Back to Journals » International Journal of Nanomedicine » Volume 19
In-Situ Grown Nanocrystal TiO2 on 2D Ti3C2 Nanosheets with Anti-Tumor Activity from Photo-Sonodynamic Treatment and Immunology
Authors Yu H , Huang Y, Nong Z, Lin X , Tang K, Cai Z, Huang K, Yu T, Lan H, Zhang Q, Wang Q, Yang L, Zhu J, Wu L , Luo H
Received 29 December 2023
Accepted for publication 6 July 2024
Published 6 August 2024 Volume 2024:19 Pages 7963—7981
DOI https://doi.org/10.2147/IJN.S457112
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dongwoo Khang
Hailing Yu,1,* Yongquan Huang,2,* Zhisheng Nong,3,* Xi Lin,1 Kexin Tang,1 Zeyu Cai,1 Kaichen Huang,4 Ting Yu,1 Huimin Lan,1 Qianqian Zhang,1 Qiang Wang,5 Lei Yang,6 Jingchuan Zhu,7 Lili Wu,8 Hui Luo1
1Guangdong Provincial Engineering Research Center of Molecular Imaging, Guangdong-Hong Kong-Macao University Joint Laboratory of Interventional Medicine, The Fifth Affiliated Hospital, Sun Yat-Sen University, Zhuhai, Guangdong, People’s Republic of China; 2Department of Ultrasound, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, Guangdong, People’s Republic of China; 3School of Materials Science and Engineering, Shenyang Aerospace University, Shenyang, Liaoning, People’s Republic of China; 4Department of Clinical laboratory, The Third People’s Hospital of Zhuhai, Zhuhai, Guangdong, People’s Republic of China; 5The Green Aerotechnics Research Institute of Chongqing Jiaotong University, Chongqing, People’s Republic of China; 6Center for Composite Materials and Structures, Harbin Institute of Technology, Harbin, Heilongjiang, People’s Republic of China; 7School of Materials Science and Engineering, Harbin Institute of Technology, Harbin, Heilongjiang, People’s Republic of China; 8Key Laboratory for Photonic and Electronic Bandgap Materials, Ministry of Education, School of Physics and Electronic Engineering, Harbin Normal University, Harbin, Heilongjiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hui Luo; Lili Wu, Email [email protected]; [email protected]
Introduction: Traditional cancer treatment strategies often have severe toxic side effects and poor therapeutic efficacy. To address the long-standing problems related to overcoming the complexity of tumors, we develop a novel nanozyme based on the in situ oxidation of 2D Ti3C2 structure to perform simultaneous phototherapy and sonodynamic therapy on tumors. Ti3C2 nanozymes exhibit multi-enzyme activity, including intrinsic peroxidase (POD) activities, which can react with H2O2 in the tumor microenvironment. This new material can construct Ti3C2/TiO2 heterostructures in vivo.
Methods: Photothermal (PTT), sonodynamic (SDT) effects, and photoacoustic (PA) image-guided synergy therapy can be achieved. Finally, anticancer immune responses occur with this nanozyme. In vivo experiments revealed that the Ti3C2/TiO2 heterostructure inhibited tumor growth.
Results: Complementarily, our results showed that the Ti3C2/TiO2 heterostructure enhanced the immunogenic activity of tumors by recruiting cytotoxic T cells, thereby enhancing the tumor ablation effect. Mechanistic studies consistently indicated that Reactive Oxygen Species (ROS) regulates apoptosis of HCC cells by modulating NRF2/OSGIN1 signaling both in vitro and in vivo. As a result, Ti3C2 nanozyme effectively inhibited tumor through its synergistic ability to modulate ROS and enhance immune infiltration of cytotoxic T cells in the tumor microenvironment.
Discussion: These findings open up new avenues for enhancing 2D Ti3C2 nanosheets and suggest a new way to develop more effective sonosensitizers for the treatment of cancer.
Keywords: nanozyme, sonodynamic therapy, Ti3C2/TiO2, ROS
Introduction
Hepatocellular carcinoma (HCC) was a dominant form of primary liver cancer and was the fifth most common malignant cancer in the world.1 Recent advances in nanotechnology have significantly accelerated the development of novel therapies for HCC, but more effective treatment for patients remains an unmet clinical need.2,3 Numerous studies show that photothermal therapy (PTT) effectively treats malignant cancers.4–6 However, PTT has been limited in clinical application due to its intrinsic drawbacks, including phototoxicity and low penetration depth into tumor regions.7,8 In addition, PTT cannot ablate the entire solid tumor or large tumors due to its penetration depth. The residual tumor after PTT might become activated and lead to tumor metastasis and recurrence.9 Thus, there is still a great unmet need to exploit novel therapies or synergistic methods to overcome these limitations.
In recent years, nanocatalytic therapies based on the generation of ROS in the tumor microenvironment (TME) have attracted much attention.10 Nanozyme-mediated catalytic tumor therapy has been widely used with the advantages of high stability, low cost, adjustable catalytic activity, and in situ catalytic reaction in vivo. Our previous study showed that nanomaterials with catalytic activity can specifically induce ROS production in tumor cells, thereby inducing mitochondrial damage.11 Among them, nanomaterials with peroxidase (POD)-like activity, which kills cancer cells by generating reactive hydroxyl radicals (⋅OH) upon reaction with H2O2, have attracted widespread attention. However, due to the generally low H2O2 concentration in the tumor microenvironment, as well as the heterogeneity of tumors, low catalytic activity limited therapeutic efficiency. Therefore, there is an urgent need for more efficient nanozymes with multiple effects that can effectively accumulate and release ROS to enhance tumor therapeutic effects.
