Back to Journals » Journal of Pain Research » Volume 18
Inflammation Factors Mediate Association of Muscle Mass and Migraine: NHANES 1999–2004 and Mendelian Randomization
Authors Jia C , Li H, Yang S, Liu Y, Liu L, Ma A , Zhang L
Received 10 January 2025
Accepted for publication 17 April 2025
Published 2 May 2025 Volume 2025:18 Pages 2269—2283
DOI https://doi.org/10.2147/JPR.S516748
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Rune Häckert Christensen
Chunyan Jia, Hong Li, Shaonan Yang, Yue Liu, Lijun Liu, Aijun Ma, Liang Zhang
Department of Neurology, The Affiliated Hospital of Qingdao University, Qingdao University, Qingdao, People’s Republic of China
Correspondence: Liang Zhang, Department of Neurology, The Affiliated Hospital of Qingdao University, 16 Jiangsu Road, Shinan District, Qingdao, Shandong, 266071, People’s Republic of China, Tel +86-178-5329-7291, Email [email protected] Lijun Liu, Department of Neurology, The Affiliated Hospital of Qingdao University, 16 Jiangsu Road, Shinan District, Qingdao, Shandong, 266071, People’s Republic of China, Tel +86-178-5329-0950, Email [email protected]
Purpose: The relationship between adipose-muscle distribution and its effect on migraine remains unclear. This study examines the association between muscle mass and migraine prevalence and evaluates potential mediation by systemic inflammatory biomarkers.
Methods: Using a cross-sectional design, we analyzed data from 10,400 participants in the National Health and Nutrition Examination Survey (NHANES) (1999– 2004). The association between appendicular lean mass normalized to body mass index (ALM/BMI) and migraine prevalence was evaluated through weighted logistic regression and subgroup analyses. Mediation analyses were conducted to examine the potential mediating roles of inflammatory markers, including C-reactive protein (CRP), white blood cell count (WBC), and neutrophils, in the relationship between ALM/BMI and migraine prevalence. Genetic causality was investigated via two-sample Mendelian randomization (MR) using genome-wide association study (GWAS) data.
Results: 20% of total participants reported migraines. A higher ALM/BMI ratio was inversely associated with migraine after full adjustment (OR = 0.243; 95% CI: 0.122– 0.487, p < 0.001). Vigorous activity reduced migraine susceptibility by 24% (OR = 0.760; 95% CI: 0.663– 0.872, p < 0.001). CRP, WBC and neutrophils mediated 2.0% (p = 0.024), 3.1% (p = 0.011), and 2.8% (p = 0.019) of the ALM/BMI-migraine association, respectively. The inverse-variance weighted approach (IVW) in MR analysis indicated that higher basal metabolic rate (BMR) reduced migraine risk (OR = 0.996, 95% CI: 0.992– 0.998, p = 0.004) and headache risk (OR = 0.998, 95% CI: 0.997– 1.000, p = 0.018). Fat-free mass also exhibited protective effects on migraines (OR = 0.997, 95% CI: 0.994– 1.000, p = 0.045).
Conclusion: Increased muscle mass is associated with reduced migraine risk, partially mediated by attenuating systemic inflammation. These findings provide us with an approach of health management to prevent migraines.
