Back to Journals » Vascular Health and Risk Management » Volume 21
Impact of White Blood Cell Count After Percutaneous Coronary Intervention on Long-Term Prognosis in Patients with Unstable Angina Pectoris: A Single-Center Retrospective Observational Cohort Study
Authors Zhang Z, Wang H, Wang R, She Z, Liang X, Liu H, Kou X, Wang S
Received 20 September 2024
Accepted for publication 10 December 2024
Published 16 January 2025 Volume 2025:21 Pages 25—37
DOI https://doi.org/10.2147/VHRM.S492059
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Akash Batta
Zhiyuan Zhang,1– 3,* Heyan Wang,4,* Ruiyu Wang,5 Zeyu She,6 Xingyue Liang,6 Huiyi Liu,7 Xuemeng Kou,1 Shipeng Wang1– 3
1Department of Cardiology, the Second Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang Province, People’s Republic of China; 2Key Laboratory of Myocardial Ischemia, Ministry of Education, Harbin Medical University, Harbin, Heilongjiang Province, People’s Republic of China; 3State Key Laboratory of Frigid Zone Cardiovascular Diseases (SKLFZCD), Harbin Medical University, Harbin, Heilongjiang Province, People’s Republic of China; 4Department of Cardiology, Central Hospital of Dalian University of Technology, Dalian, Liaoning Province, People’s Republic of China; 5Department of Cardiology, the First Affiliated Hospital of Medical School, Xi’an Jiaotong University, Xi’an, Shaanxi Province, People’s Republic of China; 6School of Basic Medicine, Harbin Medical university, Harbin, Heilongjiang Province, People’s Republic of China; 7Department of Cardiology, the First Affiliated Hospital of Peking University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shipeng Wang, Department of Cardiology, 2nd Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang Province, 150086, People’s Republic of China, Tel +86 13654504286, Fax +86 86296225, Email [email protected]
Objective: An association between white blood cell count (WBC-C) before percutaneous coronary intervention (PCI) and prognosis has been established in patients undergoing PCI. However, the effect of WBC-C after PCI on the long-term prognosis of patients with unstable angina pectoris (UA) is unclear.
Methods: A retrospective cohort study was conducted in 1811 consecutive patients with UA. The changes of WBC and subgroup counts before and in the early postoperative stages after PCI were observed by paired Wilcoxon signed-rank test. The Kaplan–Meier method and COX proportional regression model were used to evaluate the association between the incidence of 5-year endpoint events and post-PCI leukocytosis.
Results: Leukocytosis and neutrocytosis within 24 hours after PCI were observed in majority of patients with UA, while lymphocyte count significantly decreased after PCI in those patients. There were no significant differences in 5-year all-cause mortality and major adverse cardiovascular and cerebrovascular events (MACCE) between patients in the post-PCI leukocytosis and the control group. However, the 5-year incidence of major adverse cardiovascular events (MACE) was significantly increased in the post-PCI leukocytosis group (p = 0.017, Log rank test). Leukocytosis after PCI was independently associated with the occurrence of MACE (hazard ratio: 1.36; 95% confidence interval: 1.06– 1.75; p = 0.015).
Conclusion: Peripheral WBC and neutrophil counts within 24 hours after PCI significantly increased in response to PCI in patients with UA, while lymphocyte count significantly decreased after PCI in those patients. The post-PCI leukocytosis offered predictive value for an increased risk of MACE for up to 5 years in patients with UA.
Keywords: white blood cell count, percutaneous coronary intervention, unstable angina pectoris, leukocytosis, major adverse cardiovascular events
Introduction
Percutaneous coronary intervention (PCI) is the standard treatment for unstable angina (UA) patients with severe coronary stenosis. Despite the widespread availability of drug-eluting stents, there is a high risk of cardiovascular events in patients with UA. ISCHEMIA (International Study of Comparative Health Effective-ness with Medical and Invasive Approaches) studies had shown that an initial invasive strategy did not reduce the risk of ischemic cardiovascular events or death from any cause over a median of 3.2 years.1 Therefore, early warning of patients with a high risk of MACE (major adverse cardiovascular events) after PCI is important, and it is a challenging and difficult topic at present.
