Back to Journals » Infection and Drug Resistance » Volume 18
Impact of Patient-Centered Care on Treatment Outcomes of Multidrug-Resistant/Rifampicin-Resistant Tuberculosis in Xi’an
Authors Luo H, Ma J, He X, Ruan Y, Ren F, Dang L, Xu Y, Zhao A
Received 25 June 2024
Accepted for publication 22 January 2025
Published 13 March 2025 Volume 2025:18 Pages 1425—1437
DOI https://doi.org/10.2147/IDR.S484268
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sandip Patil
Hui Luo,1 Jinbao Ma,1 Xiaomou He,1 Yunzhou Ruan,2 Fei Ren,1 Liyun Dang,1 You Xu,1 Ali Zhao1
1Department of Drug-Resistance Tuberculosis, Xi’an Chest Hospital, Xi’an, 710100, People’s Republic of China; 2National Center for Tuberculosis Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, People’s Republic of China
Correspondence: Ali Zhao, Department of Drug-resistance tuberculosis, Xi’an Chest Hospital, East Section of Hang Tian Avenue, Chang’an District, Xi’an, Shaanxi, 710100, People’s Republic of China, Tel +86-18192105257, Email [email protected]
Objective: A hospital in Xi’an, Shaanxi Province, China, implemented patient-centered care services to improve the treatment outcomes of patients with multidrug-resistant/rifampicin-resistant tuberculosis. Given the high recurrence rate and treatment challenges of this disease, this study aimed to evaluate the effects of patient-centered care services compared to standard care in improving patient treatment adherence and reducing loss to follow-up.
Methods: This single-center retrospective cohort study included multidrug-resistant/rifampicin-resistant tuberculosis patients diagnosed and treated at the Xi’an Tuberculosis Prevention and Treatment Hospital from January 2018 to December 2019. Descriptive statistics, survival analysis, and multivariate Cox regression analysis were used to analyze the impact of patient-centered care services on treatment adherence. Data collection included patients’ demographic characteristics, clinical data, treatment outcomes, and reasons for loss to follow-up.
Results: A total of 429 patients were included in the final analysis, with 166 in the standard-of-care group and 263 in the patient-centered care services group. The treatment success rate in the patient-centered care services group (86.3%) was significantly higher than the standard of care group (59.0%), and the loss to follow-up rate was significantly lower (6.8% vs 30.1%). Multivariate analysis showed that patient-centered care services significantly reduced the risk of loss to follow-up (adjusted odds ratio of 0.14). The main reasons for the loss of follow-up included economic difficulties, lack of knowledge, and inadequate social support.
Conclusion: The patient-centered care services model significantly improved the treatment success rate and reduced the loss to follow-up rate for multidrug-resistant/rifampicin-resistant tuberculosis patients, demonstrating potential benefits in managing drug-resistant tuberculosis. Based on these findings, exploring and optimizing the patient-centered care services model in other high-burden areas is recommended to enhance overall treatment outcomes and quality of life for patients.
Keywords: multidrug-resistant tuberculosis, rifampicin-resistant tuberculosis, loss to follow-up, patient-centered care, survival analysis, treatment adherence
Graphical Abstract:

Introduction
Multidrug-resistant/rifampicin-resistant tuberculosis (MDR/RR-TB) is one of the major challenges in the field of global public health.1 According to the World Health Organization (WHO), approximately 480,000 people worldwide develop MDR-TB each year, with some cases progressing to extensively drug-resistant tuberculosis (XDR-TB).2,3 MDR/RR-TB not only has a long treatment duration and complex treatment regimens but also significantly increases treatment costs compared to regular tuberculosis, leading to a significant decrease in treatment adherence and completion rates among patients.4,5 China ranks third globally in the number of tuberculosis cases, accounting for approximately 8–9% of the global TB burden. Recent studies indicate that the primary mode of TB transmission in China is localized, with 23% of cases forming genomic clusters and 61.4% of MDR/RR-TB cases resulting from recent transmission of drug-resistant strains.6 In densely populated and economically underdeveloped areas, the treatment and management of MDR/RR-TB remain particularly challenging due to resource limitations and insufficient patient education.3,7,8
The treatment strategy for drug-resistant tuberculosis has undergone several changes in the past few decades. Currently, the recommended treatment by the WHO includes the combined use of multiple anti-tuberculosis drugs for up to 20 months.2,9,10 However, even under optimal treatment conditions, the success rate is much lower than that for non-drug-resistant tuberculosis. Additionally, patient attrition during treatment (Lost to Follow-Up, LTFU) is common, leading to an increased risk of treatment failure and further spread of drug resistance.11–13 Thus, improving treatment adherence and reducing LTFU rates are crucial factors in enhancing treatment outcomes.
