Back to Journals » International Journal of Nanomedicine » Volume 19
In vivo Fate of Targeted Drug Delivery Carriers
Authors Zhao F , Wang J, Zhang Y, Hu J, Li C, Liu S, Li R , Du R
Received 26 February 2024
Accepted for publication 22 June 2024
Published 9 July 2024 Volume 2024:19 Pages 6895—6929
DOI https://doi.org/10.2147/IJN.S465959
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Dongwoo Khang
Fan Zhao,1,2 Jitong Wang,1,2 Yu Zhang,1,2 Jinru Hu,1,2 Chenyang Li,3 Shuainan Liu,4,5 Ruixiang Li,1 Ruofei Du1,2
1Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, People’s Republic of China; 2Engineering Research Center of Modern Preparation Technology of TCM, Ministry of Education, Shanghai, 201203, People’s Republic of China; 3School of Pharmacy, Shenzhen University Medical School, Shenzhen University, Shenzhen, Guangdong, 518055, People’s Republic of China; 4State Key Laboratory of Bioactive Substance and Function of Natural Medicines, Key Laboratory of Polymorphic Drugs of Beijing, Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100050, People’s Republic of China; 5Diabetes Research Center of Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Ruixiang Li, Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, People’s Republic of China, Email [email protected]; Ruofei Du, Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, People’s Republic of China, Email [email protected]
Abstract: This review aimed to systematically investigate the intracellular and subcellular fate of various types of targeting carriers. Upon entering the body via intravenous injection or other routes, a targeting carrier that can deliver therapeutic agents initiates their journey. If administered intravenously, the carrier initially faces challenges presented by the blood circulation before reaching specific tissues and interacting with cells within the tissue. At the subcellular level, the carrier undergoes processes, such as drug release, degradation, and metabolism, through specific pathways. While studies on the fate of 13 types of carriers have been relatively conclusive, these studies are incomplete and lack a comprehensive analysis. Furthermore, there are still carriers whose fate remains unclear, underscoring the need for continuous research. This study highlights the importance of comprehending the in vivo and intracellular fate of targeting carriers and provides valuable insights into the operational mechanisms of different carriers within the body. By doing so, researchers can effectively select appropriate carriers and enhance the successful clinical translation of new formulations.
Plain Language Summary: Nowadays, scientists are actively researching nanocarrier drugs. After administration via injection or other methods, these drugs experience in the body and reach the target treatment site to relieve or cure symptoms. As research progresses, scientists are gaining more insights into the behavior of nanocarrier drugs in the body, which is useful in developing safer and more effective drugs. Historically, research has focused primarily on the drug itself. However, it is important to understand that the carrier that delivers and protects the drug (often described as the drug sitting in a “car” or under an “umbrella”) plays an essential role in the drug’s therapeutic effect. This paper aims to highlight the importance of the carrier’s role, which is vital for developing new drugs and advancing basic research.
Keywords: drug carriers, targeted delivery, in vivo fate, subcellular fate
Graphical Abstract:

Introduction
Over the past 70 years, significant advancements have been made in drug delivery technology. A crucial development in modern pharmacy is the invention and implementation of carrier-based drug delivery systems, specifically those using nanometer-sized particles. These carriers offer several advantages over traditional drug delivery systems, effectively addressing various challenges in disease treatment.1,2 Nanocarriers are vehicles for drugs, acting as a protective shield within the body. They shield drugs from degradation in the physiological environment throughout the pre-release process, prolonging the drug’s half-life and enabling targeted delivery. Targeted delivery drug carriers, including liposomes, lipid-based nanoparticles, protein-based nanoparticles, polymer nanoparticles, and inorganic nanoparticles, can deliver drugs to designated sites, enhancing drug efficacy, improving drug bioavailability, minimizing damage to normal tissues and cells, and reducing side effects.1,3 Traditional systemic drug delivery methods have several limitations, such as low drug-specific bioavailability, inadequate drug concentration at the target site, and significant adverse effects. As a response to these challenges, targeted therapies have emerged.3,4 Moreover, the invention and advancement of targeted delivery drug carriers hold great significance in treating diseases that require precise drug release, such as cancer. The aforementioned targeted delivery drug carriers encompass various types that have gained considerable attention and research, including nanogels, micro/nanomotors, microbubbles, polysaccharide, biomimetic nanoparticles, extracellular vesicles, yeast, and viral vectors.5–10 With continuous advancements in passive targeting, active targeting, and other targeting methods, the diversity of targeted delivery drug carriers continues to expand.
Many delivery carriers with targeting capabilities have been mentioned; however, how many nanocarriers have made it to market? We have compiled a table of the nanocarriers approved by the Food and Drug Administration (FDA) (Table 1), including those with “Prescription” and “Discontinued” status. Of these, four of the liposome-based drugs have a “Discontinued” status; only one of the micelle-based drugs was approved but is now also with a “Discontinued” status, and one of the iron oxide nanoparticles-based drugs has a “Discontinued” status. The FDA has approved 13 nanocarriers, six of which are with a “discontinued” status. The discontinuation of a marketed drug is complex and includes (1) problems with the safety, efficacy, or controllable quality of the drugs; (2) emergence of new, more efficacious, decrease in the market share; (3) owing to the control by the relevant departments, the supply of raw materials is difficult because of the environmental pollution, hindering the production; (4) low end-demand and small market share of the disease cured by the drugs; (5) increase in costs of production. In addition to production, market, policy, and other external factors, the efficacy and safety of the drug need extra attention. In the field of nanomedicines, a small proportion of the research focuses on using nanocarriers to deliver drugs. In the field of nanocarrier delivery, researchers focus more on the nature of the loaded drug than the nature of the blank nanocarriers. Additionally, there is a tendency to overly concentrate on the design of the nanocarriers themselves. Most pharmaceutical researchers design the carrier in a very subtle way and modify it to have several great properties, expecting them to work effectively in delivering drugs in vivo. However, the designed carrier may not function as intended once in the body.
 |
Table 1 Summary of FDA-Approved Nanocarrier Drugs to Date (Grouped by Nanocarrier Type, Including Discontinued Nanocarrier Drugs)a |
When developing nanocarriers, formulation design and subsequent pharmacological experiments are usually performed by different teams, which poses a major problem as front-end pharmaceutical researchers, although having theoretical knowledge and insights into carrier design, often lack a thorough understanding of the carrier’s in vivo fate and mechanisms compared to pharmacologists. The process of bringing a nanocarrier to market requires a substantial investment in terms of money, human resources, or material resources. If the drug has poor clinical effects or safety issues, it can result in significant losses owing to the need for rework or abandonment. Therefore, in the early stage of nanocarrier design, we should consider the clinical impact, which requires researchers to have sufficient knowledge about the in vivo fate of nanocarriers.
Therefore, pharmacy workers and others involved in nanocarrier research should first study the fate of the carriers in vivo, increase the investment in the study of the fate of the blank carriers in vivo, and firmly grasp the fate of the carrier in vivo. By doing so, the success rate of pharmacological experiments and clinical translation can also be improved, benefiting patients by providing drugs for their conditions promptly.
The above was also our intention while conducting this study, which aimed to provide an overview of the in vivo fate of delivery carriers with targeting capabilities that have been reported. We aimed to compare the subject matter of this study with other studies on similar topics about drug delivery carriers (Table 2).
 |
Table 2 Theme Comparison of This Review Article with Other Review Articles on Similar Topics (with Examples of Typical Articles) |
In conclusion, this article aimed to summarize the in vivo fate mechanisms of 13 major classes of targeted drug delivery carriers that have undergone some research progress. Specifically, this compilation elaborates on their major tissue distribution upon entry into the body, blood circulation time, interaction mechanisms with cell membranes, intracellular transport methods, escape capabilities, degradation and excretion pathways, and potential toxicity. Despite the existence of undefined aspects in the in vivo fate mechanism of the targeted delivery drug carriers summarized in this article, this study can provide valuable references for researchers in selecting targeted drug delivery carriers and gaining a preliminary understanding of their in vivo distribution.
Types of Targeted Drug Delivery Carriers
Presently, various carriers are used for targeted drug delivery. We broadly categorized the delivery carriers with targeting capabilities reported into 13 major categories (Figure 1). We organized their common carriers (We listed the specific carrier categories commonly used, and analyzed these commonly used carriers. The commonly used carrier categories was not listed because they are not generalized designations, belonging to a specific class of carriers, and are subsequently used directly in the analysis), meaning, property, role, the types of drugs that can be transported and their main fields of application, and created a table (Table 3).
 |
Figure 1 Schematic diagram of targeted drug delivery carriers. |
 |
Table 3 Summary of a Brief Description of the 13 Major Classes of Targeted Drug Delivery Carriers |
Biodistribution
Small molecule drugs are often quickly eliminated by the kidneys upon entering the bloodstream, potentially reducing their efficacy. However, when drugs are loaded into carriers, their circulation half-life is prolonged, leading to enhanced accumulation at the targeted site and promoting therapeutic efficacy. Various targeted drug delivery carriers exhibit unique biological distributions, and the following section discusses three main aspects of the distribution for each carrier.
Targeting
Targeted drug delivery is a promising research approach for treating diseases such as cancer, aiming to deliver therapeutic drugs precisely to the affected site and minimize damage to healthy tissue. This approach improves drug efficacy while reducing side effects.3
Targeted drug delivery can be achieved through three main strategies: 1) passive targeting, primarily based on the carrier’s size, and Enhanced Permeability and Retention (EPR) effect. The EPR effect refers to high permeability and prolonged retention of substances <200 nm in solid tumors. The lack of vascular support tissue, poor structural integrity, and large inter-endothelial cell gaps in tumors allow carriers <200 nm to easily penetrate the vascular wall and enter tumor gaps. Furthermore, tumors lack lymphatic vessels, preventing the carried drugs from being drained and enabling long-term accumulation.59–61 2) Active targeting mode: addition of targeting ligands. Targeting ligands are substances with a high affinity for the target site, including folate, transferrin, peptides, antibodies, aptamers, and oligosaccharides. These ligands bind to specific receptors overexpressed in the lesion tissue, facilitating targeted drug delivery.61,62 3) Physicochemical targeting mode: using physical and chemical methods to enable targeted formulations to exert their therapeutic effects in specific locations. The carriers prepared via this method are stimulus-sensitive carriers, which undergo rapid changes when exposed to external stimuli (such as light, magnetic fields, high temperature, ultrasound) or special target site environments (such as enzyme overexpression, low pH), releasing the drugs they carry and achieving accurate drug release at the target site.63,64
Passive Targeting
Carriers based on passive targeting patterns are liposomes,65 lipid nanoparticles (LNPs),66 albumin carriers,67 follicular dendritic cell (FDC),59 dendrimers,24 micelles/ polymeric micelles (PMs),68,69 gold nanoparticles (AuNPs),37 nanogels,70 microbubbles,71 angelica sinensis polysaccharide (ASP),43 extracellular vesicles (EVs),72 adeno-associated viral (AAV) vectors.73 Carriers that target specific sites without adding ligands belong to the passive targeting strategy. The targeting mechanism of passive targeting carriers is as follows.