It is generally accepted that coronary heart disease is a multifactorial inflammatory disease. Thus, the search of biologic markers, especially potent proinflammatory biomarkers, is very important for clinical risk assessment and prognosis of patients with UA.2 As the major immune system cells, white blood cells whose count increases in response to the presence of inflammation promote the pathogenesis of coronary heart disease and ultimately lead to ischemia.3 Monocyte and neutrophil are crucial for contributing to ruptures or erosions of atherosclerotic plaque by promoting foam cell formation. The progression of plaque leads to fatal events such as myocardial infarction and stroke, which together account for 85% of all cardiovascular deaths.4 In addition, different leukocyte populations play important roles in ischemic heart failure healing and remodeling.5 Several previous studies have shown that white blood cell count (WBC-C) was an independent risk factor for coronary heart disease.6,7 An independent relationship between WBC-C and in-hospital death was found in a study consisting of 1078 consecutive patients with ST-segment elevation myocardial infarction (STEMI) admitted for primary PCI.8 Elevated leukocyte count after PCI is also associated with adverse clinical outcomes in patients with STEMI. Stanley et al found that leukocytosis after primary PCI in patients with STEMI was directly related to myocardial infarct size and the left ventricular ejection fraction (LVEF) and was independent predictors of 180-day composite cardiac events.9 In addition to patients with STEMI, an independent correlate between elevated leukocyte count before PCI and 1-year mortality has been established in those with non-STEMI and UA.10 PCI, a mechanical way resulting in intima damage and plaque rupture in coronary artery, is related to the provocation of inflammatory response. However, whether increased leukocyte count after PCI directly reflects adverse clinical outcomes in patients with UA is not known.
Therefore, we aimed to use a large-sample dataset in the real world to evaluate the correlation between the post-PCI leukocytosis and long-term all-cause death in patients with UA. We further examined the association of the post-PCI leukocytosis with long-term major adverse cardiovascular and cerebrovascular events (MACCE) and MACE in patients with UA. In addition, we observed the changes of WBC and subgroup counts before and in the early postoperative stages after PCI and evaluated the factors that predispose to post-PCI leukocytosis in patients with UA.
Patients and Methods
Study Patients
This study was a retrospective, single-centre, observational cohort study. From March 2018 to October 2018, 3189 patients undergoing elective PCI were screened in the Second Affiliated Hospital of Harbin Medical University (Figure 1). According to the inclusion and exclusion criteria, 1168 patients were excluded, 108 patients had incomplete data, 102 patients were lost to follow-up, and a total of 1811 consecutive patients of either sex were finally enrolled. Before PCI, all patients without sufficient dual antiplatelet therapy were given 300 mg loading dose followed by 100 mg maintain dose of aspirin, and 180 mg loading dose followed by 90 mg twice daily maintain dose of ticagrelor or 300 mg loading dose followed by 75 mg maintain dose of clopidogrel. The study was performed in accordance with the Declaration of Helsinki. The study protocol was approved by Institutional Review Board of the Second Affiliated Hospital of Harbin Medical University (approval number: KY2024-010). All participants provided written informed consent.
 |
Figure 1 Patient flow chart for the study cohort. |
Diagnostic Criteria and PCI
The inclusion criteria were: (1) patients with UA treated with elective PCI; (2) patients undergoing elective PCI after acute myocardial infarction (AMI) for more than three months; (3) patients over 18 years old. Criteria for the degree of coronary stenosis treated by PCI for coronary angiography must meet at least one of the following two conditions: (1) the degree of left main stenosis is ≥50%; (2) the degree of left anterior descending artery or circumflex artery or right coronary artery stenosis is ≥75%. Patients with diagnosed myopathy or suspected myopathy supported by clinical examination (creatine phosphokinase >2 times the upper limit of normal reference value), atrial fibrillation and/or pacing rhythm and/or Wolff-Parkinson-White syndrome, severe aortic stenosis or mitral stenosis, hypertrophic obstructive cardiomyopathy, pericardial disease, LVEF <30%, severe renal insufficiency (glomerular filtration rate <30 mL/min), acute infection (such as cholecystitis, liver abscess, lung infection, skin infection, etc), or other serious systemic diseases (hematological diseases, malignant tumors, severe primary liver and kidney disease and chronic lung disease), or WBC-C greater than 10 × 109/L at admission were excluded.
Coronary angiography and PCI were performed according to standard practices. After PCI, dual anti-platelet therapy (DAPT) with aspirin (100 mg/day) and ticagrelor (90 mg, twice per day) or clopidogrel (75 mg/day) was continued. It was within the discretion of the treating physician to prescribe other drugs (comprising predominantly statins, angiotensin-converting enzyme inhibitors, betablockers or calcium channel blockers).
