Back to Journals » Research and Reports in Tropical Medicine » Volume 15
Implementation of Mass Drug Administration for Lymphatic Filariasis in Madagascar: The Progress, Effectiveness and Financial Savings of Integrating into an Existing Polio Campaign
Authors Mandrosovololona V, Rasoamihanta P, Djawe K, Mupfasoni D, Andriamino B, Rakotonavalona R , Bakajika D, Ratsimbasoa AC, Kirigia J , Musango L
Received 16 July 2024
Accepted for publication 12 December 2024
Published 27 December 2024 Volume 2024:15 Pages 123—147
DOI https://doi.org/10.2147/RRTM.S487163
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mario A. Rodríguez-Pérez
Vatsiharizandry Mandrosovololona,1 Patricia Rasoamihanta,1 Kpandja Djawe,1 Denise Mupfasoni,2 Brusa Andriamino,3 Rivomalala Rakotonavalona,3 Didier Bakajika,4 Arsène Claude Ratsimbasoa,5 Joses Kirigia,6 Laurent Musango1
1Madagascar Country Office, World Health Organization, Antananarivo, Madagascar; 2Department of Control of Neglected Tropical Diseases, World Health Organization, Geneva, Switzerland; 3Ministry of Public Health, Antananarivo, Madagascar; 4Expanded Special Project for Elimination of NTD (ESPEN), World Health Organization Regional Office for Africa, Brazzaville, Congo; 5Faculty of Medicine, University De Fianarantsoa, Antananarivo, Madagascar; 6African Sustainable Development Research Consortium (ASDRC), Nairobi, Kenya
Correspondence: Laurent Musango, WHO Country Office, P.O. Box 362, Antananarivo, 101, Madagascar, Email [email protected]
Introduction: This paper presents (a) the progress made towards achieving the 2023 Lymphatic Filariasis (LF) Mass Drug Administration (MDA) campaign goals, (b) the estimated financial savings resulting from integrating LF MDA into Polio immunization campaigns, and (c) the best practices, challenges, and recommendations.
Methods: In 2023, 21,336,057 people in 83 districts were affected by LF and required Preventive Chemotherapy (PC). The National NTD Control Programme (NTDCP) conducted three phases of LF MDA campaigns in those districts. In the first phase, 24 districts received triple therapy of Ivermectin, Diethylcarbamazine, and Albendazole (IDA), while the remaining 59 districts continued to receive dual therapy of Diethylcarbamazine and Albendazole (DA) as before. The first phase (15 districts) was not integrated, while the second phase (61 districts) was conducted simultaneously with the Polio Supplementary Immunization Activities (SIA) fourth round. The third phase (7 districts) was combined with periodic intensification of routine immunization (PIRI) and vitamin A supplementation.
Results: In Phases 2 and 3, the campaign covered 99.97% of the targeted 12,208 villages, meaning only three villages remained untreated. In contrast, Phase 1 covered all the targeted 2,847 villages, attaining 100% geographic coverage. The 68 districts (Phase 2 and 3) that implemented an integrated approach attained an average therapeutic coverage of 76.6% (STDEV=8.3) compared to 73.2% (STDEV=6.7) among the 15 districts (Phase 1) that conducted MDA for LF without integration. The p-values for geographical and therapeutic coverage were below the significance level of 0.05, leading to the conclusion that the average geographic and therapeutic coverages for districts implementing LF MDA with and without integration into Polio immunization campaigns differed significantly. Integrating the LF MDA campaign into the Polio SIA and PIRI campaigns saved US$1,431,203.
Conclusion: Incorporating LF MDA into polio immunization campaigns can improve financial efficiency and effectiveness in meeting the objectives of LF programs.
Keywords: cost effectiveness, integration, mass drug administration, financial savings
Introduction
Madagascar is the largest island country in Africa, covering an area of 587,041 square kilometers and an estimated population of 29,914,679 in 2023.1,2 It is a low-income country with a gross national income of only US$510 per capita in 2022.3 The country faces multidimensional poverty, with a Multidimensional Poverty Index (MPI) of 0.364. Around 65.7% of the population (about 20.4 million people in 2024) live in multidimensional poverty.4 It means that they are experiencing poverty in various MPI indicators, including 26.0% in nutrition, 5.5% in child mortality, 47.7% in years of schooling, 25.3% in school attendance, 65.5% in cooking fuel, 62.1% in sanitation, 51.3% in drinking water, 56.6% in electricity, 57.2% in housing, and 48.3% in assets. Neglected Tropical Diseases (NTD) significantly contribute to multidimensional poverty.5
Currently, 20 diseases and conditions are classified as NTD by the World Health Organization (WHO). The NTD are grouped into seven categories, which include helminth infections (such as lymphatic filariasis [LF], dracunculiasis, echinococcosis, foodborne trematodiases, onchocerciasis, schistosomiasis, soil-transmitted helminthiases, taeniasis and cysticercosis); protozoan infections (Chagas disease, human African trypanosomiasis, leishmaniasis); bacterial infections (Buruli ulcer, leprosy, trachoma, yaws); fungal infections (mycetoma, chromoblastomycosis and other deep mycoses); viral infections (dengue and chikungunya, rabies); ectoparasitic infections (scabies and other ectoparasitoses); and non-infectious diseases/conditions (snakebite envenoming).6 As per Hotez et al,7 NTDs are severe and debilitating infections that have a devastating impact on impoverished households and communities.