Liposomes, Dendrimers, and LNPs
Carriers with a diameter <200 nm, such as liposomes and dendrimers, are easily passively targeted to tumors because the endothelial walls of healthy tissues are tightly connected, preventing the carriers from entering. In contrast, tumors have larger inter-endothelial gaps and more porous capillaries, resulting in higher permeability. The amount of carriers taken up by tumors can be >100 times higher than that of healthy tissues.24,59,74,75
LNPs can bind with apolipoprotein E (ApoE) in plasma to form ApoE-bound lipoproteins, which are mainly cleared by the liver, allowing LNPs to reach the liver preferentially for liver targeting. This method is commonly used to deliver RNA to the liver using cationic or ionizable LNPs. The presence of ionizable lipids and intentional short acyl chain PEG lipids enhance the ability of LNPs to deliver RNA compared to liposomes. Liver targeting is insufficient for treatment, as specific cell targeting is often necessary. Therefore, Kim et al modified the overall size of LNPs by adjusting the polyethylene glycol (PEG) content, specifically targeting hepatocytes.76 Lipid nanoparticles (LNPs) can be modified to selectively target specific cell types. They offer the benefits of delivering mRNA with high transfection efficiency, high expression levels, and good stability, making them crucial carriers for mRNA vaccine development.77
Albumin Carriers and FDC
Albumin carriers can specifically bind to cell surface albumin receptor (gp60), which binds to intracellular caveolin-1, leading to membrane invagination and the formation of caveolae. Caveolae can transport albumin carriers to tumors, making carriers bind to secreted protein acidic and rich in cysteine protein overexpressed in tumors, thereby targeting the albumin carriers to the tumor.78 The tumor-targeting effect of ferritin is ten times greater than that of the EPR effect. Ferritin does not require surface modification as it naturally targets tumors through its specific affinity for transferrin receptor 1 (TfR1), which is overexpressed in malignant tumors. Ferritin is the optimal size of 12 nm for inducing the EPR effect caused by disrupting the blood and lymphatic systems in tumors.79
AuNPs, Nanogels, and ASP
AuNPs, after being injected into the body, can accumulate in inflammatory and tumor sites through the enhanced permeability and EPR effect to achieve passive targeting.37
Nanogels also accumulate at the site of lesions through the enhanced permeability and EPR effect to achieve passive targeting. However, the targeting capabilities of these nanogels are low; therefore, current nanogels are mostly designed as active targeting agents by adding ligands and other methods.70
ASP has a high affinity for the asialoglycoprotein receptor that is highly expressed in the liver, allowing for liver targeting.43
EVs, Yeast, and AAV Vectors
EVs can cross physiological barriers and target tissues through their surface protein markers.80 However, some problems are encountered in the targeting design of EVs because of the complex composition and naturally highly heterogeneous of EVs, which influence the therapeutic effect.81
The mechanism of specific targeting of phagocytes by yeast carriers is unique. The β-glucan in the yeast cell wall can specifically bind to pattern recognition receptors on phagocytes, thereby targeting phagocytic cells.55
AAV vectors, such as AAV8, target the liver, and AAV1 and AAV5 target cells in the central nervous system.73
Active Targeting
Carriers based on active targeting patterns are biomimetic nanoparticles and carriers modified with folate, transferrin protein, peptides, antibodies, aptamers, hyaluronic acid (HA), PEG, and oligosaccharides.49,62,82
Folate, transferrin protein, peptides, antibodies, aptamers, HA, and oligosaccharides can bind with specific receptors overexpressed in lesions, allowing carriers to target lesion sites. For example, folate receptor (FR) and TfR in tumors are responsible for the uptake of folate and iron, respectively. Tumors need a large amount of folic acid and iron during rapid proliferation. Folate and transferrin can bind to FR and TfR expressed at high levels in cancer cells with high affinity, mediating carriers modified with folate or transferrin to target tumor tissues, respectively.83,84 HA can bind to CD44 receptors that are significantly expressed in cancer cells, targeting carriers modified with HA to cancer cells.85
The emerging targeting strategy is to load drugs for precisely targeted delivery by using vesicles from disease cells or some cell membranes that contain receptors on their surface that bind specifically to cells at the site of the disease as carriers. For example, Yon et al86 developed a bio-compatible tumor cell-derived extracellular vesicle biomimetic porous silicon nanoparticle as a drug carrier for targeted cancer therapy (Figure 2). This carrier exhibits enhanced tumor accumulation and penetration into the deep tumor. The developmental idea of using biomimetic extracellular vesicle nanoparticles derived from tumors as drug carriers for targeted chemotherapy in cancer is a proof-of-concept and provides new design ideas for researchers developing targeted delivery drug carriers.
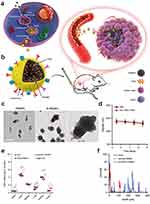 |
Figure 2 (a and b) Schematic diagram of E-PSiNPs as drug carriers for targeted cancer chemotherapy. (c) Transmission electron microscope images of PSiNPs and E-PSiNPs. (d) Hydrodynamic diameter of E-PSiNPs incubating in phosphate-buffered saline with or without 10% fetal bovine serum for different intervals. (e) DOX content in tumor tissues and major organs of H22 tumor-bearing mice 24 h after intravenous injection. (f) Penetration of DOX@PSiNPs or DOX@E-PSiNPs into tumor parenchyma. Reprinted from Yong T, Zhang X, Bie N, et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat Commun. 2019;10(1):3838. Creative Commons.86 |
Yan et al87 prepared a penetrating macrophage-based nanoformulation (MZ@PNM)-encapsulating hydrogel (MZ@PNM@GCP) that responded to the periodontitis microenvironment. The loaded nanoparticles (MZ@PNM) could specifically target porphyromonas gingivalis, a key mediator of periodontitis, through their Toll-like receptor 2/1 mimicking membrane on macrophages. Subsequently, the macrophage membrane fused with the bacterial outer membrane, and the cationic nanoparticles within MZ@PNM disrupted the bacterial structure and released metronidazole in the bacterial cytoplasm (Figure 3).
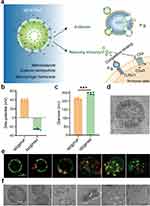 |
Figure 3 (a) Schematic diagram of the action of MZ@PNM in targeting and killing P.g. (b–c) Zeta potential and diameter characterization of MZ@PNM and MZ@PNP. ***p < 0.001. (d) Representative TEM image of the nanomacrophages targeting P.g. (e–f) Representative fluorescence image and TEM images of the destruction of P.g. by MZ@PNM over time. Reprinted from Yan N, Xu J, Liu G, et al. Penetrating Macrophage-Based Nanoformulation for Periodontitis Treatment. ACS Nano. 2022;16(11):18253–18265. Copyright © 2022, American Chemical Society.87 P.g., Porphyromonas gingivalis; TEM, Transmission electron microscope. |
Physicochemical Targeting
When the physicochemical target carriers are exposed to external stimulation or are in a target site environment, they will rapidly change and release their loaded drug to achieve the effect of precise drug release at the target site. For example, thermo-sensitive liposomes, photo-sensitive liposomes, ultrasound-guided carriers, and pH-sensitive carriers are all based on this design.82 Thermo-sensitive liposomes are sensitive to temperature, photo-sensitive liposomes are sensitive to light, and ultrasound-guided carriers are guided by ultrasound, all of which are precisely delivered to target sites under external stimulation. The mechanism of pH-sensitive carrier release is that in different pH environments inside the body, pH-sensitive carriers are sensitive to the pH of a specific environment and undergo structural or other changes, thereby releasing the loaded drugs in that location, achieving precise drug release. Generally, there are two types of pH-sensitive carriers. One type is polymers containing ionizable functional groups, which protonate owing to pH changes, resulting in changes in hydrophilic–hydrophobic balance and changes in structure or solubility. The other type has acid–labile bonds; therefore, they are cleaved in acidic environments, leading to changes in carrier structure and drug release.88 For example, the pH of plasma, the tumor microenvironment, and the pH of lysosomes and nuclei are neutral (6.5–7.0) and acidic (4.5–5.5), respectively. Therefore, carriers sensitive to an environment with a pH of 6.5–7.0 can release drugs precisely in tumor environments, achieving tumor targeting.89,90
Moreover, carriers with dual sensitivity can be combined to achieve dual targeting control, such as thermo-sensitive magnetic liposomes, which are thermally sensitive and magnetically guided, making them more suitable for the special properties of the lesion location.82
In addition to carriers with inherent targeting properties, other carriers mostly need to be modified on the surface to have targeting properties to cells and tissues. Researchers have also discovered some materials that are very suitable for developing targeted carriers, such as selenium nanoparticles (SeNPs),91 graphene and its composite materials,92 layered double hydroxide (LDHs),93 quantum dots (QDs),94 chitosan,95 adenovirus vectors,96 and lentiviral vectors.97
Different targeted drug delivery carriers have different targeting mechanisms, and selecting targeting carriers can be based on the site and characteristics of the disease to be treated. For example, we can choose a carrier that can specifically bind to the lesion or passively target the lesion, or we can modify a carrier with better performance to make it actively target the lesion. Furthermore, a responsive targeting vector can be designed to target some focal sites with special environments. If the microenvironment of a focal site has a low pH, a targeting vector sensitive to low pH can be designed so that it is released at that site but not in a normal pH environment. Precisely targeted carriers for release can also be achieved by designing carriers externally controlled or stimulated by delivering the carrier through control or stimulation such as light, heat, temperature, and magnetic field.
Major Tissue Distribution
Generally, carriers with a diameter <5 nm are easily cleared from the circulation, while carriers >50 nm accumulate non-specifically in the liver, which has fenestrations with a length of 50–100 nm. Carriers with a diameter >200 nm accumulate in the spleen, which has fenestrations with a length of 200–500 nm.2,98,99 However, the specific distribution of particles with diameters ranging from 20–150 nm is also influenced by their charge and shape. For example, among particles of this size, those shaped like cylinders or discs are more likely to distribute to the liver and spleen.98 Below is a summary of the major tissue distribution of various carriers.
Liposomes, LNPs, and Graphene Materials
Conventional liposomes and LNPs, which are carriers composed of lipids, are recognized and taken up by the reticuloendothelial system (RES) after intravenous injection, with most accumulating in the liver and spleen (RES organs), and after oral administration, they are hydrolyzed in the gastrointestinal tract, with only a few remaining carriers able to circulate to the target site.11,14,63 After LNPs are administered through the intramuscular route, they first accumulate in the muscle tissues, then enter the systemic circulation through the lymphatic system and the blood circulatory system, part of which will be taken up by the RES organs such as the liver, and the remaining will reach the corresponding target site to deliver mRNA according to the design.100
Graphene and graphene oxide are mainly distributed in the liver after entering the body and are taken up by the RES.101
EVs
EVs entering the body are primarily accumulated in the liver, followed by the spleen, gastrointestinal tract, and lungs, although the actual distribution of EVs from different cells and in different amounts may vary.102 For example, ginger-derived extracellular vesicles remain in the gastrointestinal tract for a long time after oral administration, while grape fruit-derived extracellular vesicles mainly accumulate in the lungs and brain after nasal administration.103–105 Additionally, exosomes accumulate most in the liver, followed by the spleen, gastrointestinal tract, and lungs, although excessive accumulation of exosomes in the liver is relatively low owing to the phagocytic system’s uptake threshold.106
PEG-modified carriers affect carrier distribution in tissues, reducing steric hindrance, forming a barrier to prevent the modulatory effect of serum proteins, reducing phagocytosis by the RES, and protecting the carrier from uptake by the liver.64
Blood Circulation Time
When the carriers enter the bloodstream, they will adsorb plasma proteins, such as serum albumin, lipoproteins, complement components, and immunoglobulins, and form a protein corona on its surface. This structure makes the carrier more prone to attaching to specific receptors on the surface of phagocytes for phagocytosis, thereby shortening its circulation time in the bloodstream and affecting its stability and fate in the body. Additionally, the circulation time of the carrier in the bloodstream is also related to its shape. For example, filamentous polymer micelles align with the blood flow owing to their shape, resulting in longer circulation times than spherical polymer micelles.98,107 Therefore, different targeted drug delivery carriers have different circulatory times in vivo. The following are discussions on the circulatory time of various carriers in vivo.
Liposomes, LNPs, Dendrimers, and Micelles/PMs
Conventional liposomes are easily eliminated by the RES owing to their easy uptake, resulting in a short circulatory time.63 Newly developed long-circulating liposomes, which combine liposomes with some chemically and biologically inert synthetic polymers, can prolong the circulatory time of drugs in vivo.2
NPs with a diameter of 10–100 nm are not easily engulfed by the RES and generally have longer circulatory times, while larger NPs are easily engulfed by the RES and have shorter circulatory times.2 LNPs with a diameter <200 nm are not easily taken up by the RES and are cleared by the RES, resulting in a longer circulatory time.99
Conventional dendrimers have a short circulatory time owing to their small volume of 10 nm, which makes them easily cleared in vivo.24 Micelles and PMs have a hydrophilic outer shell and are 10–100 nm in diameter, making them not easily taken up and cleared by the RES, resulting in a longer blood circulation time.68,108,109
Albumin Carriers
Endogenous albumin circulates for approximately 19 days because when it enters the endothelial cells, the FcRn receptors in the endothelial cells expel the albumin from the cells via exocytosis, and the albumin returns to the bloodstream through the lymphatic system, resulting in prolonged circulation. However, if albumin is made into albumin nanoparticles or modified into drugs, the circulation time will be reduced to an extent.67
AuNPs, Graphene Materials, QDs, Nanogels, and Microbubbles
AuNPs have a short plasma half-life, which is mainly influenced by two substances in the body that affect the carrier’s half-life, namely opsonins and dysopsonins. Opsonins (such as complement and immunoglobulin supplements) can promote the rapid engulfment of liposomes via the RES, while dysopsonins (such as serum albumin and lipoprotein) can improve the circulation half-life of liposomes by preventing engulfment.74 Opsonins are easily adsorbed onto the surface of AuNPs, promoting the clearance of AuNPs by phagocytes, resulting in a short half-life for AuNPs.89 Graphene and graphene oxide are easily cleared from the blood, resulting in a short circulatory time.110 QDs’ coating materials have certain immunogenicity, which can easily cause an immune response in the body, leading to the clearance and ineffectiveness of QDs, resulting in a short half-life. However, if low-toxicity materials such as carbon and silicon are used for preparation, their circulation time can be prolonged.111
Nanogels with a size of 20–200 nm are not easily engulfed by the RES and have a longer blood circulation time.33 Using ultrasound-mediated microbubble carriers can significantly prolong their circulation time.71
Biomimetic Nanoparticles, EVs, and Viral Vectors
Red blood cell membrane coating and platelet membrane encapsulation can reduce the uptake of nanoparticles via the RES and extend their circulation in the bloodstream.44,112 EVs have a long circulation time because they are endogenous and are not easily cleared by the immune system.72
Owing to the expression of non-deleted viral genes and ensuing immune responses to the expressed viral proteins, the circulation time of Ad vectors in the bloodstream is relatively short.73 Among them, chimpanzee-derived Ad vectors have the longest circulation time because the level of neutralizing antibodies against them in the body is the lowest.56
Moreover, modifying the carriers can also change the circulation time in the bloodstream. For example, coating the carrier surface with PEG to form a PEG-coating can provide a protective barrier to prevent carrier lipolysis when entering the gastrointestinal tract. The ethylene glycol units of PEG coating can form a hydrating layer by tightly binding with water molecules. This hydration layer can prevent protein adsorption and recognition by RES, thereby extending the half-life of drugs and increasing their circulation time.11,85,113,114 Modifying the carrier with dextran can also protect it and prolong its circulation in the bloodstream. This benefits the drug accumulation around the target tissue or cells to enhance its effectiveness.115
In addition, nanomotors116 and cell carriers117 can prolong the circulation of drugs in the blood. Since the data obtained from different studies are different, and the circulation time of some carriers is not recorded, it is inaccurate to directly state the long or short circulation time of the carriers, so we have compiled a table on the expected pharmacokinetic parameters and biodistribution of the carriers (Table 4) for the readers’ reference.
 |
Table 4 Summary of General PK and Major Tissue Distribution of Different Types of Carriers |
Fate of Targeted Drug Delivery Carriers at the Site of Lesions
In the previous section, we discussed how targeted drug delivery carriers can distribute to different tissues and organs after entering the human body. Thereafter, carriers face the problem of releasing drugs. Different carriers have different mechanisms for drug release. Some carriers may release drugs at the site of lesions without entering cells, while others may only interact with cell membranes and release drugs into the cytoplasm but not enter the cells. Some carriers can enter cells through certain pathways and be transported at the subcellular level through certain pathways. Some cannot escape the fate of degradation, while others can escape. The following is a discussion of the fate of each carrier at the site of lesions. The carriers discussed in this section are carriers in their complete form that can reach the site of lesions.