Clinical Variables
Medical history and clinical examination data, including diabetes, arterial hypertension, hypercholesterolemia, current smoking, cardiac function (NYHA (New York Heart Association) classification), chest radiography or lung CT diagnostic results and preoperative laboratory test results (cholesterol, triglyceride, CRP (C-reactive protein), liver function, renal function, myocardial enzyme and cTnI (cardiac troponin I)), were collected. Angiographic criteria of the Thrombolysis in Myocardial Infarction (TIMI) study group were used to classification of epicardial blood flow pre-PCI and post-PCI. Left ventricular ejection fraction and end-diastolic dimension were measured by echocardiography. The Chronic Kidney Disease Epidemiology Collaboration formula was used to calculate estimated glomerular filtration rate (eGFR).
Measurement of WBC-C
Blood samples were collected from patients at admission and within 4 to 20 hours after PCI. Tubes collecting blood samples contained anticoagulant. Total leukocyte counts were measured with an automated hematology analyzer (XE5000, Sysmex, Kobe, Japan). The same procedure was used for measurement in the experiment and in the control group.
Definitions and Endpoints
According to the recent fourth universal definition of myocardial infarction,11 periprocedural myocardial infarction (PMI) referred to an increase of the cTnI threshold to five times the 99th percentile upper reference limit in patients with UA. The outcome measure was all-cause death, MACE and cerebrovascular events (MACCE) within 5 years post-PCI. MACE were defined as cardiac death, hospitalization due to attacks of cardiac arrest resuscitation, heart failure and angina pectoris, myocardial infarction, stent thrombosis, and coronary revascularization (including coronary intervention and coronary artery bypass grafting). MACCE were defined as all-cause mortality, hospitalization due to attacks of cardiac arrest resuscitation, heart failure and angina pectoris, myocardial infarction, stent thrombosis, coronary revascularization (including coronary intervention and coronary artery bypass grafting) and stroke. Cardiac death referred to death caused by heart disease. Coronary revascularization was an unplanned repeat PCI or coronary artery bypass graft performed due to symptoms of coronary ischemia.
Follow-Up
Inpatient electronic medical records and telephone interview were used to follow up at 6 months, 1 year, 2 years, and 5 years after discharge. If there were clinical symptoms or records of myocardial ischemia, it was recommended that the patient should return for coronary angiography. Patients were observed from the date of PCI until the outcome event (all-cause death, MACCE, or MACE) or loss to follow-up occurred, whichever came first. All endpoint events and time to event were adjudicated centrally by two independent cardiologists.
Statistical Analysis
Statistical analysis was performed in R language (version R 4.3.1, R Foundation). The Kolmogorov–Smirnov test was used to test whether the distribution of continuous data followed a normal distribution. All continuous data exhibited a non-Gaussian distribution pattern under the Kolmogorov–Smirnov test. For paired continuous variables, Wilcoxon signed-rank test was used. The Kruskal–Wallis rank sum test was used for group comparisons. Categorical data were compared by using the chi-square test or Fisher‘s exact test. The changes of peripheral WBC-C before and after PCI were observed by scatter plot, paired box plot and cumulative plot. The correlates of leukocytosis after PCI were assessed by using the multiple logistic regression model. All variables in Tables 1 and 2, except for therapy at discharge, were entered into the model. Survival curves were evaluated by Kaplan–Meier method and the log-rank method was used for comparison. We used COX proportional hazards regression model to analyze the relationship between WBC-C in early stage after PCI and MACE in patients with UA, and the corresponding hazard ratio (HR) and 95% confidence interval (CI) were calculated. All P-values in this study were two-tailed. Comparisons between groups were considered statistically significant when P < 0.05.