Keywords: appendicular muscle mass, basal metabolic rate, migraine, inflammation, national health and nutrition examination survey, Mendelian randomization
Introduction
Migraine is a common and intricate neurological disorder marked by recurrent, unilateral, moderate-to-severe headaches. It is often accompanied by other symptoms like nausea, vomiting, photophobia, and phonophobia.1,2 Some patients experience an aura, such as visual or sensory disturbances, prior to headache onset.3,4 Migraine imposes a substantial global public health burden due to its detrimental effects on quality of life and functional capacity.5 While pharmacological therapies can alleviate acute symptoms, effective long-term prevention and management remain elusive.6
Elucidating migraine pathophysiology is critical for identifying therapeutic targets. Current evidence highlights intricate interactions across peripheral and central networks.4,7,8 Migraine pathogenesis is driven by a cascade of neurovascular and neuroinflammatory events. Peripheral sensitization arises from the activation of trigeminal nociceptors, leading to the release of calcitonin gene-related peptide (CGRP) and proinflammatory cytokines that amplify pain signals.4,7,9 Subsequent central sensitization, marked by hyperexcitability of second-order neurons in the trigeminal nucleus caudalis and thalamocortical dysrhythmia, perpetuates pain hypersensitivity and chronification.10–12 Mitochondrial dysfunction and neuroinflammation further modulate neuronal excitability, suggesting metabolic dysregulation as a contributor to migraine susceptibility.8,13,14
Despite advances in understanding these mechanisms, treatment efficacy remains suboptimal, particularly for vulnerable populations (eg, pregnant women, children) with restricted pharmacological options.15–17 Modifiable risk factors, including obesity and metabolic dysregulation, are increasingly recognized for their association with migraine severity and frequency.18 For instance, obesity elevates migraine risk, while weight loss may alleviate migraine attacks.19,20 Sarcopenic obesity (SO), characterized by reduced muscle mass and elevated adiposity,21 may exacerbate migraine through inflammatory pathways and metabolic disturbances. Factors contributing to the gradual decline in skeletal muscle mass and strength include lack of exercise, sedentary behavior, and the natural aging process. Skeletal muscle mass, particularly appendicular lean mass (ALM), has been established as a reliable marker for diagnosing sarcopenia.
Migraine management is challenged not only by its inherent pathophysiology but also by associated comorbidities and modifiable risk factors. Differentiating comorbidities (eg, obesity, depression) from contributing factors (eg, sedentary behavior, poor sleep hygiene) is essential due to divergent clinical implications. Migraine exhibits a high comorbidity burden, spanning psychiatric, cardiovascular, metabolic, and neurological domains. Systematic reviews indicate that 60–80% of migraineurs present with at least one comorbid condition, most commonly depression (OR = 2.8), anxiety (OR = 2.5), and metabolic syndrome (OR = 1.7).22 Bidirectional relationships suggest shared pathophysiological pathways. Chronic inflammation may simultaneously drive migraine chronification and mood disorders.14,23 Notably, SO exacerbates psychiatric and cardiovascular risks,24,25 while modifiable factors like physical inactivity may worsen migraine and comorbidities. Addressing these factors offers dual benefits: mitigating migraine burden and improving comorbid conditions. Despite extensive research on obesity, the role of body composition phenotypes, particularly SO, remains unclear, hindering development of lifestyle interventions targeting muscle mass to reduce migraine burden.
Emerging intervention studies support a muscle-migraine link.26,27 Skeletal muscle functions as an endocrine organ, secreting myokines with systemic anti-inflammatory and neuroprotective effects.28,29 These myokines suppress pro-inflammatory cytokines implicated in peripheral and central sensitization. This study analyzed data from the US National Health and Nutrition Examination Survey (NHANES) carried out between 1999 and 2004 to investigate the association between skeletal muscle mass and migraine, with emphasis on the mediating role of inflammatory markers in this relationship. Subsequently, Mendelian randomization (MR) analysis was employed to determine a causal connection between muscle mass and migraine. By clarifying these associations, we aim to identify non-pharmacological strategies for migraine prevention, addressing critical gaps in managing this complex disorder.
Materials and Methods
Study Population
The National Health and Nutrition Examination Survey (NHANES) is a nationally representative cross-sectional health survey conducted in the United States, collecting data through health interviews, physical examinations, and various laboratory tests, including imaging and radiological data.30 Publicly accessible data are released in two-year cycles by the National Center for Health Statistics (NCHS). Detailed information about the study methods and data collection procedures can be found on the NHANES official website (http://www.cdc.gov/nchs/nhanes.htm). The NHANES study protocol (Protocol #98-12) has been approved by the NCHS Research Ethics Review Board, with written informed consent obtained from all participants. This analysis utilized de-identified NHANES data, which was exempt from additional institutional review board (IRB) review by the Affiliated Hospital of Qingdao University Ethics Committee under US Department of Health and Human Services (HHS) guidelines.31 All research complies with the Declaration of Helsinki. For more information about the NCHS Ethics Review Board, visit https://www.cdc.gov/nchs/nhanes/about/erb.html?CDC_AAref_Val=https://www.cdc.gov/nchs/nhanes/irba98.htm
From the 1999–2004 NHANES cycles, 31,126 individuals were initially enrolled (Figure 1). After excluding participants aged <20 years (n = 15,794), those lacking migraine status data or dual-energy X-ray absorptiometry (DXA) measurements (n = 2232), and individuals with incomplete covariate data (eg, smoking status, blood samples; n = 2688), the final cohort comprised 10,400 participants (Figure 1). Pregnant individuals and those exceeding DXA weight/height limits were excluded from DXA assessments. Private health interviews, including those assessing health status, were conducted by trained Mobile Examination Center (MEC) interviewers.
