Back to Journals » Drug Design, Development and Therapy » Volume 19
Impact of Fungal Co-Infection on Teicoplanin Plasma Trough Concentration in Critically Ill Adults: A Novel Consideration for Dose Adjustment
Authors Cheng L , Wang L, You X, Xiong L, Dai Q, Wang Q
Received 15 January 2025
Accepted for publication 5 June 2025
Published 9 June 2025 Volume 2025:19 Pages 4967—4977
DOI https://doi.org/10.2147/DDDT.S516472
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Mariana Carmen Chifiriuc
Lin Cheng,1,* Lina Wang,2,* Xi You,1 Lirong Xiong,1 Qing Dai,1 Qian Wang1
1Department of Pharmacy, The First Affiliated Hospital of Army Medical University, Chongqing, People’s Republic of China; 2Department of Obstetrics and Gynecology, The First Affiliated Hospital of Army Medical University, Chongqing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qing Dai, Email [email protected] Qian Wang, Email [email protected]
Objective: The pathophysiology and disease status of critically ill patients have a significant impact on the pharmacokinetics/pharmacodynamics (PK/PD) of antimicrobial agents. However, the effect of fungal co-infection on the plasma trough concentration (Cmin) of teicoplanin in critically ill patients remains unclear.
Materials and Methods: A retrospective cohort study was carried out. Clinical data of patients admitted to the intensive care unit and receiving teicoplanin therapeutic drug monitoring were collected. Multiple linear stepwise regression analysis and binary logistic regression analysis were used to identify the factors influencing teicoplanin Cmin and the achievement of the target Cmin (≥ 15.0 μg/mL).
Results: A total of 404 teicoplanin Cmin values from 231 patients were included. The mean teicoplanin Cmin was 20.63 ± 10.40 μg/mL, and the percentage of Cmin > 30.0 μg/mL was 15.8%. In the multivariate analysis, fungal co-infection was identified as an independent factor affecting teicoplanin Cmin (B=4.056, 95% CI 2.089– 6.023; p< 0.001) and the attainment of the target Cmin (OR=3.233, 95% CI 1.538– 6.795; p=0.002). Sex, weight, teicoplanin dose, levels of direct bilirubin, blood urea nitrogen, estimated glomerular filtration rate, and uric acid were also found to be influencing factors. Patients with fungal co-infection had a higher teicoplanin Cmin (p< 0.001) and a higher percentage of Cmin > 30.0 μg/mL (20.3% vs 12.0%; p=0.022) compared to those without, despite similar teicoplanin doses (p=0.302). The percentage of patients receiving continuous renal replacement therapy was higher in the fungal co-infection cohort (p=0.016), along with an older age and a lower body weight.
Conclusion: For critically ill patients with fungal co-infections, the teicoplanin dose should be decreased, or at least not increased. This is essential for reducing the potential risk of toxicity and customizing dosing strategies to meet individual patient needs. A large-scale, multi-center, prospective study is necessary to confirm the findings related to this dosing approach.
Keywords: teicoplanin, critically ill patients, therapeutic drug monitoring, fungal infection, direct bilirubin
Introduction
Teicoplanin, a glycopeptide antibiotic, is mainly used to treat a variety of severe Gram-positive bacterial infections, such as those caused by Staphylococcus, Streptococcus, Enterococcus, and most anaerobic positive bacteria, especially in patients who cannot tolerate penicillins and cephalosporins.1 Compared with vancomycin, teicoplanin shows similar antimicrobial activity but has fewer adverse effects, such as nephrotoxicity and infusion reactions.2,3 The plasma concentration of teicoplanin is closely related to its clinical efficacy. In different diseases, the teicoplanin plasma trough concentration (Cmin) needs to reach the corresponding target value to meet the treatment requirements.4,5 For patients with severe infections, monitoring teicoplanin Cmin can improve the cure rate.6–8 In febrile neutropenic patients with hematological malignancies, achieving a teicoplanin Cmin ≥20 μg/mL at 48 hours significantly improves treatment success rates.9,10 However, sustained Cmin elevation over 10 days during therapy increases the risk of adverse events.8 For patients with renal dysfunction, prompt attainment of teicoplanin Cmin within 15–30 μg/mL is critical to optimize clinical outcomes, with comparable nephrotoxicity and hepatotoxicity incidence rates to those with Cmin <15 μg/mL.7
Extreme inter- and intra-individual pharmacokinetic (PK) variability exists in intensive care unit (ICU) patients.11 The variability in teicoplanin exposure is also significant in critically ill patients.12–15 Due to the complex pathophysiology and disease status of critically ill patients, the incidence of suboptimal teicoplanin Cmin during conventional dosing is relatively high.16,17 Thus, therapeutic drug monitoring (TDM) of teicoplanin Cmin in critically ill patients is recommended. Previous studies on teicoplanin Cmin in critically ill patients mainly focused on the influence of various physiological factors such as age and renal function on the achievement of Cmin (≥ 15–30 μg/mL).18–20 However, patients with severe infections admitted to the ICU are often associated with multiple bacterial or fungal infections and the combined use of various antimicrobial agents. In a worldwide multicenter study involving 1150 centers in 88 countries, Gram-negative bacteria were detected in 67% of ICU patients, Gram-positive bacteria in 37%, and fungal microorganisms in 16%.21 Moreover, critically ill patients usually undergo extracorporeal membrane oxygenation (ECMO), continuous renal replacement therapy (CRRT), mechanical ventilation, etc.6,22 The impact of these factors on the Cmin and standard attainment rate of teicoplanin has been rarely reported. It is necessary to investigate the effect of these factors on teicoplanin Cmin in critically ill patients.
