Back to Journals » Cancer Management and Research » Volume 16
Importance of Testing for ROS1 Rearrangements in Non-Small Cell Lung Cancer in the Era of Targeted Therapy in a Latin American Country
Authors Osorio A, Fernandez-Trujillo L , Restrepo JG, Sua LF, Proaño C, Zuñiga-Restrepo V
Received 20 December 2023
Accepted for publication 22 May 2024
Published 11 July 2024 Volume 2024:16 Pages 781—789
DOI https://doi.org/10.2147/CMAR.S455809
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Alvaro Osorio,1,2 Liliana Fernandez-Trujillo,2,3 Juan G Restrepo,1,2 Luz F Sua,2,4 Catalina Proaño,5 Valeria Zuñiga-Restrepo2
1Department of Internal Medicine, Oncology Service, Fundación Valle Del Lili, Cali, Colombia; 2Faculty of Health Sciences, Universidad Icesi, Cali, Colombia; 3Department of Internal Medicine, Pulmonology Service, Fundación Valle Del Lili, Cali, Colombia; 4Department of Pathology and Laboratory Medicine, Fundación Valle Del Lili, Cali, Colombia; 5Clinical Research Center, Fundación Valle del Lili, Cali, Colombia
Correspondence: Liliana Fernandez-Trujillo, Department of Internal Medicine, Pulmonology Service, Interventional Pulmonology, Avenida Simón Bolívar. Cra. 98 No. 18-49, Fundación Valle del Lili, Tower 6, 4th Floor, Cali, 760032, Colombia, Tel +57 3155006300, Email [email protected]; [email protected]
Purpose: Lung cancer is the leading cause of cancer-related deaths worldwide. However, with the optimization of screening strategies and advances in treatment, mortality has been decreasing in recent years. In this study, we describe non-small cell lung cancer patients diagnosed between 2021 and 2022 at a high-complexity hospital in Latin America, as well as the immunohistochemistry techniques used to screen for ROS1 rearrangements, in the context of the recent approval of crizotinib for the treatment of ROS1 rearrangements in non-small cell lung cancer in Colombia.
Methods: A descriptive cross-sectional study was conducted. Sociodemographic, clinical, and molecular pathology information from non-small cell lung cancer individuals who underwent immunohistochemistry to detect ROS1 rearrangements between 2021 and 2022 at Fundación Valle del Lili (Cali, Colombia) was recorded. The clinical outcomes of confirmed ROS1 rearrangements in non-small cell lung cancer patients were reported.
Results: One hundred and thirty-six patients with non-small cell lung cancer were included. The median age at diagnosis was 69.8 years (interquartile range 61.9– 77.7). At diagnosis, 69.8% (n = 95) were at stage IV. ROS1 immunohistochemistry was performed using the monoclonal D4D6 antibody clone in 54.4% (n = 74) of the cases, while 45.6% (n = 62) were done with the monoclonal SP384 antibody clone. Two patients were confirmed to have ROS1 rearrangements in non-small cell lung cancer using next-generation sequencing and received crizotinib. On follow-up at months 5.3 and 7.0, one patient had a partial response, and the other had oligo-progression, respectively.
Conclusion: Screening for ROS1 rearrangements in non-small cell lung cancer is imperative, as multiple prospective studies have shown improved clinical outcomes with tyrosine kinase inhibitors. Given the recent approval of crizotinib in Colombia, public health policies must be oriented toward early detection of driver mutations and prompt treatment. Additionally, future approvals of newly tested tyrosine kinase inhibitors should be anticipated.
