Back to Journals » Infection and Drug Resistance » Volume 18
In vitro Activity of Eravacycline and Risk Factors for Carbapenem-Resistant Acinetobacter Baumannii Infections in Patients with Respiratory Diseases: A Retrospective Cohort Study
Authors Guan J, Wang Z, Dong Y, Wang J, Ren Y, Lu Z, Hu S, Duan X
Received 25 February 2025
Accepted for publication 6 June 2025
Published 25 June 2025 Volume 2025:18 Pages 3127—3136
DOI https://doi.org/10.2147/IDR.S519301
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Oliver Planz
Jiahao Guan,1,* Zitong Wang,1,2,* Yihan Dong,1,2 Jun Wang,3 Yajuan Ren,4 Zifan Lu,5 Shuling Hu,1 Xianglong Duan6
1Department of Clinical Laboratory, Shaanxi Provincial People′s Hospital, Xi ′an, People’s Republic of China; 2Medical College, Yan’ an University, Yan’ an, People’s Republic of China; 3Department of Respiratory MedicineI, Shaanxi Provincial People’s Hospital, Xi ′an, People’s Republic of China; 4Department of Respiratory Medicine II, Shaanxi Provincial People’s Hospital, Xi ′an, People’s Republic of China; 5Translational medicine Center, Shaanxi Provincial People’s Hospital, Xi ′an, People’s Republic of China; 6Second Department of General Surgery, Shaanxi Provincial People’s Hospital, Xi ′an, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xianglong Duan, Second Department of General Surgery, Shaanxi Provincial People′s Hospital, No. 256, Youyi West Road, Xi’an, Shaanxi, 710068, People’s Republic of China, Tel +86 029-85251331, Email [email protected]
Background: Carbapenem-resistant Acinetobacter baumannii (CRAB) infections, particularly among respiratory patients, are associated with high mortality rates and substantial healthcare costs, exceeding $1.2 billion annually. Current the medications are recommended therapies, including Colistin and Tigecycline, face significant limitations, such as nephrotoxicity and inadequate lung tissue penetration. In contrast, Eravacycline (ERV), a novel fluorocycline, exhibits potent activity against multidrug-resistant Gram-negative pathogens and may help mitigate these limitations.
Patients and Methods: A retrospective analysis was conducted on 1071 CRAB isolates obtained from 524 respiratory patients at Shaanxi Provincial People’s Hospital in 2024. Bacterial identification was performed using mass spectrometry (Zhongyuan Co). while drug susceptibility testing was carried out using the BD Phoenix M50 (Becton Dickinson) and E-test strips (Liofilchem). Multivariable logistic regression was applied to identify independent risk factors, including age, intubation history, comorbidities, and the use of feeding tubes, with adjusted odds ratios (OR), 95% confidence intervals (CI), and P-values reported.
Results: Tracheal intubation emerged as the strongest independent risk factor for CRAB acquisition (OR=3.325, 95% CI: 2.273– 4.865, P< 0.001). Resistance to β-lactam antibiotics exceeded 96% (Ceftazidime: 96.56%, Ceftriaxone: 97.14%). C-reactive Protein (CRP) (OR=1.001, 95% CI: 0.996– 1.006, P=0.004) and interleukin-6 (IL-6) (P< 0.001) independently predicted mortality, with SAA demonstrating a strong association with risk (OR = 1.001, 95% CI: 0.999– 1.002, P = 0.006).
Conclusion: Endotracheal intubation significantly contributes to the transmission of CRAB, underscoring the necessity for employing early respiratory ventilation and ERV as a recommended therapies therapeutic strategy in environments with elevated β-lactam resistance. Serum amyloid A (SAA) and interleukin-6 (IL-6) are important prognostic biomarkers that facilitate risk stratification. The implementation of infection control measures that prioritize intubation-associated practices is essential for alleviating the burden of CRAB infections.
