Back to Journals » Journal of Pain Research » Volume 18
Impact of Propranolol and Psychologically Informed Intervention on Pain Sensitivity: Secondary Analysis from the Biopsychosocial Influence on Shoulder Pain Preclinical Randomized Trial
Authors Bishop MD , Simon CB , Huo Y, Wallace MR , Borsa PA, Fillingim RB, Staud R , Wu SS, George SZ
Received 10 October 2024
Accepted for publication 12 March 2025
Published 5 April 2025 Volume 2025:18 Pages 1837—1850
DOI https://doi.org/10.2147/JPR.S500140
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Alaa Abd-Elsayed
Mark D Bishop,1– 3 Corey B Simon,4,5 Yanan Huo,6 Margaret R Wallace,7 Paul A Borsa,8 Roger B Fillingim,2,9 Roland Staud,10 Samuel S Wu,11 Steven Z George4,5,12
1Department of Physical Therapy, University of Florida, Gainesville, FL, USA; 2Pain Research and Intervention Center of Excellence, University of Florida, Gainesville, FL, USA; 3Center for Pain Research and Behavioral Health, University of Florida, Gainesville, FL, USA; 4Department of Orthopaedic Surgery, Physical Therapy Division, Duke University, Durham, NC, USA; 5Duke Clinical Research Institute, Duke University, Durham, NC, USA; 6Gilead Sciences, Inc., Foster City, CA, USA; 7Department of Molecular Genetics and Microbiology, UF Genetics Institute, University of Florida, Gainesville, FL, USA; 8Department of Applied Physiology and Kinesiology, University of Florida, Gainesville, FL, USA; 9Department of Community Dentistry and Behavioral Science, University of Florida, Gainesville, FL, USA; 10Department of Medicine, University of Florida, Gainesville, FL, USA; 11Department of Biostatistics, University of Florida, Gainesville, FL, USA; 12Departments of Orthopaedic Surgery and Population Health Sciences, Duke University, Durham, NC, USA
Correspondence: Mark D Bishop, Department of Physical Therapy, University of Florida, 1225 Center Drive, HPNP #1139, Gainesville, FL, 32610, USA, Tel +1+352+2736112, Email [email protected]
Purpose: Measures of pain sensitivity have potential relevance for patient care. We previously identified a subgroup of people at risk for ongoing pain characterized by genetic AND psychological factors. Here, we report planned secondary analyses examining the effect of personalized interventions on pain sensitivity outcomes.
Patients and Methods: Two hundred and sixty-one healthy individuals with the COMT SNP rs6269 AA genotype and Pain Catastrophizing Scale scores of 5 or higher received exercise-induced muscle injury, followed by a randomly assigned treatment: (1) general education and placebo; (2) personalized psychological intervention and placebo; (3) general education and propranolol; or (4) personalized psychological intervention and propranolol. Pain sensitivity outcomes (pressure pain thresholds (PPT), suprathreshold heat rating, temporal summation, and conditioned pain modulation efficiency) were compared using a mixed effect model to examine difference among groups, adjusted for age, sex and race.
Results: No main effects for group assignment were noted (p > 0.05 for all), when considered as 4 groups or 2 collapsed groups (ie propranolol vs placebo or personalized psychologic vs general education). Interaction terms were then entered into our models in an exploratory fashion. For PPT outcomes interactions were noted for, sex and time, and race and time (p< 0.015). For temporal summation outcomes, interactions were noted for sex and group and race and group (p < 0.015).
Conclusion: Results indicated no statistically reliable changes in pain sensitivity when considering matched vs unmatched treatment groups. Caution is needed in this interpretation given that the trial was not powered to specifically identify these differences. Exploratory analysis of interactions among ethnic/racial and gender identities by treatment, however, showed the potential for differential effects for specific pain sensitivity measures. Significant interactions across modalities suggest analysis of higher order interactions/intersectionality could be of great interest for testing efficacy of personalized interventions in future trials.