Keywords: non-small cell lung cancer, ROS1, proto-oncogene receptor tyrosine kinase, immunohistochemistry, next generation sequencing, tyrosine kinase inhibitor
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide. However, due to the optimized screening strategies and advances in treatment, mortality has been decreasing in recent years.1–3 Within the context of lung cancer, certain genetic alterations, known as driver mutations, confer a selective advantage to oncogenic cells.4 To date, multiple driver mutations have been identified in non-small cell lung cancer (NSCLC), and some of these mutations predict responses to specific therapies, making molecular diagnosis or biomarking mandatory for treatment selection.5,6
The prevalence of proto-oncogene receptor tyrosine kinase (ROS1) rearrangements in NSCLC (ROS1-NSCLC) has been described in about 2.4% (95% confidence interval (CI) 1.5–3.7), being more frequent in adenocarcinomas than in any other histologic type, representing 2.9% (95% CI: 1.9–4.5) and 0.6% (95% CI: 0.3–1.2), respectively.7,8 Rarely, ROS1 rearrangements coexist with other driver mutations such as epidermal growth factor receptor (EGFR) mutations and mesenchymal–epithelial transition factor (MET) amplifications, among others.9–11
Immunohistochemistry (IHC) is currently accepted as a screening strategy, primarily due to its lower cost compared to molecular tests, which also require more time and technical expertise.7 The rabbit monoclonal D4D6 antibody clone (Cell Signaling Technology, Danvers, Massachusetts, USA) is the most used technique, with a sensitivity of around 100% and a variable specificity between 92% and 100%, depending on the positivity threshold.12,13 Recently, a new monoclonal antibody, SP384, has demonstrated a sensitivity of up to 91% and specificity of 100% in the ROSING study.14
IHC and molecular tests have demonstrated high sensitivity and concordance when comparing negative results, achieving a concordance rate of nearly 100%, making it possible to rule out ROS1 rearrangements when IHC is negative. However, in positive cases, the concordance rate can be as low as 79%, indicating the necessity for a confirmation test in cases where IHC is positive. Additionally, immunostaining can sometimes be heterogeneous, leading to the possibility of false-positive results.7 Confirmation can be made through FISH (fluorescent in situ hybridization), real-time polymerase chain reaction (RT-PCR), or next-generation sequencing (NGS) and Nanostring.7,15,16
Crizotinib is a tyrosine kinase inhibitor (TKI) that inhibits several enzymes, including anaplastic lymphoma kinase (ALK), cellular mesenchymal–epithelial transition factor (c-MET), and ROS1. This inhibition is associated with cell cycle arrest in the G1-S phase and the induction of apoptosis in positive cells in vitro and in vivo.17 Since 2014, the benefit of crizotinib treatment in advanced ROS1-NSCLC has been tested,18 and subsequent studies have confirmed these results.19,20
In our hospital in Colombia, both monoclonal antibodies (D4D6 and SP384) have been utilized over the past few years. Once a positive IHC result is obtained, the choice of a confirmatory test depends on the oncologist’s criteria and its availability. With the recent approval of crizotinib by the National Institute for Food and Drug Surveillance (INVIMA) in Colombia in October 2021, ROS1 testing in NSCLC has become especially relevant in determining whether targeted therapy with crizotinib can provide benefits. Therefore, in this study, we describe NSCLC patients diagnosed between 2021 and 2022 in a high-complexity hospital in Latin America, as well as the IHC techniques employed for ROS1 rearrangement screening and the trend in testing frequency over the past year.
Methods
A descriptive cross-sectional study was conducted, collecting sociodemographic, clinical, and molecular pathology information from individuals with NSCLC who underwent IHC for the detection of ROS1 rearrangements between 2021 and 2022 at Fundación Valle del Lili (FVL) in Cali, Colombia. Patients older than 18 years at any disease stage were included, excluding those who had a second primary lung tumor.
A descriptive statistical analysis was performed by determining absolute frequencies and proportions for qualitative variables. For the quantitative variables, central tendencies (mean or median) and their corresponding measures of dispersion (standard deviation or interquartile range) were reported, based on the normal distribution. All statistical analyses were performed using RStudio. The study was approved by the FVL Biomedical Research Ethics Committee (trial No. 155–2023).