In patients with severe infections, teicoplanin is often empirically combined with anti-Gram-negative antibiotics. These patients may also be frequently infected with fungi. Critically ill patients with fungal infections are often immunocompromised,23 and the influence on the PK of antimicrobial agents may be more pronounced. In fungal infections, pathogen-associated molecular patterns activate pattern recognition receptors, initiating inflammatory cytokine production.24 Systemic inflammatory response syndrome-driven cytokine overexpression enhances vascular permeability, expanding the volume of distribution for hydrophilic antimicrobials. Concurrently, these cytokines downregulate metabolic enzymes, reducing drug clearance.25 In addition, anti-fungal drugs amphotericin B exhibits pronounced nephrotoxic potential, whereas triazole antifungals pose minimal indirect nephrotoxic risks.26 Caspofungin demonstrates high albumin binding (≈97%), while voriconazole (58% binding) displays nonlinear pharmacokinetics and hepatotoxicity.27,28 These factors collectively influence teicoplanin metabolism.
Currently, the effect of fungal co-infection on teicoplanin Cmin in critically ill patients remains unclear. In this study, we explored the influencing factors of teicoplanin Cmin in critically ill adult patients using real-world data. Common physiological parameters, fungal co-infection status, combined use of potentially nephrotoxic antimicrobials, liver function indicators, and the receipt of CRRT, ECMO, and mechanical ventilation were included as influencing factors.
Patients and Methods
Patients and Study Design
This was a retrospective study. Patients who met the following criteria and were admitted to Southwest Hospital, Chongqing, China, from January 1, 2018, to December 31, 2023, were included: a) admitted to the ICU; b) aged ≥ 18 years; c) received teicoplanin TDM, and the Cmin was at steady state (measured before the next dose after at least 6 doses); d) had the required clinical data; e) was not currently pregnant.
Teicoplanin Cmin Measurement
Venous blood was drawn before the next dose after at least 6 doses of teicoplanin administration. Plasma total teicoplanin concentrations were determined using the high-performance liquid chromatography method. The linear range of teicoplanin was 3.125–100.0 µg/mL.
Data Collection
The following data were collected: a) Baseline characteristics: These included sex, age, height, weight, clinical diagnosis, Acute Physiology and Chronic Health Evaluation (APACHE) II score, details regarding whether the patient underwent CRRT or ECMO, mechanical ventilation duration, as well as the types of infected bacteria and fungi. b) Combination drug information: This involved the dosage of teicoplanin and details about the concurrent use of other antimicrobials. c) Laboratory test indices measured within 3 days before detecting the teicoplanin concentration: Liver function parameters such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), glutamyl transferase (γ-GT), alkaline phosphatase (ALP), albumin, total bile acid (TBA), total bilirubin (TBIL), direct bilirubin (DBIL) and indirect bilirubin (IBIL). Renal function indicators like serum creatinine, estimated glomerular filtration rate (eGFR), blood urea nitrogen (BUN), and uric acid.
Statistical Analysis
Sample size was calculated using the Events Per Variable principle. With 12 variables included in regression analysis and a 40% probability of suboptimal teicoplanin Cmin, the required sample size was determined as 12×10/0.4=300. Missing data were managed as follows: <20% missingness was imputed with the latest available values, followed by recalculating missing proportions. If ≥90% data remained after imputation, median substitution was applied; otherwise, cases were excluded. Variables with >20% missingness were removed entirely.