In Shaanxi Province, China, despite significant government resources being allocated to the fight against tuberculosis, the treatment of MDR/RR-TB still faces numerous challenges. Various factors contribute to this phenomenon, including economic burdens, inadequate social support, and improper management of drug side effects, all significantly affecting patient treatment adherence and outcomes.
Given the limitations of existing treatment management strategies in improving patient compliance and reducing LTFU, patient-centered care services (PCCS) have gradually gained attention globally. The PCCS model emphasizes providing personalized treatment plans and comprehensive psychosocial support to patients through interdisciplinary teamwork to enhance the overall treatment experience and outcomes.14,15
Research indicates that implementing PCCS can effectively improve patient treatment adherence and reduce the occurrence of treatment interruptions and LTFU. However, in China, especially in resource-constrained areas, the implementation and effects of PCCS have not been widely researched and validated.
The PCCS model, through multidisciplinary teamwork, provides more detailed and systematic support across key stages such as inpatient treatment, discharge preparation, community referrals, and follow-up. This personalized and comprehensive care approach significantly improves treatment success rates, reduces LTFU rates, and enhances patients’ treatment experiences and overall outcomes. This study aims to evaluate the impact of PCCS on treatment success rates and LTFU rates among MDR/RR-TB patients in Xi’an, China.
Materials and Methods
Ethical Approval and Informed Consent
This study complies with the ethical principles outlined in the Declaration of Helsinki, prioritizing the protection of participants’ rights and privacy. Ethical approval was obtained from the Xi’an Chest Hospital Ethics Committee in November 2022 (Reference No. S2022-0013). Written informed consent was exempted as it relied solely on electronic medical records with minimal risk to participants. Instead, verbal consent was obtained via phone or WeChat, ensuring ethical compliance. To protect privacy, the primary researcher assigned unique identifiers to participants and ensured they only had access to identifiable data.
Patient Population
We conducted a single-center observational retrospective analysis of all multidrug-resistant/rifampicin-resistant tuberculosis (MDR/RR-TB) patients treated at Xi’an Tuberculosis Control Hospital from January 1, 2018, to December 31, 2019. Patients were excluded if they were under 15 years old, received only short-term treatment regimens (9–12 months), had severe mental disorders or limited self-care abilities, or were transferred out of the area, preventing follow-up. Eligible patients were divided into the SoC cohort (patients receiving standard treatment in 2018) and the PCCS cohort (patients receiving PCCS intervention in 2019).
Data Collection and Definitions
We retrieved medical records of enrolled patients from the hospital’s electronic medical record system, including demographic information (age, gender, BMI, smoking history, education level, residence, marital status, occupation, medical payment method, and history of anti-tuberculosis treatment), clinical data (presence of diabetes mellitus, drug susceptibility test results, anti-tuberculosis drugs used, and adverse reactions), and treatment outcomes. MDR/RR-TB was confirmed using drug susceptibility testing (DST) results, which identified resistance to rifampicin and/or isoniazid. Patients diagnosed based on DST were included in the study. The research team utilized a double data entry system (REDCap) with error-checking functions to ensure data quality. Two independent data entry personnel entered the same dataset, with a third party performing final confirmation.
We implemented a systematic patient tracking process to minimize LTFU and data loss, using electronic case management and regular follow-ups via phone calls, SMS, and WeChat reminders. For patients unreachable by initial contact, alternative methods were used, with all attempts recorded. Treatment outcomes were classified based on WHO standards into cured, treatment completed, treatment failed, LTFU, and death. Treatment success was defined as the sum of patients cured or completing treatment. LTFU was defined as patients interrupting treatment for ≥2 months.16 Adverse events were graded using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.17
Analysis of Reasons for Treatment Interruption
In order to understand the reasons for non-adherence to treatment among some of the enrolled patients (68 individuals), nurse counselors conducted telephone interviews with the patients to identify the primary reasons for their non-adherence and to assist them in resuming treatment. Among them, 17 patients could not be contacted, including 3 patients who had died after delaying treatment. Therefore, the investigators of this study, based on feedback from the physician in charge of pulmonary tuberculosis, nurse counselors, and family members of LTFU patients, as well as medical record reviews, conducted a comprehensive assessment to determine the main reasons leading to LTFU. The main reasons contributing to prolonged non-cure were categorized into five groups: limited knowledge, financial difficulties, lack of social and psychological support, complications, and others.