Keywords: eravacycline, carbapenem-resistant Acinetobacter baumannii, multidrug resistance, hospital-acquired infections, respiratory diseases, biomarkers
Introduction
CRAB infections have become an increasingly serious public health issue in hospital settings, posing a significant threat to patient health and the medical system.1,2 According to global surveillance data, CRAB infections impose an annual economic burden exceeding $1.2 billion on healthcare systems.3 Furthermore, patients infected with CRAB incur hospitalization expenses that are 2.3 times greater than those of non-CRAB cases, primarily due to extended ICU stays and the complexity of treatments required.4 CRAB infections in patients with respiratory diseases present significant challenges. Recent systematic studies indicate that5 these patients are particularly susceptible to CRAB due to prolonged mechanical ventilation and disruption of mucosal barriers, which facilitate biofilm formation and the upregulation of efflux pump genes. However, prognostic biomarkers and region-specific resistance patterns in this population remain underexplored. Current first-line therapies for CRAB, including Colistin and Tigecycline,6,7 face challenges such as nephrotoxicity, heteroresistance, and insufficient lung tissue penetration Eravacycline(ERV),8 a novel fluorocycline, demonstrates potent activity against multidrug-resistant Gram-negative pathogens, potentially overcoming existing limitations. Global surveillance reports indicate a year-on-year increase in the detection rate of CRAB strains. In certain intensive care unit (ICU) wards, CRAB infections account for 20% to 30% of total infections. 9 Multi-drug-resistant and extensively drug-resistant strains continue to emerge. The World Health Organization (WHO) has classified CRAB as a critical drug-resistant pathogen, highlighting its significant threat to global public health. These factors underscore the urgent need for research on CRAB infections in patients with respiratory diseases.
Materials and Methods
Study Design and Sample Collection
This study is a retrospective investigation focusing on 1,071 CRAB strains isolated from clinical specimens at Shaanxi Provincial People’s Hospital between January and December 2024. The cases were categorized into two groups based on clinical diagnosis: the respiratory disease group and the non-respiratory disease group. Among these, 524 CRAB strains were isolated from respiratory diseases, while 547 were from non-respiratory diseases. The inclusion criteria for CRAB strains required resistance to at least one of the following drugs: Meropenem, Imipenem, or Ertapenem. Duplicate strains from the same site of the same patient were excluded. The quality control strains utilized in this study included Pseudomonas aeruginosa ATCC27853 and Escherichia coli ATCC25922. The inclusion criteria for all patients were as follows: (1) Detection of Acinetobacter baumannii in samples collected more than 48 hours after admission; (2) Presence of clinical signs and symptoms indicative of infection; (3) Diagnosis of a respiratory disease; (4) Possession of complete admission registration and medical course records. The exclusion criteria consisted of: (1) Absence of the patient’s clinical data; (2) Isolates obtained from the same site of the same patient; and (3) Failure to properly preserve the strains. Furthermore, based on microbiological culture, serological testing, PCR technology, and clinical diagnosis, the infection status of each included group was assessed and categorized into respiratory disease and non-respiratory disease groups. This study fully complies with the Declaration of Helsinki and Chinese ethical regulations. The retrospective anonymized data analysis exempted the need for informed consent. All procedures were designed to minimize risks to patient privacy, no commercial conflicts of interest were present.
Methods
Clinical specimens are promptly delivered to the microbiology laboratory, where inoculation and culture are completed within two hours. All procedures are conducted using aseptic techniques. The fully automated Mass Spectrometer (Zhongyuan Co.,China), operates on the principle of matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry for protein fingerprint-based identification. Quality control (QC) strains, including Pseudomonas aeruginosa ATCC 27853 and Escherichia coli ATCC 25922, are validated daily. For clinical drug sensitivity testing, the BD Phoenix M50 automated microbial drug sensitivity analyzer (Becton Dickinson, USA) and its corresponding drug sensitivity detection board were utilized. The improved broth microdilution method was employed for automatic phenotypic identification and antimicrobial susceptibility testing (AST). The version used is the Phoenix system, which supports Gram-negative bacteria, specifically the NMIC/ID-413 board. AST interpretation adheres to the CLSI 2021 guidelines.10 The Liofilchem (Italy) E-test strip was utilized for the drug sensitivity test of Eravacycline, with the minimum inhibitory concentration (MIC) defined as follows: sensitive (S): MIC ≤ 1 µg/mL; intermediate (I): MIC = 2 µg/mL; resistant (R): MIC ≥ 4 µg/mL. Daily quality control (QC) involved running ATCC strains (Pseudomonas aeruginosa ATCC 27853 and Escherichia coli ATCC 25922) alongside clinical isolates. The acceptance standard mandates that MIC results must fall within the range of QC strains as defined by CLSI. If a deviation of more than 10% is observed, the test must be repeated. Calibration of the BD Phoenix™ instrument and mass spectrometer is conducted monthly according to the manufacturer’s guidelines.