 |
Figure 1 Flow chart of population selection from NHANES. |
Migraine Definition
Migraine status was determined using self-reported data from the NHANES questionnaire’s miscellaneous pain section. The question MPQ090 asks, “During the past 3 months, did you have severe headaches or migraines?” Participants who answered “Yes” were classified as migraine sufferers. This approach aligns with validation studies from the American Migraine Prevalence and Prevention (AMPP) initiative, which indicates that self-reported severe headaches predominantly reflect migraine diagnoses.32
Dual-Energy X-Ray Absorptiometry Measurement and Sarcopenia Definitions
From 1999 to 2004, participants underwent DXA scanning, a widely used and validated technique for measuring body composition. In NHANES, to evaluate the body’s composition across the limbs, chest, and head, whole-body DXA scans were performed using the Hologic QDR-4500A fan-beam densitometer (Hologic Inc., Bedford, MA, USA). Pregnant women were excluded from these scans. Additionally, participants weighing over 300 pounds (136 kg) or taller than 6 feet 5 inches (198 cm) were excluded due to limitations of the DXA equipment.
In DXA measurements, appendicular lean mass (ALM) is characterized as the sum of lean mass in all four limbs, excluding bone mineral content. ALM was normalized to body mass index (BMI) as ALM/BMI, a validated metric for sarcopenia. According to the directives set forth by the Foundation for the National Institutes of Health (FNIH), a nonprofit affiliated with the National Institutes of Health (NIH), sarcopenia thresholds were sex-specific: ALM/BMI <0.789 for males and <0.512 for females.33
Covariables
The analysis included the following variables as covariates: age, gender, ethnicity, education level, C-reactive protein (CRP), white blood cell (WBC), neutrophil (NEU), vigorous activity, moderate activity, drinking status, smoking status, arthritis, coronary heart disease (CHD) and stroke. Ethnicity was classified into the following groups: non-Hispanic White, non-Hispanic Black, Mexican American, other Hispanic, and other race. Smoking status was evaluated according to the question SMQ020 “Have you/Has SP smoked at least 100 cigarettes in your/his/her entire life?” and the question SMQ040 “Do you/Does SP now smoke cigarettes” in the NHANES questionnaire’s “smoking - cigarette/tobacco use - adult” section. The classification of educational achievement encompassed groups of below high school, high school, and above high school. The classification of smoking status comprises the following three separate groups: participants who have never smoked (defined as those who have consumed fewer than 100 cigarettes), current smokers, and former smokers (characterized as those who have smoked more than 100 cigarettes but have afterwards ceased smoking). Drinking status was assessed using NHANES questionnaire data, according to the question “Had at least 12 alcohol drinks/lifetime?” in the alcohol use section. Physical activity was classified into two categories: exercise and daily activity. Exercise was categorized into two distinct levels according to the answer of PAD320 and PAD200 in the NHANES questionnaire’s “physical activity” section, respectively: moderate, which is characterized by participation in physical activity for a minimum of 10 minutes within the preceding 30 days that leads to mild sweating or mild to moderate elevations in breathing or heart rate; and vigorous, which is defined as engaging in physical activity for more than 10 minutes in the past 30 days resulting in significant sweating or significant increases in breathing or heart rate. Daily physical activity was classified into distinct categories according to the answer of PAD160 in the NHANES questionnaire’s “physical activity” section: sedentary behavior (sitting), standing or walking, light loads, or heavy loads. Arthritis, CHD and stroke diagnosis was determined through responses from participants to a survey inquiry in NHANES questionnaire’s medical conditions section concerning whether they had been previously informed of the condition by a medical professional. Specifically, participants who answered “Yes” to the question MCQ160A (Has a doctor or other health professional ever told you/SP that you/s/he. had arthritis?), MCQ160C (Has a doctor or other health professional ever told you/SP that you/s/he. had coronary heart disease?) and MCQ160F (Has a doctor or other health professional ever told you/SP that you/s/he. had a stroke?) were considered as arthritis, CHD and stroke, respectively.