In 2019, Madagascar lost 360 lives to NTD, which accounted for 0.2% of all deaths (164,161) in the country. Schistosomiasis was responsible for the highest number of NTD deaths (251 or 69.7%), followed by rabies (55 or 15.2%), cysticercosis (38 or 10.6%), intestinal nematode infections (6 or 1.7%), cystic echinococcosis (2 or 0.6%), dengue (0 or 0%), and other NTD (8 or 2.2%).8 Chagas disease, leishmaniasis, and African trypanosomiasis did not cause any deaths in Madagascar that year.
NTDs also caused premature mortality and non-fatal disability, resulting in a loss of 75,007 DALY, which constituted 0.7% of all the 10,725,782 DALY lost in Madagascar.8 The age distribution of NTD DALY was as follows: 8.3% were borne by those under five years old, 5–14-year-olds bore 24.1%, 15–59-year-olds bore 60.8%, and 60-year-olds and above bore 6.9%. Out of the 9,721 DALY caused by LF, those under five bore 0%, 5–14-year-olds bore 25.4%, 15–59-year-olds bore 68.7%, and the 60-year-olds and above bore 5.9%.
Intestinal nematode infections caused the highest number of DALY losses (22,854 or 30.5%), followed by schistosomiasis (19,260 or 25.7%) and LF (9,721 or 13.0%). Other diseases that caused DALY losses include cysticercosis (7,205 or 9.6%), rabies (3,502 or 4.7%), dengue (1,040 or 1.4%), leprosy (321 or 0.4%), cystic echinococcosis (89 or 0.1%), and other NTD (11,103 or 14.6%). Interestingly, despite causing no deaths, LF was the third largest cause of DALY losses.
LF is a disease that can cause abnormal enlargement of body parts, leading to pain, severe physical disability, social stigma, and mental health challenges.9 Along with the distressing symptoms, LF can also result in significant direct costs associated with diagnosis and treatment and productivity losses.10,11 Therefore, effective treatment and management of the disease is essential to minimize its impact on individuals and society.
The WHO has set global targets to be achieved by 2030 for combating NTD.6 These targets include reducing the number of people requiring interventions against NTD by 90%, reducing DALY related to NTD by 75%, eliminating at least one NTD, and eradicating at least two NTDs. Additionally, the integrated approaches for combating NTD have two cross-cutting targets for 2030: achieving a 75% integrated treatment coverage index for preventive chemotherapy and adopting and implementing integrated skin NTD strategies.
Eliminating LF can help prevent suffering and loss of DALY and contribute to achieving the United Nations Sustainable Development Goals (SDG) 1 and 3.12 SDG 1 focuses on reducing poverty, while SDG 3 aims to ensure healthy lives and well-being for all people of all ages. The spread of LF infection can be stopped through preventive chemotherapy, which involves administering an annual dose of medicines to the at-risk population. The WHO recommends mass drug administration (MDA) as the best strategy for LF elimination.6
Annual rounds of Preventive Chemotherapy (PC) with a single co-administered dose of Diethylcarbamazine and Albendazole regime (DA) against LF have been administered since 2005 to people of all ages, except for children under 2 years old and pregnant women in endemic districts of Madagascar, as reported by Garchitorena et al13 and WHO.14 Recent research in Cote D’Ivoire,15 Haiti,16 India,17 Indonesia,18 and Papua New Guinea19 has demonstrated that a triple therapy (a single co-administered dose) of Ivermectin, Diethylcarbamazine and Albendazole regime (IDA) was well-tolerated (safe) and more effective than DA in achieving sustained clearance of microfilaria (Mf) of the filarial nematode (Wuchereria bancrofti) from human blood and stopping the spread of infection.
A review conducted by Budge et al,20 covering studies published between 1985 and 2017 that reported adverse events following the treatment of LF with Ivermectin, Diethylcarbamazine, Albendazole, or any combination of these medications, revealed that although mild to moderate systemic adverse events related to the death of microfilariae are common following treatment, they are temporary and rarely severe. The evidence mentioned above has been verified by the WHO for use by national programs to speed up the progress in eliminating the LF disease.6 Madagascar decided to adopt the triple therapy (IDA) strategy in 2023.
In 2023, Madagascar developed a master plan (2023–2027) to address NTD.21 To execute the plan, the national NTDCP regularly carries out MDA campaigns to help eliminate LF by 2030. In 2023, the national NTDCP conducted three phases of LF MDA campaigns (1st in May, 2nd in October, and 3rd in December). The 2nd phase was combined with the third round of Polio Supplementary Immunization Activities (SIA), and the 3rd phase was combined with Periodic Intensification of Routine Immunization (PIRI) and vitamin A supplementation.22 The three phases of LF MDA differed from previous campaigns in that 24 districts received IDA distribution, while 59 other districts continued to receive DA distribution as before (refer to Table 1 for details).
 |
Table 1 Distribution of Target Districts in Madagascar by Phase and Regimen |
The LF MDA campaigns in 2023 had specific targets, which were: (a) to achieve a Therapeutic Coverage Rate (TCT) of over 65% in 59 districts treated with the combined DA regime, (b) to achieve a TCT of over 80% in 24 districts treated with the IDA regime, and (c) to identify all cases of morbidity related to LF and treat all individuals over 2 years of age in the LF-targeted district.22
The WHO Road Map for NTD calls for strengthening links between NTD programs and national health information systems and for better coordination among specialized programs such as those for polio and malaria vector control.6
The implementation study includes the following information: (a) the progress made in achieving the 2023 LF MDA campaign objectives, (b) the approximate financial savings resulting from integrating LF MDA into Polio immunization campaigns, and (c) a discussion of best practices, lessons learned, challenges faced during the campaign, and recommendations for the implementation of future MDA campaigns.