Results
We included 136 patients diagnosed with NSCLC between 2021 and 2022 who underwent an IHC test for the detection of ROS1 rearrangements. Among them, 51% (n = 70) were women, 62.5% (n = 85) were aged between 66 and 85 years (median age 69.8; IR 61.9–77.7). The majority of patients had adenocarcinomas (95.6%, n = 130), while the remaining percentage corresponded to squamous cell carcinoma (n = 4), one case of adeno-squamous-cell carcinoma, and one case of lymphoepithelioma-like tumor. At the time of diagnosis, 69.8% (n = 95) were at stage IV. Table 1 summarizes the demographic and clinical characteristics.
 |
Table 1 Demographics and Baseline Characteristics |
A total of 136 IHC tests for ROS1 were performed within the established timeframe. About 54.4% (n = 74) were performed using the monoclonal D4D6 antibody clone and 45.6% (n = 62) with the monoclonal SP384 antibody clone. Fifty-five tests were carried out in 2021, and 81 tests were done in 2022. The percentage of positive tests with each technique in relation to the year of testing is shown in Table 2. The median time between histological diagnosis and the IHC result was 11 days (IR 6.0–35.2). Among the 136 patients included, 33.5% (n = 45) had EGFR gene mutations, 5.2% (n = 7) had ALK rearrangements, and 25.8% (n = 34) had high expression of programmed death-ligand 1 (PD-L1) (tumor proportion score >50). Detailed information regarding molecular features, including EGFR gene mutations, ALK rearrangements, and PD-L1 expression, can be found in Table 3.
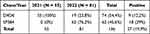 |
Table 2 IHC for ROS1 According to the Monoclonal Antibody Clone, Year of Testing and Positivity |
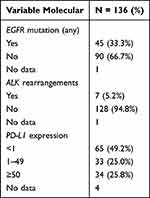 |
Table 3 Molecular Features of NSCLC |
Out of the 27 patients with positive ROS1 IHC Results, 17 were at stage IV. Among these patients, 6 underwent confirmatory testing, all of which were performed using NGS. The NGS assay utilized in our institution is FoundationOne® CDx. Two cases of ROS1-NSCLC were confirmed, and both patients received crizotinib subsequently. Additionally, one patient who tested positive on IHC without a confirmatory test received crizotinib. The remaining 4 confirmatory tests were negative. Patients with ROS1-NSCLC who received crizotinib are described in Table 4.
 |
Table 4 Clinical, Histological, and Molecular Features of ROS1-NSCLC Patients Treated with Crizotinib |
Discussion
Lung cancer treatment has evolved towards targeted therapy over the past decade. The response to treatment depends on the presence of driver mutations. Currently, the National Comprehensive Cancer Network (NCCN) guidelines recommend a panel of genetic tests for NSCLC, including EGFR, ALK, ROS1, MET, B-Raf proto-oncogene (BRAF), REarranged during transfection (RET), erythroblastic oncogene B (ERBB2), and Kirsten Rat Sarcoma viral oncogene homolog (KRAS).18,21
These driver mutations, also known as gene fusions, arise from genomic rearrangement events, including chromosomal inversions, interstitial deletions, duplications, or translocations.22 The formation of chimeric oncogenic proteins resulting from these processes enables the feasibility of selecting the optimal therapeutic approach based on the identified gene aberration.23 In the case of ROS1, translocations involving this gene are present in only 1–2% of the patient population.24
ROS1 rearrangements are more frequent in women (2.42%) than in men (1.57%) (OR = 1.54, 95% CI: 1.02–2.34, P = 0.042), as well as in young people, the non-smoking population compared to smokers (3.65% and 0.90%, respectively, [OR = 3.27, 95% CI: 1.44–7.45, P = 0.005]), and advanced stages.12 In our cohort, the two patients with ROS1-NSCLC were both 65 years or younger, had stage IV disease, had no major comorbidities, and were never smokers.