Mendelian Randomization Study Design and Data Resource
Mendelian randomization (MR) is an analytical approach that employs genetic variants as instrumental variables (IVs) to estimate causal effects of exposures on outcomes while mitigating confounding and reverse causation.34 In our study, we implemented two-sample MR to examine the causal relationships among various exposures and outcomes, employing both univariable and multivariable MR analyses based on prior epidemiological research. In this context, BMR, ALM, and fat-free mass (whole-body and limb-specific) were designated as exposure variables, while migraine/headache was defined as the outcome. Single nucleotide polymorphisms (SNPs) have been identified as instrumental variables (IVs) for subsequent analysis. This IVs analysis employs a simulation of random assignment as seen in randomized controlled trials, utilizing the random distribution of SNPs in descendants, that maintains independently of confounding factors such as age and sex.
Our MR study complies with the three fundamental assumptions: 1) all selected IVs must exhibit a strong correlation with the exposure (P < 5×10−8); 2) all selected IVs must be unaffected by confounders affecting the relationship between the exposure and the outcome; and 3) All chosen IVs must only influence the outcome via exposure and not through other pathways.
The data for MR analysis were acquired from recent genome-wide association studies (GWAS). In particular, migraine and headache GWAS data were from the Integrative Epidemiology Unit (IEU) dataset,35 encompassing a sample of 484,598 individuals (13,971 migraine cases and 470,627 controls, 4122 headache cases and 480,476 controls). Data for BMR,36 ALM,37 and five muscle mass-related traits38 were acquired from the UK Biobank. All GWAS summary statistics were harmonized and curated by the Medical Research Council (MRC) IEU at the University of Bristol.
Instrumental Variable Selection
When performing MR analysis, it is crucial to comply with three fundamental assumptions: assumption of the relevance, assumption of the independence and assumption of the exclusion restriction. Consequently, all IVs included for subsequent analysis must undergo rigorous selection. Initially, we chose SNPs linked to exposure variables (BMR and muscle mass-related traits) with a p-value of less than 5×10−8, ensuring significance and avoiding weak instrument bias. Second, to prevent bias from strong linkage disequilibrium (LD) among selected SNPs, we conducted a clustering process with parameters set to r² < 0.001 and a physical window of 10,000 kb, ensuring IV independence. Afterwards, we computed the F-statistic to evaluate the robustness of the IVs, excluding those with an F-statistic below 10 due to insufficient instrument strength. Palindromic SNPs with intermediate allele frequencies were removed after harmonizing the exposure and outcome datasets to guarantee the consistency of effect alleles. This stringent selection and harmonization process is crucial for the validity of MR analysis.
Statistical Analysis
NHANES data were analyzed using sampling weights per NCHS protocols to account for complex survey design. Participants were stratified by migraine status (present/absent). Proportions were employed to illustrate categorical data, while means and standard deviations (SD) were utilized for illustrating continuous variables. Categorical variables were analyzed using the chi-square test, whereas continuous variables were assessed using one-way analysis of variance (ANOVA). To guarantee data representativeness, all analyses were carried out utilizing the suggested NHANES data weighting methodologies. A multivariate logistic regression analysis was performed utilizing three weighting models to investigate the relationship between migraines and ALM/BMI or activity levels. Unadjusted variables were included in Model 1 (univariate logistic regression); sex, age, and race were included in Model 2; education, CRP, WBC, neutrophils, vigorous and moderate activity, drinking and smoking status, arthritis, CHD, and stroke all had been included in Model 3. Results are presented as odds ratio (OR) with 95% confidence interval (CI), using the lowest quartile as the reference. Following the adjustment for all confounding variables, we used restricted cubic splines (RCS) to assess whether there is a nonlinear relationship between migraines and ALM/BMI or activity levels. To evaluate the stability of the findings, supplemental interaction and subgroup analyses have been conducted to investigate the relationship between migraines and ALM/BMI across various demographic characteristics as well as medical statuses, with interacting examines assessing the robustness of these associations within subgroups. Statistical analyses was performed using DecisionLinc 1.1.3.8. Statistical significance was defined as p<0.05.