Fate of Carriers That Interact with Cell Membranes
There are two main types of interactions between carriers and cell membranes (Figure 4): 1) endocytosis, which refers to transporting extracellular substances into cells through deformation movements of the plasma membrane. This process requires entry into the cell, and carriers that enter cells through endocytic pathways will reach lysosomes or endosomes for degradation. Some carriers will escape after reaching endosomes or lysosomes to avoid degradation; 2) membrane fusion, which refers to the ability of the carrier’s membrane to fuse with the cell membrane, allowing the loaded drugs to be directly released into the cytoplasm without entering the cell. Drugs delivered through this process will not enter endosomes or lysosomes and do not need to undergo escape.143,144
Carriers for Cellular Entry by Endocytosis
There are several pathways for endocytosis of targeted drug delivery carriers: (1) adsorptive endocytosis; (2) clathrin-mediated endocytosis; (3) receptor-mediated endocytosis; (4) caveolae-mediated endocytosis; (5) phagocytosis.2,143,145 Not all endocytosis pathways were covered. Clathrin-mediated-mediated endocytosis and pinocytosis were not covered. Listed here are only the explicitly documented endocytosis pathways for all targeted delivery carriers summarized in this study.
In general, carriers that enter cells by endocytosis first enter the lysosomes or endosomes. The exception to this is caveolae-mediated endocytosis, in which carriers entering the cell are transported via a non-lysosomal pathway within the cell for reasons explained in detail in the section on caveolae-mediated endocytosis. Lysosomes and endosomes contain more than 60 acidic hydrolases, which are inactive enzymes that become active when drugs enter the lysosomes or endosomes and can degrade the incoming substance. This process occurs at a pH of about 5. The pH in lysosomes and endosomes is about 4.5–5.5, with pH changes occurring before and after degradation. Therefore, if the carrier is required to transport drugs to the nucleus or the degradation of carriers in lysosomes or endosomes is not desired, carriers with “endosomal/lysosomal escape” properties can be selected, or carriers can be designed to have “endosomal/lysosomal escape” capability.75 “Endosomal/lysosomal escape” may not be the only way to prevent carriers from being degraded by endosomes or lysosomes; however, it is currently the most widely used method. There are generally two mechanisms by which nanoparticles can escape the endosome or lysosome upon internalization (Figure 5): (1) Expansion-induced variation, where the nanoparticle carrier dissociates within the endosome/lysosome, causing mechanical strain that leads to rupture of the organelle, thereby releasing the carrier and its cargo into the cytoplasm, followed by cytoplasmic transport of the nanoparticles; (2) Proton sponge effect, where the pH within the endosome/lysosome decreases, allowing cationic polymers within it to capture several protons. To maintain charge and concentration balance, external Cl− and water molecules flow into the organelle, resulting in increased permeability and eventual rupture of the organelle, thereby releasing the cargo or carrier into the cytoplasm and achieving escape.146 The following is a classification of various carriers’ cellular uptake, subcellular level transportation, and drug release mechanisms, mainly based on endocytosis.
Cellular Entry Mechanism, Subcellular Level Transport Pathway, and Drug Release Mechanism of Carriers Entering Cells via Adsorptive Endocytosis
Adsorptive endocytosis is a process of cellular uptake of external substances, which are adsorbed onto the cell membrane by interacting with the charge, hydrophobicity or other physicochemical properties of the cell membrane. Subsequently, the cell membrane depresses and wraps around these substances, forming endocytosis vesicles that transport them inside the cell for further processing. It is similar to receptor-mediated endocytosis, but differs in that adsorptive endocytosis is not dependent on specific cell surface receptors.147,148 Currently, carriers that enter cells through adsorptive endocytosis are cationic liposomes147 and chitosan.149 The mechanisms are as follows.
Cationic liposomes with ionizable lipids can electrostatically bind to the negatively charged cell membrane, enter the cell through adsorptive endocytosis, and become a vesicle engulfed by the cell membrane. Thereafter, it detaches from the cell membrane and enters the cytoplasm. Subsequently, it is transported through the endosome-lysosome pathway in the cell. In the acidic environment of the endosome, cationic liposomes are protonated, allowing them to interact with ionizable lipids in the bilayer of the endosome, forming a non-bilayer hexagonal structure that disrupts the bilayer. This releases the drug in the lipid into the cytoplasm.74,147 Additionally, combining cationic liposomes with primary or secondary amines with positive charges can help them escape from lysosomes, thereby protecting drug molecules.74
Chitosan with positive charges can adhere to the negatively charged cell membrane surface via electrostatic interaction, enter cells through adsorptive endocytosis, and reach the endosomes. Owing to its positive charge, it can undergo endosomal escape, allowing the release of loaded drugs into the cytoplasm. However, the endosomal escape ability of chitosan is inefficient; therefore, researchers often modify it to enhance this capability.149
Cellular Entry Mechanism, Subcellular Level Transport Pathway, and Drug Release Mechanism of Carriers Entering Cells via Clathrin-Mediated Endocytosis
The mechanism of clathrin-mediated endocytosis is that when some receptors on the cell membrane surface are bound, the clathrin coat protein in the cytoplasm aggregates on the membrane, and the adaptor protein makes the membrane bend, enclosing the carrier into a clathrin-coated pit. Thereafter, it invaginates and enlarges and finally breaks off, allowing the carrier to be transported into the cell.144 The carriers that enter cells through clathrin-mediated endocytosis include graphene/graphene oxide,101 LDHs,93 and AAV vectors.56,73 The mechanism is as follows:
Positively charged LDHs penetrate the negatively charged cell membrane through clathrin-mediated endocytosis.93 Thereafter, LDHs enter the endosome, where they partially dissolve and release metal ions. Several water molecules in the cytoplasm enter the endosome, causing it to burst. The drug, with some LDHs, is released into the cytoplasm.34 When AAV vectors reach the cell surface, they first bind to sugars (such as sialic acid, lactose, or heparan sulfate) and AAV receptors on the cell surface and enter the cell through clathrin-mediated endocytosis. Thereafter, they reach the endosome and are transported through the late endosome and lysosome compartments. During this process, AAV vectors undergo endosome/lysosome escape. Finally, the genetic material in the carrier is transported to the nucleus and exerts its effect.56,73
Cellular Entry Mechanism, Subcellular Level Transport Pathway, and Drug Release Mechanism of Carriers Entering Cells via Receptor-Mediated Endocytosis
The mechanism of receptor-mediated endocytosis is that when the carrier reaches the cell surface, it specifically binds to receptors on the surface to form a complex. Thereafter, the membrane invaginates to form a coated pit, which detaches from the membrane to form a coated vesicle, taking up the extracellular carrier.145 The carriers that enter cells through receptor-mediated endocytosis are FDC78 and nanogels containing pullulan.33 The mechanism is as follows.
FDC specifically binds to TfR overexpressed on the surface of tumors and enters cells through TfR-mediated endocytosis. Subsequently, FDC is transported through the endosome-lysosome pathway, where FDC undergoes reversible decomposition and releases the drug in the acidic environment of the endosome and lysosome.78,79
Nanogels containing pullulan can strongly bind to salivary glycoprotein receptors and enter cells through receptor-mediated endocytosis. The mechanism of drug release from nanogels is a phenomenon called the “shape-memory effect.” In the acidic environment of the endosome, the nanogel swells, causing the endosome to rupture. Thereafter, water enters the nanogel, releasing the drug into the cytoplasm. When in the neutral cytoplasm, the nanogel shrinks to its original size, retaining unreleased drugs within the nanogel, waiting for the next release cycle. The process of drug release consists of two mechanisms: “swelling” and “permeation”.33,150
Cellular Entry Mechanism, Subcellular Level Transport Pathway, and Drug Release Mechanism of Carriers Entering Cells via Caveolae-Mediated Endocytosis
The mechanism of caveolae-mediated endocytosis is that the carrier reaches the cell membrane surface, covered by caveolin, to form a vesicle and enter the cell. This process bypasses lysosomes, avoiding drug degradation.151 Caveolae-mediated endocytosis typically allows particles sized 20–100 nm to enter the cell, with a maximum particle size limit of 200 nm. Particles larger than 200 nm may enter the cell through alternative pathways.152,153 Most endocytic processes are energy-dependent. Carriers that enter the cell through these endocytic processes are first transported to lysosomes or endosomes for degradation, destroying the carriers before they can deliver the loaded drugs to the cytoplasm or nucleus. Unless carriers can escape lysosomes/endosomes, it would be better if carriers could directly bypass lysosomes or endosomes. Caveolae-mediated endocytosis is an excellent way to achieve this. Carriers entering the cell via this pathway can bypass the lysosome-endosome pathway of cytoplasmic delivery through membrane insertion and diffusion and reach the cytoplasm directly. It can avoid degradation of carriers and loaded drugs in lysosomes or endosomes and improving drug retention and efficacy. However, the only carriers that enter the cell via caveolae-mediated endocytosis are graphene QDs (GQDs) and carbon nanotubes.154,155 GQDs are distributed in the endoplasmic reticulum and nucleus after entering the cell,155 and how drug release occurs is unclear. The fate of carbon nanotubes after reaching the cytoplasm is still unknown. This field has great development potential, and to avoid carriers degraded by lysosomes or endosomes, carriers can be designed to enter the cell by caveolae-mediated endocytosis.
Zhang et al156 have successfully designed nanoparticles that enter cells through this pathway. They developed a core-shell nanocarrier coated by cationic albumin (CNC), which can simultaneously deliver miRNA-34a and docetaxel into breast cancer cells for synergistic therapeutic effects. CNCs can effectively protect miRNA-34a from degradation by RNase and serum, and they can also enter the cytoplasm via caveolae-mediated endocytosis, avoiding degradation by endosomes or lysosomes and improving cargo utilization efficiency (Figure 6a–d). While carriers that enter the cell by other endocytosis first undergo lysosomal or endosomal transport pathways at the subcellular level (Figure 6e).157 Carriers without escape capability are easily degraded in lysosomes or endosomes, and carriers with escape capability have different ways or degrees of lysosomal or endosomal escape according to their respective escape mechanisms (Figure 6f).158
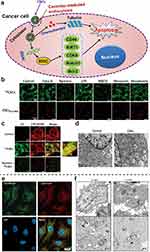 |
Figure 6 (a) Schematic illustration of CNCs co-delivering DTX and miRNA-34a. (b) Confocal laser scanning microscopy images of intracellular fluorescence of CNCs in 4T1 cells pretreated with various inhibitors. (c) Colocalization of C6CNCs with caveolae after incubation in the absence or presence of nystatin. (d) Cytosolic location of the CNCs in 4T1 cells observed using TEM after incubation. Reprinted from Zhang L, Yang X, Lv Y, et al. Cytosolic co-delivery of miRNA-34a and docetaxel with core-shell nanocarriers via caveolae-mediated pathway for the treatment of metastatic breast cancer. Sci Rep. 2017;7:46186. Creative Commons.156 (e) Confocal imaging of 200 nM FITC (green)-conjugated AfFtnAA uptake by RAW264.7 cells after 24 h of incubation. Reprinted from Ravishankar S, Nedumaran AM, Gautam A, Ng KW, Czarny B, Lim S. Protein nanoparticle cellular fate and responses in murine macrophages. NPG Asia Materials. 2023;15(1):1. Creative Commons.157 . https://creativecommons.org/licenses/by/4.0/. (f) TEM images of mouse embryonic fibroblasts incubated with listeriolysin O H311A-NPs. Reprinted from Plaza-Ga I, Manzaneda-González V, Kisovec M, et al pH-triggered endosomal escape of pore-forming Listeriolysin O toxin-coated gold nanoparticles. J Nanobiotechnology. 2019;17(1):108. Creative Commons.158 TEM, Transmission electron microscope; CNC, cationic albumin; DTX, Docetaxel. |
Cellular Entry Mechanism, Subcellular Level Transport Pathway, and Drug Release Mechanism of Carriers Entering Cells via Clathrin-Mediated Endocytosis and Phagocytosis
In addition to the above carriers, some carriers have multiple mechanisms, generally two.