Plain Language Summary: Purpose: In previous work, we identified a set of genetic and cognitive traits that were associated with persistent pain after exercise induced muscle pain. Participants and methods: In this current study, we tested whether a combination of information focused on how you think about pain and a medication tailored to a genetic risk factor would help people without clinical pain conditions recovery faster from exercise-induced muscle pain than using general information and placebo medication. Results: The study did not strongly support that interventions matched to higher risk of persistent pain made changes for all the people. What we did find is that individuals’ ethnic, racial and gender identities might be influencing sensitivity to specific measures of pain sensitivity. Conclusions: Interactions among individuals’ identities might impact pain sensitivity. These findings could be used to generate hypotheses for and/or inform future studies of individualized medicine approaches for pain.
Keywords: muscle, exercise, shoulder, genetics
Introduction
Ongoing and persistent pain after surgery or injury places a significant burden on an individual, the family, and society. On a global scale, chronic secondary musculoskeletal pain conditions rank high among health conditions in terms of prevalence, disability, and economic burden.1 We previously identified a subgroup of people at risk for ongoing pain that is characterized by both genetic AND psychological factors.2 The two key elements of this high-risk subgroup are the catechol-O-methyltransferase (COMT) gene – (responsible for encoding the COMT enzyme involved in catecholamine metabolism) and pain catastrophizing, a psychological factor that encompasses beliefs about pain. Our previous studies reported that the interaction between a COMT SNP genotype and pain catastrophizing serves as a better predictor of shoulder pain and disability outcomes compared to either factor in isolation.2,3 These findings were observed in both pre-clinical (exercise induced pain) and clinical (arthroscopic rotator cuff repair) cohorts.2,3
After promising findings from the cohort studies,2,3 a pre-clinical randomized trial tested the efficacy of interventions matched to risk factors of the high-risk subgroup. This trial did not identify additional benefits of these personalized interventions on the primary outcome, rate of recovery after exercise induced shoulder pain.4 In this current paper, we report the results of the planned secondary analyses to examine the effect of the personalized interventions on pain sensitivity outcomes.
Pain sensitivity is determined from responses to noxious thermal, mechanical, electrical, or chemical stimuli that are applied in a standard manner, commonly using quantitative sensory testing (QST) methodology. Pain sensitivity is a behavioral measure providing proxy information regarding endogenous facilitation and inhibition of sensory processing of noxious stimuli.5,6 Genetic variations in pain-related genes – such as those involved in neurotransmitter metabolism or signaling pathways – can influence an individual’s pain sensitivity;7,8 and pain sensitivity is linked to the psychological and emotional aspects of pain experiences.9 Individuals with heightened pain sensitivity may be more prone to experiencing increased pain intensity after surgery.10 Investigating pain sensitivity is therefore crucial for advancing our understanding of how individuals respond to interventions that have been personalized to genetic and psychologic risk factors.
In this planned analysis of secondary outcomes of the Biopsychosocial Influence on Shoulder Pain (BISP) pre-clinical randomized trial,11 we determined the extent to which interventions personalized to psychological and genetic factors characteristic of a group at risk of high pain intensity and longer pain duration modified pain sensitivity following acute exercise-induced muscle injury. The intervention for the psychological factor was psychologically informed education based on our work and others in people with low back pain12,13 to modify catastrophizing thoughts. A pharmaceutical intervention, propranolol, was chosen based on previous findings of reduced in pain sensitivity in people with orofacial pain that was dependent on COMT genotype.14
We hypothesized that individuals receiving the matched interventions would report greater improvement in pain sensitivity compared to those receiving unmatched interventions. Since the results of the trial were null for the primary outcome,4 we did not have hypotheses for any specific pain sensitivity measure, and investigated all measures for their response to matched interventions using a tiered approach that balanced priority of QST measure with amount of Type I error. We also explored the influence of interactions related to race and sex to fully consider whether these matched interventions have the potential for efficacy when those factors are considered.
Materials and Methods
Trial Overview
The BISP trial was prospectively registered on ClinicalTrials.gov (NCT02620579) and adhered to the reporting guidelines recommended by CONSORT and SPIRIT.15 A detailed description of the study methods can be found in our published protocol article11 and previously reported results in the primary article.4 The University of Florida Institutional Review Board approved the study, and all participants provided informed consent for eligibility screening and participation in the BISP trial in compliance with the Declaration of Helsinki.