In order to investigate the genetic relationships among exposure traits (BMR, ALM, and five muscle mass-related traits) and migraines or headaches, five distinct approaches were used: weighted median, inverse variance weighting (IVW), MR-Egger regression, simple model, and weighted model methods. IVW was regarded as the primary analysis method in this study. Additionally, other experiments were conducted to confirm the accuracy of the data. Cochrane’s Q test was done to identify heterogeneity in the associations (Supplementary Table 1). The MR-Egger intercept test and the MR-PRESSO global test were employed to assess pleiotropy (Supplementary Table 2). Furthermore, MR-PRESSO detected outliers in the associations and produced estimates subsequent to their exclusion. A leave-one-out sensitivity analysis was performed, systematically excluding each SNP to assess the impact of the remaining SNPs and ascertain if the connection was affected by any singular influential SNP. R software (version 4.4.0) were carried out for statistical studies. A p value < 0.05 was considered statistically significant.
Result
Baseline Characteristics
This study comprised a total of 10,400 individuals. Table 1 establishes the baseline characteristics stratified by migraine status. Individuals with migraines exhibited significantly lower appendicular lean mass (ALM) compared to the non-migraine group (21.30 ± 6.22 kg vs 22.54 ± 6.43 kg, p < 0.001). Similarly, the appendicular lean mass normalized to body mass index (ALM/BMI) was markedly lower in migraineurs (migraine: 0.76 ± 0.19, non-migraine: 0.82 ± 0.21, p < 0.001).
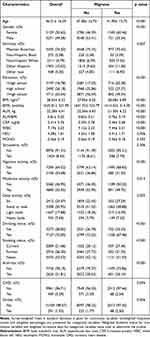 |
Table 1 Demographics and Characteristics of Study Participants From NHANES 1999–2004 |
Association Between ALM/BMI and Migraine
Multiple logistic regression models demonstrated a consistent inverse association between ALM/BMI and migraine risk (Table 2). In the fully adjusted model (Model 3), each unit increase in ALM/BMI was associated with a 75.7% reduction in migraine risk (OR = 0.243, 95% CI: 0.122–0.487, p < 0.001). Participants in the highest ALM/BMI quartile (Q4) had a 44.8% lower migraine risk compared to the lowest quartile (Q1) (OR = 0.552, 95% CI: 0.374–0.816; p for trend = 0.008).
 |
Table 2 Association Between ALM/BMI and Odds of Migraine, NHANES 1999–2004 |
Restricted cubic spline (RCS) analysis revealed no nonlinear relationship between ALM/BMI and migraine (p > 0.05; Figure 2).
Subgroup Analysis
Subgroup analyses were stratified by sex, age, smoking status, physical activity, and comorbidities. Except for arthritis (interaction p = 0.023), no statistically significant interactions were observed in any subgroups (interaction p > 0.05). The inverse association between higher ALM/BMI and reduced migraine risk was consistent among non-elderly individuals, non-smokers, and participants without cardiocerebrovascular disease (Figure 3).
 |
Figure 3 Subgroup analyses (forest plots). |
Association Between Physical Activity and Migraine
Table 3 presents the association between physical activity and the incidence of migraines. Vigorous physical activity demonstrated a significant inverse association with migraine prevalence (OR = 0.760, 95% CI: 0.663–0.872; p < 0.001). In contrast, moderate exercise shown no substantial correlation with migraine in the fully adjusted model (OR = 0.897, 95% CI: 0.777–1.035; p = 0.131). Standing/walking (OR = 0.801, 95% CI: 0.671–0.955, p = 0.016) and light-load daily activities (OR = 0.789, 95% CI: 0.659–0.943, p = 0.012) were associated with reduced migraine risk compared to sedentary behavior. However, there was no significant difference in migraine risk for the heavy loads group (p > 0.05).
