Back to Journals » Therapeutics and Clinical Risk Management » Volume 21
Impact of Onset-to-Needle Time on the Risk of Early Neurological Deterioration in Patients with Acute Ischemic Stroke Receiving Intravenous Thrombolysis
Received 17 January 2025
Accepted for publication 8 June 2025
Published 19 June 2025 Volume 2025:21 Pages 917—927
DOI https://doi.org/10.2147/TCRM.S515542
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Bo Hu, Jiewei Hua
Department of Neurology, The Fifth Hospital of Wuhan, Wuhan, Hubei, People’s Republic of China
Correspondence: Bo Hu, Department of Neurology, The Fifth Hospital of Wuhan, No. 122 Xianzheng Street, Wuhan, Hubei, 430050, People’s Republic of China, Tel +86-19971987765, Email [email protected]
Objective: This study aimed to investigate the influence of onset-to-needle time (ONT) on early neurological deterioration (END) in patients with acute ischemic stroke (AIS) undergoing intravenous thrombolysis.
Methods: Patients with AIS receiving intravenous thrombolysis at The Fifth Hospital of Wuhan between March 2021 and December 2023 were enrolled. Patients were divided into an END group (n=104) and a non-END group (n=317) based on a National Institutes of Health Stroke Scale (NIHSS) score increase of ≥ 4 points within 24 hours. Baseline and clinical data were analyzed using univariate, multivariable logistic regression, and subgroup analyses. A logistic regression model was developed to predict END, and its performance was assessed using receiver operating characteristic (ROC) curves.
Results: Univariate analysis revealed significant differences between groups in age, total cholesterol, low-density lipoprotein cholesterol (LDL-C), lipoprotein-associated phospholipase A2 (Lp-PLA2), white blood cell count, activated partial thromboplastin time (APTT), pre-admission NIHSS score, and ONT (all P< 0.05). Heart disease history, infarct location, and Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification also differed significantly (all P< 0.05). Multivariable analysis identified age (Odds Ratio [OR]=1.098, 95% Confidence Interval [CI]: 1.031– 1.169, P=0.003), LDL-C (OR=2.785, 95% CI: 1.360– 5.710, P=0.005), Lp-PLA2 (OR=1.008, 95% CI: 1.001– 1.015, P=0.045), complete anterior circulation infarction (vs lacunar; OR=8.050, 95% CI: 5.180– 12.510, P=0.023), cardioembolic stroke (vs small vessel occlusion; OR=12.810, 95% CI: 8.420– 19.530, P=0.002), and ONT (OR=1.015, 95% CI: 1.002– 1.028, P=0.028) as independent risk factors for END. Subgroup analysis by admission NIHSS score showed that for moderate and severe strokes, each minute increase in ONT raised END risk by 1.5% (95% CI: 1.002– 1.028, P=0.031) and 3.0% (95% CI: 1.009– 1.052, P=0.005), respectively.
Conclusion: Prolonged ONT is an independent risk factor for END in AIS patients, particularly those with moderate to severe strokes. Prompt thrombolysis is crucial for mitigating neurological decline.
Keywords: onset-to-needle time, acute ischemic stroke, intravenous thrombolysis, early neurological deterioration, influencing factors
Introduction
Stroke is still among the top causes of death and disability around the world, with acute ischemic stroke (AIS) representing about 80% of all stroke incidents.1 The acute phase of AIS is particularly critical, as brain tissue deprived of blood supply is highly vulnerable to irreversible damage.1 Prompt and effective intervention is essential for reducing neurological deficits and enhancing long-term outcomes. Among the different therapeutic options, intravenous thrombolysis using recombinant tissue plasminogen activator (rtPA) is one of the most commonly employed treatments, designed to dissolve the thrombus and restore blood flow to the brain.2 However, the effectiveness of rtPA is significantly time-dependent, as the therapeutic window for thrombolysis is restricted to the initial hours following symptom onset. Delayed treatment can lead to unfavorable outcomes, including a heightened risk of early neurological deterioration (END).3
END, defined as an increase in the National Institutes of Health Stroke Scale (NIHSS) score by ≥4 points within 24 hours of treatment, is a common and concerning complication following thrombolysis.4 It is associated with worse prognosis, including greater disability and increased mortality.5 The development of END is influenced by multiple factors, including the initial infarct size, collateral circulation, comorbidities, and treatment-related variables.6 However, the relationship between onset-to-needle time (ONT)—the time from symptom onset to the initiation of thrombolysis—and the risk of END remains an area of intense investigation.