Phagocytosis refers to the cell engulfing the carrier.159 LNPs are absorbed by cells through clathrin-mediated endocytosis and phagocytosis. Thereafter, they are transported through the endosome-lysosome pathway in cells.11 During the endocytosis, the positive charges on the cationic LNPs can be neutralized and disrupted by the negative lipids of the cells, leading to the release of the encapsulated drugs.160
Cellular Entry Mechanism, Subcellular Level Transport Pathway, and Drug Release Mechanism of Other Carriers Entering Cells via Endocytosis
In addition to the carriers discussed above, some carriers only enter the cell through endocytosis; however, the specific endocytic pathway is unknown.
Micelles enter the cell through endocytosis and reach the endosomes, where the micelles may degrade into monomers, may also degrade in advanced endosomes, or may escape from endosomes.151,161 EVs112 and yeast55 enter cells through endocytosis. Ad vectors can enter cells through endocytosis, reach the endosome, and then the viral shell is decomposed. Thereafter, V and VI proteins help the viral genetic material escape from the endosome and enter the cell nucleus through the nuclear envelope.56
In addition, nanocarriers containing fusogenic, pH-sensitive, or cationic lipids can escape from the endosome pathway to the cytoplasm.151
Carriers for Interaction with Cell Membranes via Membrane Fusion
The representative vectors that act with the cell membrane through membrane fusion and release the drug into the cytoplasm are lentiviral vectors.56 The glycoproteins on the envelope of lentiviral vectors bind to corresponding receptors on the cell membrane, causing the viral membrane to fuse with the cell membrane and directly release the genetic material inside into the cytoplasm.56,162 The spikes on the surface of the viral vector can also help it bind strongly to the cell membrane.163 The invasion of genetic information of lentiviral vectors mainly includes two processes: reverse transcription and integration. After the viral shell is removed, viral nucleic acids and protein complexes that enter the cytoplasm are key components for subsequent genetic information to be integrated into chromosomal DNA, often called reverse transcription complex (RTC). Viral RNA is first reverse-transcribed into double-stranded viral DNA in RTC and transported to chromosomal DNA, where it is integrated.162
Carriers for Interaction with Cells via Endocytosis and Membrane Fusion
The above describes the mechanisms by which carriers interact with cells through endocytosis and membrane fusion. These carriers belong to either of these mechanisms; however, some carriers may interact with the cell membrane, such as microbubbles, via both mechanisms. There are two main mechanisms by which microbubbles enter cells: 1) they are engulfed by the cell membrane; 2) they enter the cell through membrane fusion, where the phospholipid component of the microvesicle merges with the double-layered phospholipid of the cell membrane, directly delivering the loaded drug into the cytoplasm.71
Others
The cellular uptake of AuNPs and SeNPs is poor.89,91 Micro/nanomotors can quickly internalize into cells and are not prone to be captured and degraded by lysosomes.164 Targeting ligands influence the pathway of carrier endocytosis by binding specifically to receptors on the cell membrane. When a carrier has a cognate receptor on the target cell or is modified with a ligand that binds to a receptor on the target cell membrane, it enters the cell via receptor-mediated endocytosis. For instance, if a carrier is modified with albumin, it will bind to the albumin receptor on the target cell and enter via caveolae-mediated endocytosis. Similarly, ferritin-modified carriers bind to the TfR1 receptor on tumor cells, allowing entry through receptor-mediated endocytosis. The endocytosis pathway can vary even with the same targeting ligand if different cells are targeted. For example, entry via lipid raft-mediated endocytosis requires the presence of lipid rafts on the cell membrane, with the niche being a specific type of lipid raft.78,79
Fate of Carriers That Do Not Interact with Cell Membranes
Not all carriers can transport drugs into cells. Some carriers may change when they reach the target site and release drugs outside cells, such as neutral liposomes and physicochemical targeting carriers. Neutral liposomes have no significant interaction with cells; therefore, their drug release mainly occurs in the extracellular space. When physicochemical targeting carriers reach the target site, they undergo a certain stimulus from the external environment or are sensitive to the specific environment, and the carrier will change, allowing it to release the loaded drugs. For example, around tumors, rapid anaerobic glycolysis produces excessive protons and carbon dioxide. The tumor lacks a functional lymphatic drainage system, resulting in insufficient oxygen supply and excessive lactate secretion, leading to extracellular acidification (the Warburg effect). Therefore, the extracellular pH around tumors is generally between 6.8 and 7.0, and in some cases, even as low as 5.7. For pH-sensitive carriers that are sensitive to such pH conditions, when they reach the tumor site, their lipids, organic functional groups, or inorganic compounds can easily change shape and release the loaded drugs. Poly (methacrylic acid), N-isopropyl acrylamide, poly (diethylaminoethyl methacrylate), poly (acrylamide), and poly (acrylic acid) are key components of pH-sensitive carriers that respond to pH changes, namely “pH-responsive membrane-deshielding type polymers.” Under acidic pH conditions, pH-responsive membrane-deshielding type polymers with carboxylic groups are protonated to eliminate electrostatic forces. The hydrophobicity of the polymer increases, and the polymer transforms from a hydrophilic state to a hydrophobic state, enhancing the interaction between the carrier and cell membranes and promoting drug release.63
Clarifying how the carrier interacts with the cell membrane is also significant in the design and selection of carriers. If one does not want the carrier to be degraded by endosomes or lysosomes and to reach the nucleus, one can choose a carrier that has an endosomal/lysosomal escape capability or one that does not enter the cell via the endosome-lysosome pathway or one that is designed to have that capability.
If the carrier does not enter the cell and releases the drug directly into the cytoplasm, a carrier that interacts with the cell membrane via membrane fusion can be selected. If the carrier and drug is not required to enter the cell but only required to release the drug in the cellular microenvironment, the carrier that does not enter the cell can be selected. In conclusion, before determining the carrier, one should not only consider the carrier’s encapsulation rate, drug loading, and other characteristics but also focus on its fate in vivo. Usually, pharmacy workers focus on the targeting ability when designing a carrier, which is good but should not be ignored at the subcellular level of transport, which is related to the degradation of the carrier.
Elimination and Safety of Carriers
In the previous section, we discussed the fate of carriers at the site of lesions and how they release drugs. Thereafter, we need to focus on what happens to the carriers after drug release, whether they can be degraded, how they are eliminated from the body, whether they are safe, and whether they are toxic to the body and the specific adverse reactions they cause. The following sections will elaborate on the elimination and safety of carriers.
Once the NPs release the loaded drug, the biomolecules on their surfaces are degraded by cathepsin L. Then, NPs of different compositions undergo different degradation modes. For example, the metabolism of polymer NPs is mainly through the degradation of the polymer backbone. And different particles display different degrees and rates of degradation in the body. Finally, particles with a diameter <5 nm can be rapidly cleared by the kidneys, while metabolites of larger particles are mainly excreted through the liver and bile.2,165 However, when NPs escape from lysosomes, they may cause lysosomal damage, leading to oxidative stress, other organelle malfunctions, and the production of reactive oxygen species (ROS).151 ROS refers to chemically reactive chemicals containing oxygen, including peroxide and superoxide, which are harmful to the human body.166 Therefore, NPs are low-toxicity and can cause genetic and reproductive toxicity, inflammation, oxidative stress, and cell apoptosis, causing damage at the molecular and genetic levels.109
Liposomes and LNPs
Many studies claim that liposomes have good biodegradability and biocompatibility, and can be converted into new substances by biochemical reactions or by microorganisms such as bacteria, without accumulation in the body or additional toxicity. However, it is important to note that many studies have shown that liposomes can cause complement activation, leading to complement activation-related pseudoallergy (CARPA). This necessitates special attention in the research and development of liposomal drugs, including the addition of several evaluation metrics.15,80 LNPs are low-toxicity and biodegradable, mainly reaching the liver and degraded by lysosomal acid lipase to produce lipids, which are metabolized and excreted from the cells and finally from the systemic circulation (50% excreted in urine and feces). The elimination process does not exceed 24 h.11,49,99
Albumin Carriers, FDC, and Dendrimers
Albumin carriers are non-toxic, biodegradable, and non-immunogenic.67,78 Albumin carriers are absorbed in the proximal convoluted tubule and degraded into smaller molecular fragments by hydrolases in the tubular cells and enter the bloodstream. The amino acid components of albumin can be used for tissue repair.167 FDC has low toxicity and is also biodegradable.2
Cationic dendrimers form nanoholes when they interact with negatively charged cell membranes, leading to leakage of cellular contents and eventual cell death. Therefore, cationic dendrimers usually exhibit high cell toxicity, while anionic and neutral dendrimers exhibit low or no toxicity.168 Dendrimers are excreted through the liver and kidneys.169,170
Inorganic Nanoparticles
Metal-based nanoparticles induce DNA or oxidative damage in normal tissues.171 As reported in many studies, AuNPs can cause some degree of oxidative damage both in vivo and in vitro, with the greatest effects on the liver, spleen, and kidneys; however, the main toxicity and its extent are determined by the properties, formulation, and physicochemical properties of the AuNPs. AuNPs are eliminated from the body in urine and feces.172 Graphene and graphene oxide are toxic, and graphene oxide can potentially cause cell hemolysis and respiratory toxicity.155 Graphene and graphene oxide are mainly excreted through the kidney.173 LDHs have biodegradability and low cell toxicity.29,34 LDHs are excreted through the liver and kidney, namely urine and feces.34 QDs have high toxicity, among which carbon QDs have lower toxicity.165 The specific degradation of QDs depends on the degradability of the materials they are composed of. For example, Si QDs, composed of silicon material, are degraded into silicic acid and excreted in urine. These types of QDs are safer than traditional QDs.94 Large GQDs are non-toxic and are eliminated through the liver after drug release. GQDs administered via subcutaneous or intravenous injection are ultimately excreted in urine, while those administered orally are excreted in feces.155
Nanogels and Micro/Nanomotors
Nanogels are biodegradable and can degrade into non-toxic, biodegradable products. They may be eliminated through the kidneys; however, undegraded nanogels swell and become too large to pass through the kidneys.33,150 Nanogels containing surfactants or monomers can cause some adverse reactions.150
Chemically powered micro/nanomotors use toxic fuel, which results in significant toxicity, although the toxicity can be reduced by using endogenous substances instead of toxic fuel.164 Under ideal conditions, micro/nanomotors can be degraded into non-toxic compounds.116 The excretion pathways of micro/nanomotors are still unknown.
