Back to Journals » Infection and Drug Resistance » Volume 18
In vitro Antibacterial Effect of Reduning Combined with Polymyxin on Carbapenem Resistant Klebsiella pneumoniae
Authors Shangguan ZF, Chen HL, Li YF, Shi N, Mao QF
Received 11 September 2024
Accepted for publication 19 December 2024
Published 13 January 2025 Volume 2025:18 Pages 227—237
DOI https://doi.org/10.2147/IDR.S490029
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Zui-Fei Shangguan,1 Hong-Lei Chen,2 Yi-Fan Li,3 Na Shi,4 Qi-Fen Mao2
1Department of Clinical Laboratory, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Hangzhou, Zhejiang, 310006, People’s Republic of China; 2Department of Clinical Laboratory, Tongde Hospital of Zhejiang Province, Hangzhou, Zhejiang, 310012, People’s Republic of China; 3College of Laboratory Medicine and College of Bioengineering, Hangzhou Medical University, Hangzhou, Zhejiang, 311399, People’s Republic of China; 4Medical Laboratory, Second Sanatorium of Air Force Healthcare Center for Special Services, Hangzhou, Zhejiang, 310007, People’s Republic of China
Correspondence: Qi-Fen Mao, Department of Clinical Laboratory, Tongde Hospital of Zhejiang Province, 234#, Gucui Road, Hangzhou, Zhejiang, 310012, People’s Republic of China, Email [email protected]
Objective: This study aimed to investigate the status of carbapenem-resistant strains of Klebsiella pneumoniae isolated from the Department of Microbiology, Zhejiang Tongde Hospital between September 2023 and February 2024, and to examine the in vitro antibacterial effect of Reduning combined with polymyxin on carbapenem-resistant Klebsiella pneumoniae (CRKP), which may provide evidence on the application of Reduning in the clinical anti-infective therapy.
Methods: A total of 50 different isolates of CRKP were collected, and the minimum inhibitory concentrations (MIC) of polymyxin, Reduning and polymyxin plus Reduning were measured with microbroth dilution method. Then, the fractional inhibition concentration index (FICI) was calculated.
Results: A total of 50 strains of CRKP were isolated, sputum and clean urine were the most common source of CRKP, and intensive care unit was the most common source department. More than 90% of CRKP strains were resistant to cefepime, ceftazidime, piperacillin/tazobactam, and cefoperazone/sulbactam. The rate of resistance to levofloxacin was high, but that to tobramycin, tigecycline, and compound sulfamethoxazole was low. In addition, MIC of Reduning plus polymyxin for CRKP was lower than that of Reduning or polymyxin alone. Among 50 strains of CRKP, FICI ≤ 0.5 was noted in 7 strains, 0.5 < FICI ≤ 1.0 in 43 strains, and none had FICI > 1.0. The results showed Reduning combined with polymyxin B exerted additive effect on CRKP and conferred synergistic effect on several strains of CRKP.
Conclusion: Reduning has antibacterial effect on CRKP in vitro, and the addition of Reduning can reduce the dose of polymyxin in the treatment of CRKP.