Previous studies have suggested that delayed thrombolysis, or longer ONT, increases the likelihood of poor outcomes, including END, due to prolonged ischemia and larger infarct volumes.7 This relationship is thought to be mediated by the duration of ischemia, which results in more extensive neuronal injury and worsens the effectiveness of thrombolytic therapy.
Although there is a growing body of evidence linking ONT with clinical outcomes in AIS patients, it remains unclear how ONT specifically influences the development of END and which patients are most at risk. Understanding the precise relationship between ONT and END could inform clinical strategies for optimizing treatment protocols and patient management. A study by Kaesmacher et al8 demonstrated that each minute of delay in thrombolysis was associated with an increased risk of both poor clinical outcomes and hemorrhagic transformation. Furthermore, the duration of ischemia before thrombolysis may exacerbate neuronal injury, making timely intervention even more crucial.9
While ONT is clearly a critical determinant of treatment efficacy, its interaction with other variables—such as age, comorbid conditions (eg, hypertension, diabetes), lipid profiles, pre-admission stroke severity, and infarct location—remains underexplored. The role of these factors in conjunction with ONT may help identify high-risk patients who are more susceptible to END. For example, previous studies have found that patients with higher low-density lipoprotein cholesterol (LDL-C) levels, older age, and specific infarct locations (eg, complete anterior circulation infarction) are more likely to experience poor outcomes, including END.10,11
The present study aims to investigate the effect of ONT on the incidence of END in AIS patients undergoing intravenous thrombolysis. By examining clinical data from patients treated at The Fifth Hospital of Wuhan, this study will assess the role of ONT in predicting END, while considering other key risk factors such as age, lipid profiles, infarct location, and Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification. Furthermore, this research will help determine whether ONT’s effect on END varies according to the severity of the initial stroke, as measured by the NIHSS score at admission.
Subjects and Methods
Subjects
This study involved patients diagnosed with acute ischemic stroke (AIS) who were admitted to the Neurology Department of The Fifth Hospital of Wuhan from March 2021 to December 2023. The primary endpoint of the study was early neurological deterioration (END), defined based on the criteria established by the ECASS-I study team.4 Specifically, early neurological deterioration (END) was defined as an increase of 4 points or more in the National Institutes of Health Stroke Scale (NIHSS) score within 24 hours of stroke onset, compared to the baseline NIHSS score. Patients who exhibited an increase of 4 points or more were categorized into the END group, while those with a smaller increase in NIHSS score (less than 4 points) were classified into the non-END group.
Inclusion Criteria
- Patients who fulfilled the diagnostic criteria for acute ischemic stroke (AIS) as specified in the “Chinese Guidelines for the Diagnosis and Treatment of Acute Ischemic Stroke 2014.”
- Patients who received intravenous recombinant tissue plasminogen activator (rtPA) treatment upon admission.
- Admission within 24 hours of the onset of symptoms.
- Patients aged 18 years or older.
Exclusion Criteria
- Patients for whom essential clinical data pertinent to the study’s endpoints were unavailable or incomplete after diligent record review.
- Iatrogenic or traumatic stroke.
- Severe inflammatory or infectious diseases.
- Recent history of intracranial or intraspinal surgery.
- History of autoimmune diseases or hemorrhagic disorders.
- Liver or kidney dysfunction.
- Patients who underwent mechanical thrombectomy during the initial 24-hour observation period for END.
The study included a total of 421 patients, of whom 104 were assigned to the early neurological deterioration (END) group and 317 to the non-END group. The Ethics Committee of The Fifth Hospital of Wuhan approved the study, and written informed consent was obtained from all participants before their inclusion.
Methods
Clinical Data Collection
To comprehensively identify the potential factors influencing poor outcomes in stroke patients, this study utilized a literature review approach to identify key variables that have been shown to affect adverse stroke prognosis in previous research. This approach was employed to reduce the influence of confounding factors on the study’s results.
