Back to Journals » Infection and Drug Resistance » Volume 18
Impact of HIV Pretreatment Drug Resistance on Secondary Transmission Through Treatment Dropout: A Prospective Population-Based Study in Southwestern China
Authors Chen Y , Xu X, Chen H, Zhang X, Zhu Q, Liang S, Xing H, Liao L, Feng Y, Shao Y, Ruan Y, Lan G, Li J
Received 22 January 2025
Accepted for publication 14 April 2025
Published 6 May 2025 Volume 2025:18 Pages 2311—2327
DOI https://doi.org/10.2147/IDR.S516513
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sandip Patil
Yi Chen,1,* Xiaoshan Xu,2,* Huanhuan Chen,3,* Xiangjun Zhang,4 Qiuying Zhu,3 Shujia Liang,3 Hui Xing,2 Lingjie Liao,2 Yi Feng,2 Yiming Shao,2 Yuhua Ruan,2 Guanghua Lan,3 Jianjun Li3
1The Guangxi Academy of Medical Sciences, The People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, People’s Republic of China; 2State Key Laboratory of Infectious Disease Prevention and Control (SKLID), Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, National Center for AIDS/STD Control and Prevention (NCAIDS), Chinese Center for Disease Control and Prevention (China CDC), Beijing, People’s Republic of China; 3Guangxi Key Laboratory of Major Infectious Disease Prevention Control and Biosafety Emergency Response, Guangxi Center for Disease Control and Prevention, Nanning, People’s Republic of China; 4Center for Community Research and Evaluation, University of Memphis, Memphis, TN, USA
*These authors contributed equally to this work
Correspondence: Yuhua Ruan, Email [email protected] Jianjun Li, Email [email protected]
Objective: Discontinuation of antiretroviral treatment (ART) raised drug resistance and failure of Human Immunodeficiency Virus (HIV) virological suppression. The study aimed to assess the relationship between pretreatment drug resistance (PDR) and ART dropout, as well as the relationship between HIV treatment dropout and HIV secondary transmission.
Methods: This study included all eligible participants from a local surveillance database in southwestern China between 2014 and 2021. The PDR prevalence trend was assessed using trend Chi-square tests within a consecutive cross-sectional design (N = 3060). Cox proportional hazards model was used to investigate the relationship between PDR and the risk of treatment dropout within a cohort design. Generalized Estimating Equations model was applied to explore the association between treatment dropout and HIV secondary transmission within a longitudinal genetic network study design. (N = 5094).
Results: The overall PDR prevalence was 6.2%, analyzing a study sample of 3060 individuals with HIV/AIDS. Specifically, the prevalence of PDR to non-nucleoside reverse transcriptase inhibitors (NNRTIs), nucleoside reverse transcriptase inhibitors (NRTIs), and protease inhibitors (PIs) was 3.6%, 1.4%, and 1.1%, respectively. Yearly difference in prevalence was not identified. The independent association between PDR to NNRTIs and treatment dropout was significant (adjusted hazard ratio: 2.55, 95% CI 1.52– 4.29). Among 5094 newly diagnosed HIV cases, participants who dropped out did not show a significant difference in HIV secondary transmission compared to those not on ART (adjusted odds ratio: 1.15, 95% CI 0.74– 1.79).
Conclusion: PDR to NNRTIs may contribute to HIV secondary transmission through treatment dropout. It is imperative to offer comprehensive and advanced HIV care for all individuals with HIV, enhance treatment and medication adherence, and closely monitor PDR prevalence.
Keywords: HIV, pre-treatment drug resistance, treatment dropout, HIV secondary transmission
Introduction
Acquired immune deficiency syndrome (AIDS) is an infectious condition stemming from the human immunodeficiency virus (HIV), which targets leukocytes and leads to the deterioration of the immune system. People living with HIV are more susceptible to a wide range of illnesses, and the global prevalence of HIV is on the rise, causing medical, psychological, and social challenges for patients, their families, and the society.1 Antiretroviral therapy (ART) has been proven to effectively prevent HIV transmission among high-risk groups,2–5 and significantly reduce the overall mortality among people living with HIV/AIDS.6–8 In order to achieve the Joint United Nations Programme on HIV/AIDS (UNAIDS)’s “95–95-95” targets by 2030,9 China expanded the “treat all” policy to provide free ART medications to all individuals diagnosed with HIV, regardless of their HIV clinical stage or CD4 level in 201610 and implemented the “13th Five-Year Plan for AIDS Prevention and Treatment” aiming to enhance timely ART initiation in 2017.11 For treatment-naïve patients in China, the recommended first-line ART regimens comprise Tenofovir (TDF)/Zidovudine (AZT) + Lamivudine (3TC) + Efavirenz (EFV)/Nevirapine (NVP), in which EFV and NVP belong to non-nucleoside reverse transcriptase inhibitors (NNRTIs).12
Despite efforts to expand access to HIV care, there was concern about individuals discontinuing ART or dropping out of HIV treatment. Previous studies have found that individuals with HIV/AIDS who initiated ART with CD4 counts over 500 cells/mm³ at diagnosis or at World health organization (WHO) stages I and II demonstrated a higher risk of treatment dropout.13–15 The reasons of treatment dropout contained a complex interplay of health, socio-demographic, economic, psychosocial, and systemic factors.16–18 Discontinuation of ART can raise drug resistance and failure of HIV virological suppression.19,20 Two previous studies identified that the overall drug resistance prevalence for individuals who dropped out of ART for one month ranged from 16.0% to 19.3%, 63.0–93.1% of the dropout cases had high HIV viral load at over 1000 copies/mL at 24 months upon dropping out.19,20 Studies based on mathematical models revealed that a minor dropout rate from treatment can significantly increase the number of new HIV infections and drug resistance.21,22
Pretreatment drug resistance (PDR) may emerge in treatment naïve individuals, who have had prior exposure to ART, such as through pre-exposure prophylaxis (PrEP) and prevention of mother-to-child transmission (PMTCT), or restarted ART after a prolonged interruption.23 PDR may result in a reduced response to ART, leading to failure in achieving virological suppression and increased mortality.24–26 In China, to prevent drug resistance, newly diagnosed HIV/AIDS patients undergo follow-up visits every 3 months prior to ART initiation, and at 0.5, 1, 2, and 3 months after initiation, followed by every 3 months thereafter. During these ART follow-up visits, patients receive comprehensive care, including physical examinations, counseling services to promote treatment adherence, and education on managing side effects and maintaining a healthy lifestyle. Additionally, national guidelines require annual viral load testing for routine monitoring for patients on ART. However, there is a gap in the literature about the influence of PDR on individuals’ dropout from ART and the effect of the dropout on HIV secondary transmission.