IBM SPSS 26.0 software was employed for statistical analysis. Counting data were expressed as the rate (%), and were compared using the chi-square test. For measurement data that conform to a normal distribution, they are presented as the mean ± standard deviation, and compared by means of the t-test. Data with a non-normal distribution are shown as the median (interquartile range), and compared through the Mann‒Whitney U-test. Multiple linear stepwise regression analysis was carried out to determine the factors affecting teicoplanin Cmin. Binary logistic regression analysis was used to identify the factors influencing the attainment of the teicoplanin Cmin target. Multicollinearity was evaluated via tolerance values and variance inflation factors (VIF). Tolerance <0.1–0.2 and VIF >5–10 indicated significant collinearity issues. The covariates with a p-value of < 0.1 in the univariate analysis were included in the multivariate analysis. For critically ill patients, a teicoplanin Cmin ≥ 15.0 μg/mL was set as the research target.4,29 A p-value < 0.05 was considered to indicate a statistically significant difference.
Results
Patient Characteristics
A total of 404 teicoplanin Cmin values from 231 patients were included in the analysis (Table 1). Most patients were male, accounting for 72.3%. The age of patients ranged from 19 to 101 years. The APACHE II score of patients was 27±10, 51.9% of patients received CRRT, and 12.1% received ECMO. The median duration of mechanical ventilation was 12 days. Each patient received at least one combination of antimicrobial agents. The main antibiotics used in combination were meropenem, imipenem/cilastatin, piperacillin/tazobactam, and polymyxin B. The main antifungal agents used in combination were caspofungin and voriconazole.
 |
Table 1 Clinical Characteristics of Patients |
The dosage of teicoplanin was 676±248 mg/d, and the Cmin was 20.63±10.40 μg/mL. Teicoplanin dosages (mg/d) from 2018–2023 were: 583±254, 733±247, 720±216, 696±289, 693±239, and 672±242. Dosages from 2019–2023 were significantly higher than in 2018 (p<0.05), with no significant interannual variations between 2019–2023. The percentages of teicoplanin Cmin reaching the target from 2018 to 2023 were 24.0% (12/50), 61.1% (11/18), 65.0% (26/40), 69.4% (34/49), 75.0% (66/88), and 77.4% (123/159), respectively. The percentage of teicoplanin Cmin > 30.0 μg/mL was 15.8% (64/404). Two patients had teicoplanin Cmin > 60.0 μg/mL.
The main detected Gram-positive bacteria were Enterococcus faecium, Staphylococcus aureus, Staphylococcus haemolyticus, Staphylococcus epidermidis, Enterococcus faecalis, and Streptococcus mitis; the main detected Gram-negative bacteria were Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Escherichia coli, Enterobacter cloacae, and Proteus mirabilis; and the main detected fungi were Candida, Aspergillus, and Saccharomyces (Figure 1). Many patients received teicoplanin for empirical and combined use.
 |
Figure 1 Pathogenic microorganisms cultured. |
The laboratory data of patients are shown in Table 2. The median values of AST, γ-GT, TBIL, DBIL, IBIL, and BUN were higher than the upper limit of normal. The mean value of albumin was lower than the lower limit of normal.
 |
Table 2 Laboratory Data of Patients |
Factors Influencing Teicoplanin Cmin
In the multivariate analysis, factors influencing teicoplanin Cmin were sex, teicoplanin dose, levels of DBIL and BUN, and fungal co-infection (Table 3). In the univariate analysis, factors influencing the attainment of teicoplanin target Cmin were sex, weight, APACHE II score, teicoplanin dose, combined use of polymyxin B, amikacin, and amphotericin B, levels of TBIL, DBIL, serum creatinine, uric acid, BUN, and eGFR, and fungal co-infection (Figure 2). We also compared the teicoplanin Cmin values between patients who used caspofungin and those who did not, as well as between patients who used voriconazole and those who did not. The results showed no significant differences. For the caspofungin comparison, the values were 20.12 (14.67, 26.68) μg/mL and 19.67 (12.38, 25.45) μg/mL with a p-value of 0.172. For the voriconazole comparison, the values were 18.48 (10.84, 24.24) μg/mL and 19.74 (13.22, 26.18) μg/mL with a p-value of 0.172. The independent risk factors for the attainment of teicoplanin target Cmin were female, low weight, high teicoplanin dose, low eGFR, high uric acid, and fungal co-infection (Table 4). Our findings demonstrate that fungal co-infection independently influences both teicoplanin Cmin and achievement of target Cmin, representing a novel observation not previously documented in the literature.