Eligible participants underwent a protocol to produce exercise-induced muscle injury on day 1, followed by four consecutive days of a randomly assigned treatment delivered on-site (days 2–5) through the University Clinical Research Center. This protocol is described below. The four intervention groups were created by combining two pharmacologic conditions (propranolol or placebo) with two informational conditions (psychologic intervention or general education) that were theoretically appropriate for the high-risk subgroup. The primary endpoint of the parent trial was shoulder pain recovery and there were no differences between the personalized intervention group (propranolol and psychologic intervention) and any of the other three comparator groups.
Participants
Healthy individuals aged 18 to 65 years who were fluent in English, were confirmed to have the COMT AA genotype (correlated with heightened pain sensitivity at the rs6269 variant) and Pain Catastrophizing Scale (PCS) scores of 5 or higher were considered for study participation. All testing occurred in clinical laboratories within the University of Florida Health Science Center.
It should be noted that, compared to clinical populations, a score of 5 on the PCS is not considered “elevated.” However, this specific cutoff score was empirically determined in a pre-clinical cohort and validated in a clinical cohort meeting COMT AA genotype x PCS subgroup classification.4 Specifically, this high-risk subgroup composed of individuals with the COMT AA genotype and PCS scores of 5 or higher, demonstrated the strongest predictive power for increased shoulder pain at 12 months post-operation in a clinical cohort. Consequently, this subgroup was designated as the high-risk subgroup of interest.
Inclusion criteria were: a) ages ≥ 18 years to 65 years and b) English speaking. Exclusion criteria were identified during screening and trial enrollment and included in Supplemental Table 1.
Exercise-Induced Shoulder Injury
Research personnel, blinded to intervention assignment, performed the muscle injury protocol and subsequent follow-up assessments. Participants underwent exercise-induced shoulder muscle injury on their dominant arm. Briefly, participants were seated in a Biodex (Shirley, NY) isokinetic dynamometer. Maximum voluntary isometric contraction (MVIC) of isometric shoulder external rotation was determined. Participants then completed eccentric (lengthening)/concentric (shortening) external rotation repetitions at 60°.s−1 for 3 sets of 10 repetitions. After completing those repetitions, MVIC was retested. If they achieved 50% or less of the pre-exercise MVIC, the fatigue protocol was terminated. If they generated more than 50% of the pre-exercise MVIC, participants performed additional sets of 10 repetitions and MVIC was re-tested. This cycle was repeated until participants’ MVIC was less than 50% of the initial MVIC. The goal of the injury protocol was to induce shoulder pain intensity, reduced range of motion, and weakness in the exercised muscle. Additional details on this procedure are available in our prior publications [8,11].
Interventions
This trial was designed to determine if the high-risk subgroup we identified in prior cohort studies responded differentially to treatments tailored to characteristics of that subgroup. Accordingly, participants were randomly assigned to one of four groups created by combining two pharmacologic intervention conditions (propranolol or placebo) with two informational conditions (psychologic intervention or general education) resulting in the following cohorts: Group A – personalized psychologic and placebo (1 personalized component), Group B – general education and placebo (0 personalized components), Group C – personalized psychologic and propranolol (2 personalized components), or Group D – general education and propranolol (1 personalized component). See Supplemental Table 2 for full descriptions of the interventions and these interventions are also described in our protocol11 and primary trial results papers.4
Randomization and Allocation
A computer-generated randomization scheme was prepared by the statistical team prior to the study. Treatment assignments were accessed by study personnel through a secure website, which provided independent assignment for pharmaceutical and psychological interventions. Research staff involved in the study were blinded to the intervention assignment that they were not directly associated with. Randomization was completed after enrollment into the study before baseline measures were collected and the muscle injury protocol performed. Randomization was stratified by sex due to reported differences in pain conditions and observed sex differences in a prior study on shoulder pain.4
Outcomes
A comprehensive battery of nine pain sensitivity outcomes were collected across multiple modalities and body regions, assessing changes in static (ie thresholds) and dynamic pain sensitivity (facilitation and inhibition) at sites local and remote to the area of induced injury. Local and remote sites were chosen based on the hypothesis that systemic effects of the injury and the intervention would be indicated by changes in remote/distant measures of pain sensitivity.