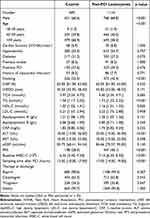 |
Table 1 Baseline Characteristics of Study Population |
 |
Table 2 Procedural Data of Study Population |
Results
Patient Classification and Baseline Data
Using WBC-C level (10 × 109/L) within 24 hours after PCI as the cutoff, 1811 patients were divided into the control group with post-PCI WBC-C level within normal limits (≤10 × 109/L) and the post-PCI leukocytosis group with elevated post-PCI WBC-C level (>10 × 109/L). Table 1 shows baseline data, and procedural characteristics are displayed in Table 2. In the post-PCI leukocytosis group, males and middle-aged adults accounted for a higher proportion, the proportion of the elderly was less, and a higher proportion of patients had a history of smoking. Triglyceride, alanine aminotransferase, and pre-PCI WBC-C levels were lower in the control group. Compared with the post-PCI leukocytosis group, patients in the control group had higher high-density cholesterol levels, fewer PMI, and shorter time from blood sampling to intervention. Patients in the post-PCI leukocytosis group had longer total length of predilated and postdilated balloons and stents, more total times of predilation and postdilation, greater maximum pressure of postdilated balloons, and more number of predilated and postdilated balloons and stents. Multivessel coronary disease was less frequent in the control group compared with the post-PCI leukocytosis group.
Correlates of Post-Procedural WBC-C
WBC-C (Figure 2A) and neutrophil count (Figure 2B) within 24 hours after PCI significantly increased compared with that before PCI; however, lymphocyte count (Figure 2C) significantly decreased after PCI and the range of monocyte count (Figure 2D) after PCI was more variable than that before PCI. The post-PCI patients had higher median counts of leukocyte (6.80 [5.90, 8.00] × 109/L versus 11.00 [8.70, 13.60] ×109 /L) (Figure 2E) and neutrophil (4.30 [3.55, 5.20] × 109/L versus 9.25 [7.12, 11.75] × 109 /L) (Figure 2F). Compared to those in baseline groups, there were lower median counts of lymphocyte (1.91 [1.54, 2.33] ×109/L versus 1.37 [1.06, 1.75] ×109 /L) (Figure 2G) and monocyte (0.36 [0.28, 0.45] × 109/L versus 0.33 [0.15, 0.50] × 109 /L) (Figure 2H) in post-PCI groups. About 61.6% of patients presented leukocytosis (WBC-C > 10 × 109/L) within 24 hours after PCI (Figure 2I) while neutrocytosis (neutrophil count > 7.0 × 109/L) was observed in 76.1% patients (Figure 2J). The cumulative distribution curve of lymphocytes (Figure 2K) after PCI showed a left shift, while the cumulative distribution curves of monocytes (Figure 2L) before and after PCI showed a cross-over. This data suggests that increased WBC recruitment occurs in response to PCI in UA.
The logistic regression model was used to assess independent correlates of leukocytosis after PCI in patients with UA. Male gender, smoking history, triglyceride, sampling time after PCI, baseline WBC-C, C-reactive protein, total times of post-dilation, PMI and multivessel disease were independently associated with elevated WBC levels after PCI (Table 3).
 |
Table 3 Independent Correlates of Post-PCI White Blood Cell Count Obtained from the Multiple Logistic Regression Analysis |
Post-Procedural WBC-C and the 5-Year Follow-Up
Overall, there were 92 deaths during the follow-up period, 54 patients (4.8%) died in the post-PCI leukocytosis group and 38 patients (5.5%) died in the control group. As shown in Figure 3A, there was no significant difference in all-cause death between patients in the post-PCI leukocytosis group and the control group, p = 0.46 (Log rank test). The 5-year incidence rate of MACCE after PCI was 23% in the post-PCI leukocytosis group and 20.4% in the control group. Although the incidence rate of MACCE of the post-PCI leukocytosis group was higher than that of the control group, there is no statistically significant difference between the two groups in Figure 3B (p = 0.29, Log rank test). However, the 5-year incidence of MACE was significantly increased in the post-intervention leukocytosis group compared with the control group (Figure 3C). The 5-year incidence rate of MACE was 18.5% in the post-PCI leukocytosis group and 13.7% in the control group. The incidence rate of MACE of the post-PCI leukocytosis group was higher than that of the control group (p = 0.017, Log rank test).
Cox Regression Analysis of MACE Events
For MACE in patients, we first performed univariate Cox regression analysis, and we included variables associated with clinical basis and coronary intervention data in univariate Cox regression analysis. In univariate analysis, postoperative leukocytosis, hypertension, diabetes, history of stroke, history of PCI, LVEF, low-density cholesterol-C, apolipoprotein B, C-reactive protein, stent restenosis, left main disease, total length and number of pre-dilated balloon, maximal pressure of pre-dilation, total time and times of pre-dilation, maximum diameter and total number of stent, total times of stent dilatation, maximum diameter and total length of post-dilated balloon, PMI, multivessel disease, and interventional therapy of multivessel were associated with MACE risk (Table 4). Thus, more risk factors for CAD, elevated inflammatory levels before and after PCI, increased CAD severity and/or procedure complexity predict elevated long-term MACE after PCI.