 |
Table 3 Influencing Factors for Teicoplanin Cmin |
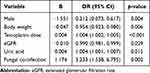 |
Table 4 Factors Influencing the Achievement of Teicoplanin Target Cmin |
 |
Figure 2 Factors influencing the attainment of teicoplanin target Cmin in the univariate analysis. *p<0.05. |
Clinical Characteristics of Patients with Fungal Co-Infection
A total of 100 (43.3%) patients had fungal co-infection. The comparison of clinical characteristics between patients with and without fungal co-infection was shown in Table 5. Patients with fungal co-infection had a higher teicoplanin Cmin compared to those without (p<0.001), although the teicoplanin doses were similar (Figure 3). Compared with patients without fungal infection, the percentage of teicoplanin Cmin >30.0 μg/mL was also higher in patients with fungal co-infection (20.3% vs 12.0%; p=0.022). Additionally, the percentage of patients receiving CRRT was higher in the fungal co-infection cohort (p=0.016), along with an older age and a lower body weight. Liver and renal functions were similar in the two cohorts (p>0.05).
 |
Table 5 Clinical Characteristics of Patients with and without Fungal Co-Infection |
Discussion
In this study, the percentages of teicoplanin Cmin reaching the target increased year by year from 2018 to 2023, ranging from 24.0% in 2018 to 77.4% in 2023. According to the latest consensus review by the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring, a target Cmin value ranging from 15 to 30 μg/mL leads to better clinical efficacy and comparable adverse effects in patients with non-complicated methicillin-resistant Staphylococcus aureus (MRSA) infections compared to a Cmin value of less than 15 μg/mL.30 This study investigated the influencing factors of teicoplanin Cmin in critically ill patients based on 6-year data and found that sex, DBIL, and fungal co-infection were new factors influencing teicoplanin Cmin, in addition to previously known factors such as dose, body weight, and renal function.17,31–33
Teicoplanin has a high plasma protein binding rate, a binding rate of 90% to 95% with albumin, and a long elimination half-life.14,34 Except for 2–3% metabolized by the liver, most is excreted by the kidney. Some studies have suggested that body weight, albumin level, and renal function play significant roles in influencing free teicoplanin Cmin and PK parameters.13,35,36 In critically ill patients, a high Sequential Organ Failure Assessment (SOFA) score and low serum albumin have been reported as risk factors for decreased teicoplanin Cmin during initial dosing.37 However, hypoalbuminemia did not seem to affect total teicoplanin concentrations.13 In this study, we measured total plasma teicoplanin concentrations and did not find an effect of serum albumin on the concentration, but DBIL significantly affected teicoplanin Cmin. The possible mechanism is that bilirubin is mainly (up to 90%) bound to proteins, which in turn causes the displacement of drugs from albumin.38
In a prospective study investigating the population PK model of teicoplanin concentrations in patients hospitalized in the ICU, eGFR was associated with systemic clearance.16,39 We also found that eGFR was an independent factor influencing the achievement of the teicoplanin Cmin target, along with other renal function indicators BUN and uric acid.
Previous studies on therapeutic monitoring of teicoplanin were mostly focused on critically ill patients infected with Gram-positive bacteria. In a prospective study evaluating the effect of CRRT on the clearance of teicoplanin, the early stage albumin level could significantly affect the initial Cmin and the eradication of Gram-positive bacteria, and also had an effect on the clearance of teicoplanin by CRRT.6 In a study focused on optimizing the antimicrobial dosing regimen for critically ill patients with MRSA infections, the dose of CRRT has an effect on the probability of reaching the target, and the CRRT modality influences the clearance of teicoplanin.40 We did not find the effect of CRRT on teicoplanin Cmin in the present study. However, the percentage of patients receiving CRRT was higher in patients with fungal co-infection, and these patients had a higher teicoplanin Cmin.