Data Analysis
Descriptive statistical analysis uses measures such as the mean, median, standard deviation (SD), quartiles, frequency, and percentages to describe continuous and categorical variables. Survival analysis employs the Kaplan-Meier (KM) method to assess the risks of treatment duration and treatment interruption, while the Cox proportional hazards model or logistic regression is used to analyze the impact of PCCS on treatment adherence while controlling for potential confounding factors.
Conducting sensitivity analysis is essential to examine the robustness of the results by varying key parameters or assumptions to assess the extent of changes in the analytical outcomes.
Statistical Analysis
Data analysis was conducted using R statistical software version 3.3.2 (R Foundation) and Free Statistics software version 1.7. All statistical tests were two-tailed, and a P-value < 0.05 was considered statistically significant. Descriptive statistics were used to summarize the basic characteristics of patients and treatment outcomes. Continuous variables were expressed as mean, standard deviation (SD), median, and interquartile range (IQR) and analyzed using t-tests or Mann–Whitney U-tests based on data distribution. Categorical variables were presented as frequencies and percentages.
The Kaplan-Meier (KM) method was employed to analyze treatment duration and risk of discontinuation. The Cox proportional hazards model was applied to assess the association between PCCS and treatment adherence, with univariate analysis used to identify potential confounders and multivariate analysis to evaluate the independent impact of PCCS on LTFU. Subgroup analyses were conducted to explore differences among patient groups, and sensitivity analysis was performed to ensure the robustness of the results. Lastly, reasons for LTFU in both cohorts were analyzed to identify changes after PCCS implementation, providing insights for treatment improvement.
Results
Analysis of Demographic Characteristics and Treatment Plans of Selected Patients
From January 2018 to December 31, 2019, 450 MDR/RR-TB patients were diagnosed and started treatment at the tuberculosis care hospital. Of these, 21 patients were excluded based on exclusion criteria, and 429 patients were included in the study, including 166 patients in the SoC cohort and 263 patients in the PCCS cohort. Please refer to Figure 1.
 |
Figure 1 Flowchart of Patient Selection for Data Analysis. |
The average median age in the SoC cohort was 35.0 years (Q1: 27.0, Q3: 51.0); among the 166 patients in the SoC cohort, 123 (74.1%) were male; 23 (13.9%) had comorbid diabetes; 84 (50.6%) were retreatment cases; 133 (80.1%) were on a fluoroquinolone-based treatment regimen, and 30 (18.1%) were on a linezolid-based treatment regimen. The average median age in the PCCS cohort was also 35.0 years (Q1: 29.5, Q3: 51.5); among the 263 patients in the PCCS cohort, 169 (64.3%) were male; 44 patients (16.7%) had comorbid diabetes; 211 patients (80.2%) were on a fluoroquinolone-based treatment regimen, and 96 patients (36.5%) were on a linezolid-based treatment regimen. Thirteen cases in both groups had not undergone fluoroquinolone resistance testing. A comparison of the two groups is provided in Table 1.
 |
Table 1 Patient Characteristics of the SoC and PCCS Cohorts |
The study results showed differences in demographic characteristics and clinical configurations between the two groups of patients, particularly in the choice of drug treatment regimens. Most patients adopted fluoroquinolone-based treatment regimens, with other alternative drugs being chosen only in cases of fluoroquinolone resistance. Importantly, the introduction of PCCS is associated with a differentiation in patient care management, including enhanced monitoring and customized treatment plans, which are crucial for meeting the complex needs of MDR/RR-TB patients. The analysis suggests that integrating patient-centered strategies into tuberculosis management protocols may significantly improve treatment adherence and outcomes, emphasizing the necessity of innovating care models in high-burden settings.