Methods
Study Area and Population
Madagascar has 23 regions, 114 districts, and 1,549 communes.23 Since 2005, preventive chemotherapy (PC) against LF has been ongoing in Madagascar. As of 2023, the total population was 21,336,057, residing in 83 endemic districts, requiring PC against LF. This population was at risk and constituted the total target population in 2023.
This report provides information on the three phases of LF MDA campaigns in these 83 LF-target districts, carried out by the national NTDCP in 2023, focusing on the second and third phases integrated with immunization activities.22
Study Design
This study was designed as an observational cross-sectional study in May-December 2023.24 Its primary goal was to provide a snapshot of the implementation of integrated LF MDA in target districts and to evaluate its effect on therapeutic coverage and financial efficiency. The study aimed to shed light on the implementation strategy but did not explore cause and effect. It assesses the current state of the implementation of the intervention rather than being designed and implemented for research purposes. As a result, it does not follow standard epidemiological research designs.
Implementation Strategies
Pre-Campaign Activities
The pre-campaign started with developing a delivery plan to make the necessary inputs and management tools available in the targeted districts. Before the LF MDA campaigns, activities included assessing input needs, procuring inputs (including medicines), packaging, providing management tools and measuring rods, and strengthening stakeholders’ capacity, including training personnel.22
MDA Campaign
The distribution for the three phases combined took place in 23 Regions encompassing 83 districts, 2,111 basic health centers (BHC), and 15,055 villages.14,22 A total of 30,110 community distributors (DCs) were mobilized and charged with responsibility for distributing inputs among the 83 districts.
Phase 1: No Integration
In May 2023, the first phase entailed the distribution and MDA of DA in 15 districts without integration with other programs.22 Notably, there were no official headquarters (HQs) or WHO teams to coordinate the activities during this phase. Instead, the national Lymphatic Filariasis Elimination Program (LFEP) technical team provided the coordination.
Phase 2: Integration of LF MDA with Polio SIA
In October 2023, during phase two, the Ministry of Health (MOH) combined LF MDA with the fourth round of Polio SIA.22 This phase covered 61 districts, 38 receiving DA and 23 receiving IDA regimens. In this phase, LF MDA and polio vaccinations were delivered to the district level using the same RCCE (Risk Communication and Community Engagement) awareness-raising campaigns, coordination, distribution, logistics/transport, infection prevention and control approach (including waste management), training facilities, supervision (by all levels of the health system, including Expanded Program on Immunization and Polio eradication teams), and monitoring and evaluation mechanisms. Additionally, an HQ was set up and integrated with the polio emergency operations center to enable quick and synchronized data collection and an effective response to public health threats.
Phase 3: Integration of LF MDA with PIRI and Distribution of Vitamin A
In the third phase, which was implemented in December 2023, LF MDA was integrated with PIRI and the distribution of vitamin A.22 This phase covered 7 districts, with 6 receiving DA and 1 receiving IDA. Like in phase two, the delivery of both LF MDA and polio vaccination inputs to the district level was done in an integrated manner. It involved utilizing the same RCCE (Risk Communication and Community Engagement) for awareness-raising campaigns, coordination, distribution, logistics/transport, training, and supervision by all levels of the health system, including Expanded Program on Immunization and Polio eradication teams, infection prevention and control approach including waste management, and monitoring and evaluation mechanism. As in the second phase, the headquarters were set up during the third phase in an integrated manner with the polio emergency operations center, providing a central location to coordinate quick and synchronized data collection and effective response to a public health threat.
Drug Administration at the Community Level
The LF MDA at the community level was accomplished using a door-to-door distribution strategy or approach. The 30,110 community distributors (CD)/agents (CA) administered and observed the consumption of medication and recorded the treatment data in the scorecard.22 The scorecard was relayed to the BHC Chief for collation and transmission to the district level.
CD used a spoon to administer medications and was also equipped with disinfectants. Generally, distribution lasted 4 to 5 days with minimal variations depending on the district. Additionally, mop-up sessions were conducted for those who had not achieved the goals.
Post Campaign Activities
Review meetings were held at district, regional, and central levels to gather supporting documents and allow teams to reflect on the factors that helped or hindered the MDA implementation process.22 The best practices, lessons learned, and recommendations identified during these meetings enhance the preparation and implementation of future MDA campaigns.
Estimating Financial Savings of Integrating LF MDA into Polio Immunization Campaigns
The financial savings of incorporating LF MDA into polio immunization campaigns were estimated from the perspective of the Ministry of Health. As the manufacturers donated the LF drugs used in the MDA campaigns, the MOH did not spend any money on them. The study focused only on the expenses associated with coordination (supervision), logistics, travel and allowances, communication, training of trainers, training of BHC community supervisors and mobilizers, and training of Community Health Workers (CHW).21,22 For each of these cost items, the financial savings per item  was the difference between potential expenditure without integration
was the difference between potential expenditure without integration  and actual expenditure with integration
and actual expenditure with integration  . Algebraically:
. Algebraically:
The total financial cost savings due to the integration of LF MDA into the polio immunization campaign’s  , equals the sum of financial expenditure savings across the seven cost items
, equals the sum of financial expenditure savings across the seven cost items  . In algebraic form:
. In algebraic form:
Data and Sources
The data on the number of deaths and DALY by cause (cited in the Introduction) were from the Global Burden of Disease Collaborative Network.8 The data on number of villages and population per LF endemic district; the number of individuals who took DA and IDA medication; lymphoedema cases and hydrocele cases; and expenditures (on communication, coordination/supervision, logistics, training CHW, training BHC, training of trainers, travel and allowances) was obtained from the national LFEP.