Quantitative sensory testing was completed every day from Day 1 (baseline) to Day 5 in the Pain Clinical Research Unit of the UF Clinical and Translational Sciences Institute by personnel blinded to intervention assignment. Pain responses to the QST were collected using the numeric pain rating scale (NRS). The NRS is a 101-point scale in which patients verbally rate their pain from 0, indicating “no pain”, to 100, indicating “the most intense pain sensation imaginable.” The NRS demonstrates sound psychometric properties. Stimulation sites were varied to prevent carryover effects due to local sensitization. All participants underwent a brief training with the stimuli to familiarize participants with the stimulus range, limit range effects in psychophysical scaling, and alleviate any participant anxiety about the upper limit of stimulus intensities to be used. Standard instructions were used for all testing.
Pressure Pain Threshold (PPT): Three Sites
Pressure pain threshold (PPT) was assessed using an AlgoMed pressure algometer (Medoc, Durham NC) with a 1 cm diameter probe. The algometer was placed vertically on three different anatomical locations, the ipsilateral deltoid, contralateral deltoid, and ipsilateral tibialis anterior (distant to the anatomic location of the pain). PPT was defined as the amount of force (applied at a rate of 1kg per second) required to produce a sensation of pain distinct from pressure or discomfort. Participants were asked to say “pain” immediately when a sensation of pain was felt. At this point, the experimenter immediately retracted the algometer and the value of pressure applied was recorded (kPa). Three PPT measures were performed on each anatomical location.
Suprathreshold Rating of Thermal Stimuli: One Measure
The thermal stimuli were delivered to the participant’s forearm using the Pathway ATS/CHEPS Model (Medoc, Durham NC) unit or the TSA Neurosensory Analyzer (Medoc, Durham NC). Temperature increased at 10°C.s−1 from a baseline temperature of 39°C to 49°C. Pain intensity was assessed with an NRS response to each stimulus. The participants rated the magnitude of any pain following the heat pulse using the NRS.
Temporal Summation of second Pain (TSSP): Two Measures
Five heat pulses were applied to the ventral forearm of the patient using a PATHWAY Contact Heat-Evoked Potential Stimulator (CHEPS; Medoc Ltd, Ramat Yishay, Israel). To ensure temporal summation, an inter-stimulus interval of 2 seconds was used with temperatures starting at 39°C and increasing to 49°C. The participants were asked to rate the magnitude of pain following each heat pulse using the NRS. Ratings of pain intensity at the 5th pulse,16 as well as the change in rating (rating of pulse 5 – rating of pulse 1) were used in subsequent analysis. TSSP reflects generally facilitatory processes.
Conditioned Pain Modulation (CPM) Protocol: Three Measures
We used a cold pressor/pressure protocol to induce and measure CPM. First, PPT on the mid-point of the contralateral tibialis anterior was assessed using the methods described above and used as the “test stimulus”. Next, participants immersed the hand on the painful side up to the wrist into a cold water bath for up to one minute (conditioning stimulus). The water was maintained at a constant temperature of 8°C by a refrigerated water circulator and was constantly circulated to prevent warming around the hand. Thirty seconds after hand immersion, participants were asked to rate the pain intensity (0–100) from the immersed hand and were instructed to maintain their hand in the water bath for as long as they could tolerate for a maximum of one minute. After removing the hand from the cold bath, a new test stimulus was delivered to the contralateral tibialis anterior. Resultant measures were CPM efficiency (prePPT – postPPT), cold tolerance (time), cold tolerance rating. CPM efficiency reflects inhibitory processes.