Our study aimed to address this research gap by investigating the prevalence of PDR in southwestern China and exploring the relationships between PDR and ART dropout, as well as the relationship between ART dropout and HIV secondary transmission. The study aim was achieved through this prospective, population-based study utilizing consecutive cross-sectional, cohort, and longitudinal genetic network methodologies.
Materials and Methods
Study Setting
The study was conducted in Qinzhou, Guangxi Zhuang Autonomous Region (referred to as “Guangxi” hereafter). Qinzhou is a prefecture located in southern Guangxi and ranks among the top three in the region for diagnosed HIV cases.27 Zhuang minority accounts for nearly 40.0% of the local people. Nearly 60.0% residents were farmers. The local HIV epidemic was primarily driven by individuals who contracted HIV through heterosexual transmission, the elderly population, and those residing in rural areas.28–30
Study Design
Our study had two parts. The first part involved investigating the trend of HIV PDR prevalence between 2014 and 2021 using a consecutive cross-sectional study design. Additionally, we explored the association between HIV PDR and dropout rate through a cohort study design. The second part of the study examined the relationship between dropout rate and secondary transmission of HIV using a longitudinal genetic network study design.
Study Participants
Our study sample included all newly diagnosed HIV/AIDS cases without ART detected between January 1, 2014, and December 31, 2021 (n = 7381). Out of these, 6214 had sufficient specimens for HIV sequencing and were included in the final analysis. In accordance with the study design, these samples were divided into two distinct datasets.
The first dataset was used to perform the analyses related to the prevalence trend of PDR and its association with the treatment dropout rate. This dataset exclusively comprised participants whose blood samples were obtained prior to ART initiation and those who had consistently undergone ART with regular follow-ups for a specific duration. Certain cases were excluded from the analysis due to the following reasons: 1) Failure of HIV amplification or sequencing, 2) Sequences with ambiguities exceeding 5%, 3) HIV pol sequence shorter than 1000 bp, 4) Age under 18 years old, 5) Duplicated cases, 6) Lack of epidemiological information, 7) Not on ART, 8) Absence of RNA sequences, and 9) less than 6 months for the duration between ART initiation and the last follow-up. Ultimately, 3060 HIV/AIDS cases with RNA sequences met the enrollment criteria for the first part of the study. The dates of ART initiation for these cases ranged from January 1, 2014, to June 30, 2021, with the endpoint as December 31, 2021. Figure 1 illustrates the process of selecting the study sample.
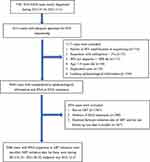 |
Figure 1 Flowchart depicting the selection process of study samples for PDR related analysis. PDR refers to pre-treatment drug resistance, ART refers to antiretroviral treatment. |
The second dataset was used to investigate the association between treatment dropout and HIV secondary transmission. From the initial pool of 6214 cases, a subset of participants was excluded for various reasons. The exclusion criteria for this dataset followed the first 1 to 6 criteria applied in the first dataset above. Following these exclusions, 5094 newly diagnosed HIV/AIDS cases between January 1, 2014, and December 31, 2021, possessing comprehensive epidemiological information and either DNA or RNA sequences, met the enrollment criteria for the second part of our study. Further details can be found in Figure 1.
Data Collection
Certain information was extracted from the Qinzhou HIV/AIDS Comprehensive Prevention and Control System for study subjects, which is a part of the national HIV/AIDS Comprehensive Prevention and Control System. The extracted data encompassed demographic information as well as epidemiological and clinical data of the participants. Demographic information included participants’ age, gender, ethnicity, education, marital status, occupation, and residence. The epidemiological and clinical data comprised information on participants’ HIV transmission route, CD4 count at diagnosis, date of blood sampling, date of HIV Western Blot testing, date of ART initiation, follow-up status such as not on ART or on ART, and dropout and corresponding dates.
Nucleic Acid Extraction Amplification and Sequencing
Blood samples were collected from eligible participants. The blood samples were processed to isolate plasma, which was subsequently transported under cold-chain conditions to the central laboratory. Using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany), DNA was extracted from a volume of 200 μL of whole blood, following the manufacturer’s protocol. RNA was extracted from 200 μL of plasma using the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany). The HIV pol region was amplified using the nested polymerase chain reaction, with the extracted DNA and RNA serving as templates. The methods employed for amplification and sequencing in this study have been previously documented.31,32 Quality control was performed concurrently to establish a more precise molecular transmission network. Sequences with a length of less than 1000 nucleotides and those with ambiguities exceeding 5% were excluded.33
Determination of Pretreatment Drug Resistance
Screening for drug resistance mutations (DRMs) was conducted and interpreted in accordance with the Stanford University Genotypic Resistance Interpretation algorithm (https:// hivdb.stanford.edu/). The degree of drug resistance was categorized into four groups based on the mutation scoring system: susceptible (<15), low-level (15–29), intermediate-level (30–59), and high-level (≥60) resistance. Any strain with a mutation score of ≥15 was considered PDR.34 Overall drug resistance to NNRTIs, nucleoside reverse transcriptase inhibitors (NRTIs), and protease inhibitors (PIs) was determined by detecting at least low-level resistance to one or more antiretroviral drugs among the 20 specified drugs listed in the Stanford HIV Resistance Database (https://hivdb.stanford.edu/hivdb/by-patterns/). A comprehensive list of the 20 antiretroviral drugs was provided in the Supplementary Table 1. The degree of drug resistance to EFV/NVP was analyzed separately.