MXene is a class of new 2D materials with excellent physical and chemical properties that have a wide range of promising applications in energy storage, wastewater treatment, and hydrogen storage.12 Ti3C2 is the typical representative of MXene, due to its good absorption in the NIR I and NIR II regions and excellent photothermal properties.13 The exfoliated Ti3C2 MXene is mildly oxidized with H2O2 to produce titanium oxide nanoclusters anchored to the substrate. TiO2 is a representative of inorganic sonosensitizers for SDT. SDT is a new non-invasive and high tissue penetration therapy that uses ultrasound to simulate sonosensitizer to generate ROS, induce tumor cell death, and achieve therapeutic purposes.14,15 Based on the deep tissue penetration ability of ultrasound, SDT can realize the treatment of deeper tumors and has broad prospects for preclinical studies.16,17 Current sonosensitizers are divided into two types, inorganic and organic sonosensitizers.18,19 The therapeutic efficacy of organic sonosensitizers, including porphyrin and its derivatives, is limited by poor bioavailability, unsatisfactory efficacy, and off-target toxicity. Compared to organic sonosensitizers, TiO2, as an important member of inorganic sonosensitizers, has excellent physiochemical stability and biocompatibility.20,21 Although the SDT performance of TiO2 nanomaterials can be improved by combining TiO2 nanomaterials with Pt, Au, and Ag to form heterostructures or oxygen defect sites in TiO2 nanomaterials, it is still necessary to improve the effect of sonosensitizers and enhance synergistic therapy.22–25 Since the surface of Ti3C2 MXenes nanosheet very easily undergoes oxidation reaction, titanium atoms on Ti3C2 can be used as nucleation sites to generate TiO2, thus forming Ti3C2/TiO2 heterostructure. Ti3C2 is an ideal nanoplatform for combining PDT and SDT to enhance the efficacy of SDT and exhibits excellent photoacoustic (PA) imaging properties.26
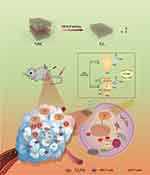
In this study, we fabricated Ti3C2 Mxene with catalytic effect by chemical etching, and Ti3C2 exhibits multi-enzyme activity, including intrinsic peroxidase (POD) activities, which can react with H2O2 in the TME and are accompanied by ROS generation. The surface defects in Ti3C2 are gently oxidized in situ by H2O2 in the TME to construct a Ti3C2/TiO2 heterostructure, integrating photothermal and sonodynamic effects to achieve image-guided tumor therapy and induce multiple anticancer immune responses. It is demonstrated that Ti3C2/TiO2 heterostructure is an excellent sonodynamic and photothermal sensitizer for tumor therapy, showing excellent photothermal/sonodynamic properties at NIR broadband and immunomodulatory function both in vivo and in vitro, as shown in Figure 1. Notably, we found that the combination of sonodynamic and photothermal therapies based on Ti3C2/TiO2 induced the TME from “cold” to “hot” in HCC mouse models. Our studies accelerate the application of Ti3C2 nanozyme-mediated sonodynamic and photothermal synergistic therapy for HCC treatment by activating the immune tumor microenvironment.
Materials and Methods
Preparation of Ti3C2 and Ti3C2/TiO2
Lithium fluoride was slowly poured into a hydrochloric acid solution and stirred by a magnetic stirrer for 10 min. Ti3C2 was prepared by etching Ti3AlC2 powder by mixing a solution of lithium fluoride and hydrochloric acid for 24 h. Then the solution was centrifuged at 8000 rpm for 10 min, and the precipitates were washed with lithium fluoride, hydrochloric acid, and water in sequence until the pH reached 7.4. The Ti3C2 solution was lyophilized and freeze-dried for further experiments. 0.1 mL H2O2 (10 mM) was added to the Ti3C2 solution (10 mg/mL) and allowed to react for 24 h, and then Ti3C2/TiO2 was obtained.
Materials
Dulbecco’s modified Eagle’s medium Dulbecco (DMEM), fetal bovine serum, penicillin-streptomycin, and trypsin-EDTA were purchased from ThermoFisher Scientific (USA). Calcein-AM/propidium iodide (PI) kit and dichlorofluorescin diacetate (DCFH-DA) probe were purchased from Beyotime Biotechnology (China). The cell counting kit (CCK-8) was purchased from Dojindo Laboratories (Japan).
DFT Calculation
Ti3C2 and the absorption of H2O2 were calculated from first principles using density functional theory (DFT) in the Cambridge Serial Total Energy Package (CASTEP) code. In these calculations, the ultra-soft pseudo-potential was used to describe the interaction between the electron and the ion, and the electron exchange-correlation function was applied by generalized gradient approximation (GGA) of Perdew Burke Ernzerhof (PBE). The cut-off energies of 380 eV were used in the calculation. The appropriate grids of the k-point mesh were selected on the basis of the Monkhorst-Pack scheme. The self-consistent first-principles calculations converged when the maximum force on the atom, the maximum displacement between cycles, the maximum stress, and the total energy changes at each atom were less than 0.01 eV/Å, 5.0 × 10−4Å, 0.02 GPa, and 5.0×10−6 eV/atom, respectively.
Cell Culture
Human hepatocellular carcinoma cells Hep3B, Bel-7402, SK-Hep-1, and human kidney cells (HEK 293T) were purchased from Jennio Biotech Co., Ltd (Guangzhou, China). All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (for Hep3B cells) or RPMI 1640 medium (for SK-Hep-1 and Bel 7402 cells) supplemented with 10% fetal bovine serum (FBS, Gibco, USA) and 1% penicillin-streptomycin (PS, Gibco, USA). They were maintained in an incubator under 5% CO2 at a constant temperature of 37°C. For sphere-forming assays, Hep3B and SK-Hep-1 cells were cultured in a 100 mm Ultra-Low Attachment Culture Dish (Corning, USA) to form tumor cell spheroids (50–100 μm).
In vitro Cytotoxicity of Ti3C2
The cytotoxicity of Ti3C2 at different concentrations was evaluated using the CCK-8 assay. Cells were plated in 96-well plates (5×103 cells per well) for 24 h and then incubated with 20 μg/mL Ti3C2 for 12 h. Then, cells were subjected to different treatments as follows: (1) Cells were exposed to ultrasound at 1 W/cm2 for different times (0, 1, 2, 3, 4 minutes). (2) Cells were exposed to ultrasound at different power levels (0, 0.5, 0.75, 1, 1.25 W/cm2) for 3 minutes. (3) Cells were exposed to laser at 1 W/cm2 for different periods (0, 1, 2, 3 minutes). (4) Cells were exposed to different laser powers (0, 0.5, 1, 1.5 W/cm2) for 2 min. (5) Cells were exposed to ultrasound (1 W/cm2, 3 min) and laser (1 W/cm2, 2 min). The cells were then cultured for a further 12 or 18 hours. Finally, the CCK-8 test was performed to measure cell viability, and absorbance at 450 nm was measured using a multimode microplate reader (PerkinElmer EnVision 2105, USA). Calcein-AM/PI staining experiments were also performed to evaluate the in vitro therapeutic efficacy of Ti3C2.