Keywords: Reduning, polymyxin, Carbapenem resistant Klebsiella pneumoniae, in vitro antibacterial activity
Introduction
Klebsiella pneumoniae (KP) is a common opportunistic pathogen that causes nosocomial infections, including lung, blood flow, and urinary tract infections, and in severe cases, it can also lead to critical illnesses such as purulent meningitis.1 Carbapenem antibiotics have the characteristics of a wide antibacterial spectrum and high antibacterial activity and have been widely used in the treatment of KP infection.2 In recent years, with the increasing use of carbapenem antibiotics in clinical practice, the detection rate of carbapenem-resistant Klebsiella pneumoniae (CRKP) and the incidence of CRKP infection are increasing over year.3 The Chinese Bacterial Resistance Monitoring Network shows that the detection rates of Klebsiella pneumoniae resistant to meropenem and imipenem are 23.6% and 22.5%, respectively, between January 2023 and December 2023.4 Klebsiella pneumoniae produces carbapenemases,5 such as KPC, NDM, OXA-48, etc., causing the ineffectiveness of carbapenem antibiotics in the treatment of Klebsiella pneumoniae infection. CRKP was generally resistant to other antibiotics, and therefore patients with CRKP infection have a high risk of death. Patients infected by CRKP usually develop severe and long-lasting symptoms, which poses a significant challenge to clinical infection control.6–8
Polymyxin is a cationic antimicrobial peptide discovered in 1949, mainly including polymyxin B and polymyxin E (myxin). Polymyxin acts on negatively charged lipids of lipopolysaccharides on cell membrane to destroy the integrity of cell membrane, exerting bactericidal effect. However, it was once abandoned due to its side effects such as neurotoxicity and nephrotoxicity.9 However, due to the emergence of multidrug-resistant bacteria, polymyxin has been reintroduced to clinical treatment of infections. To date, polymyxin has become a key antibiotic and the last resort for the treatment of carbapenem-resistant bacteria. With the increasing use of polymyxin in clinical practice, the detection rate of polymyxin-resistant pathogens has significantly increased.10 According to CHINET data in 2023, the detection rate of CRKP resistant to polymyxin is about 10.1% in China. Therefore, it is particularly crucial to identify a drug that is both efficient and low toxic and can be used in combination with polymyxin. Traditional Chinese Medicine is highly praised for its advantages of less drug resistance and minimal side effects.11 With the deepening of investigation on the efficacy of Traditional Chinese Medicine, investigators have revealed that the combination of Traditional Chinese Medicine with antibiotics can not only enhance the therapeutic effect but also help reduce the adverse reactions of drugs. Traditional Chinese Medicine directly acts on pathogens by intervening with metabolic processes in pathogens and disrupting their structure and function (such as affecting bacterial cell wall formation, hindering protein synthesis in bacteria, inhibiting nucleic acid metabolism, or interfering with other pathways important for the metabolism.12 To date, little is known about the bacterial resistance to Traditional Chinese Medicine. The combination of Traditional Chinese Medicine and antibiotics may be a new way to treat CRKP infection. As an iconic heat clearing and detoxifying Traditional Chinese Medicine, Reduning can effectively suppress inflammation and eliminate toxins in the body. In addition, Reduning has other pharmacological effects, including inhibiting viruses, eliminating bacteria, relieving allergic reactions, suppressing tumorigenesis, maintaining hepatic health, stimulating bile production, and regulating the immune system.13 Especially in the treatment of fungi and bacterial infections, Reduning has particularly outstanding effects. Therefore, we speculate that Reduning not only has the inhibitory effect on KP infection but also enhances the therapeutic effect of polymyxin and reduces the toxicity and side effects of polymyxin due to the reduction of dose of polymyxin.
This study aimed to investigate the in vitro inhibitory effect of Reduning on CRKP and explore its synergistic effect with polymyxin, in order to evaluate the clinical applicability of combined use of Reduning and polymyxin in the treatment of CRKP, which may provide evidence on the appropriate use of antibiotics in clinical practice.
Materials and Methods
Source of Strains
A total of 50 strains of CRKP were isolated from the Department of Microbiology, Zhejiang Tongde Hospital between September 2023 and February 2024. The study was approved by the ethic committee of Tongde Hospital of Zhejiang Province (2022–077), and written informed consent was obtained from each patient. The sources of these strains included sputum, urine, pleural and peritoneal fluid, throat swabs, and catheter drainage. The duplicate strains from the same patient and the same site were excluded in the collection of bacterial strains. The growth and colony morphology of different bacterial strains were observed in the culture medium, and the bacterial characteristics were compared under different culture conditions. All the strains were confirmed as KP by mass spectrometry. After strain identification, the strains were preserved for future use.
Drugs
Reduning injection (10 mL; Lot number: 230430, Jiangsu Kanion Pharmaceutical Co., Ltd) and Sulfate polymyxin B injection (500000 U; Lot number: 2308802, SPH No.1 Biochemical & Pharmaceutical Co., Ltd.) were used in the present study.
Reagents and Instrument
Following instrument and reagents were used in the present study (Table 1).
 |
Table 1 Instruments and Reagents Used in This Study |
Strain Identification and Routine Drug Susceptibility Testing
The specimens were first seeded on blood agar plates and MacConkey plates, followed by incubation for 18–24 h; Subsequently, individual colonies were screened on the MacConkey plate. All strains were identified as Klebsiella pneumoniae using a matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) identification system. In “Matching antibiotic susceptibility test”, we used the Vitek 2 system for antibiotic susceptibility testing, using Gram negative bacterial susceptibility card 335 (N335).