Treatment Outcomes of SoC and PCCS Groups
Among the 166 SoC patients, 98 (59.0%) achieved treatment success, 50 (30.1%) had LTFU, 2 (1.2%) deceased, and 16 (9.6%) treatment failure . In the PCCS cohort of 263 patients, 227 (86.3%) achieved treatment success, 18 (6.8%) had LTFU, 6 (2.3%) deceased, and 12 (4.5%) treatment failure. Many patients in both cohorts were LTFU during the intensive care phase, with 31 patients (62.0%) in the SoC cohort and 10 patients (55.6%) in the PCCS cohort. The LTFU rate was 6.8% in the PCCS cohort, lower than the rate of 30.1% in the SoC cohort. KM analysis demonstrated a statistically significant difference in LTFU. The survival curves of patients in both groups are shown in Figure 2. These results highlight the potential benefits of PCCS in improving treatment success rates and reducing LTFU in MDR/RR-TB patients.
 |
Figure 2 Survival Curves for the SoC and PCCS Groups. |
Univariate Analysis of Risk Factors for LTFU and Conclusions
Univariate analysis indicates the following risk factors are significantly associated with LTFU: compared to the PCCS group, the SoC group has a significantly increased risk of LTFU; the LTFU risk for females is significantly lower than for males; patients aged 50 and above have a significantly higher risk of LTFU compared to those aged 15–30; smokers have a significantly higher risk of LTFU compared to non-smokers; patients with primary school education level or below have a significantly higher risk of LTFU compared to those with a university degree or higher; divorced or widowed patients have a significantly higher risk of LTFU compared to married patients; farmers or unemployed patients have a significantly higher risk of LTFU compared to employed or retired patients; patients paying for medical expenses out-of-pocket have a significantly higher risk of LTFU compared to those using medical insurance; patients with a history of anti-tuberculosis treatment have a significantly higher risk of LTFU compared to newly diagnosed patients; patients with diabetes have a significantly higher risk of LTFU compared to those without diabetes as shown in Table 2. The results of univariate analysis show that several factors are significantly associated with LTFU. These factors include the type of group (SoC vs PCCS), gender, age, smoking habits, education level, marital status, occupation, payment method, history of anti-tuberculosis treatment, and comorbid diabetes. Specifically, the PCCS group has a significantly lower risk of LTFU than the SoC group, suggesting that PCCS may play an important role in reducing LTFU.
 |
Table 2 Univariate Cox Regression Analysis of LTFU |
Patient Care Methods and Their Impact on Long-Term Treatment: A Multivariate Predictive Analysis
Multivariate Cox regression analysis indicated that patient care methods significantly impact long-term treatment outcomes. Without adjusting for any confounding factors, the LTFU rate in the PCCS group was 0.19 times that of the SoC group. After adjusting for gender and age (Model 1), the LTFU rate in the PCCS group was 0.17 times that of the SoC group. Further adjustments for gender, age, treatment history, comorbidities, and adverse reactions (Model 2) also showed the PCCS group’s LTFU rate was 0.17 times that of the SoC group. Finally, after adjusting for all confounding factors, including gender, age, smoking status, education level, marital status, occupation, insurance, treatment history, comorbidities, and adverse reactions (Model 3), the LTFU rate in the PCCS group was 0.14 times that of the SoC group (Table 3). The multivariate analysis showed that PCCS (Patient-Centered Care and Support) significantly reduced the LTFU rate. Even after adjusting for various confounding factors, the LTFU rate in the PCCS group remained significantly lower than in the SoC group. It indicates that PCCS is crucial in reducing patient loss to follow-up.
 |
Table 3 Multivariate Cox Regression Analysis of PCCS Impaction on LTFU |
Subgroup Analysis and Interaction Study on the Impact of PCCS on LTFU
We conducted a subgroup analysis of the LTFU rate in the PCCS group, considering factors such as gender, age, smoking history, diabetes comorbidity, tuberculosis treatment history, education level, marital status, occupation, payment method, and the severity of adverse drug reactions (ADRs). The results showed that only the payment method had a significant interaction with PCCS on LTFU (P < 0.001) (Table 4).
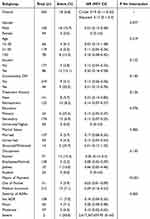 |
Table 4 Subgroup Analysis of the Impact of PCCS on Patient LTFU |
These findings indicate that the payment method significantly impacts the LTFU rate within the PCCS group. Specifically, self-paying patients benefited the most from the PCCS, with a significantly reduced LTFU rate. It may be because we provided more financial planning education and helped these patients seek additional family financial support, enhancing their confidence in treatment and improving treatment adherence.