Disease severity, concurrent antimicrobial therapies, and inflammatory responses can all have an impact on teicoplanin metabolism. They do so by influencing liver and kidney function and triggering drug-drug interactions. In the present study, the APACHE II score demonstrated a trend of influencing the teicoplanin Cmin, which was set as the research target. The combined use of polymyxin B, amikacin, and amphotericin B had a notable effect on teicoplanin Cmin. However, in the multiple regression analysis, neither the use of other antimicrobial agents nor disease severity emerged as an independent influencing factor. Additionally, there were no significant differences in teicoplanin Cmin between patients who used caspofungin and those who did not, and between patients who used voriconazole and those who did not. This suggests that a combination of multiple factors in fungal-infected critically ill patients likely affects teicoplanin concentrations. Further exploration of the underlying mechanisms connecting fungal co-infection to the altered pharmacokinetics of teicoplanin could offer valuable insights for optimizing antimicrobial regimens in complex clinical settings.
The recommended blood concentration of teicoplanin shows substantial variability depending on the severity and location of infections, rather than on the severity of the underlying illness.18 However, limited data were available for critically ill patients with fungal co-infection. Infected critically ill patients may have unfavorable outcomes due to inadequate antibiotic exposure resulting from altered PK and pharmacodynamic parameters of antibiotics. Conversely, in patients with fungal co-infection, these critically ill patients may also experience adverse outcomes owing to a compromised immune system and excessive exposure to teicoplanin.41 In our study, the proportion of patients with fungal co-infection was high, and teicoplanin Cmin were significantly higher in patients with fungal infection, as well as the percentage of teicoplanin Cmin >30.0 μg/mL at a similar teicoplanin dose. For critically ill patients with fungal co-infections, maintaining a teicoplanin Cmin above 30.0 μg/mL for an extended period may pose a high risk of toxicity. It is worth noting that patients with fungal infection were older and had a lower body weight, who may be more likely to develop adverse reactions.
Fungal products, such as beta-glucans and candidalysin, can activate the host’s immune system, thereby exacerbating liver and biliary diseases.42 Additionally, immune complexes are formed and can be deposited in the kidney tissue, resulting in inflammation and damage. Fungi are capable of directly invading the kidney tissue, causing cellular damage and triggering inflammatory reactions. Moreover, certain fungi produce toxins that exert toxic effects on kidney cells, contributing to the development of nephritis. In the context of acute kidney injury, the clearance of antimicrobials that are primarily excreted by the kidneys, like teicoplanin, is affected.
In critically ill patients, simulations have shown that the standard dosage regimen is only adequate for patients with severe renal dysfunction (eGFR ≤30 mL/min/1.73 m²) to reach the target Cmin, with a probability of target attainment (PTA) of 52.8%. When the eGFR is greater than 30 mL/min/1.73 m², adjusting the dose by increasing it and modifying the administration frequency of the loading doses are preferable strategies to achieve the target Cmin, depending on the patient’s renal function and the type of infection.16 In critically ill patients with sepsis, simulations based on a population PK model have indicated that for patients with varying renal functions, administering 3 or 5 loading doses of 12/15 mg/kg every 12 hours, followed by a maintenance dose of 12/15 mg/kg every 24 to 72 hours, is necessary to achieve a target Cmin of 15 μg/mL.39 In elderly critically ill patients with pneumonia, model-based simulations have demonstrated that a PTA of at least 85% can only be achieved with higher-dose regimens (12 mg/kg) when the minimum inhibitory concentration (MIC) is up to 0.5 mg/L.20 Optimal teicoplanin dosing under CRRT doses ≤25 mL/kg/h was determined in previous study; When CRRT doses increased to 30–35 mL/kg/h, teicoplanin dosing required a 30–40% escalation.40 However, all of these existing studies have not taken into account the influence of fungal co-infection. Hence, it is essential to establish further population PK models that specifically focus on critically ill patients with fungal co-infection, so as to optimize the teicoplanin dosage for these patients.
There were several limitations in this study. First of all, this analysis relies on data collected from a single center of critically ill patients, and there might be certain biases. Secondly, as a single-center retrospective study, our research was unable to conduct external validation or perform other types of analyses. Nevertheless, two regression analysis models were employed in this study. The outcomes of the multiple linear stepwise regression analysis and the binary logistic regression analysis demonstrated that fungal co-infection acts as both an independent influencing factor for the teicoplanin Cmin and the attainment of the target Cmin. Thirdly, disease severity may independently impact teicoplanin Cmin. In our analysis, we only incorporated the APACHE II score. Although some patients with sepsis had SOFA scores, the proportion of such patients was relatively low, and thus, they were not included in the analysis. Additionally, we did not analyze the association between teicoplanin Cmin and clinical outcome. However, many patients in our study received teicoplanin for empirical use, and it is difficult to evaluate the association between teicoplanin Cmin and clinical outcome. Future studies exploring the correlation between drug exposure and therapeutic efficacy or toxicity in critically ill patients with fungal co-infection are needed. Finally, although the sample size is adequate, a larger sample size could assist in including more covariates.