Data Collection
The study considered various factors, including underlying diseases, invasive procedures, comorbidities, immunosuppressive drugs, clinical and antibiotic treatments, laboratory results, and microbiological data. Demographic data comprised gender and age. Laboratory test results and microbiological data included sample types and resistance profiles. All results were analyzed on the same day as the CRAB-positive samples.
Statistical Analysis
Statistical analyses were conducted using SPSS 26.0 software. For normally distributed measurement data, the mean and standard deviation (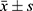 ) were utilized for statistical description, while the t-test was employed for statistical testing. In contrast, for non-normally distributed data, the median and interquartile range (M, Q) were used for description, and the Wilcoxon rank-sum test was applied for analysis. Count data were described using absolute numbers and constituent ratios (%), with the χ²-test utilized for statistical testing. Multivariate analysis was performed using logistic regression analysis.
) were utilized for statistical description, while the t-test was employed for statistical testing. In contrast, for non-normally distributed data, the median and interquartile range (M, Q) were used for description, and the Wilcoxon rank-sum test was applied for analysis. Count data were described using absolute numbers and constituent ratios (%), with the χ²-test utilized for statistical testing. Multivariate analysis was performed using logistic regression analysis.
Results
Demographic and Clinical Characteristics
The study included a total of 1,071 patients, comprising 524 in the respiratory disease group and 547 in the non-respiratory disease group. The respiratory disease group exhibited a significantly higher average age and a lower proportion of males. Notably, among the underlying conditions, the incidence of liver and kidney diseases was markedly higher in the respiratory disease group. This group also demonstrated significantly elevated rates of gastric tube use and hemodialysis catheter placement. Tracheal intubation emerged as the strongest independent risk factor for CRAB infections among patients with respiratory diseases (OR= 3.325, 95% CI=2.273–4.865, P < 0.001). This finding has not been previously reported in cohorts with comparable demographic heterogeneity, underscoring the critical role of invasive procedures in the transmission dynamics of CRAB within this population. Additionally, SAA has been identified as a novel independent predictor of mortality (OR = 1.004, 95% CI= 1.002–1.005, P < 0.001), suggesting its potential as a prognostic biomarker for patients with CRAB infections in respiratory conditions. The multifactorial regression model identified several risk factors, including increasing age (OR=0.962/year, P <0.001). Among the laboratory indicators, the respiratory disease group demonstrated lower levels of albumin, higher levels of cystatin C, and longer activated partial thromboplastin time (APTT), with P<0.001, indicating statistically significant differences. For further details, please refer to Table 1.
 |
Table 1 Demographic and Clinical Characteristics |
Antibiotic Resistance Profiles
This study demonstrates a near-universal susceptibility of CRAB isolates to ERV (>99%) in respiratory patients, contrasting sharply with the high resistance observed to β-lactams (Ceftazidime: 96.56%) and fluoroquinolones (Levofloxacin: 94.27%). Notably, minocycline resistance in the respiratory disease group (39.31%) exceeded rates reported in non-respiratory cohorts (P<0.001), underscoring the necessity to reassess the use of tetracycline-class antibiotics. To our knowledge, this is the first systematic comparison of ERV susceptibility between respiratory and non-respiratory CRAB isolates, revealing no significant intergroup differences (P=0.512). Respiratory isolates exhibited elevated resistance to Levofloxacin (94.27%) and Ciprofloxacin (96.56%) and reduced susceptibility to Sulbactam/Ampicillin (18%) compared to non-respiratory isolates. Therefore, empirical monotherapy with β-lactams or fluoroquinolones should be avoided in respiratory patients due to the high risk of treatment failure driven by resistance. However, synergistic combination regimens may still retain utility when guided by susceptibility testing. The efficacy of tigecycline was contingent on susceptibility profiles, as detailed in Table 2.
