications directly into hot drinks or meals.15 Similarly, the pattern of acid diffusion following the ingestion of solid foods also appears to be closely related to dietary temperature.70
The Biopharmaceutics Classification System (BCS) and Food Effects
The Biopharmaceutics Classification System (BCS), proposed by Amidon et al in 1995, is a classification concept utilized by regulatory agencies to determine whether in vivo bioequivalence studies can be waived for oral solid drug immediate-release formulations.71 In this section, we will explore the relationship between BCS classification and food properties based on the distinction of pharmacokinetic characteristics within the BCS framework, as well as the prediction of oral drug absorption using physiologically-based pharmacokinetic (PBPK) models (Table 2).
 |
Table 2 Biopharmaceutics Classification System(BCS) and Food Effect Trends |
BCS Class I: BCS Class I drugs are characterized by high solubility and high membrane permeability. Their absorption is generally unaffected by the site of absorption or pH. Although most BCS Class I immediate-release drugs are not influenced by high fat meals, certain ordinary preparations may see alterations in plasma concentration and time to peak in the presence of food, due to delays in gastric emptying and gastrointestinal transit time.72,75
BCS Class II: characterized by low solubility and high membrane permeability, BCS Class II drugs are profoundly influenced by postprandial changes in gastrointestinal buffering or solubilizing capacities, and often manifesting a positive food effect.72 This effect is credited to increased bile salt concentrations and micelle formation after food intake, which enhance the dissolution of lipophilic drugs in the small intestine.
However, a delay in disintegration has been observed with some BCS Class II drugs, including fosamprenavir, diclofenac, and carbamazepine, when co-administered with food. This phenomenon may be related to proteins from liquid meals absorbing onto the tablets, thereby hindering water penetration and increasing the viscosity of the intestinal lumen.14,52,53 Due to the considerable uncertainty associated with the low solubility of BCS Class II drugs, Bio-relevant dissolution media(BDM), which mimic the physiological environment of the human body, are widely used in drug formulation and development to predict the dissolution characteristics of BCS Class II drugs in vivo.
Gastric emptying is the rate-limiting step for BCS Class II drugs, which are characterized by high permeability.46 Rubbens et al demonstrated that the pharmacokinetics of diclofenac, a BCS Class II drug, were significantly influenced by delayed gastric emptying induced by high-fat meals.74
BCS Class III: Fleisher et al indicated that the bioavailability of BCS Class III (characterized by high solubility, low permeability) drugs generally decreased when taken with food.72,76 This was exemplified by Captopril, an angiotensin-converting enzyme inhibitor, which showed a marked reduction in plasma concentration after a high-fat meal.77 Similarly, hydrochloride quinine and risedronate sodium tablets formed water-insoluble complexes with food components or gastrointestinal secretions through electrostatic interactions, leading to reduced bioavailability under feeding conditions.78,79
The absorption behavior of high solubility compounds, specifically BCS Class III drugs with an “absorption window” in the proximal intestine, is highly susceptible to increases in gastrointestinal lumen viscosity. Viscous food can form a physical barrier that impedes drug mixing with gastrointestinal fluids and release from the carrier. However, non-site-specific BCS Class III drugs, such as metformin, are less affected by viscous media.13 Notably, the fat content has a minimal impact on the food effect for these types of drugs, as the presence of food significantly decreases the rate and extent of drug absorption.
BCS Class IV: BCS class IV drugs, characterized by low solubility and low permeability, present significant challenges for oral administration. Currently, there is insufficient evidence to predict the absorption behavior of these drugs reliably. Their absorption patterns are inconsistent and may be influenced by various factors, including whether the rate-limiting step is solubility or permeability, as well as the formulation of the drug product.