Conclusion
In summary, we reported for the first time that fungal co-infection was an independent risk factor influencing teicoplanin Cmin in critically ill adult patients. Patients with fungal co-infection had a higher teicoplanin Cmin and a higher percentage of the concentration >30.0 μg/mL. In critically ill patients with fungal co-infection, the teicoplanin dosage should either be lowered or, at the very least, not be increased. This is to minimize the risks of toxicity and to fine-tune individualized dosing strategies. In addition, sex and DBIL were also factors influencing teicoplanin Cmin, in addition to body weight, teicoplanin dose, and renal function indicators, which should be considered in the clinical use of teicoplanin in critically ill adult patients. Considering that the data were collected from a single center, a larger-scale, multi-center, prospective study is essential to validate the findings of this research. Developing a population PK model is crucial for optimizing the dosage of teicoplanin in critically ill patients with fungal co-infections. Additionally, further investigations focusing on elucidating the mechanism by which fungal co-infections affect the teicoplanin Cmin are highly warranted.
Data Sharing Statement
Data are available under reasonable requirements.
Ethical Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the First Affiliated Hospital of the Army Medical University (approval number: KY2024122). The Ethics Committee of the First Affiliated Hospital of the Army Medical University approved this study to be exempt from individual patient consent for publication as existing data were collected and de-identified.
Funding
This work was supported by the Key project of Chongqing Natural Science Foundation (CSTB2023NSCQ-ZDX0037, 0009), and Chongqing Clinical Pharmacy Key Specialties Construction Project, China.
Disclosure
Lin Cheng and Lina Wang contributed equally to this work as co-first authors. The authors declare no conflicts of interest in this work.
References
1. Kasai H, Tsuji Y, Hiraki Y, Tsuruyama M, To H, Yamamoto Y. Population pharmacokinetics of teicoplanin in hospitalized elderly patients using cystatin C as an indicator of renal function. J Infect Chemother. 2018;24:284–291. doi:10.1016/j.jiac.2017.12.002
2. Kato-Hayashi H, Niwa T, Ohata K, et al. Comparative efficacy and safety of vancomycin versus teicoplanin in febrile neutropenic patients receiving hematopoietic stem cell transplantation. J Clin Pharm Therapeutics. 2019;44:888–894. doi:10.1111/jcpt.13011
3. Kaur J, Mir T, Dixit P, et al. The use of vancomycin versus teicoplanin in treating febrile neutropenia: a meta-analysis and systematic review. Cureus. 2021;13:e15269. doi:10.7759/cureus.15269
4. Abdul-Aziz MH, Alffenaar JC, Bassetti M, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a Position Paper. Intensive Care Med. 2020;46:1127–1153. doi:10.1007/s00134-020-06050-1
5. Ueda T, Takesue Y, Nakajima K, et al. Clinical efficacy and safety in patients treated with teicoplanin with a target trough concentration of 20 mug/mL using a regimen of 12 mg/kg for five doses within the initial 3 days. BMC Pharmacol Toxicol. 2020;21:50. doi:10.1186/s40360-020-00424-3
6. Shi L, Zhuang Z, Duan L, et al. Dose optimization of teicoplanin for critically ill patients with renal dysfunction and continuous renal replacement therapy: experience from a prospective interventional study. Front Pharmacol. 2022;13:817401. doi:10.3389/fphar.2022.817401
7. Ueda T, Takesue Y, Nakajima K, et al. Enhanced loading regimen of teicoplanin is necessary to achieve therapeutic pharmacokinetics levels for the improvement of clinical outcomes in patients with renal dysfunction. Eur J Clin Microbiol Infect Dis. 2016;35:1501–1509. doi:10.1007/s10096-016-2691-z
8. Kim SH, Kang CI, Huh K, et al. Evaluating the optimal dose of teicoplanin with therapeutic drug monitoring: not too high for adverse event, not too low for treatment efficacy. Eur J Clin Microbiol Infect Dis. 2019;38:2113–2120. doi:10.1007/s10096-019-03652-6
