Back to Journals » Infection and Drug Resistance » Volume 18
In-Utero Maternal-to-Fetal Transmission of COVID-19: An Immunological and Virological Study in the Eastern Province of Saudi Arabia
Authors Alzayer HA, Hunasemarada BC , Alumran A , Aldossary S, Al Dossary RA
Received 4 November 2024
Accepted for publication 7 March 2025
Published 12 March 2025 Volume 2025:18 Pages 1393—1403
DOI https://doi.org/10.2147/IDR.S501533
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Oliver Planz
Hibah A Alzayer,1,2 Basavaraja C Hunasemarada,1 Arwa Alumran,3 Shaikha Aldossary,4 Reem A Al Dossary1
1Department of Microbiology, College of Medicine, Imam Abdulrahman Bin Faisal University (IAU), Dammam, Saudi Arabia; 2Clinical Laboratory Services, Dammam Medical Complex, Dammam, Saudi Arabia; 3Department of Health Information Management and Technology, College of Public Health, Imam Abdulrahman Bin Faisal University (IAU), Dammam, Saudi Arabia; 4Department of Pediatrics, College of Medicine, Imam Abdulrahman Bin Faisal University (IAU), Dammam, Saudi Arabia
Correspondence: Reem A Al Dossary, Department of Microbiology, College of Medicine, Imam Abdulrahman Bin Faisal University (IAU), P.O.Box 1982, Dammam, 31441, Saudi Arabia, Email [email protected]
Background: Coronavirus disease 2019 (COVID-19) is a respiratory illness caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2). The pandemic potential of the virus along with its severity posed a threat to all individuals particularly pregnant women. Multiple studies suggested the possibility of vertical transmission of COVID-19 with variable findings.
Aim: This study aims to assess the frequency of COVID-19 vertical transmission and identify maternal and neonatal complications.
Materials and Methods: A retrospective study of 17 months for all pregnant women attending for delivery who tested positive using SARS-CoV-2 polymerase chain reaction (PCR) (n = 80) and their neonates (n = 81) who were tested by both SARS-CoV2 PCR and viral IgG and IgM antibodies detection using immunochromatography. A matched control group of PCR negative mothers (n = 51) was included. All testing was done within 24– 48 hours, and the neonates of positive mothers were immediately and completely separated from their mothers as per the hospital policy.
Results: A total of 263 individuals were included in the study. Out of 80 SARS-CoV2 PCR positive mothers, 4 (5%) had PCR positive neonates and one (1.3%) had SARS-CoV2 IgM positive neonates. The commonest presentation of COVID-19 in mothers were cough (11.4%) and dyspnea (10%). In addition, the need for ICU admission and antibiotics usage was significantly higher in SARS-CoV2 PCR positive mothers (p value 0.042, 0.003 respectively). On the other hand, neonates of SARS-CoV2 PCR positive mothers had a higher risk of low birth weight and NICU admission (p value < 0.001).
Conclusion: This study, with its unique infection control protocol for managing SARS-CoV2 PCR-positive mothers and the use of immunological testing for neonates, provides evidence for in-utero SARS-CoV2 transmission, and interpretation of the results should be in conjunction with the WHO categorization of the timing of mother-to-fetal transmission. Further studies are needed to assess the impact of viral genetic evolution on the risk of maternal–fetal transmission.