Detection of ROS
The extracellular ROS generated by laser and ultrasound stimulation was dynamically detected by DCFH-DA, which could be oxidized by ROS to generate the fluorescence of DCF (Beyotime, China). Hep3B cells (2×105 per well) were cultured in 6-well plates with DMEM at 37 °C for 24 h. After co-incubation with 20 μg/mL Ti3C2 for 8 h, the cells were treated with 1 W/cm2 808 nm laser for 5 min; 1 W/cm2 ultrasound stimulation for 3 min, and both laser and ultrasound irradiation. The cells were treated with different ultrasound power densities (0, 0.5, 1, 1.25 W/cm2). The treated cells were cultured in Incucyte (ESSEN Bioscience, Incucyte S3, USA) for 48 h and dynamically detected by the fluorescence intensity of DCFH.
Hep3B and SK-Hep-1 mammospheres (50–100 μm) were incubated with 20 μg/mL Ti3C2 for 8 h. The mammospheres were treated with 1 W/cm2 808 nm laser for 5 min, 1 W /cm2 ultrasound for 3 min, and both laser and ultrasound. The spheroids were treated with different ultrasound power densities (0, 0.5, 1, 1.25 W/cm2). The treated mammospheres were incubated with DCFH-DA for 12 h, washed with PBS and centrifuged at 800 rpm for 3 min, then resuspended in PBS for detection by CLSM including Z-stack imaging (Carl Zeiss LSCM 880, Germany).
In vitro Photothermal Effect of Ti3C2
The in vitro photothermal effect of Ti3C2/TiO2 was measured separately under 808 nm, or 1064 nm laser irradiation (1 W/cm2) for 10 min at different concentrations (25, 50, 100, and 200 μg/mL). The photothermal stability of Ti3C2/TiO2 was tested by measuring the periodic heating under 808, or 1064 nm laser irradiation (1 W/cm2) for 10 min and the cooling process of Ti3C2/TiO2 for 5 cycles. Hep3B, Bel-7402, and SK-Hep-1 were cultured in 10 cm dishes with DMEM at 37 °C and incubated with 40 μg/mL Ti3C2/TiO2 for 8 h, then washed with PBS and centrifuged at 800 rpm for 3 min. The cell mass was collected and measured under 808, or 1064 nm laser irradiation (1 W/cm2) for 10 min. All infrared thermographs were taken using a Fluke thermal imager (Fluke, USA), and the temperature was recorded.
Cellular Uptake of Ti3C2 in vitro
Hep3B cells were seeded into the 6-well cell culture plate at a density of 1×105 cells per well. Hep3B cells were then incubated with 5-FAM-labelled Ti3C2 (20 μg/mL) for different time intervals (0, 2, 4, 6, 8, 12 h). Cells were harvested and detected by flow cytometry using the B525-FITC channel. Hep3B cells were seeded into the cover glass bottom dish at a density of 105 cells per well. Hep3B cells were incubated with Ti3C2 (20 μg/mL) labeled with Cy-5 for different time intervals (0, 2, 4, 6, 8, 12 h). The cells were then detected and photographed using laser confocal scanning microscopy.
Photoacoustic Imaging
Ti3C2 dispersed in PBS solution and photoacoustic images of Ti3C2 at different concentrations (0, 25, 50, 150, 200 μg/mL) in vitro were photographed using the Vevo FUJIFILM VisualSonics system. Ti3C2 solution was injected intravenously into the tumor-bearing mouse and photographed at different time intervals (0, 2, 4, 6, 8, 10, 12, 24 h).
Western Blot
Western blot assays were carried out as previously described. Briefly, cells were treated as indicated. Total cellular lysates were prepared and followed to SDS-PAGE. Gels were then electro transferred to PVDF membranes and blotted against the primary antibodies after being blocked with 5% milk solution. Primary antibodies against GAPDH, Cleaved caspase 3, Cleaved PARP, and P53 were purchased from Cell Signaling Technology (USA). Antibodies against NRF2 were obtained from Abcam (UK), and OSGIN1 was purchased from Proteintech (USA). HRP-conjugated antibodies were then incubated with the membranes and subjected to ECL analysis. Chemiluminescence results were captured by iBright FL1500 (Invitrogen, Thermofisher Scientific, USA).
RNA-Seq Analysis and Quantitative PCR
Briefly, cells were lysed in Trizol solution, and mRNA was obtained by Ribobio Company (Guangzhou, China). Transcriptome analyses were performed and results were analyzed by GraphPad software. Quantitative PCR experiments were conducted by RT-qPCR mix (Takara, Japan). Results from three independent experiments were statistically analyzed and graphed.
Immunohistochemistry (IHC) Assays
Tissue slides were cropped into 5 μm slices and subjected to dewaxing, H&E and IHC analysis were carried out as previously described. Briefly, slices were incubated with antibodies against CD4, CD8, TNF-α, and IFN-γ (Bioss, Beijing, China). Subsequently, they were subjected to DAB staining and scanned by 3D Histech. Representative pictures were shown from each treated group.
Immunofluorescence Staining
Cells were cultured in sterilized over slides plated in 6-well plates and treated as indicated. After the treatment cover slides were fixed with 4% paraformaldehyde and permeabilized by Triton-X100. Then, cells were stained with primary antibodies, followed by Alexafluo-488nm or 596 nm conjugated secondary antibodies. DAPI was used as counterstaining to visualize nucleus. Cells were then transferred to slides and observed under confocal laser scanning microscopes. For tissues, slides from OCT-embedded tumor tissues were prepared freshly after being excised freshly from animal models and deposited at −80 °C. Just before use, cells were rewarmed to temperature. Slides were rinsed with PBS solution and stained with the antibodies as described above.
Animal Studies
H22 cells were inoculated subcutaneously into Balb/c mice. Two weeks after inoculation, when tumors grow to palpable size. Mice were randomly divided into five groups and treated with PBS, Ti3C2, Ti3C2+ultrasound, Ti3C2+laser, Ti3C2+ultrasound+laser. For the bilateral subcutaneous model, H22 cells were inoculated on two sides on the back, proximal side of one group received Ti3C2 +ultrasound+laser treatment, while the distal tumors were not. Body weight and tumor size were recorded every four days just before each treatment. After six treatment sessions, blood was collected and the mice were anesthetized. Organs were harvested, followed by ICP testing or fixed in paraformaldehyde. Tumors were weighed and measured. Photographs were taken of each group to compare the size of each group. All tumors were fixed for further immunohistochemistry or H&E analysis. All animal studies were conducted in accordance with the National Animal Welfare Guidelines (GB/T 35892–2018) and approved by the Animal Ethics Committee of the Fifth Affiliated Hospital of Sun Yat-sen University (Approval No. 00153).
Statistical Analysis
All experiments were performed at least three times. Representative images were selected to be shown in the figures. All figures were plotted and statistically analyzed using GraphPad prism 8.0 software. Student’s t-test or two-way ANOVA was used to compare two or more groups. p<0.05 was considered statistically significant.