Analysis Considerations for QST Measures
We applied a tiered approach to analysis and interpretation of these variables, where we prioritized QST measures. In the first tier were “dynamic” measures purported to assess the modulatory aspects of pain sensitivity: suprathreshold heat, TSSP, and CPM efficiency. In the second tier were “static” measures purported to assess key aspects of pain sensitivity thermal tolerance, ipsilateral PPT and distant PPT (tibialis anterior), followed in the third tier by measures with lower priority but with relevance to this study question tolerance ratings and PPT at the contralateral deltoid. These tiers were used for type 1 error correction and explained in more detail in statistical methods.
Statistical Analysis Methods
Summary statistics were provided for baseline characteristics by intervention groups. To answer our primary question, QST outcome variables were compared across the four randomly assigned groups, adjusting for sex and self-reported racial identity. For each outcome, a mixed effect model with a random subjects effect was fitted to examine difference between the four treatment groups and within-patient change over time, adjusting for age, self-identified sex (female vs male) and self-identified race (non-White vs White). The rationale for this exploration is that it is plausible that responses to personalized intervention may differ across key sociodemographic factors like age, sex, and racial identity.17,18 Unstructured covariance was assumed. Four intervention groups comprised of pharmacologic (propranolol or placebo) or informational (psychologic intervention or general education) combinations were modeled as fixed effect with the placebo and general education as the reference group.
Propranolol effects were compared between the two placebo groups versus two propranolol groups combined (Group A+B vs Group C+D). Education effects were compared between the two education 1 groups versus two education 2 groups combined (Group A+C vs Group B+D). Two-way interaction terms (group and sex, group and race, time and sex, time and race) were examined to guide future work. Three-way interactions were not considered (eg race, group, and time). Non-significant interactions were removed from the final models.
The graphical approach to sequential statistical testing was used to adjust for multiple testing of between group differences.19 The graphical approach relies on sequentially testing hypotheses based on an a priori sequence of “relative importance”. Specifically, our outcomes were divided into three tiers (first three, second three, and last three) based on hypothesized importance of any findings as ranked by the investigator team. We equally allocated alpha to the three hypotheses corresponding to first tier outcomes (ie, test at two-tailed alpha of 0.05/3). If a hypothesis was rejected in Tier one or Tier two, half of alpha was equally distributed to the other hypotheses in the same tier while the other alpha was equally distributed to the hypothesis of the next tier. If a hypothesis is rejected in Tier three, alpha was equally distributed to the other hypotheses in the same tier. All data analysis was done in SAS software (version 9.4; SAS Institute).
Results
A total of 1363 people were screened for the presences of COMT AA and a pain catastrophizing scale score of >5 which identified 261 participants. 28 were excluded/withdrawn post-randomization for medical/safety reasons such as hypertension or tachycardia measured during the first session. For the remaining 233 eligible patients analyzed (ie number included in the primary analysis), there were 57 in the placebo and psychologic intervention group (Group A), 57 in the placebo and general education group (Reference Group B), 62 in the propranolol and psychologic intervention group (Group C), and 57 in the propranolol and general education group (Group D). Baseline characteristics were balanced across 4 groups (Refer to Table 1 for details). No serious adverse events, defined as the need to seek healthcare outside of the study, were reported.
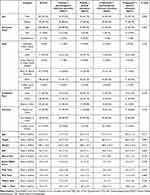 |
Table 1 Demographic and Anthropomorphic Variables |
Treatment Group Effects
Summary statistics for the nine outcomes are presented in Supplemental Table 3 by intervention group and day of visits. No main effect for group was noted for any outcome variables (p>0.05 for all). This was the case when the analysis included a “group” variable with 4 levels (ie 4 treatment groups, or a “group variable” with 2 levels (ie participants who received propranolol versus participants who received placebo drug, or between participants who received personalized psychologic and participants who received general education.
Influence of Covariates
Females had significantly lower CPM efficiency, lower average pressure pain threshold (PPT) of both ipsilateral/dominant and contralateral/non-dominant deltoid, and lower ipsilateral/dominant tibialis anterior than males (p value <0.05). White participants had lower ratings of suprathreshold heat, higher CPM efficiency, lower TSSP, and longer cold tolerance than non-White participants (p < 0.05).