Keywords: SARS-CoV-2, in-utero, transmission, IgM, PCR
Introduction
Coronavirus disease 2019 (COVID-19) is a highly communicable respiratory illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) first originated in Wuhan, China, in December 20191 and rapidly causing a pandemic declared by the WHO as public health emergency of international significance on January 31, 2020.2 This virus is mainly transmissible via respiratory droplet, or contaminated fomite.3 In addition, vertical transmission has been a hot area of research to facilitate our understanding of the risk of transmission and its complications on both the mother and the newborn and to plan efficient infection control measures.4–8
Vertical transmission of SARS-CoV-2 from pregnant mothers to their newborns can occur in utero, intrapartum or postpartum.9 In utero transmission requires the presence of viremia, which has been documented particularly in severe COVID-19 in 12.7% (27/212) of serum samples tested.10 In addition, the presence of transmembrane protease serine 2 (TMPRSS2) and cell-membrane associated angiotensin-converting enzyme 2 (ACE-2) viral receptors on placental tissue facilitate this transmission.11 Recent report of histopathological evidence of in utero transmission using autopsy analysis of a case of intrauterine death in COVID-19 infected mother demonstrated the detection of viral nucleic acid and viral antigens in multiple tissue including placenta, umbilical cord, brain, lung and liver, but direct viral cytopathological changes were not evident in these tissues.12 Therefore, the mere detection of virus in the placenta and even cord blood was not highly correlated with neonatal infection13 and the need for further testing of neonatal peripheral blood was highlighted. On the other hand, Intrapartum transmission is possible as a result of exposure to maternal blood, vaginal secretion or faecal contamination in normal vaginal delivery14,15 and even exposure to respiratory aerosol at the time of delivery. Postnatal transmission follows the same common respiratory mode of transmissions since the baby will be exposed to the external environment and is at risk for SARS-CoV-2 infection. Since the distinction between these is crucial for better understanding of the viral pathogenesis, the World Health Organization (WHO) issued the WHO Categorization of the timing of mother-to-foetal transmission of SARS-CoV-2 to improve the interpretation and guide clinical testing.9 In order to diagnose in utero, intrapartum or early postnatal transmission, three criteria must be fulfilled including evidence of maternal infection, in utero exposure, viral persistence/ immune response.9
With the accumulation of evidences to support the possibility of vertical transmission, the detection of virus in maternal blood, vagina, and stool and the published evidence of in utero, intrapartum and postnatal transmission,15 we aim to retrospectively investigate the frequency of maternal to fetal SARS-CoV-2 transmission using maternal and neonatal SARS-CoV-2 PCR and neonatal IgG and IgM antibody tests done as part of patient management plan at King Fahad Hospital of the University, AL Khobar, Saudi Arabia, between June 2020 and Oct 2021 and evaluate pregnant mothers and neonatal clinical presentations and complications associated with COVID-19 infections.
Materials and Methods
Study Design
A retrospective study of 17 months duration from June 2020 to October 2021 was conducted in King Fahad Hospital of University (KFHU), Eastern Region, Saudi Arabia, which is a secondary care hospital with 550 beds. A search in the hospital electronic patient data system identified all pregnant women attending for delivery during the study period who were tested using SARS-CoV2 polymerase chain reaction (PCR) as per the hospital policy at that time. All pregnant women who were SARS-CoV-2 PCR-positive (n = 80) and their neonates (n = 81, 1 one set of twins) were included in the study. In addition, a randomly selected matched group of SARS-CoV-2 negative mothers who presented in the same study period and whose neonates were tested using SARS-CoV-2 PCR (n = 51) were included as control group with their neonates (n = 51). All pregnant women and their neonates who were not screened using SARS-CoV-2 PCR at admission were excluded from the study.
The samples tested included maternal and neonatal nasopharyngeal swabs for SARS-CoV-2 PCR and neonatal whole blood samples for SARS-CoV-2 IgG and IgM antibody testing. Maternal screening was done upon admission, and all neonatal tests were performed simultaneously within 24–48 hours after delivery as per the hospital policy. Neonates of SARS-CoV-2 PCR negative mothers were only screened with SARS-CoV-2 PCR and those with SARS-CoV-2 PCR positive mothers were screened with both SARS-CoV-2 PCR and SARS-CoV-2 IgG and IgM antibody testing. As per the hospital policy during the pandemic, to minimize postnatal SARS-CoV-2 transmission from the mother to their newborn babies, all mothers with positive SARS-CoV-2 PCR tests were separated from their neonates for 14 days postnatally and not allowed to breast feed.
SARS-CoV-2 Molecular Testing Using Polymerase Chain Reaction (PCR)
At King Fahad Hospital of University (KFHU) the Xpert Xpress SARS-CoV-2 test using the GeneXpert Instrument Systems (Cepheid, USA) that detect the small envelope (E) glycoprotein and the nucleocapsid (N) protein was used in accordance with manufacturer’s instructions. The samples that were sent to Dammam Regional Lab were tested using a variety of PCR platforms including Kingfisher Flex Purification RNA DNA Extraction System 96 Well (Thermo Fisher, USA), Logix Smart SARSCoV-2 (COVID-K-002) Test Kit on LightCycler® Real-Time PCR Systems reverse transcriptase PCR (Roche, Switzerland) and the PowerChek™ 2019-nCoV Real-time PCR Kit (Kogene, Korea), in accordance with Saudi ministry of health regulations and manufacturers’ instructions.