9. Byrne CJ, Egan S, Fennell JP, et al. Teicoplanin use in adult patients with haematological malignancy: exploring relationships between dose, trough concentrations, efficacy and nephrotoxicity. Int J Antimicrob Agents. 2015;46:406–412. doi:10.1016/j.ijantimicag.2015.05.019
10. Wang YW, Hou HA, Lin CC, et al. Early therapeutic drug monitoring optimizes teicoplanin use in febrile neutropenic patients with hematological malignancies. Adv Ther. 2024;41:2966–2977. doi:10.1007/s12325-024-02884-z
11. Sistanizad M, Hassanpour R, Pourheidar E. Are antibiotics appropriately dosed in critically ill patients with augmented renal clearance? A narrative review. Int J Clin Pract. 2022;2022:1867674. doi:10.1155/2022/1867674
12. Byrne CJ, Roberts JA, McWhinney B, et al. Variability in trough total and unbound teicoplanin concentrations and achievement of therapeutic drug monitoring targets in adult patients with hematological malignancy. Antimicrob Agents Chemother. 2017;61:e02466–16. doi:10.1128/AAC.02466-16
13. Brink AJ, Richards GA, Lautenbach EE, et al. Albumin concentration significantly impacts on free teicoplanin plasma concentrations in non-critically ill patients with chronic bone sepsis. Int J Antimicrob Agents. 2015;45:647–651. doi:10.1016/j.ijantimicag.2015.01.015
14. Roberts JA, Stove V, De Waele JJ, et al. Variability in protein binding of teicoplanin and achievement of therapeutic drug monitoring targets in critically ill patients: lessons from the DALI Study. Int J Antimicrob Agents. 2014;43:423–430. doi:10.1016/j.ijantimicag.2014.01.023
15. Mouton JWA, De Clercq A, De Paepe P, et al. Pharmacokinetics and target attainment of teicoplanin: a systematic review. Clin Pharmacokinet. 2025;64(4):467–509. Online ahead of print. doi:10.1007/s40262-025-01483-7
16. Wang Y, Yao F, Chen S, et al. Optimal teicoplanin dosage regimens in critically ill patients: population pharmacokinetics and dosing simulations based on renal function and infection type. Drug Des Devel Ther. 2023;17:2259–2271. doi:10.2147/DDDT.S413662
17. Li H, Gao L, Zhou L, et al. Optimal teicoplanin loading regimen to rapidly achieve target trough plasma concentration in critically ill patients. Basic Clin Physiol Pharmacol. 2020;126:277–288. doi:10.1111/bcpt.13338
18. Wang L, Chen M, Ye H, et al. Higher teicoplanin blood level needed in elderly critically ill patients. Curr Drug Metab. 2021;22:1124–1131. doi:10.2174/1389200222666211122124420
19. Wang S, Lin F, Ruan J, Ye H, Wang L. Pharmacokinetics of multiple doses of teicoplanin in Chinese elderly critical patients. Expert Rev clin Pharmacol. 2018;11:537–541. doi:10.1080/17512433.2018.1449107
20. Kang SW, Jo HG, Kim D, et al. Population pharmacokinetics and model-based dosing optimization of teicoplanin in elderly critically ill patients with pneumonia. J Crit Care. 2023;78:154402. doi:10.1016/j.jcrc.2023.154402
21. Vincent JL, Sakr Y, Singer M, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323:1478–1487. doi:10.1001/jama.2020.2717
22. Karkar A, Ronco C. Prescription of CRRT: a pathway to optimize therapy. Ann Intens Care. 2020;10:32. doi:10.1186/s13613-020-0648-y
23. Pathakumari B, Liang G, Liu W. Immune defence to invasive fungal infections: a comprehensive review. Biomed Pharmacother. 2020;130:110550. doi:10.1016/j.biopha.2020.110550
24. Mancuso G, Midiri A, Gerace E, Biondo C. Role of the innate immune system in host defence against fungal infections. Eur Rev Med Pharmacol Sci. 2022;26:1138–1147. doi:10.26355/eurrev_202202_28105
25. Tanaka R. Pharmacokinetic variability and significance of therapeutic drug monitoring for broad-spectrum antimicrobials in critically ill patients. J Pharm Health Care Sci. 2025;11:21. doi:10.1186/s40780-025-00425-6
26. Tragiannidis A, Gkampeta A, Vousvouki M, Vasileiou E, Groll AH. Antifungal agents and the kidney: pharmacokinetics, clinical nephrotoxicity, and interactions. Expert Opin Drug Saf. 2021;20:1061–1074. doi:10.1080/14740338.2021.1922667