Results and Discussion
Synthesis and Characterization of Ti3C2 and Ti3C2/TiO2
Ti3C2 was synthesized by a chemical exfoliation strategy according to the previous report.27 The Ti3AlC2 power was etched by LiF and HCl solutions for removing the Al layer, and the Ti3C2 layer was obtained after centrifugation. TEM image of Ti3C2 layer has been shown in Figure 2A, and the AFM image of Ti3C2 layer revealed a 2D structure (Figure 2B). The XRD result showed that the nanostructure consists of Ti3C2, as shown in Figure 2C. Defects in Ti3C2 nanosheets can react with H2O2 to form the two-dimensional heterogeneous Ti3C2/TiO2 structure in situ. The Ti3C2/TiO2 heterostructures were prepared by the oxidation reaction of Ti3C2 and transmission electron microscopy (TEM) revealed an ultrathin layer structure of Ti3C2/TiO2, as shown in Figure 2D. Ti3C2/TiO2 has a fusiform shape and the size of the obtained nanostructure is 100–200 nm. There are some wrinkles on the nano-layer. The HRTEM images showed that the TiO2 was randomly decorated on the Ti3C2 layers (Figure 2E). The XRD pattern in Figure 2F confirmed that the composite structure was composed of Ti3C2 and TiO2. ESR measurements showed that Ti3C2 can convert H2O2 to ·OH and 1O2, and ultrasound enhances the generation of ·OH and 1O2. (Figure 2G and H). The Zeta potential is shown in Figure S4.
Figure 2I shows XPS spectra of Ti3C2 and Ti3C2/TiO2 heterostructures. The full XPS spectra show O 1s, Ti 2p, and C1s peaks at 530.2, 456.2, and 283.5 eV for both samples. The Al 1s peak appears negligibly small, indicating good removal of Al layers during the etching process. The O 1s peak intensity of Ti3C2/TiO2 heterostructure is much stronger than that of Ti3C2Tx MXene, which means that there is a significant amount of oxide in the heterostructure. Interestingly, the F 1s peak is clearly found at 683.4 eV for Ti3C2Tx, but it is drastically reduced for Ti3C2/TiO2, suggesting that most of the F functional groups on the original MXene disappear during the transformation. Focused XPS spectra of Ti 2p and C 1s are shown in Figure 2J and K. The Ti 2p3/2 components centered at 455.4, 456.3, 457.5, and 459.2 eV are attributed to Ti-C bonds, Ti-X from titanium oxycarbides or substoichiometric TiCx (x < 1), Ti ions with reduced charge state (TixOy) and Ti4+ ions (TiO2), respectively.28 After oxidation with H2O2, the relative percentage of Ti4+ ions (TiO2) increases from 6.14% to 60.60%, and the relative percentage of Ti-C bonds shows a remarkable decrease from 31.87% (for Ti3C2) to 14.91% of (for Ti3C2/TiO2), indicating that a part of Ti3C2 was converted to TiO2 by oxidation with H2O2. The C 1s XPS spectrum of Ti3C2 shows five obvious peaks at 282.3, 283.4, 284.8, 286.4, and 288.9 eV, corresponding to Ti-C, C-Ti-O, C-C, C-O and C-F bonds, respectively.29
The oxidation process by H2O2 in situ was hypothesized by DFT calculations. First, we constructed the Ti3C2 monolayer structures with and without defects, as illustrated in Figure S1. We optimized the geometries of the Ti3C2Tx (Tx=-O) monolayer structure. The DFT calculated the atomic electron density difference maps, which are displayed in Figure 2L. The blue area indicates a decrease in electron density, while the red area indicates an increase. Figure S1 shows the atomic electron density difference maps of cross-sections that include H2O2 and Ti3C2 atoms. Figure 2L presents an enlarged view of the selected cross-sections, displaying the electron density difference. The coordinates on the right indicate the electron gain and loss for each Ti3C2 atom before and after the H2O2 adsorption. “+” and “-” signs indicate electron gain and loss, respectively. The electron density difference map of the Ti3C2 with titanium vacancies indicates an enlarged electron gain and loss region, revealing increased bonding between atoms. Additionally, the presence of Ti defects enhances the electron gain ability of H2O2, indicating stronger binding interactions between H2O2 and the surface of Ti3C2. The DFT calculations showed that Ti vacancies in Ti3C2 were the primary reactive site for the oxidation of Ti3C2 to form Ti3C2/TiO2 heterostructures.
Photothermal Effect of Ti3C2 Nanoparticles
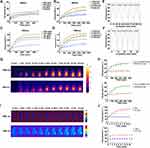
To investigate the photothermal conversion of nanoparticles, we applied a gradient concentration of Ti3C2 in a solution. We then examined its photothermal effect after irradiation with a near-infrared imager. The results demonstrate that varying concentrations of Ti3C2 caused an increase in temperature in the solution under both laser irradiations. The temperature of the Ti3C2 solution increased with 808 nm or 1064 nm laser irradiation (1 W/cm2), resulting in a rise from 40.8 °C to 53.9 °C and from 43.3 °C to 81.2 °C, respectively (Figure 3A–C). When the laser power increased from 0.5 W to 1.5 W, the Ti3C2 solution escalated from 40.6 °C to 64.8 °C (200 ppm, 808 nm, Figure 3B). Similarly, the temperature of Ti3C2 (200 ppm) increased from 45.5 °C to 84.0 °C under 1064 nm irradiation (Figure 3D). The photothermal stability was successfully demonstrated by subjecting it to either an 808 nm or 1064 nm laser (1 W/cm2) for 5 minutes and cooling it to room temperature for five cycles. No degradation was observed after 5 heating and cooling cycles, even under continuous laser irradiation (Figure 3E and F). These results indicate that Ti3C2 possesses superior photothermal stability and has the potential to be used as a photosensitizer in the NIR region. Based on the results obtained from the Ti3C2 solution, we incubated Hep3B cells with Ti3C2. We consistently observed a gradual increase in temperature after NIR light irradiation at both 808 and 1064 nm. PBS-treated cells did not display any significant temperature increase. This supports the conclusion that Ti3C2 induced a specific temperature increase in Hep3B cells, as shown in Figure 3G and H. To determine the photothermal effect of Ti3C2 in vivo, we established a subcutaneous xenograft model and analyzed the photothermal conversion of Ti3C2/TiO2. Ti3C2 was injected into the tail vein, and we used an infrared camera to detect the temperature increase induced by 808 nm or 1064 nm laser in the tumors. This experiment provides further insight into the photothermal properties of Ti3C2/TiO2 in vivo. As shown in Figure 3I and J, Ti3C2/TiO2 groups irradiated with 2 W/cm2 of 808 nm or 1064 nm irradiance recorded a significant temperature increase to 57.7 °C and 86.7 °C, respectively. Taken together, these findings demonstrate that Ti3C2/TiO2 exhibits exceptional photothermal conversion efficiency and stability, rendering it a promising candidate for tumor eradication.