SARS-CoV-2 IgM and IgG Antibody Testing Using Immunochromatography
Neonatal samples were tested using BIOSYNEX COVID-19 BSS (Whole Blood, Serum or Plasma) (Biosynex, France) at King Fahad Hospital of University (KFHU) according to the manufacturer’s instructions. It is a rapid qualitative membrane-based test that detects IgG and IgM antibody to spike protein Receptor Binding Domain (RBD). Test sensitivity for IgM and IgG is 92.6% and 100%, and specificity is 99.2%, and 99.5%, respectively.
Statistical Analysis
Data were analysed using the chi-square and Fisher’s exact tests on Excel and SPSS-23 software. The chi-squared test was used to determine whether there is a significant association between two categorical variables. In the context of our study, the chi-squared test was used to analyse the relationship between COVID-19 severity and complications in both mothers and neonate and positivity of maternal SARS-CoV2 PCR test. On the hand, Fisher’s exact tests were used when the sample size was small.
Ethical Approval
Ethics approval was obtained from the Imam Abdulrahman bin Faisal University Institutional Review Board (IRB) Committee (reference number: IRB-PGS—2022-01-149) and the study complies with the declaration of Helsinki. In this study, written informed consent was waived due to its retrospective design. The research involved the analysis of pre-existing data available prior to the study’s initiation, and obtaining consent would not have been feasible. All identifiable information was anonymized to protect the confidentiality of participants.
Results
Frequency of SARS-CoV-2 Maternal to Fetal Transmission
During the study period, 263 individuals were included in the study. This includes 131 mothers and 132 neonates (1 one set of twins). Of the 131 mothers, eighty were found to be SARS-CoV-2 PCR positive and fifty-one were negative. Both maternal groups were matched and showed no significant differences in age and comorbidities (Table 1). On the other hand, eighty-one of the neonates were born to SARS-CoV-2 PCR positive mothers and fifty-one were born to SARS-CoV-2 PCR negative mothers.
 |
Table 1 Descriptive Data of Both SARS-CoV-2 PCR Positive and Negative Postnatal Mothers |
All the fifty-one neonates of SARS-CoV-2 negative mothers were SARS-CoV-2 PCR negative, but out of the eighty-one neonates of SARS-CoV-2 PCR positive mothers, four (5%) had a positive SARS-CoV-2 PCR. In addition, 23 (28.8%) had a positive SARS-CoV-2 IgG antibody test and only one (1.3%) showed SARS-CoV-2 IgM positivity (Table 2).
 |
Table 2 Neonatal IgG and IgM Antibody Results |
Impact of the Mode of Delivery on Neonatal SARS-CoV−2 PCR Result
Among neonates delivered via normal vaginal delivery, three had positive SARS-CoV-2 PCR, and 85 were SARS-CoV-2 PCR negative. On the other hand, among those delivered via caesarean section (CS), one had positive SARS-CoV-2 PCR and 39 were negative (p = 0.875) (Table 3).
 |
Table 3 Impact of Delivery Type on Neonatal SARS-CoV −2 PCR Result |
Clinical Presentation of Maternal COVID-19
Majority were asymptomatic (n = 65, 81.3%), 12 (15%) of them had mild-to-moderate disease, and only 3 (3.8%) had severe COVID-19 symptoms. The most common clinical manifestation was cough (11.4%), and dyspnoea (10%) followed by fever (6.25%), vomiting (5.1%) and nausea (3.8%) (Table 4).
 |
Table 4 Clinical Presentation of Postnatal SARS-CoV-2 PCR-Positive Mothers |
Maternal COVID-19 Complications
Among postnatal mothers, the need for ICU admission was significantly higher among SARS-CoV-2 positive mothers (n = 7, 8.9%, p = 0.042) and use of antibiotics treatments (n = 12, 15%, p = 0.003). On the other hand, the need for mechanical ventilation and steroid therapy were only seen SARS-CoV-2 positive mothers with no statistical significance (n = 4, 5%, p = 0.095) (n = 6, 7.5%, p = 0.081), respectively (Table 5).