The data collected for analysis were categorized into four primary groups:
- Demographic information (eg, age, sex, origin, and education level; other demographic factors such as occupational status were not uniformly available for this retrospective cohort but are acknowledged as potential variables for future studies)
- Personal and past medical history (eg, history of hypertension, diabetes, smoking, and hyperlipidemia)
- Basic clinical examination indicators (eg, initial NIHSS score, Glasgow Coma Scale, and imaging findings)
- Neurological-specific indicators (eg, infarct location, stroke subtype based on the TOAST classification, and collateral circulation status)
In total, 39 factors were collected, each of which could potentially influence the risk of adverse outcomes, including END, after intravenous rtPA treatment. The pre-admission NIHSS score was collected for all patients and used for baseline severity assessment and subgroup analysis.
Statistical Methods
Statistical analyses were conducted using SPSS version 26.0 (IBM Corp., Armonk, NY, USA) for primary data management and initial analyses, and R version 4.0 software (R Foundation for Statistical Computing, Vienna, Austria) for specific analyses such as nomogram construction. The use of both software packages allowed for comprehensive statistical evaluation and graphical representation. To compare continuous variables between the END and non-END groups, independent sample t-tests were used for normally distributed data, while the Mann–Whitney U-test was utilized for data that did not meet normality assumptions. For categorical variables, chi-square (χ²) tests were employed, and Fisher’s exact tests were used when sample sizes were small.
Variables demonstrating statistical significance in the univariate analysis (p < 0.05) were considered for inclusion in the subsequent multivariable logistic regression analysis to identify independent risk factors for END. The inclusion criteria for the regression model were set at α = 0.05, and variables with p-values greater than 0.10 were excluded from the analysis. The pre-admission NIHSS score, while significant in univariate analysis, was primarily utilized for subgroup stratification to explore its moderating effect on the ONT-END relationship, rather than as a direct independent variable in the main multivariable model predicting END, to provide a more nuanced understanding of its role. Continuous data were reported as mean ± standard deviation (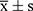 ) or median (interquartile range) [M (Q25, Q75)], while categorical data were expressed as number (%). A significance level of α = 0.05 was applied to all statistical tests.
) or median (interquartile range) [M (Q25, Q75)], while categorical data were expressed as number (%). A significance level of α = 0.05 was applied to all statistical tests.
Furthermore, the rms package in R software was used to develop a nomogram model based on the independent risk factors identified through multivariable logistic regression. This nomogram will act as a predictive tool to estimate the individual risk of early neurological deterioration for future patients based on their clinical characteristics.
Results
Univariate Analysis Results
The detailed findings from the univariate analysis are shown in Table 1. Statistically significant differences were identified between the END group and the non-END group regarding age, total cholesterol, low-density lipoprotein cholesterol (LDL-C), lipoprotein-associated phospholipase A2 (Lp-PLA2), white blood cell count, activated partial thromboplastin time (APTT), pre-admission NIHSS score, and onset-to-needle time (ONT) (all P < 0.05). Furthermore, notable differences were observed in the distribution of heart disease history, infarct location, and TOAST classification between the two groups (all P < 0.05). The average door-to-needle time was 148.5 (98.0, 201.5) minutes in the END group and 104.0 (87.0, 151.0) minutes in the non-END group (data related to ONT in Table 1). Specific data on the percentage of patients with large vessel occlusion (LVO) shows that within the TOAST classification, 30 (28.8%) patients in the END group and 75 (23.7%) in the non-END group were classified under Large Artery Atherosclerosis. Complete Anterior Circulation Infarcts, often associated with LVO, were present in 58 (55.8%) of END patients and 57 (18.0%) of non-END patients. Data on recanalization rates for LVO patients treated only with IV thrombolysis were not specifically collected for this analysis.
 |
Table 1 Comparison of Clinical Data Between END Group and Non-END Group [ |
Multivariable Analysis Results
As displayed in Table 2, the factors that were statistically significant in the univariate analysis (shown in Table 1), along with demographic characteristics such as gender, origin, education level, and body mass index, were considered for the multivariable logistic regression analysis to identify independent predictors of early neurological deterioration (END). The results indicated that age, low-density lipoprotein cholesterol (LDL-C), lipoprotein-associated phospholipase A2 (Lp-PLA2), TOAST classification, infarct location, and onset-to-needle time (ONT) were significant risk factors for END in stroke patients.