Detection of Carbapenemase Type
The enzyme immunochromatography was employed for the detection of carbapenemase type.14 In the positive specimens, the components of carbapenemase can interact with the monoclonal mouse carbapenem enol antibody coated with gold particles, forming a specific immune complex. This complex then moves onto the T region of nitrocellulose membrane and specifically binds to the pre-existing monoclonal antibody against carbapenemase, ultimately revealing a red reaction line. When the remaining gold labeled antibodies pass through the quality control line (C line), the gold labeled chicken IgY antibodies bind to pre-coated sheep anti-chicken IgY antibodies, exhibiting a red band. In the negative specimens, color is observable at the quality control line. The whole procedures are show in Figure 1.
 |
Figure 1 Detection of carbapenemase by gold standard immunochromatography. |
After evaluation, when a red line appeared in the first card, a positive result was considered if a red line was observed at any one of the KPC, NDM and IMP test lines. For the second card, when the control line was a red line, a positive result (“+”) was considered if one or more red lines were observed at the VIM and 0XA-48 test lines. If only the control line was a red line and no test lines turned red, a negative result (“-”) was considered. If there was no line at the site of C-line, the detection was invalid, and retesting was needed.
Detection of MIC of a Single Drug
According to the 2022 version of the American Society for Clinical and Laboratory Standards (CLSI) standards,15 the minimum inhibitory concentration (MIC) values of 50 experimental strains against Reduning and polymyxin B were determined using the microdilution method. Firstly, select bacterial colonies cultured for 18 to 24 hours and adjust the turbidity to 0.5 McPherson turbidity using physiological saline.Then dilute 100 times with cation adjusted MH broth (CAMHB). Dilute the ratio of Reduning to (1000, 500, 250, 125, 62.5, 31.25, 15.625, 7.8125μL/mL); Dilute the ratio of polymyxin B to (32, 16, 8, 4, 2, 1, 0.5, 0.25, 0.125μg/mL). Take 100 μL of various concentrations of prepared Reduning and polymyxin B in sequence and add them to the corresponding wells of a 96 well plate,Then add 100 μL of diluted bacterial suspension to each well, simultaneously perform negative control and culture at 37°C for 16–18 hours. The quality control strain is Escherichia coli ATCC25922. Observe the results and record the MIC values of various drugs. Repeat the experiment three times.
Study of Combination Treatment
In this study, detection was performed strictly following the standard testing procedure provided by CLSI. After dilution in a multiple ratio series, Reduning and polymyxin B were mixed in different concentration combinations. After incubation, the MIC values of the single drug and the combination of the two drugs were measured. After the final inoculation using the microbroth dilution method, the final volume of liquid in each well is 100mL, and the final bacterial concentration is 5x105cfu/mL. Based on experimental data, the effective concentration range of polymyxin B for the combined application of Reduning and polymyxin is determined to be 0.25–4μg/mL.
Detection of Fractional Inhibitory Concentration Index (FICI)
The fractional inhibition concentration index (FICI) was used to analyze the mutual influence between two drugs used for efficacy analysis.
The synergistic effect of two drugs is considered if the FICI is lower than 0.5. If the FICI ranges from 0.5 to 1.0, addictive effect is considered. If the FICI ranges from 1.0 to 2.0, no significant interaction between drugs is considered. Once the FICI is higher than 2.0, an antagonistic effect can be considered between drugs.
Results
Distribution and Drug Resistance of 50 Strains of CRKP
Sources of 50 CRKP strains: The 50 strains of CRKP were mainly separated from sputum (64%; 32/50) and urine (20%; 10/50), and the other sources of CRKP included pharyngeal swabs and catheters (16%; 8/50) (Figure 2).