 |
Table 5 Complications in Postnatal SARS-CoV-2 PCR Positive Mothers Compare to SARS-CoV-2 PCR Negative Postnatal Mothers |
Associations Between Maternal COVID-19 Severity and Chronic Diseases Such as Hypothyroidism, Diabetes Mellitus (DM), Asthma and Anemia
Among the 8 (10%) cases of hypothyroidism in the PCR-positive postnatal mothers, 2 (66%) presented with severe COVID-19 (p = 0.047). On the other hand, there were no association between severity and bronchial asthma (p = 0.941), anaemia (p = 0.855), diabetes mellitus (p = 0.724), or cardiac disease (p = 0.890) (Table 6).
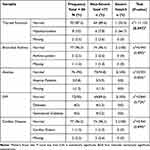 |
Table 6 Associations of COVID-19 Severity in Postnatal Mothers with Chronic Disease |
Impact of Maternal SARS-CoV-2 Positivity on Neonatal Clinical Condition
In comparison to neonate of SARS-CoV-2 PCR negative mothers, those born to SARS-CoV-2 PCR positive mother at higher risk for low birth weight and NICU admissions (p ≤ 0.001) in addition, the use of mechanical ventilation was 7.5% (n = 5) in positive PCR group compared to 3.9% (n = 2) in negative PCR group and there was one case of neonatal death seen in PCR positive group (p = 0.482, p = 1.00, respectively) (Table 7) and (Figure 1).
 |
Table 7 Comparison of Neonatal Clinical Conditions Between SARS-CoV-2 Positive and Negative Postnatal Mothers |
 |
Figure 1 Neonatal conditions based on mothers SARS-CoV2 PCR. |
Discussion
In order to assess the risk of maternal to fetal transmission of SARS-CoV-2, we followed the WHO categorization of the timing of mother-to-fetal transmission of SARS-CoV-2.9 Accordingly, we detected 4 (5%) cases of neonates with positive SARS-CoV-2 PCR test. Only one neonate (1.3%) was IgM positive, zero confirmed in utero SARS-CoV-2 transmission cases, zero possible in utero SARS-CoV-2 transmission cases, zero unlikely in utero SARS-CoV-2 Transmission cases, and five indeterminate in utero SARS-CoV-2 Transmission of SARS-CoV-2.
Another study in Malaysia on 766 neonates of 753 SARS-CoV-2 positive mothers found that the risk of SARS-CoV PCR positivity was 3% (n = 23/766), yet testing was done within 8 days after delivery, and therefore increased the chance of postnatal infections, which interfered with the result interpretation.16 On the other hand, in Italy, a higher frequency of transmission was reported, 4 out of 5 neonates of SARS-CoV-2 positive mothers tested positive,17 and no transmission was reported in 101 neonates of 100 mothers in two large academic centers in New York.18 A large meta-analysis confirmed the risk of vertical transmission and was found to be less than 3%.15 Furthermore, another systematic review reported 2.7% overall risk of SARS-CoV2 PCR positivity in babies born to COVID-19 positive mothers,19 while other studies concluded the lack of adequate evidence to support vertical transmission of COVID-19.7,20,21
Since IgG reflects the transfer of maternal immunity to neonate, its detection indicates past maternal COVID-19 infection or even vaccination, so IgG cannot be used to determine neonatal SARS-CoV-2 status. In our study, IgM antibodies were tested and only one neonate (1.3%) was positive and 28.8% (n = 23) were IgG positive. This neonate (IgM positive) was a full term with a normal birth weight of 3.5 kg, no history of IUGR, no COVID-19 symptoms, APGAR score 9/10, and negative SARS-CoV-2 PCR. The mother had cough and dyspnoea, but no molecular evidence of current or recent COVID-19 infections. Although detection of neonatal IgM is strongly suggestive of neonatal infection,22 neonatal SARS-CoV-2 PCR was negative, and therefore the diagnosis was not confirmed. In this case, the mother was clinically suspected of COVID-19 with negative PCR and the neonate showed positive IgM antibodies and again negative PCR. This could be explained by the suboptimal sensitivity of PCR or target mutation in the primer binding site rendering the PCR results negative. A multicentre study also detected IgM antibodies in five neonates of SARS-CoV-2 infected mothers’ group but not in non-infected or vaccinated mothers’ groups highlighting the diagnostic value of neonatal IgM.23
The most common clinical manifestations of maternal COVID-19 were cough (11.4%), dyspnoea (10%) followed by fever (6.25%), vomiting (5.1%) and nausea (3.8%), similar to published literature.24 Maternal SARS-CoV-2 PCR positivity was significantly associated with ICU admissions (7 out of 7 cases) and use of antibiotics compared to negative mother (p = 0.042, p = 0.003 respectively).