Polysaccharide
Polysaccharide are non-toxic or low-toxicity and have good biodegradability.85 ASP is non-toxic and have no immunogenicity,43 while dextran is non-toxic.115 Furthermore, HA is non-immunogenic and biodegradable.174 Chitosan has good biodegradability and is non-toxic, although some studies suggest that it may have a low level of toxicity. However, its toxicity is similar to its molecular weight, which means lower toxicity is related to less molecular weight and vice versa. Administration through the nasal cavity can cause slight nasal leakage, while oral administration can cause minor gastrointestinal side effects such as bloating, diarrhea, and constipation.45,175,176 Chitosan is mainly degraded by muramidase and enzymes produced by bacteria in the gastrointestinal tract.177
EVs and Viral Vectors
EVs have high heterogeneity and complex composition, making accurate in vivo dosage monitoring difficult and safety low.81 However, exosomes are relatively safe and have no cell toxicity. For example, curcumin-wrapped exosomes are safe and have no toxic effects or immune stimulation.178
After nuclear escape, AAV vectors undergo proteasome degradation and form other structures.179 However, for most vectors, drug release into the cytoplasm is sufficient; therefore, even if the vector structure changes after escaping lysosomes/endosomes, as long as it does not affect the drug, escaped vectors can increase drug delivery concentration. However, for viral vectors to be transfected, reaching the cell nucleus is their mission. AAV vector structure changes after nuclear escape, which can disrupt the cargo delivery to the nucleus, leading to reduced transfection efficiency and drug efficacy. Systemic administration of AAV vectors has certain toxicity, causing elevated transaminase levels (the most common adverse reaction), ocular inflammation, and possibly liver toxicity.180,181 However, the adverse reactions of AAV vectors are closely related to the dosage. Therefore, dosage control according to demand is necessary.181 Ad vectors have high transduction efficiency and packaging capability; however, they also cause high levels of inflammation and potentially immune toxicity, even leading to death.179 However, it should not be overly alarming. Although we emphasize “non-toxic” and “safety” when developing vectors, it is best to choose materials compatible with the body to avoid immune reactions and other adverse reactions that can affect normal bodily function. Nonetheless, while safe and non-toxic vectors are what researchers are seeking, they are necessary for treating diseases. For diseases for which there are currently only chemotherapy treatments with severe side effects or no cure, Ad vectors can be a good choice as their toxicity is lower than that of chemotherapy. Lentiviral vectors do not cause any significant adverse reactions.56
Initially, protein replacement therapies alleviated disease symptoms by replacing missing or malfunctioning proteins. These treatments required regular injections or protein supplements, which could be recognized as foreign by the patient’s immune system, potentially triggering an immune response. Subsequently, gene-based therapies emerged, offering long-lasting therapeutic effects by introducing normal genes into the patient’s body. This approach enables the patient’s own cells to produce the required proteins on a long-term or permanent basis, reducing the likelihood of immune rejection and addressing the root cause of the disease without the need for frequent treatments. Gene therapy vectors are classified into non-viral and viral vectors. Viral vectors have become popular in gene therapy research due to their superior properties, such as their natural ability to efficiently infect and deliver genes, their ability to selectively target specific sites, their high specificity, and their potential to enable long-term or permanent gene expression.182,183
Others
As mentioned earlier, vector modifications require clarification of their safety. PEG is non-biodegradable, immunogenic, and can accumulate in the body. Intravenous injection of PEGylated vectors can also cause multiple immune responses.143,184 Therefore, when using PEGylated vectors, these points must be considered. Pullulan is biodegradable, non-immunogenic, and relatively safe.33
When selecting carriers, researchers do not want them to be difficult to metabolize and accumulate in the body, causing invisible toxicity. For carriers that are not easily excreted, researchers often modify the carriers to target excretory organs such as the kidneys; however, this also means that the carriers do not easily accumulate at the target site, which reduces the therapeutic efficacy. Umeda et al have proposed a new strategy that, instead of reducing the accumulation of carriers in the RES, promotes their removal after accumulation. Radionuclide therapy is a new approach to cancer treatment that will replace chemotherapy, which uses the cytotoxicity of radionuclides to kill cancer cells and responds in real-time to the location of the drug in the body. However, because radionuclides are distributed non-specifically, they accumulate in healthy tissues and cause systemic toxicity, and using liposome-loaded radionuclides to help with their targeted delivery in research is common. However, the ability of liposomes to reduce the radionuclide’s non-specific accumulation is not very high. Therefore, Umeda et al185 discovered a ligand called ethylenedicysteine, which was modified to load radionuclides in liposomes and increased the ratio of tumor/liver distribution of radionuclides and clearance of the carriers after accumulation with relatively high efficiency (Figure 7).
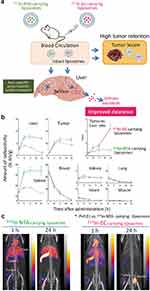 |
Figure 7 (a) Schematic illustration of the in vivo fate of radionuclide-carrying liposomes in tumor-bearing mice. (b) Time-dependent uptake, retention, and clearance of 111In radioactivity in each organ and tumor. *: p < 0.01. (c) In vivo single-photon emission computed tomographs of the two types of 111In-carrying liposomes in sarcoma 180-bearing mice. Reprinted from J Control Release, volume 361, Umeda IO, Koike Y, Ogata M, et al. New liposome-radionuclide-chelate combination for tumor targeting and rapid healthy tissue clearance. 847–855, copyright 2023, with permission from Elsevier.185 |
A point of concern is whether the degradation products of carriers can be reused for the physiological processes of the organism. For example, (1) in the aspect of “membrane production”, the lipids obtained by elimination can be reassembled and used to form the phospholipid bilayer of the cell membranes, which play an important role in the growth, repair, and division of the cell; (2) In “lipoproteins formation”, some lipids and proteins obtained by elimination can be combined to form lipoproteins, which play an important role in the transport of lipids and cholesterol; (3) In “protein synthesis”, the amino acids obtained by elimination are involved in the formation of proteins, which are essential for the structure of cells and organisms, the transport of substances, signaling, catalysis, immunity and so on; (4) In the “source of energy”, carrier upon dissociation produces organic matter such as proteins, carbohydrates, lipids, etc., which can be metabolized and release energy, which is used to maintain the basic activities of the cell and the organism. Therefore, the elimination of drug delivery carriers includes not only how they are eliminated and what their metabolites are but also other aspects, such as the previously described, the elimination products of the carriers have the potential to be involved in the natural processes of the organism, and this is a novel research angle.186–188
Conclusion
We elaborated the process that the fate of drug delivery carriers in the body and subcellular compartments undergo upon entering the body and their toxicity and side effects. The in vivo fate of drug delivery carriers specifically includes their targeting specificity, tissue distribution, circulation time, mechanism of action on cell membranes, intracellular transport mode, escape ability, degradation ability, and excretion mechanism. Although detailed articles on the in vivo fate of specific types of drug delivery carriers exist, they have only focused on the mechanism of a type of carrier and have not provided an overview of the in vivo fate mechanism for various carriers used for targeted delivery. While this article may not be as comprehensive and detailed as the literature, it is suitable for different readers. The advantage of this article lies in its broader coverage and overall nature. Researchers who have identified their research targets can refer to this article to guide their carrier design work. Researchers who have not yet identified the type of carrier to study, researchers from other fields, and even the general public who come across this article can regard it as an introduction to the in vivo fate of drug delivery carriers, providing a preliminary and comprehensive understanding of the design and fate of these carriers. As mentioned before, design should not be done for the sake of design. Pharmaceutical workers should have such awareness. Researchers involved in the development of drug delivery carriers should have a framework in mind regarding their in vivo processes, which is crucial and worth emphasizing repeatedly. Additionally, the images in this article are concise and lively. If one only looks at the captions and images without reading the text, a visual framework for targeted drug delivery carriers can be formed, adding interest and popularization to the monotonous text. Table 5 provides a clear and concise overview of the framework. Readers can directly refer to the table to understand the in vivo process of different targeted drug delivery carriers.
 |
Table 5 Summary of the Fate of Various Carriers After Intravenous Injection into the Body |
Targeted delivery systems can target diseased sites, enhance the biodistribution of drugs, and minimize harm to normal tissues and cells. These systems are extensively sought after for precise drug release in diseases like cancer. Moreover, targeted delivery systems possess significant potential.
The fate of a carrier in vivo is influenced by various factors, including its administration method, particle size, surface charge, surface ligand modification, shape and so on. While this study does not extensively discuss each of these factors, aspects of certain carriers’ fate require further research, guiding the efforts of pharmaceutical professionals.
In summary, this study provides an overview of >13 targeted drug delivery carriers that have made significant progress in understanding the mechanisms underlying their in vivo fate. This study elaborates on their tissue distribution, circulation time, mechanism of action on cell membranes, intracellular transport mode, escape ability, degradation, excretion, and potential toxicity. By enhancing pharmacological knowledge among researchers, promoting in vivo research on targeted drug delivery carriers, and improving the success rate of clinical translation of pharmaceuticals, this study plays a crucial role in advancing the field.
This article highlights several challenges faced by targeted drug delivery carriers. Nanoparticles, for instance, often struggle with inefficient delivery to target sites and require modification to enhance targeting efficiency. Liposomes, dendrimers, and similar carriers have short half-lives in vivo, making them prone to rapid clearance or degradation, which complicates treatments needing sustained effective concentrations. Additionally, metal nanoparticles and viral vectors lack sufficient research on biocompatibility and toxicity, raising concerns about their safety in practical applications. Beyond these in vivo challenges, the production of cell carriers and other advanced carriers involves high precision, complex processes, and significant costs, limiting their applicability. These examples only scratch the surface; the development of nanocarrier drugs still has a long way to go.
All the literature cited in this study was selected as references only when the following requirements were met: (1) For carriers with more research, the Journal Citation Reports (JCR) partition of the article needs to be in zones 1, 2, or 3, with a score ≥3. (2) For carriers with very little research, articles with JCR partitions in zone 4 were acceptable, with a score ≥3. (3) For articles with lower scores, observing the specific content of the article to determine whether it has the value of being cited was necessary. (4) If there was no score or partition of the article, the quality of the journal and the value of its specific content were considered to determine if it could be used as cited literature. Future studies could focus on enhancing the biocompatibility and safety profiles of drug delivery carriers by exploring novel materials and modification strategies.
Abbreviations
LNPs, Lipid-based nanoparticles; FDC, Ferritin drug carrier; TfR, Transferrin receptor; PMs, Polymeric micelles; AuNPs, Au nanoparticles; SeNPs, Selenium nanoparticles; Se, Selenium; LDHs, Layered double hydroxides; QDs, Quantum dots; ASP, Angelica sinensis polysaccharide; EVs, Extracellular vesicles; AAV, Adeno-associated viral; AD, Adenoviral; EPR, Enhanced permeability and retention; ApoE, Apolipoprotein E; PEG, Polyethylene glycol; HA, Hyaluronic acid; FR, Folate receptor; Tf, Transferrin; MZ@PNM, Penetrating macrophage-based nanoformulation; MZ@PNM-GCP, Penetrating macrophage-based nanoformulation encapsulating hydrogel; RES, Reticuloendothelial system; GQDs, Graphene quantum dots; CNC, Core-shell nanocarrier coated by cationic albumin; RTC, Reverse transcription complex; ROS, Reactive oxygen species; CARPA, Complement activation-related pseudoallergy.
Declaration on the Use of AI
We did not use AI tools to help us draft this manuscript.