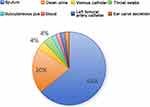 |
Figure 2 Clinical distribution of 50 CRKP strains. |
Distribution of Patients with CRKP Infection
Patients with CRKP were mainly from intensive care units, including intensive care unit in the Department of Emergency and Department of Intensive Care Medicine (48%; 24/50). The 50 CRKP strains displayed distinct resistance for different antibiotics. High resistance rate was noted in cefepime, ceftazidime, piperacillin tazobactam, and cefoperazone/sulbactam (higher than 90%). The resistance to levofloxacin was also evident. Of note, the rate of CRKP strains resistant to carbapenem antibiotics (such as meropenem and imipenem) was as high as 100%. However, the rate of CRKP strains resistant to tobramycin, tigecycline, and compound sulfamethoxazole was significantly lower (Figure 3).
 |
Figure 3 Drug resistance of 50 CRKP strains in our hospital. |
Distribution of Carbapenemase Types in 50 CRKP Strains
The colloidal gold immunochromatography was performed to detect the carbapenemase types. Our results showed that the 50 CRKP strains in our hospital mainly produced KPC. About 96% (48/50) of CRKP strains produced KPC alone, and 4% (2/50) CRKP strains could produce KPC and NDM (Figure 4).
 |
Figure 4 Distribution of carbapenemase types of 50 CRKP strains. |
MIC of Reduning, Polymyxin B and Reduning Plus Polymyxin B for CRKP Strains
In this study, broth microdilution method was used to determine the MIC of Reduning, polymyxin B, and Reduning plus polymyxin B for 50 CRKP strains. Our results showed that the MIC of Reduning plus polymyxin B for CRKP was lower than that of Reduning alone. The MIC of polymyxin B plus Reduning was reduced by 2–4 times as compared to the MIC of polymyxin B alone. (Note: concentration of Reduning: μ l/mL; concentration of Polymyxin B: μ g/mL) (Table 2).
 |
Table 2 MIC Values for Drug Monotherapy and Combination Therapy |
Synergistic Antibacterial Index of Two Drugs
Based on the MIC of Reduning, polymyxin B and Reduning plus polymyxin B for 50 CRKP strains, the FICI of Reduning, polymyxin B and Reduning plus Polymyxin B were calculated using the algorithm of inhibitory concentration index. Results reveal 7 strains had FICI lower than 0.5; 43 strains had FICI ranging from 0.5 to 1.0; no strains had FICI higher than 1.0. Our results showed Reduning and polymyxin B exhibited an additive effect on CRKP, especially several CRKP strains (Table 3 and Table 4).
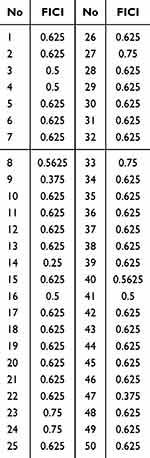 |
Table 3 FICI of Reduning Plus polymyxinB in 50 CRKP Strains |
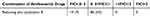 |
Table 4 FICI of Reduning Plus polymyxinB in 50 CRKP Strains (%, n) |
Discussion
Currently, the detection rate of carbapenem-resistant Klebsiella pneumoniae (CRKP) in clinical practice is continuously increasing worldwide. The Chinese Bacterial Resistance Monitoring Network shows that, from January to December 2023, the detection rate of KP resistant to meropenem and imipenem was 23.6% and 22.5%, respectively. Thus, the commonly used carbapenem is currently ineffective for CRKP.
Polymyxin eliminates bacteria by affecting negatively charged phospholipids on cell membrane, thereby disrupting the structure of cell membrane.16 However, due to its severe nephrotoxicity, polymyxin has not been commonly used since 1980s. In recent years, polymyxin has been reintroduced due to the presence of multidrug-resistant bacteria. Polymyxin can be used for the treatment of ventilator-associated pneumonia,17 bloodstream infections,18 abdominal infections,19 and urinary tract infections,20 caused by CRKP regardless of the mechanisms of drug resistance. However, the heterogeneity of bacterial population usually affects the efficacy of polymyxin alone, and the increasing use of polymyxin in clinical practice also promotes the presence of resistance to polymyxin.