 |
Table 2 Antibiotic Resistance Profiles |
Analysis of Risk Factors for Patient Mortality
This study identified ICU admission as the strongest predictor of mortality in patients with CRAB-infected respiratory conditions (OR=9.692) surpassing the risks reported in general ICU populations. Respiratory failure (OR = 3.633) and mechanical ventilation (OR = 5.641) were found to independently predict mortality. In contrast, cardiovascular comorbidities exhibited protective effects, with an (R=0.505,P = 0.016). Deep puncture (OR = 2.67, P < 0.001) and endotracheal intubation (OR = 2.435, P = 0.016) were more prevalent among the deceased group. Elevated IL-6 levels demonstrated subgroup-specific prognostic value in respiratory patients (P = 0.018), while additional predictive utility was observed for the AST/ALT ratio (OR = 1.276, P = 0.005) and the neutrophil-to-lymphocyte ratio (NLR) (OR = 1.025, P < 0.001). Despite higher utilization of peripherally inserted central catheters (PICC) (P < 0.001) and procalcitonin (PCT) levels (P < 0.05) in non-survivors, these factors lacked multivariate significance. Hemodialysis and gastric tube use demonstrated univariate associations (P < 0.001) but failed to retain significance in adjusted models. No age disparity was observed between the survival and death groups across analyses. For detailed statistical outcomes, as detailed in Tables 3 and 4.
 |
Table 3 Multivariable Analysis of Mortality Risk Factors in the Overall CRAB-Infected Cohort |
 |
Table 4 Multivariable Analysis of Mortality Risk Factors in the Respiratory Disease Subgroup with CRAB Infections |
Discussion
This study focuses on infections caused by drug-resistant Acinetobacter baumannii, The multidrug-resistant characteristics of this pathogen have resulted in a global public health crisis.11,12 Drug-resistant Acinetobacter baumannii not only poses a significant threat to patient health, leading to higher mortality and complication rates but also imposes a considerable economic burden on healthcare systems.13,14 The widespread use of antibiotics has facilitated the emergence of drug-resistant strains, complicating the treatment of infections and presenting greater challenges, particularly in cases involving multidrug-resistant strains.15 This study retrospectively analyzed 1,071 cases of CRAB infection, including 524 patients with respiratory diseases, admitted to Shaanxi Provincial People’s Hospital in 2024. For the first time, it systematically reveals the unique clinical characteristics of CRAB infection in patients with respiratory diseases. Research indicates4,16 that the spread of drug-resistant Acinetobacter baumannii is closely associated with the clinical manifestations observed in hospitalized patients.17 In this study, an investigation was performed to characterize the clinical features and antibiotic resistance profiles of CRAB infections, thereby addressing a critical knowledge gap in the extant literature regarding the attributes of such infections. The innovation of this research is reflected in two aspects: firstly, it provides a comprehensive analysis compared to previous studies, particularly the one conducted by Smith et al in 2021.18,19 This research represents the first confirmation of the long-term efficacy of novel antibiotics in treating CRAB infections in human subjects. An analysis of 524 patients with respiratory diseases and 547 patients with non-respiratory diseases identified a close association between CRAB infections and underlying diseases, invasive procedures, and laboratory indicators. The placement of central venous catheters (CVCs) and peripherally inserted central catheters (PICCs), both of which are intravascular invasive procedures, provides pathways for bacteria to enter the bloodstream.20 Prolonged placement may result in local vascular intimal injury, promoting thrombus formation, which can subsequently serve as a breeding ground for bacteria thereby contributing to increased patient mortality rates.21 Tracheal intubation and tracheostomy, as more direct invasive airway procedures, not only disrupt the normal defense mechanisms of the upper respiratory tract22,23 but also lead to poor secretion drainage post-intubation. Additionally, the easy formation of biofilms at the intubation site facilitates bacterial colonization and proliferation.24 Compared to other factors, hemodialysis is associated with a higher mortality rate. The patient’s blood must be drawn out of the body and exchanged with the external environment through a dialyzer,25 This process necessitates the establishment of vascular access, such as an arteriovenous fistula or a central venous catheter, which provides a direct pathway for bacterial invasion. For patients undergoing various invasive procedures, it is essential to strictly adhere to aseptic techniques, enhance the disinfection management of surgical instruments, standardize operational procedures, and ensure proper post-procedural care to