Acknowledgments
We would like to thank Editage for English language editing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Expenditure Budget Program of Shanghai University of Traditional Chinese Medicine (Grant No. 2021LK003,23KFL001), Shenzhen Natural Science Fund (the Stable Support Plan Program 20220811110339002), Shenzhen Science and Technology Innovation Program (JCYJ20230808105913028), Shanghai Pujiang Programme (23PJD085), CAMS Initiative for Innovative Medicine (CAMS-I2M) (NO. 2021-1-I2M-1-026), and National Natural Science Foundation of China (NO. 81973379).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Bae YH, Park K. Advanced drug delivery 2020 and beyond: perspectives on the future. Adv Drug Deliv Rev. 2020;158:4–16. doi:10.1016/j.addr.2020.06.018
2. Sun Y, Chen D, Pan Y, et al. Nanoparticles for antiparasitic drug delivery. Drug Deliv. 2019;26(1):1206–1221. doi:10.1080/10717544.2019.1692968
3. Majumder J, Taratula O, Minko T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Adv Drug Deliv Rev. 2019;144:57–77. doi:10.1016/j.addr.2019.07.010
4. Golombek SK, May JN, Theek B, et al. Tumor targeting via EPR: strategies to enhance patient responses. Adv Drug Deliv Rev. 2018;130:17–38. doi:10.1016/j.addr.2018.07.007
5. Choi H, Yi J, Cho SH, Hahn SK. Multifunctional micro/nanomotors as an emerging platform for smart healthcare applications. Biomaterials. 2021;279:121201. doi:10.1016/j.biomaterials.2021.121201
6. Jangjou A, Meisami AH, Jamali K, et al. The promising shadow of microbubble over medical sciences: from fighting wide scope of prevalence disease to cancer eradication. J Biomed Sci. 2021;28(1):49. doi:10.1186/s12929-021-00744-4
7. Chen HY, Deng J, Wang Y, Wu CQ, Li X, Dai HW. Hybrid cell membrane-coated nanoparticles: a multifunctional biomimetic platform for cancer diagnosis and therapy. Acta Biomater. 2020;112:1–13. doi:10.1016/j.actbio.2020.05.028
8. Jin K, Luo Z, Zhang B, Pang Z. Biomimetic nanoparticles for inflammation targeting. Acta Pharm Sin B. 2018;8(1):23–33. doi:10.1016/j.apsb.2017.12.002
9. Zhou X, Ling K, Liu M, et al. Targeted Delivery of Cisplatin-Derived Nanoprecursors via a Biomimetic Yeast Microcapsule for Tumor Therapy by the Oral Route. Theranostics. 2019;9(22):6568–6586. doi:10.7150/thno.35353
10. Li X, Le Y, Zhang Z, Nian X, Liu B, Yang X. Viral Vector-Based Gene Therapy. Int J Mol Sci. 2023;24(9):7736. doi:10.3390/ijms24097736
11. Qi J, Zhuang J, Lu Y, Dong X, Zhao W, Wu W. In vivo fate of lipid-based nanoparticles. Drug Discov Today. 2017;22(1):166–172. doi:10.1016/j.drudis.2016.09.024
12. Xiang J, Zhao R, Wang B, et al. Advanced Nano-Carriers for Anti-Tumor Drug Loading. Front Oncol. 2021;11:758143. doi:10.3389/fonc.2021.758143
13. Fang Z, Liu K. Plant-derived extracellular vesicles as oral drug delivery carriers. J Control Release. 2022;350:389–400. doi:10.1016/j.jconrel.2022.08.046
14. Ickenstein LM, Garidel P. Lipid-based nanoparticle formulations for small molecules and RNA drugs. Expert Opin Drug Deliv. 2019;16(11):1205–1226. doi:10.1080/17425247.2019.1669558
15. Antoniou AI, Giofrè S, Seneci P, Passarella D, Pellegrino S. Stimulus-responsive liposomes for biomedical applications. Drug Discov Today. 2021;26(8):1794–1824. doi:10.1016/j.drudis.2021.05.010
16. Large DE, Abdelmessih RG, Fink EA, Auguste DT. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv Drug Deliv Rev. 2021;176:113851. doi:10.1016/j.addr.2021.113851
17. Karim A, Gerliani N, Aïder M. Kluyveromyces marxianus: an emerging yeast cell factory for applications in food and biotechnology. Int J Food Microbiol. 2020;333:108818. doi:10.1016/j.ijfoodmicro.2020.108818
18. Eygeris Y, Gupta M, Kim J, Sahay G. Chemistry of Lipid Nanoparticles for RNA Delivery. Acc Chem Res. 2022;55(1):2–12. doi:10.1021/acs.accounts.1c00544
19. Lam K, Schreiner P, Leung A, et al. Optimizing Lipid Nanoparticles for Delivery in Primates. Adv Mater. 2023;35(26):e2211420. doi:10.1002/adma.202211420
20. Zhang Y, Sun C, Wang C, Jankovic KE, Dong Y. Lipids and Lipid Derivatives for RNA Delivery. Chem Rev. 2021;121(20):12181–12277. doi:10.1021/acs.chemrev.1c00244
21. Hong S, Choi DW, Kim HN, Park CG, Lee W, Park HH. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics. 2020;12(7):604. doi:10.3390/pharmaceutics12070604
22. Martínez-López AL, Pangua C, Reboredo C, Campión R, Morales-Gracia J, Irache JM. Protein-based nanoparticles for drug delivery purposes. Int J Pharm. 2020;581:119289. doi:10.1016/j.ijpharm.2020.119289
23. Zhang N, Yu X, Xie J, Xu H. New Insights into the Role of Ferritin in Iron Homeostasis and Neurodegenerative Diseases. Mol Neurobiol. 2021;58(6):2812–2823. doi:10.1007/s12035-020-02277-7
24. Kesharwani P, Gothwal A, Iyer AK, Jain K, Chourasia MK, Gupta U. Dendrimer nanohybrid carrier systems: an expanding horizon for targeted drug and gene delivery. Drug Discov Today. 2018;23(2):300–314. doi:10.1016/j.drudis.2017.06.009
25. Ghezzi M, Pescina S, Padula C, et al. Polymeric micelles in drug delivery: an insight of the techniques for their characterization and assessment in biorelevant conditions. J Control Release. 2021;332:312–336. doi:10.1016/j.jconrel.2021.02.031
26. Ghosh B, Biswas S. Polymeric micelles in cancer therapy: state of the art. J Control Release. 2021;332:127–147. doi:10.1016/j.jconrel.2021.02.016
27. Hwang D, Ramsey JD, Kabanov AV. Polymeric micelles for the delivery of poorly soluble drugs: from nanoformulation to clinical approval. Adv Drug Deliv Rev. 2020;156:80–118. doi:10.1016/j.addr.2020.09.009
28. Zielińska A, Carreiró F, Oliveira AM, et al. Polymeric Nanoparticles: production, Characterization, Toxicology and Ecotoxicology. Molecules. 2020;25(16):3731. doi:10.3390/molecules25163731
29. Li L, Zhang R, Gu W, Xu ZP. Mannose-conjugated layered double hydroxide nanocomposite for targeted siRNA delivery to enhance cancer therapy. Nanomedicine. 2018;14(7):2355–2364. doi:10.1016/j.nano.2017.06.006
30. Ferro C, Florindo HF, Santos HA. Selenium Nanoparticles for Biomedical Applications: from Development and Characterization to Therapeutics. Adv Healthc Mater. 2021;10(16):e2100598. doi:10.1002/adhm.202100598
31. Matea CT, Mocan T, Tabaran F, et al. Quantum dots in imaging, drug delivery and sensor applications. Int J Nanomed. 2017;12:5421–5431. doi:10.2147/ijn.S138624
32. Song W, Jia P, Zhang T, et al. Cell membrane-camouflaged inorganic nanoparticles for cancer therapy. J Nanobiotechnology. 2022;20(1):289. doi:10.1186/s12951-022-01475-w
33. Suhail M, Rosenholm JM, Minhas MU, et al. Nanogels as drug-delivery systems: a comprehensive overview. Ther Deliv. 2019;10(11):697–717. doi:10.4155/tde-2019-0010
34. Hu T, Gu Z, Williams GR, et al. Layered double hydroxide-based nanomaterials for biomedical applications. Chem Soc Rev. 2022;51(14):6126–6176. doi:10.1039/d2cs00236a
35. Grimaudo MA, Concheiro A, Alvarez-Lorenzo C. Nanogels for regenerative medicine. J Control Release. 2019;313:148–160. doi:10.1016/j.jconrel.2019.09.015
36. Zhang Y, Zou Z, Liu S, Miao S, Liu H. Nanogels as Novel Nanocarrier Systems for Efficient Delivery of CNS Therapeutics. Front Bioeng Biotechnol. 2022;10:954470. doi:10.3389/fbioe.2022.954470
37. Chandrasekaran R, Madheswaran T, Tharmalingam N, Bose RJ, Park H, Ha DH. Labeling and tracking cells with gold nanoparticles. Drug Discov Today. 2021;26(1):94–105. doi:10.1016/j.drudis.2020.10.020
38. Londhe V, Sharma P. Unfolding the future: self-controlled catalytic nanomotor in healthcare system. Mater Sci Eng C Mater Biol Appl. 2020;117:111330. doi:10.1016/j.msec.2020.111330
39. Xu T, Gao W, Xu LP, Zhang X, Wang S. Fuel-Free Synthetic Micro-/Nanomachines. Adv Mater. 2017;29(9):3250. doi:10.1002/adma.201603250
40. Fournier L, de La Taille T, Chauvierre C. Microbubbles for human diagnosis and therapy. Biomaterials. 2023;294:122025. doi:10.1016/j.biomaterials.2023.122025
41. Jamburidze A, Huerre A, Baresch D, Poulichet V, De Corato M, Garbin V. Nanoparticle-Coated Microbubbles for Combined Ultrasound Imaging and Drug Delivery. Langmuir. 2019;35(31):10087–10096. doi:10.1021/acs.langmuir.8b04008
42. Sharma D, Leong KX, Czarnota GJ. Application of Ultrasound Combined with Microbubbles for Cancer Therapy. Int J Mol Sci. 2022;23(8):4393. doi:10.3390/ijms23084393
43. Nai J, Zhang C, Shao H, et al. Extraction, structure, pharmacological activities and drug carrier applications of Angelica sinensis polysaccharide. Int J Biol Macromol. 2021;183:2337–2353. doi:10.1016/j.ijbiomac.2021.05.213
44. Xia Q, Zhang Y, Li Z, Hou X, Feng N. Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application. Acta Pharm Sin B. 2019;9(4):675–689. doi:10.1016/j.apsb.2019.01.011
45. Rashki S, Asgarpour K, Tarrahimofrad H, et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr Polym. 2021;251:117108. doi:10.1016/j.carbpol.2020.117108
46. Plucinski A, Lyu Z, Schmidt B. Polysaccharide nanoparticles: from fabrication to applications. J Mater Chem B. 2021;9(35):7030–7062. doi:10.1039/d1tb00628b
47. Yu Y, Shen M, Song Q, Xie J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: a review. Carbohydr Polym. 2018;183:91–101. doi:10.1016/j.carbpol.2017.12.009
48. Zhou S, Huang G. Preparation, structure and activity of polysaccharide phosphate esters. Biomed Pharmacother. 2021;144:112332. doi:10.1016/j.biopha.2021.112332
49. Jha A, Nikam AN, Kulkarni S, et al. Biomimetic nanoarchitecturing: a disguised attack on cancer cells. J Control Release. 2021;329:413–433. doi:10.1016/j.jconrel.2020.12.005
50. Han H, Bártolo R, Li J, Shahbazi MA, Santos HA. Biomimetic platelet membrane-coated nanoparticles for targeted therapy. Eur J Pharm Biopharm. 2022;172:1–15. doi:10.1016/j.ejpb.2022.01.004
51. Elsharkasy OM, Nordin JZ, Hagey DW, et al. Extracellular vesicles as drug delivery systems: why and how? Adv Drug Deliv Rev. 2020;159:332–343. doi:10.1016/j.addr.2020.04.004
52. Hade MD, Suire CN, Mossell J, Suo Z. Extracellular vesicles: emerging frontiers in wound healing. Med Res Rev. 2022;42(6):2102–2125. doi:10.1002/med.21918
53. Marar C, Starich B, Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat Immunol. 2021;22(5):560–570. doi:10.1038/s41590-021-00899-0
54. Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int J Nanomed. 2020;15:6917–6934. doi:10.2147/ijn.S264498
55. Tan Y, Chen L, Li K, Lou B, Liu Y, Liu Z. Yeast as carrier for drug delivery and vaccine construction. J Control Release. 2022;346:358–379. doi:10.1016/j.jconrel.2022.04.032
56. Bulcha JT, Wang Y, Ma H, Tai PWL, Gao G. Viral vector platforms within the gene therapy landscape. Signal Transduct Target Ther. 2021;6(1):53. doi:10.1038/s41392-021-00487-6
57. McCann N, O’Connor D, Lambe T, Pollard AJ. Viral vector vaccines. Curr Opin Immunol. 2022;77:102210. doi:10.1016/j.coi.2022.102210
58. Perry C, Rayat A. Lentiviral Vector Bioprocessing. Viruses. 2021;13(2):268. doi:10.3390/v13020268
59. Kalyane D, Raval N, Maheshwari R, Tambe V, Kalia K, Tekade RK. Employment of enhanced permeability and retention effect (EPR): nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater Sci Eng C Mater Biol Appl. 2019;98:1252–1276. doi:10.1016/j.msec.2019.01.066
60. Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37. doi:10.1038/nrc.2016.108
61. Gonda A, Zhao N, Shah JV, et al. Engineering Tumor-Targeting Nanoparticles as Vehicles for Precision Nanomedicine. Med One. 2019;4. doi:10.20900/mo.20190021
62. Carita AC, Eloy JO, Chorilli M, Lee RJ, Leonardi GR. Recent Advances and Perspectives in Liposomes for Cutaneous Drug Delivery. Curr Med Chem. 2018;25(5):606–635. doi:10.2174/0929867324666171009120154
63. Alrbyawi H, Poudel I, Annaji M, Arnold RD, Tiwari AK, Babu RJ. Recent Advancements of Stimuli-Responsive Targeted Liposomal Formulations for Cancer Drug Delivery. Pharm Nanotechnol. 2022;10(1):3–23. doi:10.2174/2211738510666220214102626
64. Almeida B, Nag OK, Rogers KE, Delehanty JB. Recent Progress in Bioconjugation Strategies for Liposome-Mediated Drug Delivery. Molecules. 2020;25(23):5672. doi:10.3390/molecules25235672
65. Yue X, Dai Z. Liposomal Nanotechnology for Cancer Theranostics. Curr Med Chem. 2018;25(12):1397–1408. doi:10.2174/0929867324666170306105350
66. Choi J, Rustique E, Henry M, et al. Targeting tumors with cyclic RGD-conjugated lipid nanoparticles loaded with an IR780 NIR dye: in vitro and in vivo evaluation. Int J Pharm. 2017;532(2):677–685. doi:10.1016/j.ijpharm.2017.03.007
67. Hoogenboezem EN, Duvall CL. Harnessing albumin as a carrier for cancer therapies. Adv Drug Deliv Rev. 2018;130:73–89. doi:10.1016/j.addr.2018.07.011
68. Charlie-Silva I, Fraceto LF, de Melo NFS. Progress in nano-drug delivery of artemisinin and its derivatives: towards to use in immunomodulatory approaches. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S611–s20. doi:10.1080/21691401.2018.1505739
69. Muddineti OS, Ghosh B, Biswas S. Current trends in the use of vitamin E-based micellar nanocarriers for anticancer drug delivery. Expert Opin Drug Deliv. 2017;14(6):715–726. doi:10.1080/17425247.2016.1229300
70. Hajebi S, Rabiee N, Bagherzadeh M, et al. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019;92:1–18. doi:10.1016/j.actbio.2019.05.018
71. Chaudhary A, Setia A, Singh D, Bhattacharya S. The potential of microbubbles as a cancer eradication theranostic agent. Pharm Nanotechnol. 2022. doi:10.2174/2211738510666220615154841
72. Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106(Pt A):148–156. doi:10.1016/j.addr.2016.02.006
73. Chen YH, Keiser MS, Davidson BL. Viral vectors for gene transfer. Curr Protocols Mouse Biol. 2018. doi:10.1002/cpmo.58
74. Sheikholeslami B, Lam NW, Dua K, Haghi M. Exploring the impact of physicochemical properties of liposomal formulations on their in vivo fate. Life Sci. 2022;300:120574. doi:10.1016/j.lfs.2022.120574
75. Voronin DV, Abalymov AA, Svenskaya YI, Lomova MV. Key Points in Remote-Controlled Drug Delivery: from the Carrier Design to Clinical Trials. Int J Mol Sci. 2021;22(17):9149. doi:10.3390/ijms22179149
76. Żak MM, Zangi L. Lipid Nanoparticles for Organ-Specific mRNA Therapeutic Delivery. Pharmaceutics. 2021;13(10):675. doi:10.3390/pharmaceutics13101675
77. Verbeke R, Hogan MJ, Loré K, Pardi N. Innate immune mechanisms of mRNA vaccines. Immunity. 2022;55(11):1993–2005. doi:10.1016/j.immuni.2022.10.014
78. Iqbal H, Yang T, Li T, et al. Serum protein-based nanoparticles for cancer diagnosis and treatment. J Control Release. 2021;329:997–1022. doi:10.1016/j.jconrel.2020.10.030
79. He J, Fan K, Yan X. Ferritin drug carrier (FDC) for tumor targeting therapy. J Control Release. 2019;311-312:288–300. doi:10.1016/j.jconrel.2019.09.002
80. Patel MM, Patel BM. Crossing the Blood-Brain Barrier: recent Advances in Drug Delivery to the Brain. CNS Drugs. 2017;31(2):109–133. doi:10.1007/s40263-016-0405-9
81. Wu H, Fu M, Liu J, et al. The role and application of small extracellular vesicles in gastric cancer. Mol Cancer. 2021;20(1):71. doi:10.1186/s12943-021-01365-z