Traditional Chinese Medicine therapy has a long history in our country, and Traditional Chinese Medicine is rich in medical wealth and resources. In recent years, clinicians have paid increasing attention to the efficacy of traditional Chinese medicine and their extracted components in preventing and treating infections. Traditional Chinese medicine can selectively inhibit pathogenic microorganisms, intervene with their physiological metabolism, and disrupt the biological structure and function of microorganisms (such as hindering the synthesis of bacterial cell wall, inhibiting protein production in bacteria, suppressing normal nucleic acids metabolism, and interfering with other biochemical pathways in microorganisms). In addition, traditional Chinese medicine has some advantages over other pharmacotherapeutics: it is less likely to cause drug resistance and has few side effects. Few studies have reported the resistance of bacteria to traditional Chinese medicine, indicating that the combined use of traditional Chinese medicine and antibiotics has broad application prospects in the treatment of drug-resistant bacteria.21 Reduning injection is a widely used traditional Chinese patent medicine in clinical practice, it shows excellent anti-inflammatory, heat clearing and detoxification effects and has multiple effects such as antiviral, antibacterial, anti-allergic, anti-cancer, hepatoprotective and biliary protective, and immunoregulative effects, and the main effective ingredients of Reduning include Artemisia annua, honeysuckle, and Gardenia jasminoides.22 Modern pharmaceutical study has revealed that Artemisia annua and Lonicera japonica have significant inhibitory effect on bacterial growth. Artemisia annua, as a major ingredient, can clear heat from the body, cool the blood, dissipate surface heat toxin, and had both immunosuppressive and immunoenhancing effects.23 Honeysuckle, as a ministerial ingredient, helps to clear heat, detoxify, and expel external pathogens.24 It can disrupt the peptidoglycan layer in the bacterial cell wall to change the permeability of cell membrane, thereby inhibiting bacterial growth. Gardenia is an adjunctive ingredient, can clear internal heat in the heart, lung, and stomach, and exert the anti-infective and temperature-lowering effects.25 In clinical practice, Reduning is commonly used in antibacterial and antiviral treatments and has significant therapeutic efficacy in bacterial or viral infections of upper respiratory tract.26 A study by Wang et al27 showed that the combination of Reduning injection and Imipenem Sitastatin Sodium could significantly improve the clinical efficacy in severe pneumonia patients, alleviate clinical symptoms and disease severity, improve lung ventilation, and markedly reduce inflammatory reactions. A study by Li et al28 showed that the combination of Reduning injection and piperacillin sodium/tazobactam sodium could improve the clinical efficacy in severe pneumonia patients, improve clinical symptoms and lung ventilation and reduce inflammatory reactions with favorable safety. Xiao et al29 found that, in the treatment with Reduning plus cefotaxime or cefoperazone/sulbactam, the required antibacterial concentration for combination therapy was significantly lower than that of monotherapy. Therefore, this study aimed to explore the possibility of combined use of Reduning and Polymyxin in the treatment of CRKP infection, and the therapeutic effect of Reduning plus Polymyxin was further investigated.
In the present study, among 50 CRKP strains, the majority were collected from sputum samples, particularly in patients in intensive care unit (ICU). This is likely related to the long hospital stay, poor self-resistance capability, relatively complex disease condition, and multiple invasive procedures (such as indwelling catheterization, tracheotomy, mechanical ventilation, deep vein catheterization, drainage tube placement, etc.), which increase the risk of concurrent infections.30 Studies have reported that inflammatory factors such as procalcitonin, interleukin-6, and high-sensitivity CRP are independent risk factors for bacterial pneumonia.31 In the present study, our results showed that the levels of various inflammatory indicators increased in 50 CRKP specimens, and thus timely judgments can be made on the development of disease based on the changes in these indicators.