Cellular Uptake of Ti3C2
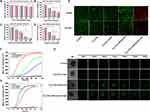
Since the uptake of nanodrugs into tumor cells is necessary for their anti-tumor effect, we analyzed the uptake of our nanoparticles in HCC cells using confocal laser scanning microscopy and flow cytometry assays. Figures 4A and S5 display increased Cy5 signals under microscopy, indicating the gradual uptake of Ti3C2 by HCC cells with extended treatment. The flow cytometry results quantified that the maximum uptake of Ti3C2 occurred at 12 hours (Figure 4B and C). This study demonstrated that Ti3C2 was internalized by HCC cells in a time-dependent manner, indicating its potential for future therapeutic applications. The depth of nanodrug penetration into tumor spheres may ensure adequate drug delivery into tumor tissues. To verify this, we incubated Hep3B tumor mammospheres with 5’FAM-labeled Ti3C2 and assessed its penetration using the Z-stack mode of confocal laser scanning microscopy. As depicted in Figure 4D, Ti3C2/TiO2 exhibits a superior penetration depth within the tumor mammospheres in comparison to 5-FAM, which only penetrates the surface. This observation validates Ti3C2 capability to penetrate the mammospheres significantly. The combination of high uptake and deep penetration contributes to the accumulation of Ti3C2/TiO2 in the core of the tumor and the therapeutic efficacy of Ti3C2/TiO2.
The excellent photoacoustic (PA) property of Ti3C2 is due to its NIR abundance and good photothermal conversion ability. The accumulation of Ti3C2/TiO2 in the tumor section was confirmed through photoacoustic imaging. Ti3C2/TiO2 at different concentrations was detected by PA tomography in Figure 4E. As the Ti3C2/TiO2 concentration was increased from 0 to 200 ppm, the brightness and intensity of the PA signal steadily increased. A direct relationship was observed and depicted in Figure 4F between the concentration of Ti3C2/TiO2 and photoacoustic signal. Based on the aforementioned findings, it is inferred that Ti3C2/TiO2 exhibits commendable photoacoustic properties. Moreover, the in vivo photoacoustic properties of Ti3C2/TiO2 were investigated in Hep3B tumor-bearing mice. Subcutaneous xenograft models were established and Ti3C2 was administered through the tail vein. Photoacoustic imaging was observed and recorded. The photoacoustic (PA) signal at the tumor site increased over time, with a maximum of 6 hours after injection (Figure 4G and H). The PA signal significantly decreased 24 hours after injection, indicating the successful accumulation of Ti3C2/TiO2 in the tumor tissue and its potential for long-term treatment. These findings demonstrate the efficient internalization of Ti3C2 into HCC cells and its ability to induce a photoacoustic effect on tumor models, supporting its potential for targeted tumor therapy.
The Anticancer Performance in vitro and the Cellular Internalization
Based on the above results analyzing the uptake of Ti3C2 in HCC tumors, we investigated its effect on cell viability. Initially, we evaluated the cytotoxicity of Ti3C2/TiO2 in vitro using the CCK-8 assay, where it was incubated with three HCC cell lines including Hep3B, SK-Hep-1, Bel-7402, and normal cell HEK-293T. As indicated in Figure 5A, cell viability remains at 80% of the control even when incubated with high concentrations of Ti3C2 (60 μg/mL) for 24 hours, implying that Ti3C2 has excellent therapeutic potential due to its low cellular toxicity. The therapeutic effect of Ti3C2/TiO2 induced by laser and ultrasound was evaluated. Cancer cell viability decreased as the laser power density increased under laser irradiation. Cell viability dropped below 40% when the laser power density reached 1.5 W/cm2 (Figure 5B). Cell viability significantly decreased as ultrasound power increased in all three cell lines, as demonstrated in Figure 5C. At the highest ultrasound power intensity (1.25 W/cm2), only about 10% of cells remained viable. Longer laser irradiation treatments resulted in more cancer cell death, as displayed in Figure 5D. Furthermore, both laser and ultrasound treatment caused concentration- or time-dependent cell death in Hep3B cells (Figure S2). Importantly, the in vitro anticancer performance of Ti3C2 was systematically investigated using Calcein AM/PI detected by confocal laser scanning microscopy (CLSM) as shown in Figure 5E. Further, ultrasound and near-infrared (NIR) laser treatments of Ti3C2 resulted in the highest rate of dead cells labeled by red staining, demonstrating the best antitumor effect induced by synergistic photothermal and sonodynamic therapies.
ROS Was Involved in Ti3C2/TiO2-Induced Photothermal and Sonodynamic Therapy
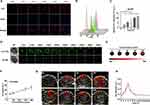
ROS has been identified as a crucial molecular trigger for sonodynamic therapy. We examined the ROS levels following treatment of Ti3C2 with synergistic ultrasound and laser. To dynamically monitor the ROS increase, we incubated Hep3B cells with 2.7-dichlorofluorescein diacetate (DCFH-DA) probes. ROS levels were monitored in real-time using the Incucyte S3 system. As illustrated in Figure 5F, the fluorescence intensity of DCF increased steadily from 5 hours of ultrasound exposure to 35 hours. The Ti3C2/TiO2-induced ROS generation via sonodynamic therapy was significantly higher under ultrasound compared to laser activation. Additionally, the ROS generation of Ti3C2 increased proportionally with the ultrasound power. Below 1 W/cm2 of ultrasound power, the ROS generation of Ti3C2/TiO2 reached its peak (Figure 5G). Importantly, we investigated the level of ROS in three-dimensional mammosphere models that recapitulate the stemness and microenvironment of the original tumor. As demonstrated in Figure 5H, the groups treated with laser irradiation and ultrasound show the strongest green fluorescent signal, covering the entire tumor mammospheres formed by Hep3B. Additionally, a comparable pattern of green signal is observed in the mammospheres treated with ultrasound. However, laser irradiation alone induced a low green signal in the mammospheres, suggesting the exceptional ROS enhancement of the Ti3C2/TiO2 heterostructure induced by ultrasound. A similar ROS alteration was observed in the three-dimensional mammospheres constituted by SK-Hep-1 cells (Figure S3). These findings support the notion that Ti3C2 can act as a proficient sonosensitizer, resulting in excellent ROS production for SDT, which is vital for its antitumor efficacy.