- Age: For every additional year, the likelihood of END increased by 9.8% (OR = 1.098, 95% CI: 1.031–1.169, P = 0.003).
- LDL-C: Each 1 mmol/L increase in LDL-C was associated with a 2.785-fold increase in the risk of END (OR = 2.785, 95% CI: 1.360–5.710, P = 0.005).
- Lp-PLA2: A 1 ng/mL increase in Lp-PLA2 correlated with a 0.8% increase in the risk of END (OR = 1.008, 95% CI: 1.001–1.015, P = 0.045).
- Infarct Location: Patients with complete anterior circulation infarction had an 8.050-fold higher risk of END compared to those with lacunar infarction (OR = 8.050, 95% CI: 5.180–12.510, P = 0.023).
- TOAST Classification: The risk of END was 12.810 times greater in patients with cardioembolic stroke compared to those with small artery occlusion (OR = 12.810, 95% CI: 8.420–19.530, P = 0.002).
- Onset-to-Needle Time (ONT): Each additional minute of ONT increased the risk of END by 1.5% (OR = 1.015, 95% CI: 1.002–1.028, P = 0.028).
 |
Table 2 Multivariable Logistic Regression Analysis for END |
Subgroup Analysis Results
According to previous studies and clinical experience, the effect of onset-to-needle time (ONT) on the risk of early neurological deterioration (END) may vary depending on the severity of the stroke at the time of admission [5]. To investigate this further, patients were divided into three subgroups based on their National Institutes of Health Stroke Scale (NIHSS) scores upon admission: mild (0–4 points), moderate (5–15 points), and severe (> 15 points). The results of the subgroup analysis are shown in Table 3.
- Moderate Subgroup: In patients with a moderate stroke severity at admission, each additional minute of ONT increased the risk of END by 1.015 times (OR=1.015, 95% CI: 1.002–1.028, P=0.031).
- Severe Subgroup: For patients with severe stroke, each additional minute of ONT raised the risk of END by 1.030 times (OR=1.030, 95% CI: 1.009–1.052, P=0.005).
 |
Table 3 Subgroup Analysis by Pre-Admission NIHSS Score |
These findings suggest that the risk of END associated with delayed treatment (ie, prolonged ONT) may be more pronounced in patients with higher stroke severity.
Nomogram and ROC Curve Analysis Results
A nomogram was created (Figure 1) utilizing the independent risk factors identified from the multivariable logistic regression analysis. This tool is designed to predict the probability of early neurological deterioration (END) based on various clinical parameters. This model calculates a cumulative score derived from these factors, providing a personalized prediction of END risk.
 |
Figure 1 Nomogram Model for END in AIS Patients. |
To evaluate the nomogram’s accuracy, a receiver operating characteristic (ROC) curve was generated (Figure 2). The area under the curve (AUC) was calculated to be 0.925 (95% CI: 0.861–0.987, P < 0.001), demonstrating a high degree of predictive accuracy. This robust performance highlights the utility of the nomogram in identifying patients at risk of END, integrating multiple relevant clinical factors into a single, accessible prediction tool.
Discussion
This study collected a comprehensive set of data from acute ischemic stroke (AIS) patients in clinical settings, including demographic and socioeconomic information, medical history, laboratory tests, and neurological examinations. The objective was to thoroughly investigate and analyze the factors that contribute to the onset of early neurological deterioration (END). The incidence of END among the patients included in this study was 24.7% (104/421), which is in line with the range reported in the literature (13.3% to 36.8%).12 Identifying the risk factors for END can assist in selecting early treatment options such as intravenous thrombolysis or endovascular thrombectomy and provide a foundation for developing a risk assessment scale for neurological function in acute stroke patients. This scale could be used by both healthcare providers and families for clinical evaluation.