 |
Table 4 Results of Univariable Cox Proportional Hazards Model Applied to Assess Predictors of Mace with Post-PCI Leukocytosis Entered into the Model |
Subsequently, all of the above variables were included in the multivariate Cox regression analysis (Table 5). With multivariate adjustment, there was a higher MACE risk in patients with post-PCI leukocytosis (adjusted hazard ratio (adjHR: 1.34; 95% CI: 1.05-1.72; p = 0.019). In addition, the MACE risk in patients with UA was significantly associated with a history of stroke (adjHR: 1.88; 95% CI: 1.35-2.63, p <0.001), apolipoprotein B (adjHR: 1.57; 95% CI: 1.19-2.07; p = 0.002), total number of pre-dilated balloon (adjHR: 1.20; 95% CI: 1.02-1.42; p = 0.033), left main disease (adjHR: 1.59; 95% CI: 1.07-2.35; p = 0.021), and maximum diameter of stent (adjHR: 0.76; 95% CI: 0.64-0.90; p = 0.002).
 |
Table 5 Results of Multivariable Cox Proportional Hazards Model Applied to Assess Predictors of Mace with Post-PCI Leukocytosis Entered into the Model |
Discussion
Our study is the largest analysis to date of the relationship between leukocyte count after coronary intervention and long-term prognosis in patients with UA. Our main findings in this study can be summarized as follows: 1) leukocyte and neutrophil count within 24 hours after PCI in majority of patients with UA was significantly increased compared with those before PCI, while lymphocyte count significantly decreased after PCI in those patients; 2) post-PCI leukocytosis was not associated with an increased risk of all-cause death and MACCE within 5 years in patients with UA; 3) post-PCI leukocytosis was independently associated with an increased risk of MACE within 5 years in patients with UA.
The increased circulating leukocytes after PCI are the result of the combination of a pre-procedural fraction and a released fraction of leukocytes from reservoir organs. Because patients with the threshold for WBC-C above 10 ×109/L were excluded in our study, leukocytosis after surgery may be induced by inflammation related to PCI. Increasing evidence suggests that coronary intervention can damage coronary blood vessels and/or myocardium, and subsequently provoke an inflammatory response and the recruitment of blood leukocytes into the damaged area. To the best of our knowledge, our study is the first report of circulating leukocytosis and neutrocytosis within 24 hours after PCI in patients with UA. Consistent with our study, previous clinical studies have shown that some WBC fraction counts in peripheral blood of patients with coronary heart disease increased within a few days after PCI.12,13 However, Daiju et al found that patients who underwent PCI for one day showed no significant increase in total WBC-C.12 Therefore, it is worth noting that post-PCI leukocytosis in patients with UA is an acute reaction within the definite scope of time and this effect is transient.
Previous studies documented that the cTnI-elevated coronary artery disease (CAD) patients14 had higher baseline leukocyte value and infiltration of leukocyte was correlated with the development of necrotic and apoptotic cell death from 6 to 24 hours post-reperfusion after the onset of AMI.15 The similar association between myocardial necrosis and WBC-C was confirmed in our study, we found that PMI was independently associated with elevated WBC-C from 6 to 24 hours after PCI. Consistent with our study, the association between smoking and elevated white blood cells has been reported in patients undergoing primary PCI.16 In addition, our study demonstrated that a higher level of coronary inflammation, multivessel lesions and increased procedural complexity were associated with post-PCI leukocytosis. All of factors responsible for WBC-C elevation after PCI help to explain the increased MACE risk in patients with UA. Our study indicated that patients with post-PCI leukocytosis had higher baseline number of leukocytes compared to the control group. It is important to note that baseline WBC-C was strong related to future development of cardiovascular disease and risk of myocardial infarction at one year in the Framingham study and the stent PAMI (primary angioplasty in myocardial infarction) trial, respectively.6,17 Increasing evidence revealed that increased number and aggregation of leukocytes in coronary artery may hinder microcirculatory flow, damage endothelial cells and cause chronic inflammation accounting for vulnerable plaque progression, ultimately resulting in serious cardiovascular events in patients with CAD.18,19