HIV Genetic Network Construction
An HIV genetic cluster refers to a group of individuals diagnosed with HIV, characterized by the presence of genetically similar strains of the virus.35 The sequences were spliced using BioEdit (Ibis Biosciences, Carlsbad, CA, United States; version 7.0.9.0) and aligned separately with the HIV align tool to generate the final sequences utilized for analysis. MEGA (version 10.0) was employed to identify the HIV-1 subtypes through phylogeny. The phylogenetic tree was constructed using RaxmlGUI (version 2.0.0). Before constructing the genetic network, the pairwise Tamura-Nei (TN93) genetic distances of the paired sequences were calculated using Hyphy (version 2.2.5). Figure 2 shows the trends of links and clusters identified in the genetic network across various TN93 genetic distance thresholds. To maximize the identification of clusters within the genetic network and to detect transmission events that occurred within recent years, we adopted threshold of 0.0050 substitutions/site to construct the molecular transmission network. This threshold corresponds to an estimated 2–3 years of viral evolution between strains, indicative of the time elapsed since a shared transmission event.35 Additionally, the number of clusters identified under this threshold is close to the maximum (See Figure 2).
Calculation of Genetic Linkages Between Baseline HIV Cases and Newly Diagnosed HIV Cases
In our previous studies, we developed a network linkage-based method to calculate genetic linkages between baseline HIV cases and newly diagnosed HIV cases.36–38 First, patients diagnosed with HIV during 2014–2016 were defined as the first baseline network, while the corresponding newly diagnosed HIV cases referred to those diagnosed in the following year (2017). The genetic linkages between newly diagnosed HIV cases in 2017 and baseline HIV cases diagnosed in 2014–2016 were subsequently calculated. Using the genetic network containing cases diagnosed during 2014–2017 as a second baseline network, we calculated the linkages between them and cases newly diagnosed in 2018. Additionally, the linkages between cases newly diagnosed in 2019, 2020, and 2021 and their corresponding baseline networks were calculated accordingly. The number of genetic linkages was equal to the number of newly diagnosed HIV patients linked to those at the corresponding baseline genetic network. Finally, the genetic linkages among individuals diagnosed between 2014 and 2016 were excluded because they only served as the fundamental baseline group of this study.
Statistical Analysis
The statistical analyses for this study were conducted using the R language and its associated environment (R version 4.3.2) and SAS (version 9.4). Chi-square tests were employed to assess the prevalence trend of PDR across time. Further, the association between PDR and treatment dropout for HIV/AIDS cases was examined using the proportional hazards model (Cox regression model). The association between treatment dropout and HIV secondary transmission was tested by the generalized estimating equation (GEE) model. The adjusted models included demographic and clinical variables as covariates.
Results
Participant Characteristics and HIV Status at Diagnosis
A total of 3060 participants were included to examine the prevalence trend of PDR and the association between PDR and treatment dropout. Participant characteristics and HIV status at diagnosis were shown in Table 1. The study sample size for each year ranged from 95 to 564 between 2014 and 2021. Nearly half of the participants were aged 50–69 (44.8%), followed by those aged 35–49 (29.4%). Male participants accounted for 72.2% of the total sample, with over 90.0% of participants were Han ethnicity. Nearly half of the study sample had attained an elementary education level, while approximately 40.0% of the participants had completed middle school education. More than 60.0% of the participants were married. Over three-quarters of the participants were farmers. Additionally, more than 90.0% of the participants contracted HIV through heterosexual contacts. Approximately 45.0% of the participants resided in Lingshan county, while 20.0% lived in Qinnan District, and another 20.0% resided in Qinbei District. More than half of the participants were infected with CRF01_AE, 30.7% with CRF08_BC, and 10.2% with CRF07_BC. Over half of the participants exhibited CD4 counts below 200 cells/mm³ at the time of HIV diagnosis, approximately one-quarter had CD4 counts ranging from 200 to 349 cells/mm³, and one-fifth possessed CD4 counts of at least 350 cells/mm³.
 |
Table 1 Demographic Characteristics and HIV Status at Diagnosis Among Participantso Newly Diagnosed with HIV from 2014 to 2021 in Qinzhou, Guangxi, China (N = 3060) |
A total of 5094 eligible participants were included in the analysis of the relationship between treatment dropout and HIV secondary transmission. Table 2 demonstrated participant characteristics and HIV status at diagnosis. The study sample size for each year ranged from 525 to 892 between 2014 and 2021. Participants aged 50–69 accounted for 43.0% of the study sample, followed by those aged 35–49 (30.0%). The male-to-female ratio was approximately 3:1. The Han ethnic group constituted 90.0% of the total sample, while the Zhuang ethnic group comprised approximately 10.0%. Half of the participants possessed an elementary education level, followed by 36.0% with a middle school education level. Approximately 60.0% of the participants were married, less than one-fourth were single, and about 20.0% were either divorced or widowed. Three-fourths of the participants were farmers. Nearly 90.0% of the participants were infected with HIV through heterosexual contact. About 45.0% of the samples were from Lingshan county. Over half of the participants possessed the HIV-1 subtype of CRF_01AE (52.0%), followed by CRF08_BC (31.8%). Of 52.5% of the participants were diagnosed with CD4 counts of less than 200 cells/mm3, and 23.6% had CD4 counts ranging from 200 to 349 cells/mm3.