Fleisher et al proposed that the food effects on immediate-release drugs could be predicted based on BCS classification.72 However, the BCS method cannot quantitatively predict the extent of changes in drug exposure. Currently, physiologically based pharmacokinetic (PBPK) models are widely used to evaluate oral drug absorption. These models incorporate food property data to predict gastrointestinal physiological responses after food intake, and in conjunction with in vitro dissolution studies to reliably predict food effects caused by solubility limitations.46,49
Despite the notable successes in predicting oral drug exposures, there are persisting gaps and limitations within current modeling applications. Lin et al provided a comprehensive overview of the development and limitations of PBPK modeling over time, highlighting the need for more comprehensive data on developmental physiology and pediatric absorption that is age-specific.80 Additionally, they also noted the insufficient research on newborns and individuals with structural organ damage, such as renal and hepatic impairments.81 Despite these challenges, PBPK models hold significant potential in addressing complex issues related to polypharmacy risks and pH-dependent drug interactions.
Discussion
Generally, regulatory authorities typically specify the dietary composition and caloric content of test meals used in food effect studies during new drug development, providing standards for high-fat and low-fat meals. However, the diversity of regional dietary cultures results in significant variations in food composition (such as proteins, fats, carbohydrates etc) and food properties (like dietary viscosity, temperature etc). These variations can undoubtedly pose risks for alterations in the pharmacokinetics and pharmacodynamics of oral drugs.
Over the past few decades, researchers have conducted in-depth studies on the interactions between food composition and drugs, yet the effects of food properties in these interactions remain unclear. Understanding the mechanisms and consequences of food-property interactions with oral drugs is crucial for both therapeutic efficacy and toxicity assessment. By summarizing the studies on food properties from 1971 to 2024, we categorize these interactions into three types:
Firstly, alterations in the physiological environment of the gastrointestinal tract (such as stomach pH and bile secretion) and gastrointestinal motility (such as gastric emptying rate and medium viscosity) can indirectly affect the pharmacodynamics and pharmacokinetics of oral drugs. This can lead to highly unpredictable variations in drug dosage. For instance, viscous foods can delay gastric emptying and reduce fluid permeability, with the acidic or alkaline foods can alter the pH of the gastrointestinal tract and urine, and food temperature can affect the diffusion rate of gastric acid.
Secondly, food can also directly interact with pharmaceutical preparations in physico-chemical ways, potentially impairing the drug’s bioavailability. For example, ibuprofen may form precipitates when exposed to viscous media, and unstable drugs or excipients may degrade when consumed with hot drinks or meals. Finally, there are complex and not yet fully clear interactions between drug properties and food, such as the intricate interplay between pharmaceutical formulations and viscous media or pH. These interactions represents an area that requires further research in the future.
From a pharmacokinetic perspective, BCS Class I to IV drugs exhibit specific reactions with food properties. Among them, food viscosity has the greatest impact on BCS class III drugs, which have site-specific absorption, while BCS class II drugs, characterized by limited solubility, are more influenced by liquid permeability. Consequently, understanding food properties is crucial for predicting oral drug absorption. By incorporating food property parameters and in vitro study data into PBPK models, the impact of food on oral drug absorption can be more reliably predicted.
The current study also has the following limitations: (1) The complex movement of the gastrointestinal tract and rapid intragastric dilution make it difficult to accurately predict lumenal viscosity postprandially; (2) Researchers have not yet reached consistent conclusions regarding the effects of food temperature and food viscosity on drug absorption; (3) PBPK models still encounter difficulties in specific populations, such as children, and in addressing complex drug-food interactions. Therefore, further investigation into the mechanisms of food properties and oral drug interactions is necessary to provide new directions for resolving these interactions.
Conclusion
In summary, the physical properties of food, such as viscosity, volume, and temperature, can directly or indirectly affect the absorption of oral drugs. BCS Class I to IV drugs exhibit specific reactions with food physical properties, which emphasizes the importance of considering the impact of food physical properties in guiding the rational use of medication. Combining PBPK models with in vitro studies can provide better predictions of oral drug absorption under the influence of food physical properties.
Acknowledgments
We thank all the contributors for their invaluable contributions to this review.
Funding
This research was supported by National High Level Hospital Clinical Research Funding (2022-PUMCH-B-118).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhang Y, Deng Z, Xu X, Feng Y, Junliang S. Application of artificial intelligence in drug-drug interactions prediction: a review. J Chem Inf Model. 2024;64(7):2158–2173. doi:10.1021/acs.jcim.3c00582
2. Koziolek M, Alcaro S, Augustijns P, et al. The mechanisms of pharmacokinetic food-drug interactions - a perspective from the UNGAP group. Eur J Pharm Sci off J Eur Fed Pharm Sci. 2019;134:31–59. doi:10.1016/j.ejps.2019.04.003
3. U.S. Food and Drug Administration. Guidance for Industry: Bioavailability and Bioequivalence Studies for Orally Administered Drug Products: General Considerations. Washington, DC: US: Food and Drug Administration; 2003.