The Critical Role of Financial Support in Reducing Loss to Follow-Up (LTFU): Advantages and Challenges of PCCS
We conducted a detailed analysis of the causes of LTFU. The results showed that in the SoC cohort, the top three factors leading to long-term LTFU were poor knowledge (31 cases, 62.0%), limited psychosocial support (11 cases, 22.0%), and financial difficulties (6 cases, 12.0%). In contrast, in the PCCS cohort, the primary factors for long-term LTFU were financial difficulties (8 cases, 44.4%), poor knowledge (7 cases, 38.9%), and comorbidities (2 cases, 11.1%) (Table 5).
 |
Table 5 Analysis of Leading Causes for LTFU – SoC Vs PCCS |
Table 5 shows that the proportion of LTFU due to poor knowledge and limited psychosocial support was significantly lower in the PCCS cohort compared to the SoC cohort. It indicates that PCCS has achieved positive results in improving patient knowledge and providing psychosocial support. However, the proportion of financial difficulties in the PCCS cohort relatively increased (44.4%), although the absolute number of patients facing financial difficulties did not significantly change between the SoC and PCCS cohorts (6 cases vs 8 cases).
These findings suggest that PCCS is crucial in reducing LTFU caused by poor knowledge and limited psychosocial support, thereby decreasing the LTFU rate due to these factors. Nevertheless, financial difficulties remain a major reason for LTFU in the PCCS cohort. Therefore, to further reduce the LTFU rate, enhancing financial support for patients is essential. Financial assistance and resources can further improve patient adherence to treatment and outcomes.
Discussion
This study aimed to investigate the impact of PCCS on the treatment outcomes of patients with MDR/RR-TB at a tuberculosis treatment hospital in Xi’an, Shaanxi Province, China. By retrospectively analyzing treatment data from patients receiving PCCS and traditional SoC between 2018 and 2019, this study examined the effects of the two nursing models on improving treatment adherence, reducing LTFU, and increasing treatment success rates. The methods and findings of this study provide a comparison of a more optimal treatment strategy to traditional care and have significant clinical value.
This study showed significant differences in treatment success rates and LTFU rates between PCCS and SoC. The data showed that the treatment success rate in the PCCS group reached 86.3%, much higher than the SoC group at 59.0%; additionally, the LTFU rate with PCCS was only 6.8% compared to 30.1% in the SoC group. These results align with other studies, such as Wang et al (2021), who also reported similar positive effects of PCCS on improving treatment success rates.18 However, compared to Li et al (2019), this study demonstrated a more significant effect in reducing LTFU rates, which may be attributed to the more systematic patient follow-up and support strategies implemented in this study.
After the implementation of PCCS, there was a significant improvement in patient treatment adherence. Multivariate Cox regression analysis demonstrated that compared to SoC, PCCS significantly reduced the risk of LTFU (adjusted hazard ratio of 0.14), indicating that PCCS can effectively enhance patients’ persistence with long-term treatment regimens. This is consistent with the findings of Zhang et al (2020), who found that a patient-centered care model significantly enhances patient compliance. However, this study further revealed the unique advantage of PCCS in reducing LTFU rates, possibly due to the personalized support and educational strategies adopted in this study.
The analysis indicated that the main reasons for LTFU included financial difficulties, limited knowledge, and inadequate social support. These findings are generally consistent with literature domestically and internationally, such as Zhao et al (2018), who also identified economic and educational factors as significant influencers on treatment adherence.19 Additionally, the study by Vyawahare et al (2023) further highlights the significant impact of economic hardship, low body mass index (BMI), nutritional status, and tobacco use on the occurrence of drug-resistant tuberculosis.20 In this study, although PCCS effectively reduced the LTFU rate, economic difficulties and lack of social support remained the main reasons for patients discontinuing treatment. WHO’s multicenter studies have also identified low-income levels and insufficient social support as critical factors contributing to MDR-TB treatment failure globally. Therefore, economic support and educational interventions can significantly improve treatment adherence. Future efforts should explore personalized care strategies tailored to different regions.