 |
Table 3 Association Between Physical Activity and Odds of Migraine, NHANES 1999–2004 |
Mediation Effect of Inflammatory Markers on Muscle Mass-Migraine Associations
Mediation analyses quantified the proportion of associations between muscle mass/physical activity and migraine mediated by inflammatory markers (Figure 4). C-reactive protein (CRP) mediated 2.0% and 6.1% of the associations between ALM/BMI, vigorous activity and migraine, respectively (Figure 4A–E). Also, white blood cell (WBC) and neutrophil illustrated a mediation effect on those associations, whereas monocyte showed no significant mediation (mediation <1%, p > 0.10) (Figure 4).
MR: Muscle Mass-Related Factors on Migraine
As shown in Figure 5, the results of univariable Mendelian randomization (UVMR) illustrated the causal relationship between basal metabolic rate (BMR), ALM, five muscle mass-related traits, and migraine risk. Information regarding significant single nucleotide polymorphisms (SNPs) and their correlations with additional variables is available in Supplementary Tables 3–7 All identified SNPs had adequate strength (minimum F-statistic > 10) and suggested the appropriate direction of causality.
 |
Figure 5 Associations of genetically predicted risk factors with migraine were assessed using the random effects IVW method. |
UVMR analysis identified a genetically predicted causal relationship between increased BMR and reduced migraine risk (ORIVW = 0.996; 95% CI = 0.992–0.998; p = 0.004). Similar protective trends were observed for fat-free mass (FFM) and ALM. Furthermore, following the elimination of outliers by MR-PRESSO analysis, the correlation between BMR and migraines persisted strongly, as seen by non-significant distorting results (p > 0.05). Cochran’s Q test revealed heterogeneity during MR analysis (Q < 0.05); however, the results from the random-effects model were consistent with MR estimates. The MR Egger intercept analysis indicated an absence of directional pleiotropy (Supplementary Table 2). Supplementary Figures S1–S9 present scatter graphs illustrating the impacts of BMR, ALM, five muscle mass-related traits, and migraine-associated SNPs.
Multivariable MR (MVMR) found no causal link between FFM traits and migraine risk (Supplementary Figure S10, Supplementary Table 8).
MR: Muscle Mass-Related Factors on Headache
Figure 6 presents the causal relationships between BMR, ALM, muscle mass-related traits, and headache risk, as analyzed through UVMR. The SNP information used to evaluate the causal relationships of other exposure factors with headaches is provided in Figure 6 and Supplementary Tables 9–13 Genetically predicted increases in BMR (ORIVW = 0.998, 95% CI: 0.997–1, p = 0.018) and ALM (ORIVW = 0.999, 95% CI: 0.998–1, p = 0.047) were inversely associated with headache risk (Figure 6). No significant heterogeneity or pleiotropy was detected (p > 0.05; Supplementary Tables 1 and 2). Scatter plots of the effects of BMR, ALM, five muscle mass-related traits, and headache-associated SNPs are shown in Supplementary Figures S11–S19.
 |
Figure 6 Associations of genetically predicted risk factors with headache were assessed using the random effects IVW method. |
We also conducted a MVMR analysis to examine the causal relationship between FFM traits and headaches (Supplementary Table 14). Supplementary Figure S20 presents the results of the MVMR analysis, indicating no genetic causal relationship between FFM and headaches.
Discussion
This study investigated the association between muscle mass, physical activity, and the incidence of migraines in adults. We examined data from 10,400 participants during three biennial cycles of the National Health and Nutrition Examination Survey (NHANES) survey. Upon adjusting for confounding variables, our findings revealed a substantial correlation between appendicular lean mass adjusted for body mass index (ALM/BMI), vigorous physical activity, and the occurrence of migraines. Mediation analyses revealed that systemic inflammation partially explained these associations, with C-reactive protein (CRP) and white blood cell (WBC) count mediating the ALM/BMI-migraine link. Mendelian randomization (MR) analyses further demonstrated genetically predicted causal links: elevated basal metabolic rate (BMR) and higher appendicular lean mass (ALM) were protective against both migraines and headaches. These findings suggest that increasing muscle mass and physical activity may mitigate migraine risk, partially through attenuating systemic inflammation.