In this study, IHC tested positive in 12.2% (9/74) using the D4D6 clone, and 29% for the SP384 clone (18/62). Concordance was not evaluated in this study. Although confirmation of ROS1 rearrangements can be achieved through various techniques, the most commonly used method in our hospital is NGS (n = 6). NGS, along with other innovative methods such as nCounter RNA testing, are considered more promising techniques for concurrently detecting ALK, ROS1, and EGFR alterations in a single analysis.25
In this study, the frequency of ROS1-NSCLC was 1.4% (n = 2). These results align with previous studies reporting frequencies between 1% and 2%.8,26–28 Notably, both patients with ROS1-NSCLC in this study received immune chemotherapy while awaiting ROS1 confirmation since confirmation test results in Colombia are obtained several weeks after histological diagnosis, and the risk of disease progression is a concern.
Over the past decade, multiple trials have evaluated TKIs in advanced ROS1-NSCLC. The Food and Drug Administration (FDA) has approved crizotinib, entrectinib,29 ceritinib,30 and lorlatinib31,32 for the treatment of advanced ROS1-NSCLC. Repotrectinib is currently under FDA review for approval, based on the results of the Phase 1/2 TRIDENT-1 trial.33 In Colombia, crizotinib was the first TKI to be approved by the National Institute for Food and Drug Surveillance, making it the only available TKI for ROS1-NSCLC.
Crizotinib efficacy studies in ROS1-NSCLC have involved relatively few patients given its low prevalence. In the 62.6-month analysis of the PROFILE 1001 trial, the objective response rate (ORR) and progression-free survival (PFS) of 53 enrolled patients was 72% (95% CI, 58% to 83%) and 19.3 months (95% CI, 15.2–39.1), with 15% of patients being monitored for progression at that time.20 In a Phase II study conducted in East Asia, the ORR among 127 patients was 72% (95% CI, 63% to 79%), the median PFS was 15.9 months (95% CI, 12.9–24.0), and the median duration of response (DOR) was 19.7 months (95% CI, 14.1 to NR).34 In a smaller phase II study in France, the ORR among 37 patients was 69.4% (95% CI, 53–82). The median PFS in this study was 5.5 months (95% CI, 4.6–9.1), with an overall survival (OS) of 17.2 months (95% CI 6.8–32.8).35 Variations in PFS among these three trials may be influenced by the relatively small samples.
Patients who benefit from crizotinib therapy for ROS1-NSCLC are generally those with advanced NSCLC.19,20,36 In our hospital, IHC screening is performed on all patients with NSCLC regardless of staging. However, the request for a confirmatory test is made based on the oncologist’s criteria and the clinical context, although a confirmatory test is always preferred. In our case, 11 patients did not undergo a confirmatory test due to the co-occurrence of ROS1 and EGFR mutations (EGFR::ROS1) present in 8 cases, in which therapeutic priority was given to this mutation. One patient was in an advanced disease stage and compromised condition, prompting a decision to prioritize the initiation of treatment with crizotinib, even in the absence of a confirmatory test. The 2 remaining cases had an Eastern Cooperative Oncology Group (ECOG) > 2, and due to their clinical condition, palliative care was indicated.
To our knowledge, this is the first study to evaluate the prevalence of ROS1 rearrangements in Colombian NSCLC patients and to describe the clinical outcomes with TKI treatment. At the six-month follow-up, one patient showed a partial response, while the other patient had oligoprogressive disease (a pulmonary nodule). The patient who received crizotinib without a confirmatory test was already in an advanced stage of their disease. Unfortunately, they could only be followed up for 10 days from the day of their first dose due to the deterioration of their condition, ultimately leading to their death. In this case, Conclusions regarding the efficacy of TKI treatment cannot be drawn, highlighting the importance of confirmatory testing among TKI-eligible patients. It is worth noting that both ROS1-NSCLC patients had deep venous thrombosis, consistent with the increased incidence of venous thromboembolism (including pulmonary embolism, deep venous thrombosis, renal vein thrombosis, internal jugular thrombosis, and peripheral inserted central catheter-related) reported in ROS1-NSCLC in recent studies.37,38
Despite the small sample of ROS1-NSCLC patients in this study and our emerging experience in treatment with TKI treatment in this context, the clinical outcomes have been optimistic. In this regard, this study reinforces the importance of ROS1 rearrangement screening in a Latin American country where crizotinib was recently approved for ROS1-NSCLC (October 2022). More investigation is warranted to establish better evidence regarding clinical outcomes in our population, which may differ from previous cohorts mainly comprising white and Asian individuals, as well as to assess the concordance of emerging IHC techniques in our population.