minimize the risk of infection. Additionally, it is important to closely monitor patients’ AST/ALT, NLR, PCT, and other indicators to promptly detect signs of infection and take appropriate measures.26,27 An increase in the AST/ALT ratio may indicate the progression of infection to multiple organ dysfunction. In this study, the NLR was identified as an independent predictor of mortality, suggesting that the immune status of patients infected with CRAB is a crucial factor influencing prognosis. PCT, a hormone-free glycoprotein composed of 116 amino acids, is released by systemic tissues during bacterial infections and exhibits a significant increase during CRAB infections. PCT is nearly undetectable in healthy individuals but begins to rise 2~3 hours after the onset of bacterial infection, with a rapid increase observed within 6~8 hours, peaking at 12~48 hours. PCT demonstrates excellent stability and high specificity; its concentration changes are significantly correlated with the severity of infection and disease progression, thus providing a reliable basis for early diagnosis and monitoring of treatment efficacy in infectious diseases. For hemodialysis patients, it is crucial to strengthen the maintenance and disinfection of dialysis equipment, strictly standardize the dialysis operational procedures, closely monitor the condition of the patient’s vascular access, and unnecessary catheters should be removed as soon as possible. Ultimately achieve effective implementation of infection prevention and control measures.
In comparison to previous studies, such as that by Taj et al (2020), which emphasized the widespread presence of CRAB in hospital infections and its drug-resistance characteristics,28,29 our results indicate that factors such as tracheal intubation and underlying diseases are significantly associated with CRAB infection. This finding provides a new perspective for future interventions. Additionally, our study identified the potential roles of SAA and IL-6 as biomarkers, which align with the findings of Gou et al (2022) regarding biomarkers in drug-resistant bacterial infections.30 Research has demonstrated that IL-6 serves not only as a biomarker for the presence of inflammatory responses in the body but also reflects the intensity of these reactions, thereby providing a basis for evaluating treatment efficacy. SAA is a highly sensitive acute-phase reactant associated with numerous chronic inflammatory diseases. During systemic inflammatory responses, hepatic SAA primarily associates with high-density lipoprotein. In cases of acute infection, SAA concentrations can increase by more than 1000-fold compared to baseline levels. The implications of this study are significant for clinical practice, particularly in managing patients in ICU. Our research reveals that the mortality rate of patients infected with CRAB is influenced by multiple factors, including tracheal intubation, respiratory failure, and invasive procedures during hospitalization. Furthermore, the study demonstrates that in the respiratory disease group, the sensitivity of ERV to CRAB is 99.24%, while in the non-respiratory disease group, its sensitivity is 99.27%. These rates are significantly higher than those of other commonly used antibiotics such as Gentamicin, Meropenem, and Ceftazidime, illustrating a strong inhibitory effect on CRAB and presenting a new effective option for clinical treatment. Moreover, studies conducted by Jackson et al31 found that among patients with respiratory-associated pneumonia caused by CRAB who were treated with ERV, 71% of evaluable patients exhibited a reduction in pathogenic bacterial counts in follow-up cultures.32,33 This result is consistent with the efficacy observed in this study, further validating the effectiveness and clinical application potential of ERV in treating CRAB infections. These findings suggest that clinicians should pay greater attention to these risk factors when formulating treatment plans, enabling early intervention and improved patient outcomes. Additionally, our study underscores the importance of the rational use of new antibiotics, especially in the context of increasing drug resistance. These new antibiotics can provide clinicians with additional treatment options, thereby contributing to a reduction in mortality associated with CRAB infections. This paper investigates the impact of clinical maneuvers on CRAB infections by integrating theoretical experiments with empirical clinical data. Compared to similar research findings, this study presents specific strategies to mitigate CRAB infections and validates the effectiveness of the proposed methods through detailed experimental data. Minimizing the risk of bacterial infection associated with each invasive procedure offers new insights for subsequent interventions. The findings from this research can serve as a valuable reference for future treatments of CRAB infections, thereby enhancing various therapeutic approaches.