Data Analysis
The Excel Software developed by Microsoft was used to estimate the LF MDA geographical coverage and PC coverage. Also, the same software was used to estimate Equations 1 and 2.
Hypothesis Testing
In this section, we aim to determine if there is a statistically significant difference between (a) the average geographic coverage of LF MDA with and without integration into Polio immunization campaigns; (b) the average therapeutic coverage of LF MDA with and without integration into Polio immunization campaigns; and (c) the average number of LF morbidity cases reported with and without integration into Polio immunization campaigns. Therefore, we tested the following three sets of hypotheses at a 5% level of significance (95% confidence level):
Geographic Coverage
Null hypothesis (H0): Average geographic coverage of LF MDA with  and without
and without  integration into Polio immunization campaigns is the same.
integration into Polio immunization campaigns is the same.
Alternative hypothesis (H1): Average geographic coverage of LF MDA with  and without
and without  integration into Polio immunization campaigns is different.
integration into Polio immunization campaigns is different.
The parameters used to test this hypothesis are in Table 2 below.
 |
Table 2 Test of Hypothesis Related to Geographic Coverage of Villages in Madagascar with LF MDA |
Therapeutic Coverage
Null hypothesis (H0): Average therapeutic coverage of LF MDA with  and without
and without  integration into Polio immunization campaigns is the same.
integration into Polio immunization campaigns is the same.
Alternative hypothesis (H1): Average therapeutic coverage of LF MDA with  and without
and without 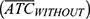 integration into Polio immunization campaigns is different.
integration into Polio immunization campaigns is different.
The parameters used to test this hypothesis are in Table 3 below.
 |
Table 3 Test of Hypothesis Related to Therapeutic Coverage of Target Population in Madagascar with LF MDA |
Morbidity Cases Detected
Null hypothesis (H0): Average number of LF cases detected with  and without
and without  integration of LF MDA into Polio immunization campaigns is the same.
integration of LF MDA into Polio immunization campaigns is the same.
Alternative hypothesis (H1): Average number of LF cases detected with  and without
and without  integration of LF MDA into Polio immunization campaigns is different.
integration of LF MDA into Polio immunization campaigns is different.
The parameters used to test this hypothesis are in Table 4 below.
 |
Table 4 Test of Hypothesis Related to LF Morbidity (Cases) Detected per District in Madagascar with and without LF MDA |
The two samples (ie, with and without integration) were assumed to be independent and unequal. The specific level of significance (α):  . The decision rule based on
. The decision rule based on  approach is:
approach is:  and
and  .
.
We tested the above hypotheses employing the online “365Datascience difference of means calculator” available at: https://365datascience.com/calculators/difference-in-means-calculator/.
Ethical Clearance
The Ministry of Public Health Biomedical Research Ethics Committee of the Republic of Madagascar has approved the study referenced in the uploaded letter (Reference No. 85 MSANP/SG/AMM/CNPV/CERBM). The study was carried out per scientific standards, procedures, and regulations and complies with ethical standards. The Biomedical Research Committee approved the production of this scientific article. The committee noted that the study relied solely on analyzing existing secondary data recorded by LF program implementers in a way that ensures the subjects cannot be identified. The data was obtained from the Madagascar National Lymphatic Filariasis Elimination Program as part of the integration of these two campaigns.
Results
Geographic Coverage
Overall Geographic Coverage
In 2023, a report on the geographic coverage of LF treatments administered in 83 target districts indicated that out of the 15,055 villages located in these districts, 15,052 (99.98%) were treated. All villages were treated in 82 out of the 83 districts (98.8%) (Figure 1a and Figure 1b). However, treatment did not occur in 3 out of 142 villages in one district.
Figure 1 Continued. Figure 1 Geographic coverage of LF treatments administered in 2023 reported among 83 target districts.

Geographic Coverage with Integration
In October and December 2023, the integration targeted 12,208 villages spread across 68 districts (Figure 2). Out of the targeted villages, 12,205 (99.98%) were treated. However, treatment did not occur in 3 out of 142 villages in one district. Thus, the average geographic coverage among the 68 districts with integration was 99.97% and a standard deviation of 0.243.
 |
Figure 2 Reported geographical coverage of LF MDA with integration among 68 districts of Madagascar. |
Therapeutic Coverage
Overall Therapeutic Coverage
Figure 3a and Figure 3b illustrates the therapeutic coverage of LF treatments administered in 2023 among 83 target districts. In 83 target endemic districts, 16,130,212 (75.60%) out of 21,336,057 people targeted in 15,055 villages were treated with either DA or IDA.
Figure 3 Continued. Figure 3 Therapeutic coverage LF treatments administered in 2023 as reported among 83 target districts.

Therapeutic Coverage with Integration
In October 2023 (phase 2) and December 2023 (phase 3), the average therapeutic coverage of LF MDA with integration was at 76.6% with a standard deviation of 8.3% among 68 districts of Madagascar (Figure 4). Of the 17,322,503 people targeted in the integrated districts, 13,322,871 people (76.9%) received either DA or IDA treatment.
 |
Figure 4 Therapeutic Coverage of LF MDA with Integration among 68 districts of Madagascar. |
Coverage of Combined DA Regimen in 59 Districts
The 2023 geographic and DA therapy coverages can be found in Supplementary Table 1. Of the 11,339 villages targeted for DA therapy, 11,336 (99.97%) received the treatment. However, three villages did not receive treatment. In the 59 districts that were targeted for DA therapy, out of 16,479,815 people residing in 11,339 villages, 12,268,975 people (74.45%) were treated.