Conclusions
Screening for ROS1 rearrangements in NSCLC is imperative, as multiple prospective studies have demonstrated improved clinical outcomes with TKIs. Given the recent approval of crizotinib in Colombia, public health policies must be oriented to focus on early detection of driver mutations and prompt treatment. Additionally, efforts should be directed towards the potential approval of newly tested TKIs in the future.
Abbreviations
NSCLC, Non-Small Cell Lung Cancer; ROS1, Proto-Oncogene Receptor Tyrosine Kinase; ROS1-NSCLC, ROS1 Rearrangements in NSCLC; CI, Confidence Interval; EGFR, Epidermal Growth Factor Receptor; MET, Mesenchymal–Epithelial Transition Factor; IHC, Immunohistochemistry; FISH, Fluorescent In Situ Hybridization; RT-PCR, Real-Time Polymerase Chain Reaction; NGS, Next-Generation Sequencing; TKI, Tyrosine Kinase Inhibitor; ALK, Anaplastic Lymphoma Kinase; c-MET, cellular mesenchymal–epithelial transition factor; INVIMA, National Institute for Food and Drug Surveillance; FVL, Fundación Valle del Lili; PD-L1, Programmed Death-Ligand 1; NCCN, National Comprehensive Cancer Network; BRAF, B-Raf Proto-Oncogene; RET, REarranged during Transfection; ERBB2, Erythroblastic oncogene B; KRAS, Kirsten Rat Sarcoma viral oncogene homolog; FDA, Food and Drug Administration; ORR, Objective Response Rate; PFS, Progression-Free Survival; DOR, Duration of Response; OS, Overall Survival; EGFR ROS1, ROS1 and EGFR mutations; ECOG, Eastern Cooperative Oncology Group.
Data Sharing Statement
Datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This manuscript was written in compliance with the ethical standards of the institutional ethics committee and with the 1964 Helsinki Declaration. We have the approval of the Ethics Committee in Biomedical Research from Fundación Valle del Lili. This is supported in letter No. 155 of 2023, which is available with the Corresponding Author if needed. According to Colombian regulations for research involving human subjects, since this is a retrospective observational study, informed consent signatures are not required; this was approved by the ethics committee.
Consent for Publication
According to Colombian regulations for research involving human subjects, since this is a retrospective observational study, informed consent signatures are not required; this was approved by the ethics committee. The authors declare that all sensitive and confidential patient data were handled with confidentiality in compliance with the requirements of the ethics committee.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Dr Alvaro Osorio reports personal fees from Fundación Valle de lili, personal fees from Universidad Icesi, personal fees from Pfizer, personal fees from Roche, personal fees from MSD, personal fees from Bristol, personal fees from AstraZeneca, personal fees from Ipsen, personal fees from Astellas, personal fees from Tecnofarma, outside the submitted work. The authors declare that they have no other competing interests.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;2:1–41.
2. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503–513. doi:10.1056/NEJMoa1911793
3. Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640–649. doi:10.1056/NEJMoa1916623
4. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Kinzler KW. Cancer genome landscapes. Science. 2013;340(6127):1546–1558. doi:10.1126/science.1235122
5. Swanton C, Govindan R, Longo DL. Clinical implications of genomic discoveries in lung cancer. N Engl J Med. 2016;374(19):1864–1873. doi:10.1056/NEJMra1504688
6. Reck M, Rabe KF, Longo DL. Precision diagnosis and treatment for advanced non–small-cell lung cancer. N Engl J Med. 2017;377(9):849–861. doi:10.1056/NEJMra1703413
7. Yang J, Pyo JS, Kang G. Clinicopathological significance and diagnostic approach of Ros1 rearrangement in non-small cell lung cancer: a meta-analysis: ros1 in non-small cell lung cancer. Int J Biol Markers. 2018;33(4):520–527. doi:10.1177/1724600818772194
8. Zhu Q, Zhan P, Zhang X, Lv T, Song Y. Clinicopathologic characteristics of patients with ROS1 fusion gene in non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res. 2015;4(3):300–309. doi:10.3978/j.issn.2218-6751.2015.05.01
9. Lin JJ, Ritterhouse LL, Ali SM, et al. ROS1 fusions rarely overlap with other oncogenic drivers in non–small cell lung cancer. J Thorac Oncol. 2017;12(5):872–877. doi:10.1016/j.jtho.2017.01.004
10. Park S, Ahn BC, Lim SW, et al. Characteristics and outcome of ROS1-positive non–small cell lung cancer patients in routine clinical practice. J Thorac Oncol. 2018;13(9):1373–1382. doi:10.1016/j.jtho.2018.05.026
11. Zhu Y, Wang W, Xu C, et al. A novel co-existing ZCCHC8-ROS1 and de-novo MET amplification dual driver in advanced lung adenocarcinoma with a good response to crizotinib. Cancer Biol Ther. 2018;19(12):1097–1101. doi:10.1080/15384047.2018.1491506
12. Cha YJ, Lee JS, Kim HR, et al. Screening of ROS1 rearrangements in lung adenocarcinoma by immunohistochemistry and comparison with ALK rearrangements. PLoS One. 2014;9(7):1–10. doi:10.1371/journal.pone.0103333
13. Rimkunas VM, Crosby KE, Li D, et al. Analysis of receptor tyrosine kinase ROS1-positive tumors in non-small cell lung cancer: identification of a FIG-ROS1 fusion. Clin Cancer Res. 2012;18(16):4449–4457. doi:10.1158/1078-0432.CCR-11-3351
14. Conde E, Hernandez S, Martinez R, et al. Assessment of a new ROS1 Immunohistochemistry Clone (SP384) for the identification of ROS1 rearrangements in patients with non–small cell lung carcinoma: the ROSING study. J Thorac Oncol. 2019;14(12):2120–2132. doi:10.1016/j.jtho.2019.07.005
15. Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors guideline from the college of American pathologists, the international association for the study of lung cancer, and the a. Arch Pathol Lab Med. 2018;142(3):321–346. doi:10.5858/arpa.2017-0388-CP
16. Pavlakis N, Cooper C, John T, et al. Australian consensus statement for best practice ROS1 testing in advanced non-small cell lung cancer. Pathology. 2019;51(7):673–680. doi:10.1016/j.pathol.2019.08.006
17. Of IA. IASLC Atlas of ALK and ROS1 Testing in Lung Cancer. FL: North Fort Myers; 2016. Available from: https://www.iaslc.org/research-education/publications-resources-guidelines/iaslc-atlas-alk-and-ros1-testing-lung-cancer.
18. Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371(21):1963–1971. doi:10.1056/NEJMoa1406766
19. Mazières J, Zalcman G, Crinò L, et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol. 2015;33(9):992–999. doi:10.1200/JCO.2014.58.3302
20. Shaw AT, Riely GJ, Bang YJ, et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol. 2019;30(7):1121–1126. doi:10.1093/annonc/mdz131
21. Conde E, Rojo F, Gómez J, et al. Molecular diagnosis in non-small-cell lung cancer: expert opinion on ALK and ROS1 testing. J Clin Pathol. 2021. doi:10.1136/jclinpath-2021-207490
22. Pisapia P, Pepe F, Sgariglia R, et al. Methods for actionable gene fusion detection in lung cancer: now and in the future. Pharmacogenomics. 2021;22(13):833–847. PMID: 34525844. doi:10.2217/pgs-2021-0048
23. Malapelle U, Muscarella LA, Pisapia P, Rossi A. Targeting emerging molecular alterations in the treatment of non-small cell lung cancer: current challenges and the way forward. Expert Opin Investig Drugs. 2020;29(4):363–372. PMID: 32073317. doi:10.1080/13543784.2020.1732922
24. Muminovic M, Carracedo Uribe CR, Alvarez-Pinzon A, Shan K, Raez LE. Importance of ROS1 gene fusions in non-small cell lung cancer. Cancer Drug Resist. 2023;6(2):332–344. PMID: 37457125; PMCID: PMC10344718. doi:10.20517/cdr.2022.105
25. Pisapia P, Lozano MD, Vigliar E, et al. ALK and ROS1 testing on lung cancer cytologic samples: perspectives. Cancer Cytopathol. 2017;125(11):817–830. PMID: 28743163. doi:10.1002/cncy.21899
26. Bergethon K, Shaw AT, Ou SH. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863–870. doi:10.1200/JCO.2011.35.6345
27. Davies KD, Le AT, Theodoro MF, et al. Identifying and targeting ROS1 gene fusions in non-small cell lung cancer. Clin Cancer Res. 2012;18(17):4570–4579. doi:10.1158/1078-0432.CCR-12-0550
28. Zhang Q, Wu C, Ding W, et al. Prevalence of ROS1 fusion in Chinese patients with non-small cell lung cancer. Thorac Cancer. 2019;10(1):47–53. doi:10.1111/1759-7714.12899
29. Drilon A, Siena S, Dziadziuszko R, et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1–2 trials [published correction appears in Lancet Oncol. 2020 Feb;21(2):e70] [published correction appears in Lancet Oncol. 2020 Jul; 21(7):e341]. Lancet Oncol. 2020;21(2):261–270. doi:10.1016/S1470-2045(19)30690-4
30. Lim SM, Kim HR, Lee JS, et al. Open-label, multicenter, phase II study of ceritinib in patients with non-small-cell lung cancer harboring ROS1 rearrangement. J Clin Oncol. 2017;35(23):2613–2618. doi:10.1200/JCO.2016.71.3701
31. Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global Phase 2 study. Lancet Oncol. 2018;19(12):1654–1667. doi:10.1016/S1470-2045(18)30649-1
32. Shaw AT, Solomon BJ, Chiari R, et al. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: a multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2019;20(12):1691–1701. doi:10.1016/S1470-2045(19)30655-2
33. Cho BC, Lin J. Pivotal topline data from the phase 1/2 TRIDENT-1 trial of repotrectinib in patients with ROS1+ advanced non-small cell lung cancer (NSCLC). Eur J Cancer. 2022;15(16):30. doi:10.1016/S0959-8049(22)00812-7
34. Wu YL, Yang JC, Kim DW, et al. Phase II study of Crizotinib in East Asian patients with ROS1-positive advanced non-small-cell lung cancer. J Clin Oncol. 2018;36(14):1405–1411. doi:10.1200/JCO.2017.75.5587
35. Moro-Sibilot D, Cozic N, Pérol M, et al. Crizotinib in c-MET- or ROS1-positive NSCLC: results of the AcSé phase II trial. Ann Oncol. 2019;30(12):1985–1991. doi:10.1093/annonc/mdz407
36. Sahu A, Prabhash K, Noronha V, Joshi A, Desai S. Crizotinib: a comprehensive review. South Asian J Cancer. 2013;2(2):91–97. doi:10.4103/2278-330X.110506
37. Chiari R, Ricciuti B, Landi L, et al. ROS1-rearranged non-small-cell lung cancer is associated with a high rate of venous thromboembolism: analysis from a phase II, Prospective, Multicenter, Two-arms Trial (METROS). Clin Lung Cancer. 2020;21(1):15–20. doi:10.1016/j.cllc.2019.06.012
38. Muñoz-Unceta N, Zugazagoitia J, Manzano A, et al. High risk of thrombosis in patients with advanced lung cancer harboring rearrangements in ROS1. Eur J Cancer. 2020;141:193–198. doi:10.1016/j.ejca.2020.10.002
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