END may occur in AIS patients following intravenous thrombolysis, significantly affecting their prognosis.13 A multicenter study on stroke in 2021 reported that 10% to 30% of patients may experience END, with most cases linked to reocclusion of the arterial pathway.14 In the univariate analysis of this study, age was a significant factor, with older patients demonstrating poorer tolerance to neurological stress and a higher risk of END. This finding is consistent with multiple studies that highlight advanced age as a key determinant of stroke outcome.10,11 Previous research15,16 has shown that elevated hemoglobin A1c levels are associated with worsening neurological function (OR = 1.476, 95% CI: 1.129 to 1.928), and that diabetes may increase oxidative stress and reactive oxygen species levels. However, in this study, blood glucose levels did not show a statistically significant difference between the two groups, which may be attributed to the skewed distribution of the data, or potentially effective acute glucose management in the cohort.
Lp-PLA2 has been shown to promote the expression of inflammatory cytokines and leukocyte chemotaxis, leading to the formation of atherosclerotic plaques, which increase plaque instability and contribute to thrombosis and cardiovascular events.17,18 One earlier study reported that patients with high levels of Lp-PLA2 had a 2.99-fold higher risk of END compared to those with low levels (95% CI: 1.26 to 5.73).19 Similarly, LDL-C, which reflects lipid levels, has been associated with an increased risk of cardiovascular diseases.20,21 The current study found that elevated LDL-C was also a significant risk factor for END, aligning with findings that dyslipidemia contributes to endothelial dysfunction and atherothrombosis, thereby potentially exacerbating ischemic injury and hampering response to thrombolysis.10
Among the different causes of stroke, those due to complete anterior circulation infarction were found to have the highest risk of END, a finding consistent with related studies.22 In patients with large artery occlusion in the anterior circulation, combined endovascular thrombectomy has demonstrated greater efficacy compared to best medical therapy alone, including intravenous rt-PA, across nine randomized trials.23 This underscores the importance of timely and appropriate intervention to reduce the risk of END and improve overall stroke outcomes. The higher risk associated with complete anterior circulation infarcts likely reflects larger infarct volumes and greater initial neurological deficit, making these patients more vulnerable to deterioration.
In this study, patients with varying degrees of stroke severity showed different sensitivities to ONT (onset-to-needle time). Mild stroke patients were less affected by the ONT in terms of whether they developed END, possibly because such patients are less likely to experience END in the first place. For patients with moderate to severe stroke, however, ONT proved to be a more critical factor. Research has shown that patients with longer admission-to-thrombolysis times (≥55 minutes) have a 1.48-fold higher risk of death compared to those who receive treatment more promptly (<55 minutes) (95% CI: 1.15 to 1.88).24 In our study, each additional minute of ONT increased the risk of END by 1.5% to 3.0%. These findings align with previous research that suggests reducing ONT time can significantly lower the risk of poor outcomes. A randomized controlled trial25 also indicated that longer ONT times correlate with poorer health outcomes, as measured by the European Five-Dimensional Health Scale and the Visual Analog Pain Score, reflecting reduced health status and increased suffering. The principle “time is brain” is strongly reinforced by these observations, emphasizing that every minute saved in initiating reperfusion therapy can translate into better neurological outcomes, especially in more severe strokes where the ischemic penumbra is often larger and more vulnerable.8,9 In China, the establishment of stroke centers and green channels has successfully reduced the median ONT time to 175 minutes, which has been associated with improved outcomes.26 Standardized treatment protocols, including streamlined diagnostic and examination processes, can further reduce ONT and contribute to better patient outcomes.27
In this study, the final set of screened risk factors included demographic and socioeconomic data, laboratory test results, and neurological examination findings. Specifically, LDL-C, total cholesterol, and Lp-PLA2 were included to assess their impact on END, with LDL-C emerging as the most significant factor among lipid markers in the predictive model. These indicators collectively offer a comprehensive assessment of patient conditions and may serve as useful biomarkers in clinical settings. The binary logistic regression model developed in this study achieved an accuracy rate of 92.5% (95% CI: 0.861 to 0.987), highlighting the robustness and predictive power of the identified risk factors for END.