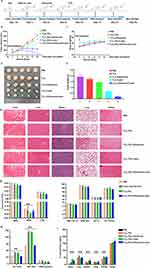
Ti3C2 Inhibited Tumor Growth in Xenograft Models
To examine the synergistic effect of Ti3C2/TiO2 photothermal and sonodynamic therapy on tumor ablation in vivo, we administered Ti3C2-induced SDT and PTT treatment to tumor models. Subcutaneous H22 cells were inoculated in Balb/c mice which were then divided into five groups. The mice were treated with either PBS or Ti3C2, and ultrasound and laser treatment modalities were applied as depicted in Figure 6A. The tumors were treated at four-day intervals and observed for 21 days. The tumor volume for each group was recorded and graphed against the treatment intervals. The group treated with laser plus ultrasound had significantly smaller tumors than other groups, indicating a synergistic effect of photothermal and sonodynamic therapy (Figure 6B). Body weight slightly increased in all groups instead of decreasing, showing no obvious toxic effect of photothermal and sonodynamic therapy in vivo (Figure 6C). Finally, all mice were sacrificed, and tumors were harvested. Representative tumor images from each group were displayed in Figure 6D. Tumor growth was inhibited after ultrasound or laser treatment alone, while tumors treated with both ultrasound and laser were the most inhibited. Figure 6E displays the tumor weights from each group, demonstrating that the combined strategy of photothermal and sonodynamic treatment is the most effective in inhibiting tumor growth. All of these outcomes demonstrate that the use of Ti3C2/TiO2-enhanced sonodynamic therapy increases the inhibitory impact on tumor growth, implying significant potential for the combined therapy of photothermal and sonodynamic effects.
Furthermore, hearts, livers, spleens, kidneys, and lungs were collected from each group after 21 days. The H&E staining results revealed no discernible damage or toxic effects. It was demonstrated that Ti3C2/TiO2 and its associated therapies do not have any evident side effects in vivo (Figure 6F). Notably, no noteworthy changes in parameters, such as WBC, NEUT, LYM, RBC, LHGB, HCT, or PLT, were observed in routine blood tests (Figure 6G). Furthermore, liver and kidney function tests did not show significant damage to these organs after treatment with Ti3C2 and its associated ultrasound and laser treatment (Figure 6H). ICP results demonstrate that the accumulation of Ti3C2/TiO2 mostly occurs in the liver, suggesting that it is predominantly metabolized by this organ (see Figure 6I). These findings suggest strong blood biocompatibility of Ti3C2/TiO2 in vivo Figure 6I.
Ti3C2/TiO2 Perturbs Oxidative Homeostasis and is Associated with Apoptosis in HCC Tumor
We confirmed the impact of Ti3C2-based SDT therapies on HCC cell viability and ROS levels. These results prompted an investigation into the relationship between Ti3C2/TiO2-regulated signaling pathways and cell death. We executed a series of experiments to unravel the molecular mechanisms underlying this effect. Initially, we treated Hep3B or SK-Hep-1 cells with or without laser irradiation and ultrasound treatment. Total RNA lysates were prepared and subjected to RNA-seq analysis. Differences in gene expression between the control and PTT/SDT groups were depicted via volcano plots (Figure 7A and B). Genes that exhibited differential expression in both cell lines post-treatment were identified and counted. The expression of 228 or 332 genes significantly changed after treatment in Hep3B or SK-Hep-1 cells, respectively, as evinced by the Venn diagram (Figure 7C). To eliminate the distinction between the cell lines treated with sonodynamic therapy, we selected 15 co-differentially expressed genes in both cell lines. Among them, nine genes exhibited a consistently up-regulated pattern. The up-regulated expression pattern of these nine genes is presented as a heat map in Figure 7D. Upon analyzing these genes, we identified a potential correlation between oxidative stress-induced growth inhibitor 1 (OSGIN1) and ROS. OSGIN1, also known as OKL38, encodes a protein that responds to oxidative stress and regulates cell death.30 This prompted us to further investigate the relationship between OSGIN1 and SDT after Ti3C2/TiO2 treatment. We examined the expression of genes related to apoptosis following Ti3C2/TiO2 treatment in the presence of laser or ultrasound treatment. We observed that sonodynamic plus photothermal therapy induced by Ti3C2/TiO2 led to the expression of cleaved caspase 3 and cleaved PARP, indicating apoptosis of HCC cells. OSGIN1 and its upstream regulator NRF2 also increased in the SDT-treated group (Figure 7E). To clarify the role of ROS in apoptosis of HCC cells induced by combinatorial therapies, we pre-treated cells with Manganese (III) Tetrakis (1-Methyl-4-Pyridyl) Porphyrin (MnTmPyP), a superoxidase mimic, to eliminate ROS in the case of Ti3C2/TiO2 treatment with laser and ultrasound. Figure 7F clearly shows that the elimination of ROS reversed the increase in OSGIN1 expression. The expression of cleaved caspase 3 and cleaved PARP was reversed upon scavenging ROS via MnTmPyP treatment. Correspondingly, silencing OSGIN1 and NRF2 abolished the expression of cleaved caspase 3 and cleaved PARP. This demonstrates that ROS-modulated OSGIN1 signaling is necessary for SDT-induced apoptosis mediated by Ti3C2 (Figure 7G). Taken together, these results demonstrate that OSGIN1 signaling regulated by ROS, played a critical role in the apoptosis of HCC cells induced by Ti3C2/TiO2-mediated sonodynamic and photothermal combination therapies.
Finally, our conclusion was validated on xenograft animal models. Treatment with Ti3C2/TiO2 in combination with ultrasound and laser therapy resulted in a significant increase of ROS in tumor tissues from xenograft mouse models. (bottom panel of Figure 7H). Furthermore, OSGIN1 and its upstream regulator NRF2 were upregulated in the SDT and PTT treated groups (upper panel of Figure 7H). Ti3C2/TiO2-induced photothermal and sonodynamic therapies were found to induce apoptosis in tumor tissues, indicated by the strongest staining of cleaved caspase 3 and PARP in comparison to PBS and other groups (middle panel of Figure 7H). The results obtained from in vitro cell lines are in accordance with in vivo findings, further supporting the conclusion that ROS-modulated OSGIN1 signaling plays a critical role in Ti3C2/TiO2-induced tumor inhibition in both HCC cells and mouse models.