Conclusion
This study identifies endotracheal intubation as the primary risk factor for the acquisition of CRAB, advocating for early rescue ventilation and ERV as the medications are recommended in settings characterized by high β-lactam resistance. SAA and IL-6 are identified as independent prognostic biomarkers, with SAA serving as a predictor of mortality and IL-6 guiding interventions specific to subgroups. Implementing infection control measures that focus on intubation-associated practices is critical to mitigating the burden of CRAB infections.
Ethics Approval
The study protocol was approved by the Ethics Committee of Shaanxi Provincial People’s Hospital [Approval No. (2024) Ethics Review No. (R131)], and is a retrospective analysis. In this study, informed consent from patients is not required. The patient data in this research have been anonymized, and sensitive personal information and commercial interests are not involved in the data processing and analysis. According to the relevant legal provisions of the “Notice on Issuing the Measures for Ethical Review of Life Science and Medical Research Involving Humans” (Guowei Kejiaofa [2023] No. 4), this study meets the situation specified in Point (ii) of Article 32, Chapter III of the Measures, and is exempted from ethical review.
Acknowledgment
The authors are grateful to the staff of the microbiology lab for their assistance in identifying bacteria and performing antimicrobial susceptibility testing.
Funding
This study was completed with the support of the following funds: Key Research and Development Program of Shaanxi (No. 2020GXLH-Y-019, 2022KXJ-141). Key Research and Development Program of Shaanxi (S2024-YF-YBSF-0702). Innovation Capability Support Program of Shaanxi (No. 2019GHJD-14, 2021TD-40). Science and Technology Talent Support Program of Shaanxi Provincial People’s Hospital (No. 2021LJ-05). Technology Innovation Leading Program of Shaanxi (No.2022KXJ-141). Science and Technology Program of Xi’an (No. 23ZDCYJSGG0037-2022).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ibrahim S, Al-Saryi N, Al-Kadmy IMS, Aziz SN. Multidrug-resistant Acinetobacter baumannii as an emerging concern in hospitals. Mol Biol Rep. 2021;48(10):6987–6998. doi:10.1007/s11033-021-06690-6
2. Abdi SN, Ghotaslou R, Ganbarov K, et al. Acinetobacter baumannii efflux pumps and antibiotic resistance. Infect Drug Resist. 2020;13:423–434. doi:10.2147/IDR.S228089
3. Pogue JM, Zhou Y, Kanakamedala H, Cai B. Burden of illness in carbapenem-resistant Acinetobacter baumannii infections in US hospitals between 2014 and 2019. BMC Infect Dis. 2022;22(1):36. doi:10.1186/s12879-021-07024-4
4. Nguyen M, Joshi SG. Carbapenem resistance in Acinetobacter baumannii, and their importance in hospital-acquired infections: a scientific review. J Appl Microbiol. 2021;131(6):2715–2738. doi:10.1111/jam.15130
5. Onishi R, Shigemura K, Osawa K, et al. Impact on quinolone resistance of plasmid-mediated quinolone resistance gene and mutations in quinolone resistance-determining regions in extended spectrum beta lactamase-producing Klebsiella pneumoniae isolated from urinary tract infection patients. Pathog Dis. 2022;80(1):ftac030. doi:10.1093/femspd/ftac030
6. Kaye KS, Shorr AF, Wunderink RG, et al. Efficacy and safety of sulbactam-durlobactam versus colistin for the treatment of patients with serious infections caused by Acinetobacter baumannii-calcoaceticus complex: a multicentre, randomised, active-controlled, Phase 3, non-inferiority clinical trial (ATTACK). Lancet Infect Dis. 2023;23(9):1072–1084. doi:10.1016/S1473-3099(23)00184-6