However, this study has several limitations. First, it is a single-center study, and while efforts were made to ensure data completeness, some patients’ basic information or examination results might have had inherent limitations typical of retrospective data collection, potentially introducing information bias. We attempted to mitigate this by defining clear exclusion criteria for substantially incomplete records. Additionally, the sample size was relatively small, and the proportions of certain types of stroke in the END group were uneven. Future research could involve larger cohort studies across multiple centers, with more comprehensive patient follow-up, to obtain more reliable and generalizable results. Furthermore, detailed data on recanalization status post-IV thrombolysis, especially for LVO cases not proceeding to mechanical thrombectomy, were not systematically available, which could be a valuable addition to future investigations.
Conclusion
This study confirms that prolonged onset-to-needle time (ONT) is an independent and significant risk factor for early neurological deterioration in patients with acute ischemic stroke receiving intravenous thrombolysis. The detrimental impact of increased ONT is particularly pronounced in patients presenting with moderate to severe stroke severity. Alongside ONT, older age, elevated LDL-C and Lp-PLA2 levels, complete anterior circulation infarction, and cardioembolic stroke subtype were also identified as independent predictors of END. These findings underscore the critical importance of expediting the process from symptom onset to the administration of thrombolytic therapy. Efforts to shorten ONT, including optimizing pre-hospital care, streamlining in-hospital stroke protocols, and raising public awareness about early stroke recognition and action, are paramount to reducing the risk of END and improving patient outcomes. The developed predictive model, incorporating these risk factors, shows good accuracy and may aid in identifying high-risk patients for more intensive monitoring or adjunctive therapeutic considerations.
Data Sharing Statement
Data is provided within the manuscript files, further enquiries can be directed to the corresponding author.
Ethics Approval and Consent to Participate
The study was approved by the Ethics Committee of The Fifth Hospital of Wuhan (NO. 2024-42A). All Patients and their families participated voluntarily and signed informed consent forms, and the study was performed in accordance with the Helsinki II declaration. Informed consent was obtained from all the study subjects before enrollment.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding has been received for the study.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Campbell BCV, De Silva DA, Macleod MR, et al. Ischaemic stroke. Nat Rev Dis Primers. 2019;5(1):70. doi:10.1038/s41572-019-0118-8
2. Grotta JC. Intravenous thrombolysis for acute ischemic stroke. Continuum. 2023;29(2):425–442. doi:10.1212/CON.0000000000001207
3. Zi W, Song J, Kong W. Tirofiban for stroke without large or medium-sized vessel occlusion. New Engl J Med. 2023;388(22):2025–2036. doi:10.1056/NEJMoa2214299
4. Antos A, Członkowska A, Smolinski L, et al. Early neurological deterioration in Wilson’s disease: a systematic literature review and meta-analysis. Neurol Sci. 2023;44(10):3443–3455. doi:10.1007/s10072-023-06895-6
5. Li H, Zhang JT, Zheng Y, et al. Risk factors and prognosis of early neurological deterioration in patients with posterior circulation cerebral infarction. Clin Neurosurg. 2023;228:107673. doi:10.1016/j.clineuro.2023.107673
6. Tian T, Wang L, Xu J. Prediction of early neurological deterioration in acute ischemic stroke patients treated with intravenous thrombolysis. J Cereb Blood Flow Metab. 2023;43(12):2049–2059. doi:10.1177/0271678X231200117
7. Han Q, You S, Maeda T, et al. Predictors of early versus delayed neurological deterioration after thrombolysis for ischemic stroke. Cerebrovascular Dis;2024. 1–9. doi:10.1159/000539322
8. Kaesmacher J, Cavalcante F, Kappelhof M, et al. Time to treatment with intravenous thrombolysis before thrombectomy and functional outcomes in acute ischemic stroke: a meta-analysis. JAMA. 2024;331(9):764–777. doi:10.1001/jama.2024.0589