82. Cui D. Pharmaceutics. China Med Sci Press. 2017.
83. Carron PM, Crowley A, O’Shea D, et al. Targeting the Folate Receptor: improving Efficacy in Inorganic Medicinal Chemistry. Curr Med Chem. 2018;25(23):2675–2708. doi:10.2174/0929867325666180209143715
84. Mojarad-Jabali S, Mahdinloo S, Farshbaf M, et al. Transferrin receptor-mediated liposomal drug delivery: recent trends in targeted therapy of cancer. Expert Opin Drug Deliv. 2022;19(6):685–705. doi:10.1080/17425247.2022.2083106
85. Barclay TG, Day CM, Petrovsky N, Garg S. Review of polysaccharide particle-based functional drug delivery. Carbohydr Polym. 2019;221:94–112. doi:10.1016/j.carbpol.2019.05.067
86. Yong T, Zhang X, Bie N, et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat Commun. 2019;10(1):3838. doi:10.1038/s41467-019-11718-4
87. Yan N, Xu J, Liu G, et al. Penetrating Macrophage-Based Nanoformulation for Periodontitis Treatment. ACS Nano. 2022;16(11):18253–18265. doi:10.1021/acsnano.2c05923
88. Zheng Z, Tang W, Lu W, et al. Metabolism and Biodegradation of β-Glucan in vivo. Front Vet Sci. 2022;9:889586. doi:10.3389/fvets.2022.889586
89. Li W, Cao Z, Liu R, et al. AuNPs as an important inorganic nanoparticle applied in drug carrier systems. Artif Cells Nanomed Biotechnol. 2019;47(1):4222–4233. doi:10.1080/21691401.2019.1687501
90. Xu J, Liao K, Jiang H, Zhou W. Research progress of novel inorganic nanometre materials carriers in nanomedicine for cancer diagnosis and treatment. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S492–s502. doi:10.1080/21691401.2018.1499665
91. Khurana A, Tekula S, Saifi MA, Venkatesh P, Godugu C. Therapeutic applications of selenium nanoparticles. Biomed Pharmacother. 2019;111:802–812. doi:10.1016/j.biopha.2018.12.146
92. Sharma H, Mondal S. Functionalized Graphene Oxide for Chemotherapeutic Drug Delivery and Cancer Treatment: a Promising Material in Nanomedicine. Int J Mol Sci. 2020;21(17):6280. doi:10.3390/ijms21176280
93. Naz S, Shamoon M, Wang R, Zhang L, Zhou J, Chen J. Advances in Therapeutic Implications of Inorganic Drug Delivery Nano-Platforms for Cancer. Int J Mol Sci. 2019;20(4):965. doi:10.3390/ijms20040965
94. Kargozar S, Hoseini SJ, Milan PB, Hooshmand S, Kim HW, Mozafari M. Quantum Dots: a Review from Concept to Clinic. Biotechnol J. 2020;15(12):e2000117. doi:10.1002/biot.202000117
95. Zhang X, Yang X, Ji J, Liu A, Zhai G. Tumor targeting strategies for chitosan-based nanoparticles. Colloids Surf B Biointerfaces. 2016;148:460–473. doi:10.1016/j.colsurfb.2016.09.020
96. Lorincz R, Curiel DT. Advances in Alpha-1 Antitrypsin Gene Therapy. Am J Respir Cell Mol Biol. 2020;63(5):560–570. doi:10.1165/rcmb.2020-0159PS
97. Kasaraneni N, Chamoun-Emanuelli AM, Wright GA, Chen Z. A simple strategy for retargeting lentiviral vectors to desired cell types via a disulfide-bond-forming protein-peptide pair. Sci Rep. 2018;8(1):10990. doi:10.1038/s41598-018-29253-5
98. Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–951. doi:10.1038/nbt.3330
99. Shankar R, Joshi M, Pathak K. Lipid Nanoparticles: a Novel Approach for Brain Targeting. Pharm Nanotechnol. 2018;6(2):81–93. doi:10.2174/2211738506666180611100416
100. Carrasco MJ, Alishetty S, Alameh MG, et al. Ionization and structural properties of mRNA lipid nanoparticles influence expression in intramuscular and intravascular administration. Commun Biol. 2021;4(1):956. doi:10.1038/s42003-021-02441-2
101. Nordström R, Malmsten M. Delivery systems for antimicrobial peptides. Adv Colloid Interface Sci. 2017;242:17–34. doi:10.1016/j.cis.2017.01.005
102. Wiklander OP, Nordin JZ, O’Loughlin A, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316. doi:10.3402/jev.v4.26316
103. Zhuang X, Teng Y, Samykutty A, et al. Grapefruit-derived Nanovectors Delivering Therapeutic miR17 Through an Intranasal Route Inhibit Brain Tumor Progression. Mol Ther. 2016;24(1):96–105. doi:10.1038/mt.2015.188
104. Zhang M, Wang X, Han MK, Collins JF, Merlin D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine (Lond). 2017;12(16):1927–1943. doi:10.2217/nnm-2017-0196
105. Teng Y, Ren Y, Sayed M, et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe. 2018;24(5):637–52.e8. doi:10.1016/j.chom.2018.10.001
106. Parayath NN, Amiji MM. Therapeutic targeting strategies using endogenous cells and proteins. J Control Release. 2017;258:81–94. doi:10.1016/j.jconrel.2017.05.004
107. Beris AN, Horner JS, Jariwala S, Armstrong MJ, Wagner NJ. Recent advances in blood rheology: a review. Soft Matter. 2021;17(47):10591–10613. doi:10.1039/d1sm01212f
108. Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52–79. doi:10.1016/j.ejpb.2015.03.018
109. Ferrari R, Sponchioni M, Morbidelli M, Moscatelli D. Polymer nanoparticles for the intravenous delivery of anticancer drugs: the checkpoints on the road from the synthesis to clinical translation. Nanoscale. 2018;10(48):22701–22719. doi:10.1039/c8nr05933k
110. Lu X, Xu P, Ding HM, Yu YS, Huo D, Ma YQ. Tailoring the component of protein Corona via simple chemistry. Nat Commun. 2019;10(1):4520. doi:10.1038/s41467-019-12470-5
111. Jahangir MA, Gilani SJ, Muheem A, et al. Quantum Dots: next Generation of Smart Nano-Systems. Pharm Nanotechnol. 2019;7(3):234–245. doi:10.2174/2211738507666190429113906
112. Yurkin ST, Wang Z. Cell membrane-derived nanoparticles: emerging clinical opportunities for targeted drug delivery. Nanomedicine (Lond). 2017;12(16):2007–2019. doi:10.2217/nnm-2017-0100
113. Dos Reis SB, de Oliveira Silva J, Garcia-Fossa F, et al. Mechanistic insights into the intracellular release of doxorubicin from pH-sensitive liposomes. Biomed Pharmacother. 2021;134:110952. doi:10.1016/j.biopha.2020.110952
114. Kapoor M, Lee SL, Tyner KM. Liposomal Drug Product Development and Quality: current US Experience and Perspective. Aaps j. 2017;19(3):632–641. doi:10.1208/s12248-017-0049-9
115. Huang S, Huang G. Preparation and drug delivery of dextran-drug complex. Drug Deliv. 2019;26(1):252–261. doi:10.1080/10717544.2019.1580322
116. Tezel G, Timur SS, Kuralay F, et al. Current status of micro/nanomotors in drug delivery. J Drug Target. 2021;29(1):29–45. doi:10.1080/1061186x.2020.1797052
117. Yu H, Yang Z, Li F, Xu L, Sun Y. Cell-mediated targeting drugs delivery systems. Drug Deliv. 2020;27(1):1425–1437. doi:10.1080/10717544.2020.1831103
118. Yellepeddi VK, Joseph A, Nance E. Pharmacokinetics of nanotechnology-based formulations in pediatric populations. Adv Drug Deliv Rev. 2019;151-152:44–55. doi:10.1016/j.addr.2019.08.008
119. Mérian J, Boisgard R, Bayle PA, Bardet M, Tavitian B, Texier I. Comparative biodistribution in mice of cyanine dyes loaded in lipid nanoparticles. Eur J Pharm Biopharm. 2015;93:1–10. doi:10.1016/j.ejpb.2015.03.019
120. Mui BL, Tam YK, Jayaraman M, et al. Influence of Polyethylene Glycol Lipid Desorption Rates on Pharmacokinetics and Pharmacodynamics of siRNA Lipid Nanoparticles. Mol Ther Nucleic Acids. 2013;2(12):e139. doi:10.1038/mtna.2013.66
121. Dennis MS, Zhang M, Meng YG, et al. Albumin binding as a general strategy for improving the pharmacokinetics of proteins. J Biol Chem. 2002;277(38):35035–35043. doi:10.1074/jbc.M205854200
122. Roopenian DC, Low BE, Christianson GJ, Proetzel G, Sproule TJ, Wiles MV. Albumin-deficient mouse models for studying metabolism of human albumin and pharmacokinetics of albumin-based drugs. MAbs. 2015;7(2):344–351. doi:10.1080/19420862.2015.1008345
123. Liang M, Fan K, Zhou M, et al. H-ferritin-nanocaged doxorubicin nanoparticles specifically target and kill tumors with a single-dose injection. Proc Natl Acad Sci U S A. 2014;111(41):14900–14905. doi:10.1073/pnas.1407808111
124. Yellepeddi VK, Ghandehari H. Pharmacokinetics of oral therapeutics delivered by dendrimer-based carriers. Expert Opin Drug Deliv. 2019;16(10):1051–1061. doi:10.1080/17425247.2019.1656607
125. Bagheri M, van Nostrum CF, Kok RJ, Storm G, Hennink WE, Heger M. Utility of Intravenous Curcumin Nanodelivery Systems for Improving In Vivo Pharmacokinetics and Anticancer Pharmacodynamics. Mol Pharm. 2022;19(9):3057–3074. doi:10.1021/acs.molpharmaceut.2c00455
126. Han LM, Guo J, Zhang LJ, Wang QS, Fang XL. Pharmacokinetics and biodistribution of polymeric micelles of paclitaxel with Pluronic P123. Acta Pharmacol Sin. 2006;27(6):747–753. doi:10.1111/j.1745-7254.2006.00340.x
127. Kinnear C, Moore TL, Rodriguez-Lorenzo L, Rothen-Rutishauser B, Petri-Fink A. Form Follows Function: nanoparticle Shape and Its Implications for Nanomedicine. Chem Rev. 2017;117(17):11476–11521. doi:10.1021/acs.chemrev.7b00194
128. Wang J, Bai R, Yang R, et al. Size- and surface chemistry-dependent pharmacokinetics and tumor accumulation of engineered gold nanoparticles after intravenous administration. Metallomics. 2022;14(5):31. doi:10.1093/mtomcs/mfac031
129. Rao L, Ma Y, Zhuang M, Luo T, Wang Y, Hong A. Chitosan-decorated selenium nanoparticles as protein carriers to improve the in vivo half-life of the peptide therapeutic BAY 55-9837 for type 2 diabetes mellitus. Int J Nanomed. 2014;9:4819–4828. doi:10.2147/ijn.S67871
130. Zhang P, Gou YQ, Gao X, et al. The pharmacokinetic study of rutin in rat plasma based on an electrochemically reduced graphene oxide modified sensor. J Pharm Anal. 2016;6(2):80–86. doi:10.1016/j.jpha.2015.12.003
131. Qin L, Xue M, Wang W, et al. The in vitro and in vivo anti-tumor effect of layered double hydroxides nanoparticles as delivery for podophyllotoxin. Int J Pharm. 2010;388(1–2):223–230. doi:10.1016/j.ijpharm.2009.12.044
132. Fang J, Liu Y, Chen Y, Ouyang D, Yang G, Yu T. Graphene quantum dots-gated hollow mesoporous carbon nanoplatform for targeting drug delivery and synergistic chemo-photothermal therapy. Int J Nanomed. 2018;13:5991–6007. doi:10.2147/ijn.S175934
133. Azadi A, Rouini MR, Hamidi M. Neuropharmacokinetic evaluation of methotrexate-loaded chitosan nanogels. Int J Biol Macromol. 2015;79:326–335. doi:10.1016/j.ijbiomac.2015.05.001
134. Xie S, Mo C, Cao W, et al. Bacteria-propelled microtubular motors for efficient penetration and targeting delivery of thrombolytic agents. Acta Biomater. 2022;142:49–59. doi:10.1016/j.actbio.2022.02.008