There was a clear association with hypothyroidism and severe disease, but severity was not associated with bronchial asthma, diabetes mellitus or cardiac disease. Among the 8 (10%) cases of hypothyroidism, 2 (66%) had severe COVID-19 (p = 0.047). One of them needed ICU admission and was on mechanical ventilator, with a history of confirmed SARS-CoV-2 positive PCR 8 days before admission. She was on regular thyroxine medication and had a preterm delivery at the gestational age of 26 weeks. The neonate was SARS-CoV-2 PCR negative, IgG positive and IgM negative who eventually died due to prematurity and respiratory distress. In the second case, the mother had severe COVID-19 and needed ICU admission. She had a preterm delivery at the gestational age of 33 weeks. The neonate needed ICU admission and was on continuous positive airway pressure (CPAP). This observation is in line with published studies of association between hypothyroidism and poor outcome.25–27
Neonates born to SARS-CoV-2 positive mother had low birth weight with 18 (22.5%) neonates from SARS-CoV-2 PCR-positive mothers compared to 11 (21.6%) from SARS-CoV-2 PCR-negative mothers (p = 0.001) similar finding was also reported in China reporting increased risk of low birth weight28 and also in a large nationwide study in Korea29 yet other studies found no association between maternal COVID-19 and low birth weight.30 On the other hand, there was no association with IUGR or need for mechanical ventilation.
Our study is one of the significant studies with relatively big sample size compared to published studies and systematic review31–33 which collectively reported small sample size. In addition, it is a unique study in clearly defining the time of testing and ensuring complete separation of neonate of COVID-19 positive mothers from their neonate immediately after birth and not allowing any contact even during breast-feeding. This was based on the hospital policy at that time to minimise the transmission and to prevent neonatal COVI-19. We also used two types of testing for neonates (PCR and antibody detection) providing a better accuracy and confirmation of testing and evaluation of neonatal conditions. In addition, we included a matched control group of COVID-19 negative mother as a baseline reference to improve statistical analysis, provide external validation and reduce bias.34
Limitations of the study include the inability to fulfil the WHO criteria for categorization of the timing of mother-to-fetal transmission due to the lack of availability of tests needed to confirm the diagnosis in routine microbiology diagnostic settings, which is a challenge that has been recognized and reported by other studies35 and the lack of follow-up for neonates of COVID-19 positive mothers for long-term sequels, which was unavoidable during the pandemic. Yet, this study demonstrates the possibility of in utero maternal-to-foetal transmission of SARS-CoV-2 shown by the detection of viral nucleic acid within the first 24–48 hours of birth in 4 neonates and neonatal IgM, which is highly suggestive of in utero COVID-19 transmission.
Conclusion
Given the strict infection control protocol used in the hospital, we found that the risk of maternal-to-fetal COVI-19 transmission was about 6.3% (5% positive SARS-CoV2 PCR and 1.3% SARS-CoV2 IgM positive). Maternal Covid-19 severity was significantly associated with hypothyroidism (p = 0.047) and there was no impact of the mode of delivery on the risk of transmission. In addition, neonatal low birth weight and ICU admission were significantly associated with maternal positivity. With its unique design and use of molecular and antibody testing, this study provides evidence of possible COVID-19 vertical transmission during the earliest stage of the pandemic, shedding light on the importance of continuous surveillance to assess the impact of viral evolution on transmission and pathogenicity of SARS-CoV2.