 |
Table 2 Demographic Characteristics and HIV Status at Diagnosis Among Participants Newly Diagnosed with HIV from 2014 to 2021 in Qinzhou, Guangxi, China (N = 5094) |
The Trend of PDR Prevalence Over the years
The most commonly used ART regimen was TDF+3TC+EFV (72.0%) for treatment-naïve patients in our study. The overall PDR prevalence across time for the study sample was 6.2% (191/3060), with yearly prevalence ranging from 5.3% to 8.4% between 2014 and 2021. Specifically, the prevalence of PDR to NNRTIs (containing EFV/NVP), EFV/NVP, NRTIs, and PIs were 3.6% (2.5%–5.5%), 1.7% (1.0%–3.1%), 1.4% (0.0%–2.1%) and 1.1% (0.8–1.8%), respectively, during 2014–2021. The prevalence of PDR to NNRTIs increased dramatically from 2.5% in 2014 to 5.5% in 2018. However, there were no significant differences in trend of PDR prevalence between 2014 and 2021 regarding the overall resistance, NNRTIs, EFV/NVP, NRTIs, and PIs. Table 3 displayed the trend of PDR prevalence and PDR to specific inhibitors over the years.
 |
Table 3 Prevalence of Pre-Treatment Drug Resistance Among Participants Newly Diagnosed with HIV from 2014 to 2021 in Qinzhou, Guangxi, China |
Association Between Stratified PDR Status and Treatment Dropout
Figure 3 and Table 4 showed the results of the three Cox regression models, which examined the associations between PDR and treatment dropout. The controlled variables in the adjusted models included age, gender, ethnicity, education, marital status, occupation, HIV transmission route, location, HIV subtype, and CD4 counts at HIV diagnosis. The first model indicated no difference in dropout between participants with PDR to any antiretroviral drugs and those without PDR. The second model revealed that participants with PDR to solely NNRTIs had a higher risk of dropout compared to those without PDR (AHR:2.55, 95% CI:1.52–4.29). Participants with PDR to either only NRTIs or only PIs did not show significant differences in dropout risks compared to those without PDR. The third model disclosed that participants with intermediate or high resistance to EFV/NVP had higher dropout risks compared to those without PDR (AHR:2.56, 95% CI:1.04–6.32). However, participants with other degrees of PDR did not exhibit significant differences in the dropout risks compared to those without PDR.
 |
Table 4 Subgroup Results of Associations Between Pre-Treatment Drug Resistance and Dropout in Participants Newly Diagnosed with HIV Between 2014 and 2021 |
Association Between Treatment Dropout and HIV Secondary Transmission
Table 5 illustrates the associations between treatment dropout and HIV secondary transmission, which was indicated by the HIV genetic linkages between baseline cases and newly diagnosed cases in the following year. The results showed that individuals who were receiving ART had a lower risk of HIV secondary transmission compared to those not on ART (AOR: 0.65, 95% CI:0.51–0.84). Participants who dropped out did not show a significant difference in HIV secondary transmission compared to those not on ART (AOR: 1.15, 95% CI: 0.74–1.79). Further, participants aged over 50 years exhibited a higher risk of HIV secondary transmission compared to their younger counterparts [“35–49” vs “18–24”: AOR = 1.56 (1.02–2.38); “50–69” vs “18–24”: AOR = 3.36 (2.20–5.12); “≥70” vs “18–24”: AOR = 4.23 (2.68–6.67)]. Participants with middle school education [AOR: 0.75 (0.58–0.98)] or high school education and above [AOR: 0.60 (0.43–0.85)] exhibited a lower risk of HIV secondary transmission compared to those who were illiterate. Participants with a heterosexual transmission route exhibited a significantly higher risk of HIV secondary transmission compared to those infected through the drug-injection transmission route [AOR: 1.94 (1.37–2.75)]. The risk of HIV secondary transmission was lower among participants residing in Lingshan county [AOR: 0.62 (0.53–0.74)] and Pubei county [AOR: 0.64 (0.52–0.79)], compared to those living in Qinnan District. Participants residing in Qinbei District showed a significantly higher risk of HIV secondary transmission compared to those located in Qinnan District [AOR: 1.22 (1.02–1.46)]. The risk of HIV secondary transmission was significantly higher for participants contracting with CRF07_BC [AOR: 1.85 (1.52–2.27)] and CRF08_BC [AOR: 1.63 (1.40–1.89)] compared to those infected with CRF01_AE.
 |
Table 5 HIV Genetic Linkage Between HIV Cases at Baseline in 2014–2016 and Newly Diagnosed Cases During 2017–2021 in Qinzhou, Guangxi, China |
Discussion
This study reported a 6.2% of overall PDR prevalence in Qinzhou between 2014 and 2021, specifically, the prevalence of PDR only to NNRTIs, EFV/NVP, NRTIs, and PIs were 3.6%, 1.7%, 1.4%, and 1.1%, respectively. The overall HIV PDR prevalence, as well as those for PDR to NNRTIs, EFV/NVP, NRTIs, and PIs, did not show significant differences between 2014 and 2021. Previous studies reported that the overall prevalence of PDR in Guangxi was around 5.3–8.3%.39,40 According to the WHO’s criteria,41 this was considered moderate, aligning with the national level in China (7.4% in 2022).8 Notably, this was lower than the PDR prevalence in Africa (11.0%),42 Mexico (14.4%)43 and the United States (20.0%–54.0%).44 For a broader context, our findings are comparatively lower when aligned with global data, highlighting the regional variations in PDR prevalence.