7. Neupane L, Sah AK, Rayamajhee B, Pokhrel A, Singh A. Detection of blaoxa-23 gene from carbapenem-resistant Acinetobacter Baumannii. J Nepal Health Res Counc. 2023;20(4):899–905. doi:10.33314/jnhrc.v20i4.4257
8. Lee YR, Burton CE. Eravacycline, a newly approved fluorocycline. Eur J Clin Microbiol Infect Dis. 2019;38(10):1787–1794. doi:10.1007/s10096-019-03590-3
9. O’Donnell JN, Putra V, Maring BL, Ozer EA, Belfiore GM, Rhodes NJ. Effect of omadacycline alone and in combination with meropenem against carbapenem-resistant Acinetobacter baumannii isolates. J Glob Antimicrob Resist. 2022;29:147–149. doi:10.1016/j.jgar.2022.03.006
10. Humphries R, Bobenchik AM, Hindler JA, Schuetz AN. Overview of changes to the clinical and laboratory standards institute performance standards for antimicrobial susceptibility testing, M100, 31st edition. J Clin Microbiol. 2021;59(12):10–128.
11. Jean SS, Harnod D, Hsueh PR. Global threat of carbapenem-resistant gram-negative bacteria. Front Cell Infect Microbiol. 2022;12:823684. doi:10.3389/fcimb.2022.823684
12. Mea HJ, Yong PVC, Wong EH. An overview of Acinetobacter baumannii pathogenesis: motility, adherence and biofilm formation. Microbiol Res. 2021;247:126722. doi:10.1016/j.micres.2021.126722
13. Sana F, Hussain A, Hussain W, et al. Frequency and clinical spectrum of multidrug resistant Acinetobacter Baumannii as a significant nosocomial pathogen in intensive care unit patients. J Ayub Med Coll Abbottabad. 2021;33(4):S752–S756.
14. Mancuso G, De Gaetano S, Midiri A, Zummo S, Biondo C. The challenge of overcoming antibiotic resistance in carbapenem-resistant gram-negative bacteria: “attack on titan”. Microorganisms. 2023;11(8):1912. doi:10.3390/microorganisms11081912
15. Ramirez MS, Bonomo RA, Tolmasky ME. Carbapenemases: transforming Acinetobacter baumannii into a yet more dangerous menace. Biomolecules. 2020;10(5):720. doi:10.3390/biom10050720
16. Nasr P. Genetics, epidemiology, and clinical manifestations of multidrug-resistant Acinetobacter baumannii. J Hosp Infect. 2020;104(1):4–11. doi:10.1016/j.jhin.2019.09.021
17. Cavallo I, Oliva A, Pages R, et al. Acinetobacter baumannii in the critically ill: complex infections get complicated. Front Microbiol. 2023;14:1196774. doi:10.3389/fmicb.2023.1196774
18. Smith AR, Vowles M, Horth RZ, et al. Infection control response to an outbreak of OXA-23 carbapenemase-producing carbapenem-resistant Acinetobacter baumannii in a skilled nursing facility in Utah. Am J Infect Control. 2021;49(6):792–799. doi:10.1016/j.ajic.2020.11.012
19. Wang J, Zhang J, Wu ZH, et al. Clinical characteristics and prognosis analysis of Acinetobacter baumannii bloodstream infection based on propensity matching. Infect Drug Resist. 2022;15:6963–6974. doi:10.2147/IDR.S387898
20. Zhang X, Cui X, Jiang M, Huang S, Yang M. Nebulized colistin as the adjunctive treatment for ventilator-associated pneumonia: a systematic review and meta-analysis. J Crit Care. 2023;77:154315. doi:10.1016/j.jcrc.2023.154315
21. Huang L, Tang J, Tian G, Tao H, Li Z. Risk factors, outcomes, and predictions of extensively drug-resistant Acinetobacter baumannii nosocomial infections in patients with nervous system diseases. Infect Drug Resist. 2023;16:7327–7337.