The specific socio-economic and cultural context of Xi’an City had a certain impact on the effectiveness of treatment and care models for MDR/RR-TB. Compared to studies in other regions, such as Liu et al (2022) in Beijing, the economic challenges and lack of social support faced by patients in this research area were more severe. This regional difference highlights the need for tailored nursing strategies to adapt to local characteristics and patient needs in diverse treatment environments.21–23
This study confirms the effectiveness of PCCS in improving the treatment success rate and reducing the LTFU rate for MDR/RR-TB. This finding has significant implications for clinical practice and provides a basis for promoting similar care models in high-burden areas in the future. However, the study has certain limitations. First, the retrospective design may introduce selection bias, affecting the generalizability of the results. Second, treatment information relied on electronic medical records and follow-up data, possibly subject to recall bias and incomplete data issues. These limitations could impact the analysis of certain variables, and the findings should be interpreted cautiously. Additionally, as the study sample was primarily limited to a single hospital in Xi’an, the applicability of the results requires further validation in broader regions and patient populations.
Future research should consider employing prospective, multicenter designs and expanding the sample size to validate PCCS’s effectiveness and applicability. Exploring how to optimize PCCS strategies to meet local needs in different socioeconomic and cultural contexts will enhance its potential for widespread adoption. Furthermore, investigating the long-term impact of PCCS on patients’ quality of life will provide a more comprehensive understanding of its clinical and social significance.
Conclusion
This study demonstrates that implementing PCCS in a TB hospital in Xi’an, China, significantly improved treatment outcomes for MDR/RR-TB patients. Compared to traditional SoC, PCCS resulted in higher treatment success rates and significantly reduced LTFU. The findings indicate that PCCS effectively reduces LTFU and improves cure rates, optimizing the management of drug-resistant TB.
The scientific value of this study lies in providing a new approach to MDR-TB treatment. PCCS not only deepens the understanding of TB treatment strategies but also offers a practical solution to enhance adherence and success rates in clinical practice.
Data Sharing Statement
The data that supports the findings of this study are available on request from the corresponding author.
Ethics Approval and Consent to Participate
This study complies with the ethical principles outlined in the Declaration of Helsinki, prioritizing the protection of participants’ rights and privacy. Ethical approval was obtained from the Xi’an Chest Hospital Ethics Committee in November 2022 (Reference No. S2022-0013). Written informed consent was exempted as it relied solely on electronic medical records with minimal risk to participants. Instead, verbal consent was obtained via phone or WeChat, ensuring ethical compliance. To protect privacy, the primary researcher assigned unique identifiers to participants and ensured they only had access to identifiable data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Shaanxi Provincial Key Research and Development Program Project of Key Industrial Innovation Chain, “Research on the Construction of All-round Multidrug-Resistant Tuberculosis Prevention and Control System under the end TB strategy” (2023-ZDLSF-01), and Medical Research Project of Science and Technology in Xi’an (21YXYJ0001).
Disclosure
The authors declare no conflict of interest.
References
1. Silitonga P, Jiang W, Wyatt S, Burhan E, Kes EFM, Long Q. Factors affecting time to treatment initiation after diagnosis for multidrug-resistant/rifampicin-resistant tuberculosis patients: a mixed-methods study in Jakarta, Indonesia. Trop Med Int Health. 2023;28(1):43–52. doi:10.1111/tmi.13838
2. Vanino E, Granozzi B, Akkerman OW, et al. Update of drug-resistant tuberculosis treatment guidelines: a turning point. Int J Infect Dis. 2023;130(Suppl 1):S12–S15. doi:10.1016/j.ijid.2023.03.013
3. Dheda K, Mirzayev F, Cirillo DM, et al. Multidrug-resistant tuberculosis. Nat Rev Dis Primers. 2024;10(1):22. doi:10.1038/s41572-024-00504-2