Exploration of Interactions
Interaction terms (group and sex, group and race, time and sex, time and race) were added to models after consideration of the main effects. These two-way interactions provide additional information for future planning of trials of personalized medicine approaches17,20 and are shown in Table 2. For ipsilateral deltoid PPT, interactions across sex and time (p < 0.001), and race and time (p = 0.015) were noted in which thresholds in Whites became greater (less sensitive) at the shoulder compared to non-Whites, and females became more sensitive to PPT at local and distal testing sites over time compared to men. An interaction between race and group was observed on the ipsilateral deltoid and tibialis anterior. In both these measures, Whites in Group C (propranolol and psychological education) were more sensitive on average than non-Whites in Group B (placebo and general education).
 |
Table 2 Mixed Model ANOVA Table with Interactions |
Interactions between sex and group, and race and group were retained in the expanded models for temporal summation. The responses to the interventions differed such that females in Group C (placebo medication and general education demonstrated greater temporal summation than females in Group B (propranolol and psychological education). Whites in Group C had elevated TSSP compared to Whites in other groups and non-Whites.
No interactions were noted for any cold pressor-related tasks and measures (CPM, cold tolerance, or cold pressor ratings; p > 0.05).
Discussion
The primary findings of this planned secondary analysis of the BISP pre-clinical trial indicated no change in pain sensitivity after receiving the treatment matched to the characteristics of the high-risk subgroup (propranolol and psychologic intervention) when compared to unmatched treatment. These findings suggest no general benefit of matched care for pain sensitivity measures; this aligns with findings from the primary trial and the primary outcome of pain recovery after the exercise induced muscle injury.4 This finding in the secondary analysis was not due to lack of overall responsiveness, as on average, most measures of pain sensitivity changed over time; rather, the change was independent of random group assignment. This is surprising given effects of propranolol on pain sensitivity measures in other studies.14,21 However, the effect on pain sensitivity is not consistent. For example, propranolol administration affected heart rate variability but not TSSP or CPM in healthy people.22
While the analyses did not yield potential benefit for the matched treatment with regards to pain sensitivity, we also wanted to fully explore potential treatment effect modifiers. The rationale for this exploration is that it is plausible that personalized intervention may differ on key sociodemographic factors like age, sex, and racial identity.17,18 Unfortunately, our sample was too homogenous on age to explore that factor, but we did have enough variability to explore potential treatment effect moderation in sex and racial identity. Collectively, exploratory analysis of interactions among racial and gender identities and treatment group showed some differential effects. These effects are the remaining focus of the discussion, but we caution readers to interpret with caution due to a) lack of statistical power and b) the exploratory nature of these analyses.
White participants demonstrated greater reductions in sensitivity at the shoulder than non-White participants with respect to PPT over the study participation duration. For our analysis of racial identity, we used White and non-White groups based on participants’ self report, resulting in approximately 50% of participants in each group. Closer inspection of non-White participants indicated a majority identified as Asian or of Asian heritage. This preliminary finding aligns with work by Ahn et al 2017,23 where people of Asian heritage demonstrated greater pain sensitivity to experimental stimuli than non-Hispanic Whites.
Pain sensitivity also correlates with variation in the COMT gene. For example, people with the AA rs6269 genotype have demonstrated heightened sensitivity to pain in the masseter muscle.24 Moreover, other studies suggest propranolol’s analgesic efficacy is modified by genetic variation in the COMT gene.25 While speculative at this point, it is notable that our sample included 43% of people who identified as Asian and expressed the AA genotype; while Whites in the matched treatment group had higher temporal summation and were more sensitive to PPT than non-Whites in placebo (sham medication and general education) group.
Other findings related to interactions that did not include time are more difficult to interpret. For example, females in the matched treatment group had greater temporal summation than females in the group receiving placebo medication and general education. Overall, the combination of findings suggests consideration of three-way and four-way analyses would be of great interest (ie, sex by group by time); however, this would require much larger samples of participants across the intersection of genetic haplotypes and racial and ethnic and gender identities.