4. Hirose R, Sugano K. Effect of food viscosity on drug dissolution. Pharm Res. 2024;41(1):105–112. doi:10.1007/s11095-023-03620-y
5. Kong F, Singh RP. Disintegration of solid foods in human stomach. J Food Sci. 2008;73(5):R67–80. doi:10.1111/j.1750-3841.2008.00766.x
6. Dikeman CL, Murphy MR, Fahey GC. Dietary fibers affect viscosity of solutions and simulated human gastric and small intestinal digesta. J Nutr. 2006;136(4):913–919. doi:10.1093/jn/136.4.913
7. Liu W, Jin W, Wilde PJ, Jin Y, Pan Y, Han J. Understanding the mechanism of high viscosity food delaying gastric emptying. Food Funct. 2024;15(10):5382–5396. doi:10.1039/d4fo00319e
8. Zhu Y, Hsu WH, Hollis JH. The impact of food viscosity on eating rate, subjective appetite, glycemic response and gastric emptying rate. PLoS One. 2013;8(6):e67482. doi:10.1371/journal.pone.0067482
9. Jin Y, Wilde PJ, Li C, Jin W, Han J, Liu W. Impact of food viscosity on in vitro gastric emptying using dynamic and semi-dynamic models. Food Hydrocoll. 2023;137:108410. doi:10.1016/j.foodhyd.2022.108410
10. Marciani L, Gowland PA, Spiller RC, et al. Gastric response to increased meal viscosity assessed by echo-planar magnetic resonance imaging in humans. J Nutr. 2000;130(1):122–127. doi:10.1093/jn/130.1.122
11. Faas H, Steingoetter A, Feinle C, et al. Effects of meal consistency and ingested fluid volume on the intragastric distribution of a drug model in humans--a magnetic resonance imaging study. Aliment Pharmacol Ther. 2002;16(2):217–224. doi:10.1046/j.1365-2036.2002.01154.x
12. Radwan A, Amidon GL, Langguth P. Mechanistic investigation of food effect on disintegration and dissolution of BCS class III compound solid formulations: the importance of viscosity. Biopharm Drug Dispos. 2012;33(7):403–416. doi:10.1002/bdd.1798
13. Cvijić S, Parojčić J, Langguth P. Viscosity-mediated negative food effect on oral absorption of poorly-permeable drugs with an absorption window in the proximal intestine: in vitro experimental simulation and computational verification. Eur J Pharm Sci off J Eur Fed Pharm Sci. 2014;61:40–53. doi:10.1016/j.ejps.2014.04.008
14. Manrique YJ, Lee DJ, Islam F, et al. Crushed tablets: does the administration of food vehicles and thickened fluids to aid medication swallowing alter drug release? J Pharm Pharm Sci Publ Can Soc Pharm Sci Soc Can Sci Pharm. 2014;17(2):207–219. doi:10.18433/j39w3v
15. Batchelor HK. Influence of food on paediatric gastrointestinal drug absorption following oral administration: a review. Child Basel Switz. 2015;2(2):244–271. doi:10.3390/children2020244
16. Atkin J, Devaney C, Yoshimatsu Y, Smithard D. Modified medication use in dysphagia: the effect of thickener on drug bioavailability-a systematic review. Eur Geriatr Med. 2024;15(1):19–31. doi:10.1007/s41999-023-00896-6
17. Nissen LM, Haywood A, Steadman KJ. Solid medication dosage form modification at the bedside and in the pharmacy of Queensland hospitals. J Pharm Pract Res. 2009;39(2):129–134. doi:10.1002/j.2055-2335.2009.tb00436.x
18. Bravo-José P, Sáez-LLeó C, Moreno-Guillamont E. Combining liquid oral drugs with thickener: compatibility and changes in viscosity. Dysphagia. 2022;37(4):889–899. doi:10.1007/s00455-021-10348-7
19. Zaheer K, Langguth P. Formulation strategy towards minimizing viscosity mediated negative food effect on disintegration and dissolution of immediate release tablets. Drug Dev Ind Pharm. 2018;44(3):444–451. doi:10.1080/03639045.2017.1397685