This experiment conducted in vitro antibacterial experiments on CRKP using both Reduning and polymyxin to determine their MIC values. The combination of Reduning and polymyxin was then used to determine the MIC values of the two drugs using the micro-chessboard dilution method, and the fractional inhibitory concentration index (FICI) was calculated. The experimental results indicate that Reduning can inhibit the growth of CRKP, and when combined with polymyxin, the MIC values of CRKP are generally lower than those when used separately. Among the 50 clinically isolated CRKP samples, 43 showed additive effects, 7 showed synergistic effects, and no antagonistic phenomena were found. Compared to the MIC value when using a single drug, combination therapy can reduce the MIC value by 2 to 4 times. As a pure traditional Chinese medicine preparation, Reduning has shown significant antibacterial effects when used alone, and its combination therapy can also produce effective antibacterial effects. The research results of Gao et al32 show that the combination of Reduning Injection with polymyxin B and meropenem can significantly reduce the levels of procalcitonin (PCT), c-reactive protein (CRP), and serum amyloid protein (SAA) in patients with multidrug-resistant Gram negative pulmonary infections. This indicates that the combined use of Reduning Injection can indeed significantly improve patient prognosis and enhance clinical efficacy. The clinical use of high-dose and high concentration antibiotics may cause toxicity to the liver and kidneys of patients, and long-term use may also promote bacterial resistance.33 A study of Gao et al32 found that the incidence of adverse reactions in patients after combination therapy did not increase the risk of nephrotoxicity and central neurotoxicity caused by polymyxin B injection, indicating good drug safety. This indicates that Reduning can enhance the efficacy of polymyxin in treating CRKP without causing nephrotoxicity. Multiple studies34–36 have shown that Reduning can effectively shorten treatment time, reduce treatment costs, and has significant economic advantages. Therefore, Reduning has practical value in the treatment of refractory CRKP infections.
In our study, some results also showed discrepancies with the overall results, which may be ascribed to the physiological heterogeneity of bacterial colonies of tested strains, resulting in the dispersion of MIC to different extents. If the test drug is set under multiple ratio conditions, the true MIC of a specific strain may be between two gradient ratios, and the concomitant subjective observation of turbidity with naked eyes may bias the experimental results to a certain extent. To standardize the results, a 12 × 8 matrix agar dilution was employed using the principle of agar dilution to determine the results based on colony formation. At the same time, the manual operation also lacked training and systematic quality control guarantees, and the aseptic operation was not fully implemented. The freshness of M-H broth may also cause experimental errors. In our study, the MIC of polymyxin against CRKP obtained by the broth microdilution method was higher than that detected with the instrument. However, Xie et al37 found in their study that the sensitivity of tigecycline to KP measured by the instrument was significantly lower than that measured with the manual method, and the evaluated indicators were out of the acceptable range. Their results showed consistency of results between two methods was very poor, which was similar to the conclusions from other two studies.38,39 These findings suggest that detection with instruments may bias the results to a certain extent. Thus, it is better to compare the results determined with instrument with those with the manual method, and data detected with two methods were subjected to further statistical analysis, which may provide better scientific support for the experimental results. The strains used in this study were separated from the same hospital, and the sample size was relatively small. Therefore, the accuracy of our results still needs further validation in more multicentered studies with large sample size.
This study preliminarily indicates the antibacterial activity of Reduning in vitro and highlights the necessity for further investigation into the effects of individual components and combination therapy on their metabolism in vivo. This study presents the potential of traditional Chinese medicine in preventing and treating infections and provides new directions and methods for the investigation of treatment targeting drug-resistant bacteria in the future.
Conclusions
Reduning can inhibit the growth of CRKP, and when combined with polymyxin B, the MIC values of CRKP are generally lower than those when used separately. So, Reduning has antibacterial effect on CRKP in vitro, and the addition of Reduning can reduce the dose of polymyxin B in the treatment of CRKP.
Acknowledgments
The study was financially supported by the Project of Zhejiang Medical and Health Science and Technology Plan from the Health Commission of Zhejiang Province (No: 2020KY197), and the Chinese Medicine Research Program from the Administration of Traditional Chinese Medicine of Zhejiang Province (No: 2020ZA041, 2023ZL332).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016;80(3):629–661. doi:10.1128/MMBR.00078-15
2. Braykov NP, Eber MR, Klein EY, et al. Trends in resistance to Carbapenems and third-generation cephalosporins among clinical isolates of Klebsiella pneumoniae in the United States, 1999–2010. Infect Control Hosp Epidemiol. 2013;34(3):259–268. doi:10.1086/669523
3. Wang N, Zhan M, Liu J, et al. Prevalence of Carbapenem-Resistant Klebsiella pneumoniae infection in a Northern Province in China: clinical characteristics, drug resistance, and geographic distribution. Infect Drug Resist. 2022;15:569–579. doi:10.2147/IDR.S347343
4. CHINET China Bacterial Resistance Testing Network. Results of bacterial resistance monitoring in 2023. 2024 March 5, [2024 May 6]. Available from: http://www.chinets.com.