The average therapeutic coverage among the 59 districts that received DA was 73.89%. According to the records, 55 districts had a therapeutic coverage rate of over 65%, while four districts had therapeutic coverage below 65%, as shown in Figure 5.
 |
Figure 5 DA therapy coverage across 59 target Madagascar districts in 2023. |
Coverage of Combined IDA Regimen in 24 Districts
The data presented in Supplementary Table 2 shows that 24 districts underwent IDA treatment. Every one of the 3,716 villages (100%) in these districts received the same treatment. The villages were home to a combined population of 4,856,242, among whom 3,861,237 received the IDA therapy, resulting in an overall IDA therapeutic coverage rate of 79.51%. Of the 24 districts, 10 achieved an IDA therapeutic coverage rate of over 80%, which makes up 41.67% of all the districts (see Figure 6). Regrettably, the remaining 14 districts did not reach the campaign target of achieving an IDA therapeutic coverage rate of over 80%.
 |
Figure 6 IDA therapy coverage across 24 target districts of Madagascar in 2023. |
Results of Geographical and Therapeutic Coverage by Phase
Phase 1 Results
The information provided relates to the outcomes of Phase 1 of LF MDA conducted in May 2023 across 15 districts in Madagascar. During this phase, the targets were 2,847 villages with a population of 4,013,554 people. All villages attained 100% geographic coverage, indicating that they all received LF MDA.
During the treatment using the DA regimen, 2,902,937 people were treated. The average therapeutic coverage was 72.3% (standard deviation = 6.7), with a median of 72.1%. As shown in Figure 7, the coverage ranged from a high of 88% in Analalava to a low of 64% in Mandritsara district. Specifically, 13.3% of the districts achieved therapeutic coverage (TC) of 80% and above, 46.7% achieved TC between 70 and 79%, and 40% achieved TC between 60 and 69%.
 |
Figure 7 Phase 1 - Therapeutic Coverage of LF MDA without Integration in May 2023 among 15 districts of Madagascar. |
Phase 2 Results
In Phase 2, 10,762 villages with a population of 13,896,892 people were scheduled for LF MDA. The average geographic coverage was 99.97%, and all the targeted villages in 60 districts were reached, except for the district of Vondrozo (Atsimo Atsinanana region), where MDA did not occur in three out of the 142 villages.
Regarding therapy, 10,694,174 people received treatment, with an average therapeutic coverage of 77.0% (standard deviation = 8.6) and a median of 76.5%. Therapeutic coverage varied, with a maximum of 125.5% in Betioky Atsimo and a minimum of 59.1% in Tsaratanana Nord Et Sud, as shown in Figure 8.
 |
Figure 8 Phase 2 - Therapeutic Coverage of LF MDA with Integration in October 2023 among 61 districts of Madagascar. |
Out of 61 districts in Phase 2, 19 (31.1%) had therapeutic coverage of 80% and above. 34 (55.7%) had coverage between 70% and 79%. Seven (11.5%) had coverage between 60% and 69%, and one (1.6%) had coverage under 60%.
Phase 3 Results
Phase 3 aimed to provide LF preventive chemotherapy to 1,446 villages in seven districts across Analamanga, Itasy, and Bongolava administrative regions in December 2023. The treatment successfully covered all the target villages, achieving a geographical coverage of 100%. Six of the seven districts (Ambohidratrimo, Arivonimamo, Fenoarivobe, Miarinarivo, Soavinandriana, and Tsiromandidy) received DA therapy, while Tana Nord district received an IDA regimen.
The total population of the seven target districts was 3,425,677. Of this total, 2,628,697 people received IDA or DA combined therapy. The average therapeutic coverage was 76.7% (STDEV= 4.9), with a median of 76.0%. Figure 9 shows that therapeutic coverage varied from 82.6% in Soavinandriana to a minimum of 70.3% in Fenoarivobe. Two districts had coverage above 80%, four had coverage between 71% and 78%, and one district had a coverage of 70%.
 |
Figure 9 LF MDA therapeutic coverage (%) across the phase 3 seven districts of Madagascar in December 2023. |
Cases of Morbidity Linked to LF
Without Integration
In 2023, the 15 endemic districts of Madagascar that were not integrated reported 2,540 LF cases (Supplementary Table 3). Among these cases, 42.8% were lymphoedema cases, and 57.2% were hydrocele cases (see Figure 10). The average number of cases per district was 169, with a standard deviation of 140.4 and a median of 145. The number of cases varied from 0 in Mananara Avaratra and Soanierana-Ivongo to a maximum of 396 in Mandritsara. Five districts (Analalava, Antsohihy, Befandriana Avaratra, Mandritsara, and Vohemar) accounted for 64.2% of the LF cases. Nine of the 15 non-integrated districts recorded 100 cases or more, while the remaining six recorded fewer than 100 cases.
 |
Figure 10 Number of LF cases recorded in 2023 in the 15 endemic districts of Madagascar without integration. |
With Integration
In 2023, the 68 LF-endemic districts of Madagascar reported a total of 18,085 cases of LF (Figure 11). Among these cases, 51.5% were lymphedema cases, and 48.5% were hydrocele cases. The average number of LF cases per district was 266, with a standard deviation of 464.2 and a median of 47.5 cases per district. The number of cases varied from 0 in five districts (Ambohidratrimo, Arivonimamo, Betsoka, Fenerive Est [Fenoarivo-Atsinanana], Tana Nord [Antananarivo-Renivohitra], Tsiroanomanoidy) to 2,078 in one district (Ikongo). Among the 68 districts, 6 recorded 1000 or more LF cases, 6 recorded 500–999 cases, 15 recorded 100–499 cases, and 41 recorded fewer than 100 cases.
 |
Figure 11 Number of LF cases recorded in 2023 in the 68 endemic districts of Madagascar with integration. |
Hypotheses Test Results
Geographic Coverage
The extremely low p-value of 4.6613E-42 is well below the standard significance level of 0.05. Consequently, we reject the null hypothesis (H0) as the p-value exceeds the significance level. Based on this finding, we conclude that the average geographic coverage for districts implementing LF MDA with and without integration into Polio immunization campaigns differs significantly.