Acknowledgment
We want to thank the DSR for supporting the research. In addition, we thank the microbiology laboratory staff at King Fahd Hospital of the University, AL Khobar, Saudi Arabia, for their help and guidance during the project, and also extend our thanks to the information technology (IT) department for their valuable support in data collection.
Funding
This project was funded by the Deanship of Scientific Research (DSR) at Imam Abdulrahman bin Faisal University as a postgraduate research project (PGS—2022-01-149).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Sun P, Lu X, Xu C. et al. Understanding of COVID-19 based on current evidence. J Med Virol. 2020;92(6):548–551. doi:10.1002/jmv.25722
2. Sohrabi C, Alsafi Z, O’Neill N, et al. World health organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19). Int J Surg. 2020;76:71–76. doi:10.1016/j.ijsu.2020.02.034
3. Stadnytskyi V, Anfinrud P, Bax A. Breathing, speaking, coughing or sneezing: what drives transmission of SARS-CoV-2? J Intern Med. 2021;290(5):1010–1027. doi:10.1111/joim.13326
4. Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12(2):194. doi:10.3390/v12020194
5. Berumen-Lechuga MG, Molina-Pérez CJ, Leaños-Miranda A. COVID-19 during pregnancy: a narrative. Rev Med Inst Mex Seguro Soc. 2020;58(Supl 2):S187–193. doi:10.24875/RMIMSS.M20000130
6. Samadi P, Alipour Z, Ghaedrahmati M, et al. Relationships between fetal and neonatal outcomes and spectrum of COVID-19 disease in pregnant women. Tanaffos. 2024;23(1):73–82.
7. Palaska E, Golia E, Zacharogianni E, et al. Risk of transmission of COVID-19 from the mother to the foetus: a systematic review. J Mother Child. 2024;28(1):94–101. doi:10.34763/jmotherandchild.20242801.d-24-00032
8. Mirbeyk M, Saghazadeh A, Rezaei N. A systematic review of pregnant women with COVID-19 and their neonates. Arch Gynecol Obstet. 2021;304(1):5–38. doi:10.1007/s00404-021-06049-z
9. World Health O. Definition and categorization of the timing of mother-to-child transmission of SARS-CoV-2: scientific brief, 8 February 2021. World Health Organization 2021.
10. Andersson MI, Arancibia-Carcamo CV, Auckland K, et al. SARS-CoV-2 RNA detected in blood products from patients with COVID-19 is not associated with infectious virus. Wellcome Open Res. 2020;5:181. doi:10.12688/wellcomeopenres.16002.2
11. Gengler C, Dubruc E, Favre G, et al. SARS-CoV-2 ACE-receptor detection in the placenta throughout pregnancy. Clin Microbiol Infect. 2021;27(3):489–490. doi:10.1016/j.cmi.2020.09.049
12. Mohor CI, Oprinca GC, Oprinca-Muja A, et al. Vertical transmission of SARS-CoV-2 from mother to fetus. Pathology. 2024;56(1):107–110. doi:10.1016/j.pathol.2023.06.011
13. Zamaniyan M, Ebadi A, Aghajanpoor S, et al. Preterm delivery, maternal death, and vertical transmission in a pregnant woman with COVID-19 infection. Prenat Diagn. 2020;40(13):1759–1761. doi:10.1002/pd.5713
14. Carosso A, Cosma S, Borella F, et al. Pre-labor anorectal swab for SARS-CoV-2 in COVID-19 pregnant patients: is it time to think about it? Eur J Obstet Gynecol Reprod Biol. 2020;249:98–99. doi:10.1016/j.ejogrb.2020.04.023
15. van Doorn AS, Meijer B, Frampton CMA, et al. Systematic review with meta-analysis: SARS-CoV-2 stool testing and the potential for faecal-oral transmission. Aliment Pharmacol Ther. 2020;52(8):1276–1288. doi:10.1111/apt.16036
16. Lim SB, See KC, Law KB, et al. Characteristics and outcomes of SARS-CoV-2 positivity in neonates born to mothers with COVID-19 in Klang Valley, Malaysia: a retrospective observational study. IJID Reg. 2022;5:146–153. doi:10.1016/j.ijregi.2022.10.001
17. Olivini N, Calò Carducci FI, Santilli V, et al. A neonatal cluster of novel coronavirus disease 2019: clinical management and considerations. Ital J Pediatr. 2020;46(1):180. doi:10.1186/s13052-020-00947-9
18. Dumitriu D, Emeruwa UN, Hanft E, et al. Outcomes of neonates born to mothers with severe acute respiratory syndrome coronavirus 2 infection at a large medical center in New York City. JAMA Pediatr. 2021;175(2):157–167. doi:10.1001/jamapediatrics.2020.4298
19. Allotey J, Chatterjee S, Kew T, et al. SARS-CoV-2 positivity in offspring and timing of mother-to-child transmission: living systematic review and meta-analysis. BMJ. 2022;376(e067696). doi:10.1136/bmj-2021-067696.