Our study revealed that PDR to NNRTIs was the most prevalent type and its prevalence increased from 2.5% in 2014 to 5.5% in 2018. The literature had highlighted that the primary driver behind the increased prevalence of PDR in treatment-naïve HIV/AIDS individuals was NNRTI resistance.8,45 A dynamic transmission modeling study suggested that extensive ART coverage can cause a significant increase in pretreatment NNRTI resistance.46 One possible reason was the adoption of NNRTIs-based regimens as first-line antiretroviral drugs in China.47 Further investigation is needed to fully understand NNRTI resistance.8 A previous study investigated HIV medication resistance among 30 countries between 2014 and 2020.48 It found that among 21 of these 30 countries, over 10% of HIV/AIDS cases initiating first-line ART exhibited primary drug resistance to EFV/NVP, which is part of NNRTIs.48 Therefore, the 2016 WHO ART Guidelines recommended implementing supplementary first-line ART strategies when PDR prevalence reached or exceeded 10% for NNRTIs in individuals initiating first-line ART.49 In China, the 2023 National Free HIV Antiviral Treatment Drug Treatment Manual incorporated Etravirine into the first-line ART regimens to prevent and postpone the high resistance to NNRTIs.50 As NNRTIs remain crucial in HIV-1 treatment due to their safety and long-lasting effects,51 it is vital to remain vigilant to prevent or delay any further escalation in PDR to NNRTIs, despite the current low prevalence of PDR to NNRTIs in Guangxi.
Although the latest HIV treatment guidelines have expanded inclusion criteria for ART initiation, these extra included cases were likely to experience a significantly higher risk of treatment dropout.13–15 Our study indicated PDR to NNRTIs was associated with the discontinuation of ART. It was agreed with former study which revealed ART regimens containing NNRTIs was associated with relatively lower adherence to ART.52 The underlying mechanism of PDR to NNRTIs leading to ART dropout may involve increased risks of virologic failure, treatment failure, and the emergence of new resistance mutations.7,53,54 A low adherence rate can result in drug resistance, and increased mortality.52 To address this challenge, it is crucial to implement a comprehensive monitoring system for PDR, which includes regular drug resistance testing and real-time data analysis. Moreover, since achieving treatment adherence rates of over 95.0% can effectively control the HIV epidemic,52 it is imperative to implement a multifaceted approach that includes personalized interventions tailored to individual needs,55 establishment of reminder systems,56 and adoption of simplified treatment regimens57 to enhance treatment adherence.
Our study found that cases dropout from ART cannot significantly reduced HIV secondary transmission compared to those not on ART. The reason may be that dropout can lead to higher viral loads, thereby increasing the risk of HIV transmission.58 The current study suggested that dropout of ART may potentially serve as a mediator between PDR to NNRTIs and HIV secondary transmission. As China expanded its ART coverage, it is crucial to actively prevent PDR to ensure the effectiveness of first-line ART treatments. To achieve this, several key measures should be implemented. Firstly, conducting HIV viral load testing before initiating ART is essential for selecting appropriate regimens,50 preventing drug resistance, and improving treatment outcomes. This requires strengthening laboratory capacity, deploying point-of-care testing devices in resource-limited areas, and ensuring healthcare providers are trained to act on results promptly. Secondly, enhancing the drug resistance monitoring system and ensuring reliable drug supply chains are vital to facilitate a prompt transition to second-line ART in case of treatment failure,46 thereby guaranteeing treatment effectiveness. Lastly, strengthening patient-family bonds and promoting provider-patient collaboration through counseling, support groups, and mentorship can further improve treatment adherence.59
This study also found that individuals with certain characteristics have a higher risk of HIV secondary transmission, such as older adults, those with less education, and heterosexually infected HIV patients. Previous research showed that the HIV epidemic in Guangxi was primarily driven by heterosexual transmissions since 2013.60 With this transmission route, unprotected sexual contact between elderly men and street-based commercial sex workers emerged as a major concern.28,30 It is noted that most of these elder individuals were farmers with lower education levels.28,30 Since 2015, elderly HIV/AIDS cases aged 50 and above made up a significant proportion of newly diagnosed cases in Guangxi, with higher incidence than younger age groups.29,61 Therefore, it is crucial to design and implement targeted interventions for these high-risk groups, incorporating personalized HIV/AIDS education programs to enhance knowledge and awareness, accessible psychological counseling services to address mental health needs, and streamlined access to simplified treatment regimens to improve medication adherence. These interventions should be culturally sensitive and community-based, ensuring their effectiveness and acceptance among the intended population.
Lingshan and Pubei counties used to be major HIV hotspots in Qinzhou. However, this study found that HIV secondary transmission risk was lower in Lingshan and Pubei counties comparing with Qinnan District, while Qinbei District residents possessing higher risk than those in Qinnan. The findings help to guide future HIV intervention resources allocation in Qinzhou. HIV cases with subtypes of CRF01_AE were less transmittable than cases with other subtypes, potentially due to CRF01_AE’s high virulence and rapid disease progression limiting transmission efficiency.62
This study had a few limitations that should be acknowledged. First, due to reasons such as failed HIV amplification or sequencing, missing epidemiological information, and absence of RNA sequences, not all eligible cases could be included, potentially leading to underrepresentation of the study samples. In addition, the findings of this study may not be generalizable to the entire province or to China as a whole. The study population was drawn from specific regions, which may not fully represent the diversity of HIV/AIDS cases across different geographic, demographic, and socioeconomic contexts. Future studies should aim to include a more diverse and representative sample to enhance the external validity of the findings. Second, although this study cannot detect a direct relationship between PDR and HIV secondary transmission, the association between PDR and treatment dropout was identified. This suggests that PDR may indirectly influence transmission dynamics by affecting treatment adherence and outcomes. However, the mechanisms underlying this relationship remain unclear. Future research could further explore the underlying links between PDR, HIV secondary transmission, and treatment dropout using a larger sample and advanced analytical methods, such as machine learning models, to better understand the causal pathways and interactions. Third, the Sanger sequencing test used to detect drug-resistant strains in this study has a risk of failing to identify up to 20% of PDR strains with lower prevalence.63 This limitation may lead to an underestimation of the PDR prevalence among the study samples. Although Sanger sequencing remains a widely used method due to its cost-effectiveness and accessibility,7,8 emerging technologies such as next-generation sequencing (NGS) offer higher sensitivity for detecting low-frequency variants and could provide a more accurate assessment of PDR. Additionally, integrating NGS with Sanger sequencing could help validate findings and improve the robustness of resistance profiling. We have planned to perform NGS for our future studies.