Therapeutic Coverage
The p-value is 0. Since this value is less than the specified significance level  of 0.05, we reject the null hypothesis. This finding supports the alternative hypothesis, indicating that the average therapeutic coverage for the districts that implemented LF MDA with integration was statistically different from that for districts that did not integrate LF MDA into Polio immunization campaigns.
of 0.05, we reject the null hypothesis. This finding supports the alternative hypothesis, indicating that the average therapeutic coverage for the districts that implemented LF MDA with integration was statistically different from that for districts that did not integrate LF MDA into Polio immunization campaigns.
Morbidity Case Detection
The p-value is 0.1529. Since the p-value is greater than the specified significance level  of 0.05, we cannot reject the null hypothesis (H0). In other words, based on the reported data and chosen significance level, there was not enough evidence to suggest that the average number of LF cases detected with integration differed from the average number of LF cases detected without integrating LF MDA into Polio immunization campaigns.
of 0.05, we cannot reject the null hypothesis (H0). In other words, based on the reported data and chosen significance level, there was not enough evidence to suggest that the average number of LF cases detected with integration differed from the average number of LF cases detected without integrating LF MDA into Polio immunization campaigns.
Financial Savings of Integrating LF MDA into Polio Immunization Campaigns(s)
In Table 5, you can find the total expenditure with integration, estimated expenditure without integration, and estimated financial savings for 68 districts that integrated LF MDA into polio immunization campaigns. The total expenditure with integration was $424,719, the estimated expenditure without integration was $1,855,922, and the estimated financial savings were $1,431,203. The result means the financial savings per person in the population of the 68 districts is $0.083. The distribution of estimated total financial savings were as follows: 40.7% in travel and allowances, 25.1% in the training of CHW, 10.2% in communication, 8.1% in coordination/supervision, 7.2% in the training of trainers, 4.9% in CSB training, and 3.8% in logistics.
 |
Table 5 Actual Total Expenditure with Integration, Estimated Expenditure Without Integration, and Financial Savings in Madagascar |
Discussion
Key Findings
There were three main findings from the study:
- In Phases 2 and 3 (October and December 2023), 12,208 villages with a population of 17,322,503 people were targeted in 68 districts that implemented an integrated LF MDA approach. The achieved geographic coverage was 99.98%. In contrast, Phase 1 (May 2023) covered 15 districts implementing LF MDA without integration, targeting 2,847 villages with a population of 4,013,554 people, and attained 100% geographic coverage, meaning all the target villages in Phase 1 received LF MDA. The p-value was below the significance level of 0.05, leading to the conclusion that the average geographic coverage for districts implementing LF MDA with and without integration into Polio immunization campaigns differed significantly.
- In the 68 districts that implemented an integrated approach, the average therapeutic coverage of LF MDA was 76.6% (STDEV=8.3), with a median of 76.5%. On the other hand, the 15 districts that conducted MDA for LF without integration achieved an average therapeutic coverage of 73.2% (STDEV=6.7) and a median of 72.1%. The result showed that the p-value was less than the specified significance level (α) of 0.05, indicating that the average therapeutic coverage for the districts that implemented LF MDA with integration was statistically different from the average therapeutic coverage for the districts that did not integrate LF MDA into Polio immunization campaigns.
- The 68 districts that implemented an integrated approach detected 18,085 LF cases (STDEV=464.2), with an average of 266 cases and a median of 47.5 per district. The 15 districts that conducted MDA for LF without integration reported 2,540 LF cases (STDEV=140.4), with an average of 169 cases and a median of 145 cases per district. Based on the reported data and chosen significance level, there was not enough evidence to suggest that the average number of LF cases detected with integration differed from the average number of LF cases detected without integrating LF MDA into Polio immunization campaigns.
- Finally, the estimated financial cost of implementing the MDA campaign without integration was US$1,855,922, while the actual financial cost of integrating the MDA for the LF and Polio campaign was US$424,719. The estimated financial savings from integration was US$1,431,203.
Best Practices
Financial savings were realized through sharing coordination, supervision, logistics, travel allowances, training and communication materials between the LF MDA and polio campaigns.22
Coordination
Coordination and supervision were enhanced using WhatsApp groups shared between the LF MDA and polio campaign teams. It facilitated information exchange and helped implement the previously developed micro-plan, which included a timeline for all activities. Having a single headquarters for monitoring and evaluating activities and reporting and using the same supervision teams at the regional health offices and districts, along with integrated training for polio campaign vaccinators and drug distributors for NTDs, increased effectiveness and financial efficiency.
Technical Aspects
The same tools were used for polio and LF campaigns. The Open Data Kit (ODK) was utilized,25 and daily debriefing and assessments were integrated to identify side effects and complications such as elephantiasis, hydrocele for LF, and Acute Flaccid Paralysis for poliomyelitis. Even though the Geospatial Tracking System (GTS) was not used, sketches of the intervention areas and itineraries were identified and drawn up for both campaigns. Mop-up plans were also developed for areas needing to increase vaccination coverage for polio, therapeutic coverage for LF, and geographic coverage for both campaigns.
Logistics
In terms of logistics, the same vehicles transported vaccines and LF drugs, time markers, and other management tools required for both campaigns. Sharing of transport helped to reduce transport costs substantially.