As with all pre-clinical studies, there are limitations to consider. First, the participants who enrolled were predominantly young healthy individuals who did not have the health condition being studied. This limits the external validity of the findings to people. Second, exercise-induced injury creates pain that resolves over time and therefore may not be the best model for chronic pain development. Third, while we did have a placebo arm, we did not include a natural history/control are in this trial so we cannot report directly on QST responses that occur over time with no intervention or perception of intervention. Fourth, the trial was not explicitly powered using pain sensitivity outcomes and therefore may have been underpowered for the secondary analyses. Additionally, analyses that included interaction terms were exploratory and also likely to be underpowered. Finally, given the pre-clinical nature of this study there are no established thresholds for determining how clinically meaningful the observed changes were for any of these measures. Therefore, these findings have low clinical relevance but could be used to inform mechanistic responses to these interventions.
Conclusion
In this secondary analysis of the BISP pre-clinical randomized trial, there was no evidence that treatment matched to a previously validated high-risk subgroup resulted in better pain sensitivity outcomes. These results align with the primary outcomes of the pre-clinical trial. In order to fully explore the potential for personalized approaches we also considered interactions with race and sex for pain sensitivity outcomes. The interactions of sex and race with time, while significant were separate from intervention group effects. These results indicate the potential for differential responses based on these factors but should be interpreted with caution and only used to generate future hypotheses. However, interpreting our hypotheses, that personalized interventions would modulate pain sensitivity, is difficult. Nevertheless, these exploratory findings suggest more complex statistical models may be needed that include race, sex, intervention and time to address personalized interventions.
Data Sharing Statement
A minimal dataset will be hosted at clinicaltrials.gov. A de-identified, analyzable dataset and the code used for analyses will also be provided upon request to the corresponding author. All requests will be reviewed by the study team.
Funding
Research reported in this publication was supported by the National Institutes of Health (NIAMS AR055899 and AR081796) and the University of Florida Clinical and Translational Science Institute (supported in part by the NIH National Center for Advancing Translational Sciences under award number UL1TR001427). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure
Dr Steven Z George reports personal fees from APTA and Rehab Essentials, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Perrot S, Cohen M, Barke A, et al. The IASP classification of chronic pain for ICD-11: chronic secondary musculoskeletal pain. Pain. 2019;160(1):77–82. doi:10.1097/j.pain.0000000000001389
2. George SZ, Wu SS, Wallace MR, et al. Biopsychosocial influence on shoulder pain: influence of genetic and psychological combinations on twelve‐month postoperative pain and disability outcomes. Arthritis Care Res. 2016;68(11):1671–1680. doi:10.1002/acr.22876
3. George SZ, Wallace MR, Wu SS, et al. Biopsychosocial influence on shoulder pain: risk subgroups translated across preclinical and clinical prospective cohorts. Pain. 2015;156(1):148–156. doi:10.1016/j.pain.0000000000000012
4. George SZ, Bishop MD, Wu SS, et al. Biopsychosocial influence on shoulder pain: results from a randomized preclinical trial of exercise-induced muscle injury. Pain. 2023;164(2):305–315. doi:10.1097/j.pain.0000000000002700
5. Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain. 2009;10(6):556–572. doi:10.1016/j.jpain.2009.02.002
6. Backonja M, Attal N, Baron R, et al. Value of quantitative sensory testing in neurological and pain disorders: neuPSIG consensus. Pain. 2013;154(9):1807–1819. doi:10.1016/j.pain.2013.05.047