Numerous clinical studies demonstrated that increase in the pre-procedural leukocyte count was associated with greater myocardial necrosis area,20 worse cardiac function and prognosis in patients with acute myocardial infarction.21–23 An elevation of the WBC-C in acute coronary syndromes (ACS) was also a predictor of a higher risk of acute clinical ischemic events.24,25 Moreover, accumulating data indicated that leukocytes were predictive of mortality in patients with stable CAD, ACS and AMI.10,26–28 A recent meta-analysis including 62904 participants reported that a higher WBC count was a significant predictor of long-term all-cause mortality (HR 1.09, 95% CI 1.07 to 1.12) and long-term cardiovascular mortality (HR 1.05, 95% CI 1.02 to 1.07).29 The associations between 5-year changes in leukocyte counts with incident cardiovascular events have been investigated.30 The results indicated that participants in the increased group had 14% higher risk for cardiovascular disease than those in the stable group. In addition to the increase in the pre-procedural leukocyte counts, the elevation of leukocyte counts after primary PCI in patients with acute myocardial infarctions was significantly associated with myocardial infarct size and an independent predictor of cardiovascular events.9 In line with the above studies, our study revealed that elevation of leukocyte counts in the early stage after PCI had a predictive value for long-term MACE events in patients with UA. In addition, the present study extends the role of WBC-C in predicting the prognosis of ACS patients to the entire perioperative period. Previous clinical studies documented that elevated baseline WBC-C was an independent predictive factor for long-term mortality in patients undergoing primary PCI with acute myocardial infarction.31,32 However, our study did not find significant association between post-PCI leukocytosis and all-cause death in UA, which was inconsistent with previous findings. This suggests that post-PCI elevation of WBC-C in patients with UA is more suitable for predicting the risk of coronary ischemia events than in those with AMI.
Our results provide a better knowledge of the inflammatory processes during PCI and suggest a new therapeutic target. The potential application of this well recognized and readily available inflammatory marker is beneficial for high-risk patients undergoing PCI who should receive particular attentive monitoring of the cardiovascular risk factors by the treating physician. Future, larger cohort studies are required to determine whether anti-inflammatory strategies should be recommended during elective PCI for any patient with UA.
The limitations of our study require consideration of the following aspects. First, our study is an analysis of retrospective cohort data and is thus susceptible to the limitations inherent in such studies. Second, malignant arrhythmias and peripheral vascular disease were not included in our composite endpoint of observation, and future studies should seek to explore the full spectrum of cardiovascular outcomes in such studies. Furthermore, due to database restrictions, serial testing of WBC-C levels after PCI was not performed, resulting in the possibility that the precise peak value of post-procedural WBC-C may have been missed in a number of patients.
Conclusions
Peripheral WBC and neutrophil counts within 24 hours after PCI significantly increased in response to PCI in patients with UA, while lymphocyte count significantly decreased after PCI in those patients. Although the post-PCI leukocytosis was not associated with mortality and MACCE, leukocytosis after PCI offered predictive value for an increased risk of MACE for up to 5 years in patients with UA. We expect this study to provide a practical basis for stratified management of high-risk groups of patients after PCI and bring more aggressive management at an earlier stage in those patients.
Acknowledgments
We are very grateful to Dr Zhuozhong Wang for our study assistance.
Funding
This research was supported by grants from Heilongjiang Province Postdoctoral Science Fund (LBH-Z18212) and Innovation Fund for Scientific Research Led by Young and Middle-aged People in the Second Affiliated Hospital of Harbin Medical University (KYCX2019-17).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382:1395–1407. doi:10.1056/NEJMoa1915922
2. Khuseyinova N, Koenig W. Biomarkers of outcome from cardiovascular disease. Curr Opin Crit Care. 2006;12:412–419. doi:10.1097/01.ccx.0000244119.16377.75
3. Hoffman M, Blum A, Baruch R, Kaplan E, Benjamin M. Leukocytes and coronary heart disease. Atherosclerosis. 2004;172:1–6. doi:10.1016/S0021-9150(03)00164-3
4. World Health Organization. Cardiovascular diseases (CVDs) fact sheet. 2017. Available from: http://www.who.int/en/news-room/fact-sheets/detail/ cardiovascular-diseases-(cvds).