Communication
The authorities effectively communicated by launching campaigns to convey messages. Social mobilization was successful with the participation of local authorities such as village chiefs, religious leaders, school directors, and traditional practitioners. They all worked together to raise awareness among the population. This communication and social mobilization significantly increased awareness of the two campaigns and fostered trust between the population and health professionals. As a result, there was a high demand for free (donated) Lymphatic Filariasis drugs among the population.
Partnerships
Regarding partnerships, both the technical and financial partners, especially those involved in the Global Polio Eradication Initiative (GPEI), played a significant role from the planning phase to implementation, including supervision, evaluation, and data analysis. The Government of Madagascar also greatly valued and appreciated the financial support provided by GPEI partners.
Lessons Learnt
Five vital lessons ought to inform the planning of future integrated MDA campaigns. First, effective communication is crucial for encouraging beneficiaries to accept and participate in LF MDA strategies. Second, involving political, administrative, and religious authorities, village chiefs, and community leaders helps raise awareness, reduce participation refusals, and optimize treatment coverage. Third, good coordination at all levels during the campaign implementation increases the chances of success for integrated MDA strategies. Fourth, integrating campaigns can enhance effectiveness (achieving coverage objectives) and efficiency (minimizing costs such as health workforce, community time, transport, logistics, coordination, and other health system resources).
Finally, integration has positive side effects (spillovers).22 Joint preparatory and follow-up meetings between the NTD and immunization teams, joint training, and joint field supervision foster collaboration within the Ministry of Health, contribute to the capacity strengthening of the technical teams and promote experience sharing.
Implementation Challenges
The intervention encountered a few notable challenges.22 First, there were some communication-related hiccups. For instance, there were no visual communication aids such as posters and banners; there was a late start of the implementation of communication activities around the campaign; there was low mobilization of local resources in the districts/regions during the campaign; and there was delay in payment of arrears for the 2021 campaign demotivating stakeholders.
Second, there was a delay in starting the campaign in certain districts due to late delivery of medicines and inputs (ie, logistics and input management issues). Third, due to the insufficient number of MDA teams’ treatment did not occur in three villages of the Vondrozo district (ie, implementation of the MDA issue). In a few places, some people refused to take medication right away.
Fourth, data feedback-related problems included delayed transmission of administrative data due to the instability of the internet connection and lack of telephone airtime credit, which hampered BHC access; incomplete data; and a dearth of data analysis capacities at BHC/districts.
Limitations of the Study
The current study has a few limitations that need to be acknowledged. Firstly, the study was not designed and conducted when the integrated LF MDA was being developed and implemented. Secondly, the scope of the study was limited to estimating geographic LF MDA coverage, PC coverage, and financial savings anticipated from integrating LF MDA into polio vaccination campaigns. It did not estimate the effect of LF MDA on morbidity, specifically in clearing the LF parasites. Thirdly, the estimation of financial costs with and without integration was done from the perspective of the Ministry of Health. Therefore, the study did not consider the potential impact of LF MDA on direct and indirect costs incurred by individuals and communities.
Conclusions
The study successfully demonstrated that incorporating LF MDA into polio immunization campaigns can make the efforts more effective and financially efficient. These findings could encourage other LF-endemic countries to adopt this approach and enhance their disease control efforts.
Additionally, the study emphasizes the importance of collaboration between NTDCP and routine immunization programs to improve the delivery of preventive chemotherapy for NTD. The lack of step-by-step guidance for this collaboration constitutes an opportunity for NTD-endemic countries and partners to innovate and design implementation studies throughout the entire cycle of designing, planning, implementing, monitoring, and evaluating integrated disease interventions.
This study offers valuable insights and practical lessons for policymakers and public health practitioners to optimize disease control efforts and improve health outcomes for communities at risk of NTD.
Abbreviations
CA, Community agent; CD: Community distributor; CHW, Community Health Worker; CSB, Basic Health Centre; DA, Diethylcarbamazine and Albendazole; DALY, Disability-adjusted life years; GPEI, Global Polio Eradication Initiative; IDA, Ivermectin, Diethylcarbamazine and Albendazole; LF, Lymphatic Filariasis; MDA, Mass Drug Administration; MPI, Multidimensional Poverty Index; NTD, Neglected Tropical Diseases; NTDCP, Neglected Tropical Disease Control Program; Polio, Poliomyelitis; PC, Preventive Chemotherapy; LFEP, Lymphatic Filariasis Elimination Program; SDGs, United Nations Sustainable Development Goals; SIA, Supplementary Immunization Activities; TCT, Therapeutic Coverage Rate; US$, United States Dollar; WHO, World Health Organization.
Data Sharing Statement
The paper and its supporting supplementary tables include all relevant data analyzed.
Ethical
The study complies with the Declaration of Helsinki.
Funding
The World Health Organization funded the study.
Disclosure
Prof. Laurent Musango, Dr. Vatsiharizandry Mandrosovololona, Dr. Patricia Rasoamihanta, Dr. Kpandja Djawe, and Dr. Denise Mupfasoni are WHO employees. However, the Organization did not influence the study’s findings. The authors report no other potential conflicts of interest in this work.
References
1. WorldAtlas.com. Island countries of the world. Available from: http://www.worldatlas.com/articles/which-are-The-island-countries-of-The-world.html.
2. Madagascar Institut National de la Statistique (INSTAT). Troisième Recensement Général de la Population et de l’habitation (RGPH-3). Antananarivo: INSTAT; 2023.