Synergistic Therapy Induced an Immune-Active TME
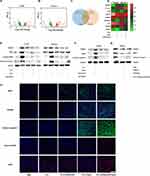
ROS are reported to be involved in various aspects of immune modulation, including T-cell activation, inflammation, and antigen presentation in the hypoxic tumor microenvironment.31 It was crucial to reveal the molecular mechanisms by elucidating the role of Ti3C2/TiO2-induced ROS in the immune milieu.30 The tumor microenvironment (TME), known to have immune-suppressive properties, is linked to the recurrence and deterioration of malignant cancers.32 Reversing immunosuppression has gained attention for improving the efficacy of tumor ablation.33,34 Our RNA-seq results (Figure 7D) also demonstrate the modulation of inflammation-associated molecules IL24 by Ti3C2/TiO2 treatment. We are currently investigating the anti-tumor immune response of synergistic therapy for HCC treatment. Following treatment with various therapies, spleen and blood lymphocytes were collected from mice and analyzed via flow cytometry. It is noteworthy that the spleen’s CD8+ T cell percentage significantly increased in the group receiving synergistic therapy compared to untreated mice (PBS group) (21.5% versus 13.0%). Interestingly, the synergistic group exhibited stronger activation phenotypes and greater TNF-α production (21.5% versus 6.02%) in splenic CD8+ T cells when compared to other groups. Notably, TNF-α and IFN-γ in CD4+ T cells were also increased, elevating from 4.06% to 14.4% and 1.49% to 4.78%, respectively (refer to Figure 8A). Significant enhancement of T cells anti-tumor activity in the blood of the synergistic group was noted. Consistently, TNF-α levels were increased in both CD8+ and CD4+ T cells (from 7.02% to 19.4% and 5.09% to 9.95%, respectively) while IFN-γ levels were increased in CD4+ T cells (from 6.43% to 24.7%) in the group treated with both laser and ultrasound, compared with other groups (Figure 8B).
Additionally, an increase of CD4+ and CD8+ T cells in tumor tissues was observed through immunohistochemical staining. The production of functional cytokines, such as IFN-γ by CD8+ T cells, was elevated in tumor tissues following Ti3C2/TiO2 activation using laser and ultrasound (Figure S6). Importantly, investigation of immune-modulatory effect of Ti3C2 on tumor growth and tumor-draining lymph nodes were investigated in bilateral tumor models. As shown in Figure S7A and B, Ti3C2/TiO2 treatment along with laser and ultrasound elicited dendritic cell maturation reflected by an increase of CD80+/CD86+ cells implicating activation of the immune system in tumor-draining lymph nodes. This modulatory effect of tumor immune microenvironment elicited by sonodynamic and photothermal therapy of proximal tumor might cause suppression of tumor growth of distal tumor growth (Figure S7D and E). Consistently, P53 as pivotal upstream regulator of NRF2/OSGIN1 signaling, was found to be upregulated after Ti3C2/TiO2 treatment plus ultrasound and laser (Figure S7C). These findings suggest that Ti3C2 can transform the immune microenvironment from “immunosuppressive” to “antitumor” and enhance tumor elimination by activating adaptive antitumor immunity.
Conclusions
Ti3C2 was successfully synthesized and characterized in the study. It exhibits multi-enzyme activity, such as intrinsic peroxidase (POD) activities that react with H2O2 in the tumor microenvironment, constructing a Ti3C2/TiO2 heterostructure in vivo. This integrates photothermal and sonodynamic effects for image-guided cancer therapy and induces multifaceted anticancer immune responses. We observed an ultrasound-induced surge of ROS in Ti3C2/TiO2-treated HCC cells and tumor tissues resulting from sonodynamic therapy. In HCC cells and animal models, we observed synergistic effects of the photothermal and sonodynamic effects of Ti3C2/TiO2. Notably, Ti3C2/TiO2 induced immune-mediated destruction of tumor tissues through recruitment of CD4+ and CD8+ T cells and cytokine secretion, complementing the conventional apoptosis-inducing effect of photothermal and sonodynamic therapies. Our study presents a new approach for ablating HCC tumors using Ti3C2/TiO2 to regulate the tumor microenvironment. This is done by adjusting the ROS balance through the NRF2/OSGIN1 pathway and promoting immune activation. Major findings in different treatments by Ti3C2/TiO2 were summarized in Table S1.
Data Sharing Statement
All the data supporting this study are available from the corresponding authors upon reasonable request.
Ethical Approval Statement
All animal experiments were approved by the Animal Ethics Committee of the Fifth Affiliated Hospital, Sun Yat-sen University. Detailed information can be found in the “Materials and Methods” Section.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Guangdong Basic and Applied Basic Research Foundation (2023A1515010620, 2022A1515012482), Youth Program of National Natural Science Foundation of China 82001837, Zhuhai basic and applied basic research project ZH22017003210009PWC, Excellent Young Researchers Program of the 5th Affiliated Hospital of SYSU WYYXQN-2021010, and the Guangdong-Hong Kong-Macao University Joint Laboratory of Interventional Medicine Foundation of Guangdong Province (2023LSYS001).
Disclosure
Hailing Yu, Yongquan Huang and Zhisheng Nong are co-first authors for this study. The authors declare no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022, CA. Cancer J Clin. 2022;72:7–33. doi:10.3322/caac.21708
2. Villanueva A, Longo DL, Longo DL. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450–1462. doi:10.1056/NEJMra1713263
3. Miller KD, Nogueira L, Devasia T, et al. Cancer treatment and survivorship statistics, 2022, CA. Cancer J Clin. 2022;72:409–436. doi:10.3322/caac.21731
4. Kadkhoda J, Tarighatnia A, Barar J, Aghanejad A, Davaran S. Recent advances and trends in nanoparticles based photothermal and photodynamic therapy. Photodiagn Photodyn Ther. 2022;37:102697. doi:10.1016/j.pdpdt.2021.102697
5. Zhu L, Li Y, Jiang M, et al. Self-assembly of precisely fluorinated albumin for dual imaging-guided synergistic chemo–photothermal–photodynamic cancer therapy. ACS Appl Mater Inter. 2023;15:2665–2678—.