135. Li SY, Guo SL. Optimal construction and pharmacokinetic study of CZ48-loaded poly (lactic acid) microbubbles for controlled drug delivery. Colloids Surf B Biointerfaces. 2019;178:269–275. doi:10.1016/j.colsurfb.2019.02.047
136. Bellaire S, Walzer M, Wang T, Krauwinkel W, Yuan N, Marek GJ. Safety, Pharmacokinetics, and Pharmacodynamics of ASP3662, a Novel 11β-Hydroxysteroid Dehydrogenase Type 1 Inhibitor, in Healthy Young and Elderly Subjects. Clin Transl Sci. 2019;12(3):291–301. doi:10.1111/cts.12618
137. Zhang C, Qu G, Sun Y, et al. Pharmacokinetics, biodistribution, efficacy and safety of N-octyl-O-sulfate chitosan micelles loaded with paclitaxel. Biomaterials. 2008;29(9):1233–1241. doi:10.1016/j.biomaterials.2007.11.029
138. Zheng X, Yu X, Wang C, et al. Targeted co-delivery biomimetic nanoparticles reverse macrophage polarization for enhanced rheumatoid arthritis therapy. Drug Deliv. 2022;29(1):1025–1037. doi:10.1080/10717544.2022.2057616
139. Kim DH, Kothandan VK, Kim HW, et al. Noninvasive Assessment of Exosome Pharmacokinetics In Vivo: a Review. Pharmaceutics. 2019;11(12):649. doi:10.3390/pharmaceutics11120649
140. Chowdhury EA, Ahuja M, Wu S, et al. Pharmacokinetics of AAV9 Mediated Trastuzumab Expression in Rat Brain Following Systemic and Local Administration. J Pharm Sci. 2024;113(1):131–140. doi:10.1016/j.xphs.2023.08.023
141. Kim CY, Park SH, Jeong M, et al. Preclinical studies for pharmacokinetics and biodistribution of Ad-stTRAIL, an adenovirus delivering secretable trimeric TRAIL for gene therapy. Exp Mol Med. 2011;43(10):580–586. doi:10.3858/emm.2011.43.10.065
142. Croyle MA, Callahan SM, Auricchio A, et al. PEGylation of a vesicular stomatitis virus G pseudotyped lentivirus vector prevents inactivation in serum. J Virol. 2004;78(2):912–921. doi:10.1128/jvi.78.2.912-921.2004
143. Cardoso RV, Pereira PR, Freitas CS, Paschoalin VMF. Trends in Drug Delivery Systems for Natural Bioactive Molecules to Treat Health Disorders: the Importance of Nano-Liposomes. Pharmaceutics. 2022;14(12):2808. doi:10.3390/pharmaceutics14122808
144. Kaksonen M, Roux A. Mechanisms of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2018;19(5):313–326. doi:10.1038/nrm.2017.132
145. Gonda A, Kabagwira J, Senthil GN, Wall NR. Internalization of Exosomes through Receptor-Mediated Endocytosis. Mol Cancer Res. 2019;17(2):337–347. doi:10.1158/1541-7786.Mcr-18-0891
146. Bus T, Traeger A, Schubert US. The great escape: how cationic polyplexes overcome the endosomal barrier. J Mater Chem B. 2018;6(43):6904–6918. doi:10.1039/c8tb00967h
147. Lee Y, Thompson DH. Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(5):145. doi:10.1002/wnan.1450
148. Sharma N, Zahoor I, Sachdeva M, et al. Deciphering the role of nanoparticles for management of bacterial meningitis: an update on recent studies. Environ Sci Pollut Res Int. 2021;28(43):60459–60476. doi:10.1007/s11356-021-16570-y
149. Zhang E, Xing R, Liu S, Qin Y, Li K, Li P. Advances in chitosan-based nanoparticles for oncotherapy. Carbohydr Polym. 2019;222:115004. doi:10.1016/j.carbpol.2019.115004
150. Shah S, Rangaraj N, Laxmikeshav K, Sampathi S. Nanogels as drug carriers - Introduction, chemical aspects, release mechanisms and potential applications. Int J Pharm. 2020;581:119268. doi:10.1016/j.ijpharm.2020.119268
151. Butt AM, Abdullah N, Rani N, Ahmad N, Amin M. Endosomal Escape of Bioactives Deployed via Nanocarriers: insights Into the Design of Polymeric Micelles. Pharm Res. 2022;39(6):1047–1064. doi:10.1007/s11095-022-03296-w
152. Cheng JPX, Nichols BJ. Caveolae: one Function or Many? Trends Cell Biol. 2016;26(3):177–189. doi:10.1016/j.tcb.2015.10.010
153. Lolo FN, Walani N, Seemann E, et al. Caveolin-1 dolines form a distinct and rapid caveolae-independent mechanoadaptation system. Nat Cell Biol. 2023;25(1):120–133. doi:10.1038/s41556-022-01034-3
154. Cui X, Wan B, Yang Y, Ren X, Guo LH. Length effects on the dynamic process of cellular uptake and exocytosis of single-walled carbon nanotubes in murine macrophage cells. Sci Rep. 2017;7(1):1518. doi:10.1038/s41598-017-01746-9
155. Henna TK, Pramod K. Graphene quantum dots redefine nanobiomedicine. Mater Sci Eng C Mater Biol Appl. 2020;110:110651. doi:10.1016/j.msec.2020.110651
156. Zhang L, Yang X, Lv Y, et al. Cytosolic co-delivery of miRNA-34a and docetaxel with core-shell nanocarriers via caveolae-mediated pathway for the treatment of metastatic breast cancer. Sci Rep. 2017;7:46186. doi:10.1038/srep46186
157. Ravishankar S, Nedumaran AM, Gautam A, Ng KW, Czarny B, Lim S. Protein nanoparticle cellular fate and responses in murine macrophages. NPG Asia Materials. 2023;15(1):1. doi:10.1038/s41427-022-00453-w
158. Plaza-Ga I, Manzaneda-González V, Kisovec M, et al. pH-triggered endosomal escape of pore-forming Listeriolysin O toxin-coated gold nanoparticles. J Nanobiotechnology. 2019;17(1):108. doi:10.1186/s12951-019-0543-6
159. Mylvaganam S, Freeman SA, Grinstein S. The cytoskeleton in phagocytosis and macropinocytosis. Curr Biol. 2021;31(10):R619–r32. doi:10.1016/j.cub.2021.01.036
160. Tenchov R, Bird R, Curtze AE, Zhou Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano. 2021;15(11):16982–17015. doi:10.1021/acsnano.1c04996
161. Cai Y, Qi J, Lu Y, He H, Wu W. The in vivo fate of polymeric micelles. Adv Drug Deliv Rev. 2022;188:114463. doi:10.1016/j.addr.2022.114463
162. Milone MC, O’Doherty U. Clinical use of lentiviral vectors. Leukemia. 2018;32(7):1529–1541. doi:10.1038/s41375-018-0106-0
163. Tong Q, Qiu N, Ji J, Ye L, Zhai G. Research Progress in Bioinspired Drug Delivery Systems. Expert Opin Drug Deliv. 2020;17(9):1269–1288. doi:10.1080/17425247.2020.1783235
164. Lin R, Yu W, Chen X, Gao H. Self-Propelled Micro/Nanomotors for Tumor Targeting Delivery and Therapy. Adv Healthc Mater. 2021;10(1):e2001212. doi:10.1002/adhm.202001212
165. Zhou Y, Peng Z, Seven ES, Leblanc RM. Crossing the blood-brain barrier with nanoparticles. J Control Release. 2018;270:290–303. doi:10.1016/j.jconrel.2017.12.015
166. Srinivas US, Tan BWQ, Vellayappan BA, Jeyasekharan AD. ROS and the DNA damage response in cancer. Redox Biol. 2019;25:101084. doi:10.1016/j.redox.2018.101084
167. Molitoris BA, Sandoval RM, Yadav SPS, Wagner MC. Albumin uptake and processing by the proximal tubule: physiological, pathological, and therapeutic implications. Physiol Rev. 2022;102(4):1625–1667. doi:10.1152/physrev.00014.2021
168. Janaszewska A, Lazniewska J, Trzepiński P, Marcinkowska M, Klajnert-Maculewicz B. Cytotoxicity of Dendrimers. Biomolecules. 2019;9(8):330. doi:10.3390/biom9080330
169. Saluja V, Mishra Y, Mishra V, Giri N, Nayak P. Dendrimers based cancer nanotheranostics: an overview. Int J Pharm. 2021;600:120485. doi:10.1016/j.ijpharm.2021.120485
170. Chis AA, Dobrea C, Morgovan C, et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules. 2020;25(17):3982. doi:10.3390/molecules25173982
171. Cai SS, Li T, Akinade T, Zhu Y, Leong KW. Drug delivery carriers with therapeutic functions. Adv Drug Deliv Rev. 2021;176:113884. doi:10.1016/j.addr.2021.113884
172. Sani A, Cao C, Cui D. Toxicity of gold nanoparticles (AuNPs): a review. Biochem Biophys Rep. 2021;26:100991. doi:10.1016/j.bbrep.2021.100991
173. Liao C, Li Y, Tjong SC. Graphene Nanomaterials: synthesis, Biocompatibility, and Cytotoxicity. Int J Mol Sci. 2018;19(11):3564. doi:10.3390/ijms19113564
174. Zhu J, Tang X, Jia Y, Ho CT, Huang Q. Applications and delivery mechanisms of hyaluronic acid used for topical/transdermal delivery - A review. Int J Pharm. 2020;578:119127. doi:10.1016/j.ijpharm.2020.119127
175. Kravanja G, Primožič M, Ž K, Leitgeb M. Chitosan-based (Nano)materials for Novel Biomedical Applications. Molecules. 2019;24(10):1960. doi:10.3390/molecules24101960
176. Dmour I, Islam N. Recent advances on chitosan as an adjuvant for vaccine delivery. Int J Biol Macromol. 2022;200:498–519. doi:10.1016/j.ijbiomac.2021.12.129
177. Su C, Liu Y, Li R, Wu W, Fawcett JP, Gu J. Absorption, distribution, metabolism and excretion of the biomaterials used in Nanocarrier drug delivery systems. Adv Drug Deliv Rev. 2019;143:97–114. doi:10.1016/j.addr.2019.06.008
178. Oskouie MN, Aghili Moghaddam NS, Butler AE, Zamani P, Sahebkar A. Therapeutic use of curcumin-encapsulated and curcumin-primed exosomes. J Cell Physiol. 2019;234(6):8182–8191. doi:10.1002/jcp.27615
179. Shirley JL, de Jong YP, Terhorst C, Herzog RW. Immune Responses to Viral Gene Therapy Vectors. Mol Ther. 2020;28(3):709–722. doi:10.1016/j.ymthe.2020.01.001
180. Brody H. Gene therapy. Nature. 2018;564(7735):S5. doi:10.1038/d41586-018-07639-9
181. Mendell JR, Al-Zaidy SA, Rodino-Klapac LR, et al. Current Clinical Applications of In Vivo Gene Therapy with AAVs. Mol Ther. 2021;29(2):464–488. doi:10.1016/j.ymthe.2020.12.007
182. Nienhuis AW, Nathwani AC, Davidoff AM. Gene Therapy for Hemophilia. Mol Ther. 2017;25(5):1163–1167. doi:10.1016/j.ymthe.2017.03.033
183. High KA, Roncarolo MG. Gene Therapy. N Engl J Med. 2019;381(5):455–464. doi:10.1056/NEJMra1706910
184. Mahajan S, Patharkar A, Kuche K, et al. Functionalized carbon nanotubes as emerging delivery system for the treatment of cancer. Int J Pharm. 2018;548(1):540–558. doi:10.1016/j.ijpharm.2018.07.027
185. Umeda IO, Koike Y, Ogata M, et al. New liposome-radionuclide-chelate combination for tumor targeting and rapid healthy tissue clearance. J Control Release. 2023;361:847–855. doi:10.1016/j.jconrel.2023.07.060
186. Feingold KR. Lipid and Lipoprotein Metabolism. Endocrinol Metab Clin North Am. 2022;51(3):437–458. doi:10.1016/j.ecl.2022.02.008
187. Paulusma CC, Lamers WH, Broer S, van de Graaf SFJ. Amino acid metabolism, transport and signalling in the liver revisited. Biochem Pharmacol. 2022;201:115074. doi:10.1016/j.bcp.2022.115074
188. MacCannell AD, Roberts LD. Metabokines in the regulation of systemic energy metabolism. Curr Opin Pharmacol. 2022;67:102286. doi:10.1016/j.coph.2022.102286
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