3. The World Bank. Data. GNI per capita, Atlas method (current US$) – Madagascar. Available from: https://data.worldbank.org/indicator/NY.GNP.PCAP.CD?locations=MG.
4. United Nations Development Programme (UNDP) and Oxford Poverty and Human Development Initiative (OPHDI). Global Multidimensional Poverty Index 2023. Unstacking global poverty: data for high-impact action. New York: UNDP & OPHDI; 2023.
5. Magalhães AR, Codeço CT, Svenning JC, et al. Neglected tropical diseases risk correlates with poverty and early ecosystem destruction. Infect Dis Poverty. 2023;18:32. doi:10.1186/s40249-023-01084-1
6. World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals a Road Map for Neglected Tropical Diseases 2021–2030. Geneva: World Health Organization; 2021.
7. Hotez PJ, Aksoy S, Brindley PJ, Kamhawi S. What constitutes a neglected tropical disease? PLoS Negl Trop Dis. 2020;14(1):e0008001. doi:10.1371/journal.pntd.0008001
8. Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results. Seattle, United States: Institute for Health Metrics and Evaluation (IHME); 2020. Available from https://vizhub.healthdata.org/gbd-results/.
9. World Health Organization. Lymphatic filariasis. Available from: https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis.
10. Kirigia JM, Mburugu GN. The monetary value of human lives lost due to neglected tropical diseases in Africa. Infect Dis Poverty. 2017;6:165. doi:10.1186/s40249-017-0379-y
11. Kastner RJ, Sicuri E, Stone CM, Matwale G, Onapa A, Tediosi F. How much will it cost to eradicate lymphatic filariasis? An analysis of the financial and economic costs of intensified efforts against lymphatic filariasis. PLoS Negl Trop Dis. 2017;11(9):e0005934. doi:10.1371/journal.pntd.0005934
12. UN United Nations (UN). Transforming our world: the 2030 agenda for sustainable development. General assembly resolution A/RES/70/1. New York: UN; 2015.
13. Garchitorena A, Raza-Fanomezanjanahary EM, Mioramalala SA, et al. Towards elimination of lymphatic filariasis in southeastern Madagascar: successes and challenges for interrupting transmission. PLoS Negl Trop Dis. 2018;12(9):e0006780. doi:10.1371/journal.pntd.0006780
14. World Health Organization. Global health observatory. lymphatic filariasis (Elephantiasis). Available from: https://www.who.int/data/gho/data/themes/topics/lymphatic-filariasis.
15. Edi C, Bjerum CM, Ouattara AF, et al. Pharmacokinetics, safety, and efficacy of a single co-administered dose of diethylcarbamazine, albendazole and ivermectin in adults with and without Wuchereria bancrofti infection in Cote d’Ivoire. PLoS Negl Trop Dis. 2019;13(5):e0007325. doi:10.1371/journal.pntd.0007325
16. Dubray CL, Sircar AD, Beau de Rochars VM, et al. Safety and efficacy of co-administered diethylcarbamazine, albendazole and ivermectin during mass drug administration for lymphatic filariasis in Haiti: results from a two-armed, open label, cluster-randomized, community study. PLoS Negl Trop Dis. 2020;14(6):e0008298. doi:10.1371/journal.pntd.0008298
17. Jambulingam P, Kuttiatt VS, Krishnamoorthy K, et al. An open label, block randomized, community study of the safety and efficacy of co-administered ivermectin, diethylcarbamazine plus albendazole vs. diethylcarbamazine plus albendazole for lymphatic filariasis in India. PLoS Negl Trop Dis. 2021;15(2):e0009069. doi:10.1371/journal.pntd.0009069
18. Supali T, Djuardi Y, Christian M, et al. An open label, randomized clinical trial to compare the tolerability and efficacy of ivermectin plus diethylcarbamazine and albendazole vs. diethylcarbamazine plus albendazole for treatment of brugian filariasis in Indonesia. PLoS Negl Trop Dis. 2021;15(3):e0009294. doi:10.1371/journal.pntd.0009294
19. Tavul L, Laman M, Howard C, et al. Safety and efficacy of mass drug administration with a single-dose triple-drug regimen of albendazole + diethylcarbamazine + ivermectin for lymphatic filariasis in Papua New Guinea: an open-label, cluster-randomized trial. PLoS Negl Trop Dis. 2022;16(2):e0010096. doi:10.1371/journal.pntd.0010096
20. Budge PJ, Herbert C, Andersen BJ, Weil GJ. Adverse events following single dose treatment of lymphatic filariasis: observations from a review of the literature. PLoS Negl Trop Dis. 2018;12(5):e0006454. doi:10.1371/journal.pntd.0006454
21. Ministere De La Santé Publique. Plan Directeur De Lutte Contre Les Maladies Tropicales Négligées 2023-2027. Antananarivo: Programme National De Lutte Contre Les Maladies Tropicales Négligées; 2023.
22. World Health Organization. Rapport Technique Des Campagnes De Distribution De Masse De Medicaments Contre La Filariose Limphatique 2023 Madagascar. Antananarivo: World Health Organization; 2023.
23. Republic of Madagascar. Madagascar National Report to the UN-Habitat III Conference, May 2015. Antananarivo: State Ministry of Presidential Projects, Country Planning and Equipment; 2015.
24. Hwang S, Birken SA, Melvin CL, Rohweder CL, Smith JD. Designs and methods for implementation research: advancing the mission of the CTSA program. J Clin Transl Sci. 2020;4:159–167. doi:10.1017/cts.2020.16
25. Hartung C, Lerer A, Anokwa Y, Tseng C, Brunette W, Borriello G. Open data kit: tools to build information services for developing regions. In:
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.