Conclusion
The development of PDR to NNRTIs could potentially facilitate HIV secondary transmission via treatment dropout. It is crucial to provide comprehensive and advanced HIV care to all individuals living with HIV/AIDS, strengthen treatment adherence, and rigorously monitor and manage the prevalence of PDR.
Data Sharing Statement
The HIV sequences and related epidemiological and clinical data used in this study were collected by our team from Qinzhou, Guangxi, China. These data and related datasets are not publicly available but can be obtained upon reasonable request and approval from the Chinese Center for Disease Control and Prevention. Requests for accessing these data and datasets should be directed to YR via email: [email protected].
Ethics Statement
This human-subject study was thoroughly reviewed and approved by the National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention in China. Prior to participation, all individuals involved in this study provided their written informed consent. The study complied with the Declaration of Helsinki.
Acknowledgments
We extend our gratitude to the Qinzhou Center for Disease Control and Prevention for their invaluable contribution to data collection.
Funding
This work was supported by the National Natural Science Foundation of China (Grant no. 82260670), Guangxi Natural Science Foundation Project (Grant no. 2025GXNSFAA069868), National Natural Science Foundation of China (Grant no. 82160636), Ministry of Science and Technology of China (Grant no. 2022YFC2305205, 2022YFC2305201), Ministry of Science and Technology of China (2018ZX10721102-006), Guangxi Key Laboratory of AIDS Prevention Control and Translation (Grant no. ZZH2020010), Guangxi Bagui Honor Scholarship, Guangxi Medical and Health Key Discipline Construction Project.
Disclosure
All authors have no conflict of interest to declare.
References
1. Hasan M, Jamil KF, Darmawi D. et al. Quality of life and its predictors among people living with HIV in Muslim majority region: a cross-sectional study in Aceh. Narra J. 2023;3:e202.
2. Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi:10.1056/NEJMoa1105243
3. Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375(9):830–839. doi:10.1056/NEJMoa1600693
4. Jia Z, Mao Y, Zhang F, et al. Antiretroviral therapy to prevent HIV transmission in serodiscordant couples in China (2003–11): a national observational cohort study. Lancet. 2013;382(9899):1195–1203. doi:10.1016/S0140-6736(12)61898-4
5. Chen H, Yang X, Zhu Q, et al. Treatment for HIV prevention study in southwestern areas of China. Infect Dis Model. 2018;3:249–255. doi:10.1016/j.idm.2018.09.007
6. Zhao Y, Wei L, Dou Z, et al. Changing mortality and patterns of death causes in HIV infected patients - China, 2013-2022. China CDC Wkly. 2023;5(48):1073–1078. doi:10.46234/ccdcw2023.201
7. Beck IA, Levine M, McGrath CJ, et al. Pre-treatment HIV-drug resistance associated with virologic outcome of first-line NNRTI-antiretroviral therapy: a cohort study in Kenya. EClinicalMedicine. 2020;18:100239. doi:10.1016/j.eclinm.2019.100239
8. Chen H, Hao J, Hu J, et al. Pretreatment HIV drug resistance and the molecular transmission network among hiv-positive individuals in China in 2022: multicenter observational study. JMIR Public Health Surveill. 2023;9:e50894. doi:10.2196/50894
9. UNAIDS. Fast-track: ending the AIDS epidemic by 2030. 2014. Available from: https://www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf.
10. Cai WP, Chen XJ, Li HQ, et al. Manual of the National Free Antiretroviral Treatment.
11. General Office of the State Council. Notification of the General Office of the State Council on Printing and Issuing the 13th Five-Year Action Plan for HIV Prevention and Treatment in China; 2017.
12. China Center for Disease Control and Prevention. National Center for STD/AIDS Control and Prevention. Handbook of National Free Antiretroviral Therapy for HIV/AIDS.
13. Tang Z, Pan SW, Ruan Y, et al. Effects of high CD4 cell counts on death and attrition among HIV patients receiving antiretroviral treatment: an observational cohort study. Sci Rep. 2017;7(1):3129.