4. Maier C, Chesov D, Schaub D, et al. Long-term treatment outcomes in patients with multidrug-resistant tuberculosis. Clin Microbiol Infect. 2023;29(6):751–757. doi:10.1016/j.cmi.2023.02.013
5. Gao J, Gao M, Du J, et al. A pragmatic randomized controlled trial to evaluate the efficacy and safety of an oral short-course regimen including bedaquiline for the treatment of patients with multidrug-resistant tuberculosis in China: study protocol for PROSPECT. Trials. 2024;25(1):227. doi:10.1186/s13063-024-07946-9
6. Liu D, Huang F, Li Y, et al. Transmission characteristics in Tuberculosis by WGS: nationwide cross-sectional surveillance in China. Emerg Microbes Infect. 2024;13(1):2348505. doi:10.1080/22221751.2024.2348505
7. Lu J, Xu Y, Li Z, Chen X, Lin H, Zhao Q. Diagnosis and treatment pathway of MDR/RR-TB in Taizhou, Zhejiang Province, China. Trop Med Infect Dis. 2023;8(2):79. doi:10.3390/tropicalmed8020079
8. Lecai J, Mijiti P, Chuangyue H, et al. Predictors and Trends of MDR/RR-TB in Shenzhen China: a Retrospective 2012-2020 Period Analysis. Infect Drug Resist. 2021;14:4481–4491. doi:10.2147/IDR.S335329
9. Seifert SA, Armitage JO, Sanchez EE. Snake Envenomation. N Engl J Med. 2022;386(1):68–78. doi:10.1056/NEJMra2105228
10. Tiberi S, Utjesanovic N, Galvin J, et al. Drug resistant TB - latest developments in epidemiology, diagnostics and management. Int J Infect Dis. 2022;124(Suppl 1):S20–S25. doi:10.1016/j.ijid.2022.03.026
11. Alsayed SSR, Gunosewoyo H. Tuberculosis: pathogenesis, current treatment regimens and new drug targets. Int J mol Sci. 2023;24(6):5202. doi:10.3390/ijms24065202
12. Williams G, Hahn D, Stephens JH, Craig JC, Hodson EM. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2023;4(4):CD001321. doi:10.1002/14651858.CD001321.pub6
13. Ruan Y, Chen L, She D, Chung Y, Ge L, Han L. Ketogenic diet for epilepsy: an overview of systematic review and meta-analysis. Eur J Clin Nutr. 2022;76(9):1234–1244. doi:10.1038/s41430-021-01060-8
14. Zhu QQ, Wang J, Sam NB, Luo J, Liu J, Pan HF. Factors associated with non-adherence for prescribed treatment in 201 patients with multidrug-resistant and rifampicin-resistant Tuberculosis in Anhui Province, China. Med Sci Monit. 2022;28:e935334. doi:10.12659/MSM.935334
15. Shin JE, Jeon D, Mok J, et al. Compliance with new drug use and the effect of discrepant drug susceptibility testing on MDR/RR-TB treatment. Int J Tuberc Lung Dis. 2024;28(2):86–92. doi:10.5588/ijtld.23.0237
16. Linh NN, Viney K, Gegia M, et al. World health organization treatment outcome definitions for tuberculosis: 2021 update. Eur Respir J. 2021;58(2):2100804. doi:10.1183/13993003.00804-2021
17. Gilbert A, Piccinin C, Velikova G, et al. Linking the European organisation for research and treatment of cancer item library to the common terminology criteria for adverse events. J Clin Oncol. 2022;40(32):3770–3780. doi:10.1200/JCO.21.02017
18. Agarwal RN, Aggarwal R, Nandarapu P, et al. COVID-19 vaccination drive in a low-volume primary care clinic: challenges & lessons learned in using homegrown self-scheduling web-based mobile platforms. Vaccines. 2022;10(7):1072. doi:10.3390/vaccines10071072
19. Deng YL, Hsu CS, Hsu CY, et al. Predictors for self-discontinuation of anti-osteoporosis medication: a hospital-based real-world study. PLoS One. 2022;17(9):e0275020. doi:10.1371/journal.pone.0275020
20. Vyawahare C, Mukhida S, Khan S, Gandham NR, Kannuri S, Bhaumik S. Assessment of risk factors associated with drug-resistant tuberculosis in pulmonary tuberculosis patients. Indian J Tuberc. 2024;71(Suppl 1):S44–S51. doi:10.1016/j.ijtb.2023.07.007
21. Zhou M, Peng Y, Liu K, et al. Direct medical expenses and influencing factors of MDR/RR-TB in Eastern China: based on data from multi-hospital information systems. Risk Manag Healthc Policy. 2023;16:1955–1965. doi:10.2147/RMHP.S420082
22. Puerto GM, Castro CM, Rubio VV, Fadul S, Montes F. Tuberculosis multirresistente en Colombia, 2013-2018: estudio de casos y controles. Drug-resistant tuberculosis in Colombia, 2013-2018: case-control study. Biomedica. 2023;43(4):447–456. doi:10.7705/biomedica.6842
23. Zhao B, Liu C, Fan J, et al. Transmission and drug resistance genotype of multidrug-resistant or rifampicin-resistant mycobacterium tuberculosis in Chongqing, China. Microbiol Spectr. 2022;10(5):e0240521. doi:10.1128/spectrum.02405-21
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