20. Jaffe JM, Colaizzi JL, Barry H. Effects of dietary components on GI absorption of Acetaminophen tablets in man. J Pharm Sci. 1971;60(11):1646–1650. doi:10.1002/jps.2600601111
21. Juvonen KR, Purhonen AK, Salmenkallio-Marttila M, et al. Viscosity of oat bran-enriched beverages influences gastrointestinal hormonal responses in healthy humans. J Nutr. 2009;139(3):461–466. doi:10.3945/jn.108.099945
22. Moore JG, Christian PE, Brown JA, et al. Influence of meal weight and caloric content on gastric emptying of meals in man. Dig Dis Sci. 1984;29(6):513–519. doi:10.1007/BF01296271
23. Weiner K, Graham LS, Reedy T, Elashoff J, Meyer JH. Simultaneous gastric emptying of two solid foods. Gastroenterology. 1981;81(2):257–266. doi:10.1016/S0016-5085(81)80056-X
24. Christian PE, Moore JG, Sorenson JA, Coleman RE, Weich DM. Effects of meal size and correction technique on gastric emptying time: studies with two tracers and opposed detectors. J Nucl Med off Publ Soc Nucl Med. 1980;21(9):883–885.
25. Rosenberg HA, Bates TR. The influence of food on nitrofurantoin bioavailability. Clin Pharmacol Ther. 1976;20(2):227–232. doi:10.1002/cpt1976202227
26. Bianchine JR, Shaw GM. Clinical pharmacokinetics of levodopa in Parkinson’s disease. Clin Pharmacokinet. 1976;1(5):313–338. doi:10.2165/00003088-197601050-00001
27. Shinkuma D, Hamaguchi T, Kobayashi M, Yamanaka Y, Mizuno N. Effects of food intake and meal size on the bioavailability of sulpiride in two dosage forms. Int J Clin Pharmacol. 1990;28(10):440–442.
28. Khosla R, Feely LC, Davis SS. Gastrointestinal transit of non-disintegrating tablets in fed subjects. Int J Pharm. 1989;53(2):107–117. doi:10.1016/0378-5173(89)90234-2
29. Bennink R, Peeters M, Van den Maegdenbergh V, et al. Evaluation of small-bowel transit for solid and liquid test meal in healthy men and women. Eur J Nucl Med. 1999;26(12):1560–1566. doi:10.1007/s002590050495
30. Kaufman PN, Richter JE, Chilton HM, et al. Effects of liquid versus solid diet on colonic transit in humans. Evaluation by standard colonic transit scintigraphy. Gastroenterology. 1990;98(1):73–81. doi:10.1016/0016-5085(90)91293-f
31. V. Schönfeld J, Evans DF, Goebell H, Wingate DL. Comparison of the small bowel motor response to solid and liquid meals in man. Digestion. 1997;58(4):402–406. doi:10.1159/000201474
32. Kunz P, Feinle C, Schwizer W, Fried M, Boesiger P. Assessment of gastric motor function during the emptying of solid and liquid meals in humans by MRI. J Magn Reson Imaging JMRI. 1999;9(1):75–80. doi:10.1002/(sici)1522-2586(199901)9:1<75::aid-jmri10>3.0.co;2-i
33. Grimm M, Scholz E, Koziolek M, Kühn JP, Weitschies W. Gastric water emptying under fed state clinical trial conditions is as fast as under fasted conditions. Mol Pharm. 2017;14(12):4262–4271. doi:10.1021/acs.molpharmaceut.7b00623
34. Kambayashi A, Shirasaka Y. Food effects on gastrointestinal physiology and drug absorption. Drug Metab Pharmacokinet. 2023;48:100488. doi:10.1016/j.dmpk.2022.100488
35. Kiyota T, Kambayashi A, Takagi T, Yamashita S. Importance of gastric secretion and the rapid gastric emptying of ingested water along the lesser curvature (“magenstraße”) in predicting the in vivo performance of liquid oral dosage forms in the fed state using a modeling and simulation. Mol Pharm. 2022;19(2):642–653. doi:10.1021/acs.molpharmaceut.1c00778