20. Bui MT, Nguyen Le CA, Duong KL, et al. Transplacental transmission of SARS-CoV-2: a narrative review. Medicina. 2024;60(9). doi:10.3390/medicina60091517.
21. Urbina-Alvarez CA, Sifuentes-Alvarez JC, Moreno-Bocanegra JF, et al. SARS-CoV-2 infection during pregnancy: clinical characteristics and vertical transmission in a referral hospital in Peru. Rev Peru Med Exp Salud Publica. 2024;41(2):178–184. doi:10.17843/rpmesp.2024.412.13293
22. Blumberg DA, Underwood MA, Hedriana HL, et al. Vertical transmission of SARS-CoV-2: what is the optimal definition? Am J Perinatol. 2020;37(8):769–772. doi:10.1055/s-0040-1712457
23. Beharier O, Plitman Mayo R, Raz T, et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Invest. 2021;131(13). doi:10.1172/JCI154834.
24. Al-Matary A, Almatari F, Al-Matary M, et al. Clinical outcomes of maternal and neonate with COVID-19 infection - multicenter study in Saudi Arabia. J Infect Public Health. 2021;14(6):702–708. doi:10.1016/j.jiph.2021.03.013
25. Damara FA, Muchamad GR, Ikhsani R, et al. Thyroid disease and hypothyroidism are associated with poor COVID-19 outcomes: a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2021;15(6):102312. doi:10.1016/j.dsx.2021.102312
26. Permana H, Soeriadi EA, Damara FA, et al. The prognostic values of thyroid disorders in predicting COVID-19 composite poor outcomes: a systematic review and meta-analysis. Diabetes Metab Syndr. 2022;16(5):102464. doi:10.1016/j.dsx.2022.102464
27. Permana H, Soeriadi EA, Damara FA, et al. The prognostic properties of thyroid disorders, hypothyroidism, and hyperthyroidism in predicting COVID-19 poor outcomes: a systematic review and diagnostic meta-analysis. Indian J Endocrinol Metab. 2022;26(6):510–517. doi:10.4103/ijem.ijem_20_22
28. Wen J. Impact of COVID-19 pandemic on birth outcomes: a retrospective cohort study in Nanjing, China. Front Public Health. 2022;10:923324. doi:10.3389/fpubh.2022.923324
29. Hwang J, Moon S, Cho KD, et al. Changes in preterm birth and birthweight during the SARS-CoV-2 pandemic: a nationwide study in South Korea. Sci Rep. 2022;12(1):16288. doi:10.1038/s41598-022-20049-2
30. Du T, Zhang Y, Zha X, et al. Association of SARS-CoV-2 infection during late pregnancy with maternal and neonatal outcomes. BMC Pregnancy Childbirth. 2024;24(1):632. doi:10.1186/s12884-024-06816-1
31. Juan J, Gil MM, Rong Z, et al. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol. 2020;56(1):15–27. doi:10.1002/uog.22088
32. Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020;99(7):823–829. doi:10.1111/aogs.13867
33. Yu N, Li W, Kang Q, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020;20(5):559–564. doi:10.1016/S1473-3099(20)30176-6
34. Kim S. Overview of clinical study designs. Clin Exp Emerg Med. 2024;11(1):33–42. doi:10.15441/ceem.23.036
35. De Luca D, Vauloup-Fellous C, Benachi A, et al. Transmission of SARS-CoV-2 from mother to fetus or neonate: what to know and what to do? Semin Fetal Neonatal Med. 2023;28(1):101429. doi:10.1016/j.siny.2023.101429
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