5. Poller WC, Nahrendorf M, Swirski FK. Hematopoiesis and cardiovascular disease. Circ Res. 2020;126:1061–1085. doi:10.1161/CIRCRESAHA.120.315895
6. Kannel WB, Anderson K, Wilson PWF. White blood cell count and cardiovascular disease - Insights from the Framingham Study. J Am Med Assoc. 1992;267:1253–1256. doi:10.1001/jama.1992.03480090101035
7. Gillum RF, Ingram DD, Makuc DM. White blood cell count, coronary heart disease, and death: the NHANES I epidemiologic follow-up study. Am Heart J. 1993;125:855–863. doi:10.1016/0002-8703(93)90181-8
8. Kruk M, Przyluski J, Kalinczuk L, et al. Association of non-specific inflammatory activation with early mortality in patients with ST-elevation acute coronary syndrome treated with primary angioplasty. Circ J. 2008;72:205–211. doi:10.1253/circj.72.205
9. Chia S, Nagurney JT, Brown DFM, et al. Association of leukocyte and neutrophil counts with infarct size, left ventricular function and outcomes after percutaneous coronary intervention for ST-elevation myocardial infarction. Am J Cardiol. 2009;103:333–337. doi:10.1016/j.amjcard.2008.09.085
10. Ndrepepa G, Braun S, Iijima R, et al. Total leucocyte count, but not C-reactive protein, predicts 1-year mortality in patients with acute coronary syndromes treated with percutaneous coronary intervention. Clin Sci. 2009;116:651–658. doi:10.1042/CS20080298
11. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. J Am Coll Cardiol. 2018;72:2231–2264. doi:10.1016/j.jacc.2018.08.1038
12. Fukuda D, Shimada K, Tanaka A, Kawarabayashi T, Yoshiyama M, Yoshikawa J. Circulating monocytes and in-stent neointima after coronary stent implantation. J Am Coll Cardiol. 2004;43:18–23. doi:10.1016/j.jacc.2003.08.026
13. Inoue T, Sata M, Hikichi Y, et al. Mobilization of CD34-positive bone marrow-derived cells after coronary stent implantation - impact on restenosis. Circulation. 2007;115:553–561. doi:10.1161/CIRCULATIONAHA.106.621714
14. Chen S, Zhang SL, Luan HX, Zeng XL, Li YZ, Yuan H. Correlation between extended leukocyte differential count and coronary artery disease. J Cardiovasc Pharmacol. 2018;71:359–366. doi:10.1097/FJC.0000000000000582
15. Zhao ZQ, Nakamura M, Wang NP, et al. Reperfusion induces myocardial apoptotic cell death. Cardiovasc Res. 2000;45:651–660. doi:10.1016/S0008-6363(99)00354-5
16. Prasad A, Stone GW, Stuckey TD, et al. Relation between leucocyte count, myonecrosis, myocardial perfusion, and outcomes following primary angioplasty. Am J Cardiol. 2007;99:1067–1071. doi:10.1016/j.amjcard.2006.11.063
17. Pellizzon GG, Dixon SR, Stone GW, et al. Relation of admission white blood cell count to long-term outcomes after primary coronary angioplasty for acute myocardial infarction (The Stent PAMI Trial). Am J Cardiol. 2003;91:729–731. doi:10.1016/S0002-9149(02)03416-1
18. Koenig W, Rosenson RS. Acute-phase reactants and coronary heart disease. Semin Vasc Med. 2002;2:417–428. doi:10.1055/s-2002-36770
19. Ono M, Tomaniak M, Koenig W, et al. Impact of white blood cell count on clinical outcomes in patients treated with aspirin-free ticagrelor monotherapy after percutaneous coronary intervention: insights from the GLOBAL LEADERS trial. Eur Heart J-Cardiovasc Pharmacother. 2022;8:39–47. doi:10.1093/ehjcvp/pvaa110
20. Kirtane AJ, Bui A, Murphy SA, Barron HV, Gibson CM. Association of peripheral neutrophilia with adverse angiographic outcomes in ST-elevation myocardial infarction. Am J Cardiol. 2004;93:532–536. doi:10.1016/j.amjcard.2003.11.013
21. Barron HV, Cannon CP, Murphy SA, Braunwald E, Gibson CM. Association between white blood cell count, epicardial blood flow, myocardial perfusion, and clinical outcomes in the setting of acute myocardial infarction - A thrombolysis in myocardial infarction 10 substudy. Circulation. 2000;102:2329–2334. doi:10.1161/01.CIR.102.19.2329