27. Cheng L, Xiang R, Liu F, et al. Therapeutic drug monitoring and safety of voriconazole in elderly patients. Int Immunopharmacol. 2020;78:106078. doi:10.1016/j.intimp.2019.106078
28. Cheng L, You X, Wang X, Yu M, Jia C. The role of plasma trough concentration of voriconazole and voriconazole N-oxide in its hepatotoxicity in adult patients. Drug Des Devel Ther. 2024;18:3617–3628. doi:10.2147/DDDT.S475706
29. Hanai Y, Takahashi Y, Niwa T, et al. Optimal trough concentration of teicoplanin for the treatment of methicillin-resistant Staphylococcus aureus infection: a systematic review and meta-analysis. J Clin Pharm Therapeutics. 2021;46:622–632. doi:10.1111/jcpt.13366
30. Hanai Y, Takahashi Y, Niwa T, et al. Clinical practice guidelines for therapeutic drug monitoring of teicoplanin: a consensus review by the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J Antimicrob Chemother. 2022;77:869–879. doi:10.1093/jac/dkab499
31. Byrne CJ, Roberts JA, McWhinney B, et al. Population pharmacokinetics of teicoplanin and attainment of pharmacokinetic/pharmacodynamic targets in adult patients with haematological malignancy. Clin Microbiol Infect. 2017;23:674e7–e13. doi:10.1016/j.cmi.2017.02.032
32. Byrne CJ, Parton T, McWhinney B, et al. Population pharmacokinetics of total and unbound teicoplanin concentrations and dosing simulations in patients with haematological malignancy. J Antimicrob Chemother. 2018;73:995–1003. doi:10.1093/jac/dkx473
33. Kontou A, Sarafidis K, Begou O, et al. Population pharmacokinetics of teicoplanin in preterm and term neonates: is it time for a new dosing regimen? Antimicrob Agents Chemother. 2020;64:e01971–19. doi:10.1128/AAC.01971-19
34. Ogami C, Tsuji Y, Muraki Y, Mizoguchi A, Okuda M, To H. Population pharmacokinetics and pharmacodynamics of teicoplanin and C-reactive protein in hospitalized patients with gram-positive infections. Clin Pharmacol Drug Dev. 2020;9:175–188. doi:10.1002/cpdd.684
35. Yano R, Nakamura T, Tsukamoto H, et al. Variability in teicoplanin protein binding and its prediction using serum albumin concentrations. Ther Drug Monit. 2007;29:399–403. doi:10.1097/FTD.0b013e3180690755
36. Emoto C, Johnson TN, Yamada T, Yamazaki H, Fukuda T. Teicoplanin physiologically based pharmacokinetic modeling offers a quantitative assessment of a theoretical influence of serum albumin and renal function on its disposition. Eur J Clin Pharmacol. 2021;77:1157–1168. doi:10.1007/s00228-021-03098-w
37. Yoshida T, Yoshida S, Okada H, et al. Risk factors for decreased teicoplanin trough concentrations during initial dosing in critically ill patients. Die Pharmazie. 2019;74:120–124. doi:10.1619/ph.2019.8731
38. Weiss JS, Gautam A, Lauff JJ, et al. The clinical importance of a protein-bound fraction of serum bilirubin in patients with hyperbilirubinemia. New Engl J Med. 1983;309:147–150. doi:10.1056/NEJM198307213090305
39. Chen CY, Xie M, Gong J, et al. Population pharmacokinetic analysis and dosing regimen optimization of teicoplanin in critically ill patients with sepsis. Front Pharmacol. 2023;14:1132367. doi:10.3389/fphar.2023.1132367
40. Chen J, Li S, Wang Q, et al. Optimizing antimicrobial dosing for critically ill patients with MRSA infections: a new paradigm for improving efficacy during continuous renal replacement therapy. Pharmaceutics. 2022;14:842. doi:10.3390/pharmaceutics14040842
41. Nakamura A, Takasu O, Sakai Y, et al. Development of a teicoplanin loading regimen that rapidly achieves target serum concentrations in critically ill patients with severe infections. J Infect Chemother. 2015;21:449–455. doi:10.1016/j.jiac.2015.02.002
42. Hartmann P, Schnabl B. Fungal infections and the fungal microbiome in hepatobiliary disorders. J Hepatol. 2023;78:836–851. doi:10.1016/j.jhep.2022.12.006
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