7. Fontanillas P, Kless A, Bothmer J, Tung JY, 23 and Me Research Team. Genome-wide association study of pain sensitivity assessed by questionnaire and the cold pressor test. Pain. 2022;163(9):1763–1776. doi:10.1097/j.pain.0000000000002568
8. Korczeniewska OA, Kuo F, Huang CY, et al. Genetic variation in catechol-O-methyltransferase is associated with individual differences in conditioned pain modulation in healthy subjects. J Gene Med. 2021;23(11):e3374. doi:10.1002/jgm.3374
9. Cruz-Almeida Y, Fillingim RB. Can quantitative sensory testing move us closer to mechanism-based pain management? Pain Med. 2014;15(1):61–72. doi:10.1111/pme.12230
10. Weissman-Fogel I, Granovsky Y, Crispel Y, et al. Enhanced presurgical pain temporal summation response predicts post-thoracotomy pain intensity during the acute postoperative phase. J Pain. 2009;10(6):628–636. doi:10.1016/j.jpain.2008.12.009
11. George SZ, Staud R, Borsa PA, et al. Biopsychosocial influence on shoulder pain: rationale and protocol for a pre-clinical trial. Contemp Clin Trials. 2017;56:9–17. doi:10.1016/j.cct.2017.03.005
12. George SZ, Childs JD, Teyhen DS, et al. Brief psychosocial education, not core stabilization, reduced incidence of low back pain: results from the Prevention of Low Back Pain in the Military (POLM) cluster randomized trial. BMC Med. 2011;9:128. doi:10.1186/1741-7015-9-128
13. George SZ, Zeppieri G, Cere AL, et al. A randomized trial of behavioral physical therapy interventions for acute and sub-acute low back pain (NCT00373867). Pain. 2008;140(1):145–157. doi:10.1016/j.pain.2008.07.029
14. Tchivileva IE, Lim PF, Smith SB, et al. Effect of catechol-O-methyltransferase polymorphism on response to propranolol therapy in chronic musculoskeletal pain: a randomized, double-blind, placebo-controlled, crossover pilot study. Pharmacogenet Genomics. 2010;20(4):239–248. doi:10.1097/FPC.0b013e328337f9ab
15. Hopewell S, Boutron I, Chan AW, et al. An update to SPIRIT and CONSORT reporting guidelines to enhance transparency in randomized trials. Nat Med. 2022;28(9):1740–1743. doi:10.1038/s41591-022-01989-8
16. Valencia C, Fillingim RB, Bishop M, et al. Investigation of central pain processing in postoperative shoulder pain and disability. Clin J Pain. 2014;30(9):775–786. doi:10.1097/AJP.0000000000000029
17. Nijs J, George SZ, Clauw DJ, et al. Central sensitisation in chronic pain conditions: latest discoveries and their potential for precision medicine. Lancet Rheumatol. 2021;3(5):e383–e392. doi:10.1016/S2665-9913(21)00032-1
18. Campbell CM, Edwards RR. Ethnic differences in pain and pain management. Pain Management. 2012;2(3):219–230. doi:10.2217/pmt.12.7
19. Bretz F, Maurer W, Brannath W, Posch M. A graphical approach to sequentially rejective multiple test procedures. Stat Med. 2009;28(4):586–604. doi:10.1002/sim.3495
20. Edwards RR, Haythornthwaite JA, Tella P, Max MB, Raja S. Basal heat pain thresholds predict opioid analgesia in patients with postherpetic neuralgia. Anesthesiology. 2006;104(6):1243–1248. doi:10.1097/00000542-200606000-00020
21. Schweinhardt P, Abulhasan YB, Koeva V, et al. Effects of intravenous propranolol on heat pain sensitivity in healthy men. Eur J Pain. 2013;17(5):704–713. doi:10.1002/j.1532-2149.2012.00231.x
22. Petersen KK, Andersen HH, Tsukamoto M, Tracy L, Koenig J, Arendt-Nielsen L. The effects of propranolol on heart rate variability and quantitative, mechanistic, pain profiling: a randomized placebo-controlled crossover study. Scand J Pain. 2018;18(3):479–489. doi:10.1515/sjpain-2018-0054
23. Ahn H, Weaver M, Lyon DE, et al. Differences in clinical pain and experimental pain sensitivity between Asian Americans and Whites with knee osteoarthritis. Clin J Pain. 2017;33(2):174–180. doi:10.1097/AJP.0000000000000378
24. Zubieta JK, Heitzeg MM, Smith YR, et al. COMT val 158 met genotype affects µ-opioid neurotransmitter responses to a pain stressor. Science. 2003;299(5610):1240–1243. doi:10.1126/science.1078546
25. Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51(4):593–628.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