36. Koziolek M, Grimm M, Schneider F, et al. Navigating the human gastrointestinal tract for oral drug delivery: uncharted waters and new frontiers. Adv Drug Deliv Rev. 2016;101:75–88. doi:10.1016/j.addr.2016.03.009
37. Schulze K. Imaging and modelling of digestion in the stomach and the duodenum. Neurogastroenterol Motil. 2006;18(3):172–183. doi:10.1111/j.1365-2982.2006.00759.x
38. Camilleri M, Malagelada JR, Brown ML, Becker G, Zinsmeister AR. Relation between antral motility and gastric emptying of solids and liquids in humans. Am J Physiol. 1985;249(5 Pt 1):G580–585. doi:10.1152/ajpgi.1985.249.5.G580
39. Horowitz M, Dent J, Fraser R, Sun W, Hebbard G. Role and integration of mechanisms controlling gastric emptying. Dig Dis Sci. 1994;39(12 Suppl):7S–13S. doi:10.1007/BF02300360
40. Collins PJ, Horowitz M, Maddox A, Myers JC, Chatterton BE. Effects of increasing solid component size of a mixed solid/liquid meal on solid and liquid gastric emptying. Am J Physiol. 1996;271(4 Pt 1):G549–554. doi:10.1152/ajpgi.1996.271.4.G549
41. Horowitz M, Maddox A, Bochner M, et al. Relationships between gastric emptying of solid and caloric liquid meals and alcohol absorption. Am J Physiol. 1989;257(2 Pt 1):G291–298. doi:10.1152/ajpgi.1989.257.2.G291
42. Collins PJ, Horowitz M, Cook DJ, Harding PE, Shearman DJ. Gastric emptying in normal subjects--a reproducible technique using a single scintillation camera and computer system. Gut. 1983;24(12):1117–1125. doi:10.1136/gut.24.12.1117
43. Houghton LA, Read NW, Heddle R, et al. Relationship of the motor activity of the antrum, pylorus, and duodenum to gastric emptying of a solid-liquid mixed meal. Gastroenterology. 1988;94(6):1285–1291. doi:10.1016/0016-5085(88)90665-8
44. Sharma S, Prasad B. Meta-analysis of food effect on oral absorption of efflux transporter substrate drugs: does delayed gastric emptying influence drug transport kinetics? Pharmaceutics. 2021;13(7):1035. doi:10.3390/pharmaceutics13071035
45. Sidery MB, Macdonald IA, Blackshaw PE. Superior mesenteric artery blood flow and gastric emptying in humans and the differential effects of high fat and high carbohydrate meals. Gut. 1994;35(2):186–190. doi:10.1136/gut.35.2.186
46. Winter F, Schick P, Weitschies W. Bridging the gap between food effects under clinical trial conditions and real life: modeling delayed gastric emptying of drug substances and gastric content volume based on meal characteristics. Mol Pharm. 2023;20(2):1039–1049. doi:10.1021/acs.molpharmaceut.2c00782
47. Reynaud Y, Buffière C, David J, et al. Temporal changes in postprandial intragastric pH: comparing measurement methods, food structure effects, and kinetic modelling. Food Res Int Ott Ont. 2020;128:108784. doi:10.1016/j.foodres.2019.108784
48. Malagelada JR, Go VL, Summerskill WH. Different gastric, pancreatic, and biliary responses to solid-liquid or homogenized meals. Dig Dis Sci. 1979;24(2):101–110. doi:10.1007/BF01324736
49. Pentafragka C, Symillides M, McAllister M, Dressman J, Vertzoni M, Reppas C. The impact of food intake on the luminal environment and performance of oral drug products with a view to in vitro and in silico simulations: a PEARRL review. J Pharm Pharmacol. 2019;71(4):557–580. doi:10.1111/jphp.12999
50. Clark GWB, Jamieson IR, Hinder RA, et al. The relationship between gastric pH and the emptying of solid, semisolid and liquid meals. Neurogastroenterol Motil. 1993;5(4):273–279. doi:10.1111/j.1365-2982.1993.tb00131.x