9. Schwarz F, Schuler G, Katus H, et al. Intracoronary thrombolysis in acute myocardial infarction: duration of ischemia as a major determinant of late results after recanalization. Am J Cardiol. 1982;50(5):933–937. doi:10.1016/0002-9149(82)90398-8
10. Jin D, Yang J, Zhu H, et al. Risk factors for early neurologic deterioration in single small subcortical infarction without carrier artery stenosis: predictors at the early stage. BMC Neurol. 2023;23(1):83. doi:10.1186/s12883-023-03128-3
11. Tan C, Zhao L, Dai C, et al. Risk factors related to early neurological deterioration in lacunar stroke and its influence on functional outcome. Int J Stroke. 2023;18(6):681–688. doi:10.1177/17474930221145259
12. Seners P, Turc G, Oppenheim C, Baron JC. Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg. 2015;86(1):87–94. doi:10.1136/jnnp-2014-308327
13. Zhang X, Zhong W, Xue R, et al. Argatroban in patients with acute ischemic stroke with early neurological deterioration: a randomized clinical trial. JAMA Neurol. 2024;81(2):118–125. doi:10.1001/jamaneurol.2023.5093
14. Boulenoir N, Turc G, Henon H, et al. Early neurological deterioration following thrombolysis for minor stroke with isolated internal carotid artery occlusion. Eur J Neurol. 2021;28(2):479–490. doi:10.1111/ene.14541
15. Wang L, Cheng Q, Hu T, et al. Impact of stress hyperglycemia on early neurological deterioration in acute ischemic stroke patients treated with intravenous thrombolysis. Front Neurol. 2022;13:870872. doi:10.3389/fneur.2022.870872
16. Tyagi S, Singh N, Virdi JK, Jaggi AS. Diabetes abolish cardioprotective effects of remote ischemic conditioning: evidences and possible mechanisms. J Physiol Biochem. 2019;75(1):19–28. doi:10.1007/s13105-019-00664-w
17. Lu X, Xu X, Zhang Y, Zhang Y, Wang C, Huo X. Elevated inflammatory Lp-PLA2 and IL-6 link e-waste Pb toxicity to cardiovascular risk factors in preschool children. Envir pollution. 2018;234:601–609. doi:10.1016/j.envpol.2017.11.094
18. Zhang F, Guo J, Yang F, Zhou Y. Lp-PLA2 evaluates the severity of carotid artery stenosis and predicts the occurrence of cerebrovascular events in high stroke-risk populations. J Clin Lab Anal. 2021;35(3):e23691. doi:10.1002/jcla.23691
19. Wang Y, Hu S, Ren L, et al. Lp-PLA(2) as a risk factor of early neurological deterioration in acute ischemic stroke with TOAST type of large arterial atherosclerosis. Neurological Res. 2019;41(1):1–8. doi:10.1080/01616412.2018.1493850
20. Navarese EP, Robinson JG, Kowalewski M, et al. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA. 2018;319(15):1566–1579. doi:10.1001/jama.2018.2525
21. Das Pradhan A, Glynn RJ, Fruchart JC, MacFadyen JG, Zaharris ES, Everett BM. Triglyceride lowering with pemafibrate to reduce cardiovascular risk. New Engl J Med. 2022;387(21):1923–1934. doi:10.1056/NEJMoa2210645
22. Lee SJ, Lee DG. Distribution of atherosclerotic stenosis determining early neurologic deterioration in acute ischemic stroke. PLoS One. 2017;12(9):e0185314. doi:10.1371/journal.pone.0185314
23. Hurford R, Sekhar A, Hughes TAT, Muir KW. Diagnosis and management of acute ischaemic stroke. Pract Neurol. 2020;20(4):304–316. doi:10.1136/practneurol-2020-002557
24. Groot AE, van Schaik IN, Visser MC, et al. Association between i.v. thrombolysis volume and door-to-needle times in acute ischemic stroke. J Neurol. 2016;263(4):807–813. doi:10.1007/s00415-016-8076-5
25. Sajobi TT, Arimoro OI, Ademola A. Quality of life after intravenous thrombolysis for acute ischemic stroke: results from the AcT randomized controlled trial. Stroke. 2024;55(3):524–531. doi:10.1161/STROKEAHA.123.044690
26. Chao BH, Yan F, Hua Y, et al. Stroke prevention and control system in China: CSPPC-Stroke Program. Int J Stroke. 2021;16(3):265–272. doi:10.1177/1747493020913557
27. Bahnasy WS, Ragab OAA, Elhassanien ME. Stroke onset to needle delay: where these golden hours are lost? An Egyptian center experience. eNeurologicalSci. 2019;14:68–71. doi:10.1016/j.ensci.2019.01.003
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