6. Wang X, Wang C, Xu Y, et al. The multifunctional Prussian blue/graphitic carbon nitride nanocomposites for fluorescence imaging-guided photothermal and photodynamic combination therapy. RSC Adv. 2023;13:335–343. doi:10.1039/D2RA07022G
7. Deng X, Shao Z, Zhao Y. Solutions to the drawbacks of photothermal and photodynamic cancer therapy. Adv. Sci. 2021;8. doi:10.1002/advs.202002504
8. Hu H, Feng W, Qian X, Yu L, Chen Y, Li Y. Emerging nanomedicine‐enabled/enhanced nanodynamic therapies beyond traditional photodynamics. Adv Mater. 2021;33. doi:10.1002/adma.202005062
9. Fan Z, Zhuang C, Wang S, Zhang Y. Photodynamic and photothermal therapy of hepatocellular carcinoma. Front Oncol. 2021;11. doi:10.3389/fonc.2021.787780
10. Xu K, Chang M, Wang Z, et al. Multienzyme-mimicking lacoo 3 nanotrigger for programming cancer-cell pyroptosis. Adv Mater. 2023;35:e2302961. doi:10.1002/adma.202302961
11. Yu H, Tang K, Cai Z, et al. Carbon Dots-Based Nanozyme for Drug-Resistant Lung Cancer Therapy by Encapsulated Doxorubicin/siRNA Cocktail. Int J Nanomed. 2023;18:933–948. doi:10.2147/IJN.S390984
12. Chen J, Fu W, Jiang F-L, Liu Y, Jiang P. Recent advances in 2D metal carbides and nitrides (MXenes): synthesis and biological application. J Mat Chem B. 2023;11:702–715—.
13. Du W, Chen J, Wang L, et al. Oxygen-deficient titanium dioxide-loaded black phosphorus nanosheets for synergistic photothermal and sonodynamic cancer therapy. Biomater Adv. 2022;136:212794. doi:10.1016/j.bioadv.2022.212794
14. Um W, K PKE, Lee J, Kim CH, You DG, Park JH. Recent advances in nanomaterial-based augmented sonodynamic therapy of cancer. Chem Commun. 2021;57:2854–2866. doi:10.1039/D0CC07750J
15. Lafond M, Yoshizawa S, Umemura S-I. Umemura, Sonodynamic Therapy: Advances and challenges in clinical translation. J Ultrasound Med. 2019;38:567–580. doi:10.1002/jum.14733
16. Li Y, Yang J, Sun X. Reactive oxygen species-based nanomaterials for cancer therapy. Front Chem. 2021;9:650587
17. Wu M, Ding Y, Li L. Recent progress in the augmentation of reactive species with nanoplatforms for cancer therapy. Nanoscale. 2019;11:19658–19683. doi:10.1039/C9NR06651A
18. Guo J, Pan X, Wang C, Liu H. Molecular Imaging-Guided Sonodynamic Therapy. Bioconjugate Chem. 2021;33:993–1010. doi:10.1021/acs.bioconjchem.1c00288
19. Sun L, Wang P, Zhang J, et al. Design and application of inorganic nanoparticles for sonodynamic cancer therapy. Bio Sci. 2021;9:1945–1960. doi:10.1039/D0BM01875A
20. Son S, Kim JH, Wang X, et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem Soc Rev. 2020;49:3244–3261. doi:10.1039/C9CS00648F
21. Wang C, Tian Y, Wu B, Cheng W. Recent progress toward imaging application of multifunction sonosensitizers in sonodynamic therapy. Int j Nanomed. 2022;17:3511–3529. doi:10.2147/IJN.S370767
22. Balou S, Shandilya P, Priye A. Carbon dots for photothermal applications. Front Chem. 2022;10. doi:10.3389/fchem.2022.1023602
23. Xiao Y, Pandey K, Nicolás-Boluda A, et al. Synergic Thermo- and pH-sensitive hybrid microgels loaded with fluorescent dyes and ultrasmall gold nanoparticles for photoacoustic imaging and photothermal therapy. ACS Appl Mater Inter. 2022;14:54439–54457. doi:10.1021/acsami.2c12796
24. Paściak A, Marin R, Abiven L, et al. Quantitative Comparison of the Light-to-Heat Conversion Efficiency in Nanomaterials Suitable for Photothermal Therapy. ACS Appl Mater Inter. 2022;14:33555–33566. doi:10.1021/acsami.2c08013
25. Zhang L, Yang A, Ruan C, et al. Copper-Nitrogen-Coordinated Carbon Dots: transformable Phototheranostics from Precise PTT/PDT to Post-Treatment Imaging-Guided PDT for Residual Tumor Cells. ACS Appl Mater Inter. 2023;15:3253–3265—.
26. Ge EJ, Bush AI, Casini A, et al. Connecting copper and cancer: from transition metal signalling to metalloplasia. Nat Rev Cancer. 2022;22:102–113. doi:10.1038/s41568-021-00417-2
27. Yue Q, Sun J, Chen S, et al. Hierarchical Mesoporous MXene–NiCoP Electrocatalyst for Water-Splitting. ACS Appl Mater Inter. 2020;12:18570–18577. doi:10.1021/acsami.0c01303
28. Rakhi RB, Ahmed B, Hedhili MN, Anjum DH, Alshareef HN. Effect of Postetch Annealing Gas Composition on the Structural and Electrochemical Properties of Ti2CTx MXene Electrodes for Supercapacitor Applications. Chem Mater. 2015;27:5314–5323. doi:10.1021/acs.chemmater.5b01623
29. My Tran N, Thanh Hoai Ta Q, Noh J-S. Unusual synthesis of safflower-shaped TiO2/Ti3C2 heterostructures initiated from two-dimensional Ti3C2 MXene. Appl Surf Sci. 2021;538:148023. doi:10.1016/j.apsusc.2020.148023
30. Huang J, Xiao Z, An Y, et al. Nanodrug with dual-sensitivity to tumor microenvironment for immuno-sonodynamic anti-cancer therapy. Biomaterials. 2021;269:120636. doi:10.1016/j.biomaterials.2020.120636
31. Zhao C, Deng H, Chen X. Harnessing immune response using reactive oxygen Species-Generating/Eliminating inorganic biomaterials for disease treatment. Adv Drug Delivery Rev. 2022;188:114456. doi:10.1016/j.addr.2022.114456
32. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nature Med. 2013;19:1423–1437. doi:10.1038/nm.3394
33. Kim M, Lee JS, Kim W, et al. Aptamer-conjugated nano-liposome for immunogenic chemotherapy with reversal of immunosuppression. J Control Rel. 2022;348:893–910. doi:10.1016/j.jconrel.2022.06.039
34. Yin Y, Jiang T, Hao Y, et al. Cascade catalytic nanoplatform based on ions interference strategy for calcium overload therapy and ferroptosis. Int J Pharm. 2021;606:120937. doi:10.1016/j.ijpharm.2021.120937
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.