5. Zarakolu P, Eser ÖK, Otlu B, et al. In-vitro activity of fosfomycin against Escherichia coli and Klebsiella pneumoniae bloodstream isolates and frequency of OXA-48, NDM, KPC, VIM, IMP types of carbapenemases in the carbapenem-resistant groups. J Chemother. 2022;34(4):235–240. doi:10.1080/1120009X.2021.1963618
6. Xue J, Xie M, Zhou T. Analysis of all-cause mortality in patients with carbapenem resistant Klebsiella pneumoniae infection. Chin J Clin Pharmacol. 2018;34(18):2220–2223.
7. Liu S, Zhang P, Liu Y, et al. Metabolic regulation protects mice against Klebsiella pneumoniae lung infection. Exp Lung Res. 2018;44(6):302–311. doi:10.1080/01902148.2018.1538396
8. Xu L, Sun X, Ma X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clinic Microbiol Antimicrob. 2017;16(1):18. doi:10.1186/s12941-017-0191-3
9. Zhang J, Hu Y, Shen X, et al. Risk factors for nephrotoxicity associated with polymyxin B therapy in Chinese patients. Int J Clin Pharm. 2021;43(4):1109–1115. doi:10.1007/s11096-020-01225-8
10. Wu Z, Liu H, Chen H, et al. Antibacterial activity of polymyxin B in combination with several antimicrobial agents against carbapenem-resistant Klebsiella pneumoniae in vitro. J Wenzhou Med Univ. 2017;47(12):898–901.
11. Fu Y, Shan Y, Zhang N, et al. Analysis of in vitro antibacterial activity of baicalin combined with tobramycin against carbapenem-resistant Pseudomonas aeruginosa. Chin J Health Lab Technol. 2023;33(7):807–10+14.
12. Li J, Bao X, Bao D, et al. Study on the antibacterial effect of Jieredu injection. Pharmacol Clini Chinese Mater Med. 2001;01:30–32.
13. Gao Q, Wang Y, Guo S, et al. Retrospective analysis of clinical efficacy and safety of Reduning Injection in children with community-acquired pneumonia. Drug Eval Res. 2024;47(02):377–382.
14. Wang J, Li Q, Qiu X, et al. Application of enzyme immunochromatography and carbapenemase inhibitor enhancement test in the detection of carbapenemase type. Chin J Health Lab Technol. 2023;33(03):271–4+81.
15. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing[S].M100-S32. Wayne, PA: Clinical and Laboratory Standards Institute; 2022.
16. Zhao Z. Study on the antibacterial activity of phosphomycin combined with polymyxin B against multidrug-resistant Pseudomonas aeruginosa in vitro and in vivo. Zhenzhou Univ. 2020.
17. Bento Talizin T, Dantas de Maio Carrilho CM, Magalhães Carvalho Grion C, et al. Polymyxin for treatment of ventilator-associated pneumonia in a setting of high carbapenem resistance. PLoS One. 2020;15(8):e0237880. doi:10.1371/journal.pone.0237880
18. Yu X, Pan J, Zhou Z, et al. TDM-guided medication of polymyxin B in a patient with CRKP-induced bloodstream infection: a case report. Eur J Clin Microbiol Infect Dis. 2021;40(1):201–204. doi:10.1007/s10096-020-03945-1
19. Tian X, Chen Z, He H, et al. The effect of polymyxin B hemoperfusion on prognosis of patients with sepsis and septic shock: a meta-analysis. Chin J Respir Critical Care Med. 2020;19(01):16–21.
20. Huen KH, Nik-Ahd F, Chen L, et al. Neomycin-polymyxin or gentamicin bladder instillations decrease symptomatic urinary tract infections in neurogenic bladder patients on clean intermittent catheterization. J Pediatr Urol. 2019;15(2):178.e1.–e7. doi:10.1016/j.jpurol.2018.12.001
21. Ma D, Tao Q, Qi H. In vitro antibacterial test of Shuanghuanglian against pan resistant Klebsiella pneumoniae. Lab Med. 2017;32(03):242–244.