14. Bock P, Fatti G, Ford N, et al. Attrition when providing antiretroviral treatment at CD4 counts >500 cells/μL at three government clinics included in the HPTN 071 (PopART) trial in South Africa. PLoS One. 2018;13(4):e0195127. doi:10.1371/journal.pone.0195127
15. Mayasi N, Situakibanza H, Mbula M, et al. Retention in care and predictors of attrition among HIV-infected patients who started antiretroviral therapy in Kinshasa, DRC, before and after the implementation of the ‘treat-all’ strategy. PLOS Glob Public Health. 2022;2(3):e0000259. doi:10.1371/journal.pgph.0000259
16. Liao B, Zhang XW, Wang JY, et al. Analysis of factors associated with dropping-out from HIV antiretroviral therapy in Kunming City, China. BMC Infect Dis. 2019;19(1):1043. doi:10.1186/s12879-019-4658-z
17. Asiimwe SB, Kanyesigye M, Bwana B, et al. Predictors of dropout from care among HIV-infected patients initiating antiretroviral therapy at a public sector HIV treatment clinic in sub-Saharan Africa. BMC Infect Dis. 2015;16(1):43. doi:10.1186/s12879-016-1392-7
18. Ahmed S, Autrey J, Katz IT, et al. Why do people living with HIV not initiate treatment? A systematic review of qualitative evidence from low- and middle-income countries. Soc Sci Med. 2018;213:72–84. doi:10.1016/j.socscimed.2018.05.048
19. Liu L, Xiao L, Tang J, et al. Drug resistance in HIV/AIDS patients who stopped ART in Yuexi and Zhaojue of Liangshan, Sichuan. China Trop Med. 2020;20(5):402–408. (In Chinese).
20. Liu L, Zuo Z, Liao L, et al. The drug resistance in HIV/AIDS patients who had stopped ART in 2016. J Trop Med. 2018;18(12):1613–1618. (In Chinese).
21. Shen M, Xiao Y, Rong L, et al. The impact of attrition on the transmission of HIV and drug resistance. AIDS Lond Engl. 2023;37(7):1137–1145. doi:10.1097/QAD.0000000000003528
22. Lai H, Li R, Li Z, et al. Modelling the impact of treatment adherence on the transmission of HIV drug resistance. J Antimicrob Chemother. 2023;78(8):1934–1943. doi:10.1093/jac/dkad186
23. World Health Organization. Global fund, US centers for disease control and prevention. HIV drug resistance report 2017. Geneva: World Health Organization, 2017.
24. Takem EN, Coox C, Shang J, et al. The association between HIV pretreatment drug resistance and virological outcomes in children and adults in sub-Saharan Africa: a systematic review and meta-analysis. PLoS One. 2024;19:e0300456. doi:10.1371/journal.pone.0300456
25. Li M, Song C, Hu J, et al. Impact of pretreatment low-abundance HIV-1 drug resistance on virological failure after 1 year of antiretroviral therapy in China. J Antimicrob Chemother. 2023;78(11):2743–2751. doi:10.1093/jac/dkad297
26. Chen H, Hu J, Song C, et al. Molecular transmission network of pretreatment drug resistance among human immunodeficiency virus-positive individuals and the impact of virological failure on those who received antiretroviral therapy in China. Front Med. 2022;9:965836. doi:10.3389/fmed.2022.965836
27. Guangxi Public Health Department. Annual report on provincial AIDS/STD in 2023. Guangxi: Guangxi Public Health Department; 2024.
28. Chen Y, Abraham Bussell S, Shen Z, et al. Declining inconsistent condom use but increasing HIV and syphilis prevalence among older male clients of female sex workers. Medicine. 2016;95(22):e3726. doi:10.1097/MD.0000000000003726
29. Chen H, Wu X, Chen L, et al. Rapidly spreading human immunodeficiency virus epidemic among older males and associated factors: a large-scale prospective cohort study in rural Southwest China. Sex Transm Dis. 2019;46(4):234–239. doi:10.1097/OLQ.0000000000000957
30. Chen X, Qin C, Chen R, et al. Epidemiological profile and molecular genetic characterization of HIV-1 among female sex workers and elderly male clients in Guangxi, China. Emerg Microbes Infect. 2021;10(1):384–395. doi:10.1080/22221751.2021.1888659
31. Xing H, Ruan Y, Hsi JH, et al. Reductions in virological failure and drug resistance in Chinese antiretroviral-treated patients due to lamivudine-based regimens, 2003-12. J Antimicrob Chemother. 2015;70(7):2097–2103. doi:10.1093/jac/dkv078
32. Liao L, Xing H, Shang H, et al. The prevalence of transmitted antiretroviral drug resistance in treatment-naive HIV-infected individuals in China. JAIDS J Acquir Immune Defic Syndr. 2010;53(Supplement 1):S10–4. doi:10.1097/QAI.0b013e3181c7d363
33. Wertheim JO, Kosakovsky Pond SL, Forgione LA, et al. Social and genetic networks of HIV-1 transmission in New York City. PLOS Pathog. 2017;13(1):e1006000. doi:10.1371/journal.ppat.1006000
34. Xu X, Luo L, Song C, et al. Survey of pretreatment HIV drug resistance and the genetic transmission networks among HIV-positive individuals in southwestern China, 2014–2020. BMC Infect Dis. 2021;21(1):1153. doi:10.1186/s12879-021-06847-5
35. USCDC. Detecting and Responding to HIV Transmission Clusters - a Guide for Health Departments. USA: CDC; 2018.
36. Chen Y, Cao Z, Li J, et al. HIV transmission and associated factors under the scale-up of HIV antiretroviral therapy: a population-based longitudinal molecular network study. Virol J. 2023;20(1):289. doi:10.1186/s12985-023-02246-1
37. Kang R, Li J, Chen H, et al. Using longitudinal genetic-network study to understand HIV treatment-as-prevention. AIDS. 2021;35(6):947–955. doi:10.1097/QAD.0000000000002812
38. Ruan Y, Lan G, Chen J, et al. Genetic network analysis of human immunodeficiency virus sexual transmission in rural Southwest China after the expansion of antiretroviral therapy: a population-based study. Front Microbiol. 2022;13:962477. doi:10.3389/fmicb.2022.962477
39. Kang RH, Liang SJ, Ma YL, et al. Pretreatment HIV drug resistance in adults initiating antiretroviral therapy in China, 2017. Infect Dis Poverty. 2020;9(1):54. doi:10.1186/s40249-020-00668-5
40. Zhang F, Liang B, Liang X, et al. Using molecular transmission networks to reveal the epidemic of pretreatment HIV-1 drug resistance in Guangxi, China. Front Genet. 2021;12.