22. Yan XN, Jin JL, Zhang M, et al. Differential leukocyte counts and cardiovascular mortality in very old patients with acute myocardial infarction: a Chinese cohort study. BMC Cardiovasc Disord. 2020;20:12. doi:10.1186/s12872-020-01743-3
23. Núñez JE, Núñez E, Bertomeu V, et al. Prognostic value of baseline white blood cell count in patients with acute myocardial infarction and ST segment elevation. Heart. 2005;91:1094–1095. doi:10.1136/hrt.2004.043174
24. Friedman GD, Klatsky AL, Siegelaub AB. The leukocyte count as a predictor of myocardial infarction. New Engl J Med. 1974;290:1275–1278. doi:10.1056/NEJM197406062902302
25. Ernst E, Hammerschmidt DE, Bagge U, Matrai A, Dormandy JA. Leukocytes and the risk of ischemic diseases. J Am Med Assoc. 1987;257:2318–2324. doi:10.1001/jama.1987.03390170074031
26. Sabatine MS, Morrow DA, Cannon CP, et al. Relationship between baseline white blood cell count and degree of coronary artery disease and mortality in patients with acute coronary syndromes - A TACTICS-TIMI 18 substudy. J Am Coll Cardiol. 2002;40:1761–1768. doi:10.1016/S0735-1097(02)02484-1
27. Cannon CP, McCabe CH, Wilcox RG, Bentley JH, Braunwald E, Investigators O-T. Association of white blood cell count with increased mortality in acute myocardial infarction and unstable angina pectoris. Am J Cardiol. 2001;87:636–639. doi:10.1016/S0002-9149(00)01444-2
28. Mueller C, Neumann FJ, Perruchoud AP, Buettner HJ. White blood cell count and long term mortality after non-ST elevation acute coronary syndrome treated with very early revascularisation. Heart. 2003;89:389–392. doi:10.1136/heart.89.4.389
29. Park C, Yoo K, Lee S, et al. The prognostic significance of leukocyte count on all-cause and cardiovascular disease mortality: a systematic review and meta-analysis. Am J Cardiol. 2023;203:226–233. doi:10.1016/j.amjcard.2023.06.119
30. Wang Q, Guo Q, Zhou L, et al. Associations of baseline and changes in leukocyte counts with incident cardiovascular events: the Dongfeng-Tongji cohort study. J Atheroscler Thromb. 2022;29:1040–1058. doi:10.5551/jat.62970
31. Palmerini T, Mehran R, Dangas G, et al. Impact of leukocyte count on mortality and bleeding in patients with myocardial infarction undergoing primary percutaneous coronary interventions analysis from the harmonizing outcome with revascularization and stent in acute myocardial infarction trial. Circulation. 2011;123:2829–2837. doi:10.1161/CIRCULATIONAHA.110.985564
32. Gurm HS, Bhatt DL, Lincoff AM, et al. Impact of preprocedural white blood cell count on long term mortality after percutaneous coronary intervention: insights from the EPIC, EPILOG, and EPISTENT trials. Heart. 2003;89:1200–1204. doi:10.1136/heart.89.10.1200
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Relation of Red Cell Distribution Width to Glucose Metabolism and Adverse Long-Term Prognosis in Patients with Acute Coronary Syndrome
Xiong K, Xu C, Shou X, Dong M
Diabetes, Metabolic Syndrome and Obesity 2023, 16:61-70
Published Date: 11 January 2023
Association of Systemic Inflammatory Response Index and Pan-Immune-Inflammation-Value with Long-Term Adverse Cardiovascular Events in ST-Segment Elevation Myocardial Infarction Patients After Primary Percutaneous Coronary Intervention
Liu Y, Liu J, Liu L, Cao S, Jin T, Chen L, Wu G, Zong G
Journal of Inflammation Research 2023, 16:3437-3454
Published Date: 14 August 2023
Nomogram to Predict Outcomes After Staged Revascularization in ST-Segment Elevation Myocardial Infarction and Multivessel Coronary Artery Disease
Wang H, Ma A, Wang T
International Journal of General Medicine 2024, 17:1713-1722
Published Date: 29 April 2024
The Predictive Value of the Systemic Immune-Inflammation Index for Cardiovascular Events in Chronic Total Occlusion Patients Who Prior Coronary Artery Bypass Grafting
Zhao Y, Zhao S, Shi Y, Ma Q, Zheng Z, Wang P, Liu J
Journal of Inflammation Research 2024, 17:8611-8623
Published Date: 9 November 2024