22. Ge W, Li H, Yu Y, et al. Research progress on chemical constituents, pharmacological action, and clinical application of Reduning Injection. Chin Traditional Herbal Drugs. 2017;48(05):1027–1036.
23. Li C, Sun D, Chen W, et al. Research progress on artemisinin and its derivatives in the treatment of rheumatoid arthritis. Chin J Rheumatol. 2024;28(06):402–406.
24. Gao X, Zhang Y, Han L. Honeysuckle oral liquid in the treatment of common novel coronavirus pneumonia: clinical observation. Chin Clin Case Achieve Database. 2022;04(01):58–59.
25. Deng Y, Sun S. Effects of different processing mechanisms on the chemical composition and anti-inflammatory effect of gardenia. Int Med Health Guid News. 2021;27(20):3123–3127.
26. Jia L, Qingchun T. Experimental study of antibacterial test on Reduning combined with other drugs resistant to carbapenem-resistant Acinetobacter baumannii in vitro. Beijing J Tradit Chinese Med. 2019;38(01):35–37.
27. Wang S, Ji F, Li P, et al. Clinical study on reduning injection combined with imipenem and cilastatin in treatment of severe pneumonia. Drugs Clinic. 2023;38(12):3066–3070.
28. Li M, Shao H. Clinical study on reduning injection combined with piperacillin sodium and tazobactam sodium in treatment of severe pneumonia. Drugs Clinic. 2022;37(12):2790–2794.
29. Xiao Y, Liao B, Lu Q, et al. Experimental study on bacteriostatic effect of reduning injection combined with Western Medicine on Carbapenem-resistant Pseudomonas aeruginosa in vitro. Res Integr Trad Chin West Med. 2022;14(05):304–308.
30. Chen X, Zhang N, Yuan C, et al. Inhibitory effect of baicalin combined with tobramycin on biofilm of carbapenem-resistant Pseudomonas aeruginosa. Chin J Health Lab Technol. 2023;33(09):1071–1074.
31. Li X, Zhu H. Therapeutic efficacy of Cefoperazone Sulbactam combined with inhaled budesonide in bacterial pneumonia and the effect on the expression of inflammatory factors in patients. Clin Res. 2024;32(02):88–91.
32. Gao S, Tian J, Ning Y, et al. Clinical efficacy and economic analysis of reduning injection combined with meropenem and Polymyxin B in the treatment of pulmonary infection of MDR-GNB. Evaluation and Analysis of Drug-Use in Hospitals of China. 2023;23(11):1309–12+16.
33. Liu P, Wang Z. Susceptibility test in vitro on Carbapenem-resistant Acinetobacter baumannii. Evaluation and analysis of drug-use in hospitals of China. 2014;14(08):705–707.
34. Huang X, Duan X, Zhu Y, et al. Comparative efficacy of Chinese herbal injections for the treatment of community-acquired pneumonia: a Bayesian network meta-analysis of randomized controlled trials. Phytomed Int J Phytother Phytopharmacol. 2019;63:153009. doi:10.1016/j.phymed.2019.153009
35. Shao X, Le Z, Liu R, et al. Efficacy and safety of reduning injection combined with Salmeterol Fluticasone in the treatment of patients with acute exacerbation of chronic obstructive pulmonary disease. World J Integr Trad West Med. 2023;18(01):93–7+103.
36. Gao J. The effect and safety of reduning injection combined with interferon atomization in the treatment of hand, foot and mouth disease. Syst Med. 2022;7(02):147–150.
37. Xie G, Zhou L, Liang B, et al. Performance of different antibiotic susceptibility tests for testing susceptibility of carbapenem-resistant gram-negative bacteria to tigecycline. Int J Lab Med. 2023;44(18):2187–2191.
38. Dai X, Huang S, Li H. Comparision of three susceptibility testing methods of tigecycline against multidrug resistant Acinetobacter baumannii. J Modern Lab Med. 2020;35(03):103–106.
39. Yang L, Liu J, Zhang Z, et al. Evaluation of different in vitro tigecycline susceptibility tests for carbapenem-resistant Klebsiella pneumoniae. ChiN J Antibiotics. 2019;44(08):963–967.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.