41. HIV drug resistance report 2019. World Health Organization. 2019.
42. Crowell TA, Danboise B, Parikh A, et al. Pretreatment and acquired antiretroviral drug resistance among persons living with HIV in Four African countries. Clin Infect Dis. 2021;73(7):e2311–22. doi:10.1093/cid/ciaa1161
43. García Morales C, Tapia Trejo D, Matías Florentino M, et al. HIV pretreatment drug resistance trends in Mexico city, 2017–2020. Pathogens. 2021;10(12):1587. doi:10.3390/pathogens10121587
44. Benson C, Wang X, Dunn KJ, et al. Antiretroviral adherence, drug resistance, and the impact of social determinants of health in HIV-1 patients in the US. AIDS Behav. 2020;24(12):3562–3573. doi:10.1007/s10461-020-02937-8
45. Ntamatungiro AJ, Kagura J, Weisser M, Francis JM. Pre-treatment HIV-1 drug resistance in antiretroviral therapy-naive adults in Eastern Africa: a systematic review and meta-analysis. J Antimicrob Chemother. 2022;77(12):3231–3241. doi:10.1093/jac/dkac338
46. Riou J, Dupont C, Bertagnolio S, et al. Drivers of HIV-1 drug resistance to non-nucleoside reverse-transcriptase inhibitors (NNRTIs) in nine Southern African countries: a modelling study. BMC Infect Dis. 2021;21(1):1042. doi:10.1186/s12879-021-06757-6
47. Zhang F, WangY, Wang J, et al. National Free AIDS Antiviral Drug Treatment Manual. Beijing: People’s Medical Publishing House; 2007.
48. HIV drug resistance report 2021. World Health Organization. 2021.
49. World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach.
50. Chinese Center for Disease Control and Prevention. National free AIDS antiviral drug treatment manual
51. Rancan I, Cassol C, Graziani L, et al. Trend over time of HIV −1 drug resistance to nonnucleoside reverse transcriptase inhibitors (NNRTIs) and their drivers: a cohort study from Antiviral Response Cohort Analysis (ARCA). HIV Med. 2023;24(11):1150–1157. doi:10.1111/hiv.13525
52. Senu E, Sakyi SA, Ayisi Boateng NK, et al. Factors associated with anti-retroviral therapy (ART) adherence among adult people living with HIV (PLWH): a 5-year retrospective multi-centre study in Kumasi, Ghana. Dialogues Health. 2022;1:100082. doi:10.1016/j.dialog.2022.100082
53. Bertagnolio S, Hermans L, Jordan M, et al. Clinical impact of pretreatment human immunodeficiency virus drug resistance in people initiating nonnucleoside reverse transcriptase inhibitor–containing antiretroviral therapy: a systematic review and meta-analysis. J Infect Dis. 2020;224(3):377–388. doi:10.1093/infdis/jiaa683
54. Milne RS, Silverman RA, Beck IA, et al. Minority and majority pretreatment HIV-1 drug resistance associated with failure of first-line nonnucleoside reverse-transcriptase inhibitor antiretroviral therapy in Kenyan women. AIDS. 2019;33(6):941–951. doi:10.1097/QAD.0000000000002134
55. Mbuagbaw L, Sivaramalingam B, Navarro T, et al. Interventions for enhancing adherence to antiretroviral therapy (ART): a systematic review of high quality studies. AIDS Patient Care STDs. 2015;29(5):248–266. doi:10.1089/apc.2014.0308
56. Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet Lond Engl. 2010;376(9755):1838–1845. doi:10.1016/S0140-6736(10)61997-6
57. Nachega JB, Parienti JJ, Uthman OA, et al. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: a meta-analysis of randomized controlled trials. Clin Infect Dis off Publ Infect Dis Soc Am. 2014;58(9):1297–1307. doi:10.1093/cid/ciu046
58. Pecoraro A, Mimiaga M, O’Cleirigh C, et al. Depression, substance use, viral load, and CD4+ count among patients who continued or left antiretroviral therapy for HIV in St. Petersburg, Russian federation. AIDS Care. 2015;27(1):86–92. doi:10.1080/09540121.2014.959464
59. Mao Y, Qiao S, Li X et al. Depression, social support, and adherence to antiretroviral therapy among people living with HIV in Guangxi, China: a longitudinal study. AIDS Educ Prev. 2019;31(1):38–50. doi:10.1521/aeap.2019.31.1.38
60. Chen H, Luo L, Pan SW, et al. HIV epidemiology and prevention in Southwestern China: trends from 1996-2017. Curr HIV Res. 2019;17(2):85–93. doi:10.2174/1570162X17666190703163838
61. Ge XM, Yang WM, Zhu QY, et al. Epidemiological characteristics of HIV/AIDS in Guangxi Zhuang Autonomous Region, 2010-2017. Zhonghua Liu Xing Bing Xue Za Zhi Zhonghua Liuxingbingxue Zazhi. 2019;40(3):315–321. (In Chinese). doi:10.3760/cma.j.issn.0254-6450.2019.03.011
62. Cui H, Geng W, Sun H, et al. Rapid CD4+ T-cell decline is associated with coreceptor switch among MSM primarily infected with HIV-1 CRF01_AE in Northeast China. AIDS. 2019;33(1):13. doi:10.1097/QAD.0000000000001981
63. Li M, Liang S, Zhou C, et al. HIV drug resistance mutations detection by next-generation sequencing during antiretroviral therapy interruption in China. Pathogens. 2021;10(3):264. doi:10.3390/pathogens10030264
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