22. Russotto V, Rahmani LS, Parotto M, Bellani G, Laffey JG. Tracheal intubation in the critically ill patient. Eur J Anaesthesiol. 2022;39(5):463–472. doi:10.1097/EJA.0000000000001627
23. Chorath K, Hoang A, Rajasekaran K, Moreira A. Association of early vs late tracheostomy placement with pneumonia and ventilator days in critically Ill patients: a meta-analysis. JAMA Otolaryngol Head Neck Surg. 2021;147(5):450–459. doi:10.1001/jamaoto.2021.0025
24. Pisano A, Yavorovskiy A, Verniero L, Landoni G. Indications for tracheal intubation in patients with coronavirus disease 2019 (COVID-19). J Cardiothorac Vasc Anesth. 2021;35(5):1276–1280. doi:10.1053/j.jvca.2020.11.062
25. Abdelsalam M, Demerdash TM, Assem M, et al. Improvement of clinical outcomes in dialysis: no convincing superiority in dialysis efficacy using hemodiafiltration vs high-flux hemodialysis. Ther Apher Dial. 2021;25(4):483–489. doi:10.1111/1744-9987.13492
26. Dhondge RH, Agrawal S, Kumar S, Acharya S, Karwa V. A comprehensive review on serum ferritin as a prognostic marker in intensive care units: insights into ischemic heart disease. Cureus. 2024;16(3):e57365. doi:10.7759/cureus.57365
27. Karatas M, Keles N, Parsova KE, et al. High AST/ALT ratio is associated with cardiac involvement in acute COVID-19 patients. Medicina. 2023;59(6):1163. doi:10.3390/medicina59061163
28. Pormohammad A, Mehdinejadiani K, Gholizadeh P, et al. Global prevalence of colistin resistance in clinical isolates of Acinetobacter baumannii: a systematic review and meta-analysis. Microb Pathog. 2020;139:103887. doi:10.1016/j.micpath.2019.103887
29. Taj Z, Rasool MH, Almatroudi A, Saqalein M, Khurshid M. Extensively drug-resistant Acinetobacter baumannii belonging to international clone II from a pet cat with urinary tract Infection; the first report from Pakistan. Pol J Microbiol. 2020;69(2):1–4. doi:10.33073/pjm-2020-017
30. Gou X, Xu W, Liu Y, et al. IL-6 prevents lung macrophage death and lung inflammation injury by inhibiting GSDME- and GSDMD-mediated pyroptosis during pneumococcal pneumosepsis. Microbiol Spectr. 2022;10(2):e0204921. doi:10.1128/spectrum.02049-21
31. Jackson MNW, Wei W, Mang NS, Prokesch BC, Ortwine JK. Combination eravacycline therapy for ventilator-associated pneumonia due to carbapenem-resistant Acinetobacter baumannii in patients with COVID-19: a case series. Pharmacotherapy. 2024;44(4):301–307.
32. Alosaimy S, Morrisette T, Lagnf AM, et al. Clinical outcomes of eravacycline in patients treated predominately for carbapenem-resistant Acinetobacter baumannii. Microbiol Spectr. 2022;10(5):e0047922. doi:10.1128/spectrum.00479-22
33. Huang PY, Hsu CK, Tang HJ, Lai CC. Eravacycline: a comprehensive review of in vitro activity, clinical efficacy, and real-world applications. Expert Rev Anti Infect Ther. 2024;22(6):387–398. doi:10.1080/14787210.2024.2351552
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