51. Ogata H, Aoyagi N, Kaniwa N. BIavailability of metronidazole from sugar-coated tablets in humans. I. Effect of gastric acidity and correlation with in vitro dissolution rate. Int J Pharm. 1985;23(3):277–288. doi:10.1016/0378-5173(85)90156-5
52. Brouwers J, Tack J, Augustijns P. Parallel monitoring of plasma and intraluminal drug concentrations in man after oral administration of fosamprenavir in the fasted and fed state. Pharm Res. 2007;24(10):1862–1869. doi:10.1007/s11095-007-9307-3
53. Abrahamsson B, Albery T, Eriksson A, Gustafsson I, Sjöberg M. Food effects on tablet disintegration. Eur J Pharm Sci off J Eur Fed Pharm Sci. 2004;22(2–3):165–172. doi:10.1016/j.ejps.2004.03.004
54. Varum FJO, Hatton GB, Basit AW. Food, physiology and drug delivery. Int J Pharm. 2013;457(2):446–460. doi:10.1016/j.ijpharm.2013.04.034
55. Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc. 1995;95(7):791–797. doi:10.1016/S0002-8223(95)00219-7
56. Cao JJ, Johnson LK, Hunt JR. A diet high in meat protein and potential renal acid load increases fractional calcium absorption and urinary calcium excretion without affecting markers of bone resorption or formation in postmenopausal women. J Nutr. 2011;141(3):391–397. doi:10.3945/jn.110.129361
57. Reddy ST, Wang CY, Sakhaee K, Brinkley L, Pak CYC. Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis off J Natl Kidney Found. 2002;40(2):265–274. doi:10.1053/ajkd.2002.34504
58. Freudenthaler S, Meineke I, Schreeb KH, Boakye E, Gundert-Remy U, Gleiter CH. Influence of urine pH and urinary flow on the renal excretion of memantine. Br J Clin Pharmacol. 1998;46(6):541–546. doi:10.1046/j.1365-2125.1998.00819.x
59. Hertrampf R, Gundert-Remy U, Beckmann J, Hoppe U, Elsässer W, Stein H. Elimination of flecainide as a function of urinary flow rate and pH. Eur J Clin Pharmacol. 1991;41(1):61–63. doi:10.1007/BF00280108
60. Abuhelwa AY, Williams DB, Upton RN, Foster DJR. Food, gastrointestinal pH, and models of oral drug absorption. Eur J Pharm Biopharm Off J Arbeitsgemeinschaft Pharm Verfahrenstechnik EV. 2017;112:234–248. doi:10.1016/j.ejpb.2016.11.034
61. Adeva MM, Souto G. Diet-induced metabolic acidosis. Clin Nutr Edinb Scotl. 2011;30(4):416–421. doi:10.1016/j.clnu.2011.03.008
62. Lange D, Pavao JH, Jacqmin P, Woestenborghs R, Ding C, Klausner M. The effect of coadministration of a cola beverage on the bioavailability of itraconazole in patients with acquired immunodeficiency syndrome. Curr Ther Res. 1997;58(3):202–212. doi:10.1016/S0011-393X(97)80016-1
63. Aoi W, Zou X, Xiao JB, Marunaka Y. Body fluid pH balance in metabolic health and possible benefits of dietary alkaline foods. eFood. 2020;1(1):12–23. doi:10.2991/efood.k.190924.001
64. Dias CB, Zhu X, Thompson AK, Singh H, Garg ML. Effect of the food form and structure on lipid digestion and postprandial lipaemic response. Food Funct. 2019;10(1):112–124. doi:10.1039/c8fo01698d
65. Sun WM, Houghton LA, Read NW, Grundy DG, Johnson AG. Effect of meal temperature on gastric emptying of liquids in man. Gut. 1988;29(3):302–305. doi:10.1136/gut.29.3.302
66. Verhagen MAMT, Luijk HD, Samsom M, Smout AJPM. Effect of meal temperature on the frequency of gastric myoelectrical activity. Neurogastroenterol Motil. 1998;10(2):175–181. doi:10.1046/j.1365-2982.1998.00089.x
67. Kagawa-Busby KS, Heitkemper MM, Hansen BC, Hanson RL, Vanderburg VV. Effects of diet temperature on tolerance of enteral feedings. Nurs Res. 1980;29(5):276–280. doi:10.1097/00006199-198009000-00003
68. Mishima Y, Amano Y, Takahashi Y, et al. Gastric emptying of liquid and solid meals at various temperatures: effect of meal temperature for gastric emptying. J Gastroenterol. 2009;44(5):412–418. doi:10.1007/s00535-009-0022-1
69. Hamid N, Malik MO, Hajira B, Shah I, Azhar M. Food temperature altered macronutrients induced changes in satiety hormones; glucagon - like peptide −1 and cholecystokinin and their correlation with subjective satiety. J Fam Community Med. 2024;31(3):237–243. doi:10.4103/jfcm.jfcm_356_23
70. Marcotte M, Grabowski S, Karimi Y, Nijland P. Acid diffusion in solid foods. Int J Food Eng. 2012;8(4). doi:10.1515/1556-3758.1300
71. U.S. Food and Drug Administration.Guidance for Industry: Waiver of in vivo Bioavailability and Bioequivalence Studies for ImmediateRelease Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System. Center for Drug Evaluation and Research; 2000.
72. Fleisher D, Li C, Zhou Y, Pao LH, Karim A. Drug, meal and formulation interactions influencing drug absorption after oral administration. Clinical implications. Clin Pharmacokinet. 1999;36(3):233–254. doi:10.2165/00003088-199936030-00004
73. Charalabidis A, Sfouni M, Bergström C, Macheras P. The biopharmaceutics classification system (BCS) and the biopharmaceutics drug disposition classification system (BDDCS): beyond guidelines. Int J Pharm. 2019;566:264–281. doi:10.1016/j.ijpharm.2019.05.041
74. Rubbens J, Brouwers J, Tack J, Augustijns P. Gastric and duodenal diclofenac concentrations in healthy volunteers after intake of the FDA standard meal: in vivo observations and in vitro explorations. Mol Pharm. 2019;16(2):573–582. doi:10.1021/acs.molpharmaceut.8b00865
75. U.S. Food and Drug Administration. Guidance for industry: Assessing the effects of food on drugs in inds and ndas-clinical pharmacology considerations. 2019.
76. Cheng L, Wong H. Food effects on oral drug absorption: application of physiologically-based pharmacokinetic modeling as a predictive tool. Pharmaceutics. 2020;12(7):672. doi:10.3390/pharmaceutics12070672
77. Indireshkumar K, Faas H, Brasseur JG, et al. Relative contribution of “pressure pump” and “peristaltic pump” to gastric emptying. Gastroenterology. 1998;Supplement 1(114):A770. doi:10.1016/S0016-5085(98)83148-X
78. Ogura Y, Gonsho A, Cyong JC, Orimo H. Clinical trial of risedronate in Japanese volunteers: a study on the effects of timing of dosing on absorption. J Bone Miner Metab. 2004;22(2):120–126. doi:10.1007/s00774-003-0459-x
79. Fujii Y, Takahashi M, Morita H, Kikuchi H, Aramaki Y, Amidon GL. Characteristics of gastrointestinal absorption of DX-9065a, a new synthetic anticoagulant. Drug Metab Pharmacokinet. 2007;22(1):26–32. doi:10.2133/dmpk.22.26
80. Lin W, Chen Y, Unadkat JD, Zhang X, Wu D, Heimbach T. Applications, challenges, and outlook for PBPK modeling and simulation: a regulatory, industrial and academic perspective. Pharm Res. 2022;39(8):1701–1731. doi:10.1007/s11095-022-03274-2
81. Zhang W, Zhang Q, Cao Z, Zheng L, Hu W. Physiologically based pharmacokinetic modeling in neonates: current status and future perspectives. Pharmaceutics. 2023;15(12):2765. doi:10.3390/pharmaceutics15122765
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