Migraines are generally induced by a confluence of intricate elements, marked by elevated incidence and recurrence rates, with considerable individual diversity.19,39–42 Numerous studies have established a significant correlation between obesity and the incidence of migraines, indicating that obesity is associated with an elevated risk of migraines.20,39–41 Research indicates that body fat percentage also correlates weakly but significantly migraine frequency.39 However, limited research exists on the relationship between specific obesity subtypes—particularly sarcopenic obesity (SO)—and migraines remains underexplored. Our study addresses this gap by focusing on the protective role of muscle mass against migraines. Moreover, Our findings also highlight vigorous physical activity as a promising non-pharmacological intervention, reducing migraine incidence by 24%, which is consistent to the previous studies.26,27 A meta-analysis by Lemmens et al reported that aerobic exercise reduces migraine frequency by 30–50%.26 Unlike previous work emphasizing moderate aerobic exercise, our study specifically identifies vigorous activity as beneficial. An randomized control trial (RCT) revealed that active exercise may have an effect on desensitization to mitigate migraine,27 mediation analyses in our research suggested inflammatory markers (eg, CRP, WBC) partially explain these associations, offering mechanistic insights.
To address confounding and reverse causality inherent in observational studies, we employed MR, leveraging genetic variants as instrumental variables.43 MR minimizes bias by simulating randomized trial conditions, ensuring genetic variants are independent of confounders. Furthermore, all instrumental variables (IVs) employed in the MR study were meticulously chosen to guarantee the precision of the results. Finally, we employed multiple methods to test sensitivity and horizontal pleiotropy. Both MR-Egger and MR-PRESSO analyses demonstrated no indication of directional pleiotropy, highlighting the robustness and trustworthiness of our MR methodology. Since migraine attacks may transiently limit activity resulting in reduced parameters of muscle mass-related traits reversely, Bidirectional MR further ruled out reverse causation, showing no effect of migraines on muscle mass traits (data not shown).
Various factors influence the onset and advancement of sarcopenia, such as aging, neurological disorders, inflammation, inactivity, and malnutrition.44,45 Both mitochondrial dysfunction and systemic inflammation are central to sarcopenia pathogenesis,14,44,46–48 and these mechanisms also contribute to migraine pathophysiology. Several mechanisms may underlie the association between decreased muscle mass and migraine development.
First, inflammation is a prevalent factor contributing to the onset of sarcopenia and is linked to numerous neurological disorders.24,49 CRP, Interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF-α) are elevated in sarcopenia.50–52 Physical activity can reduce these inflammatory markers, whereas sedentary behavior increases the risk of inflammation.53,54 Neuroinflammation, a key driver of migraines,55,56 may thus be amplified by sarcopenia-related inflammation, increasing migraine susceptibility. Second, Mitochondrial impairment in sarcopenia disrupts cellular energy production, leading to metabolic insufficiency.46 Impaired energy metabolism is linked to the occurrence of migraines, and optimizing energy metabolism may reduce the frequency and severity of migraine attacks.57–60 Higher muscle mass correlates with elevated BMR, enhancing energy availability to meet the brain’s high demand.58 Furthermore, studies demonstrate that mitochondrial dysfunction may substantially impact migraine pathophysiology by influencing calcium permeability, promoting excessive free radical generation, diminishing mitochondrial membrane potential, and reducing oxidative phosphorylation levels.57,61 These changes result in neuronal energy depletion and apoptosis, lowering the pain threshold and increasing the likelihood of migraine attacks.57 Other studies indicate that supplementation with specific nutrients, such as riboflavin, thiamine, magnesium, and coenzyme Q10,62 may support mitochondrial function and help reduce the frequency and severity of migraines.48,63
This study possesses multiple limitations. First, the cross-sectional analyses are based on American adults, and the MR analyses were conducted using genetic data from individuals of European ancestry, limiting generalizability to other ethnic or geographic groups. Second, although we utilized the MR-intercept and MR-PRESSO global tests to identify and address pleiotropy in genetic variations, confounding variables between exposure and outcome, such as personality traits and mental conditions, may still exist, potentially resulting in biased findings. Last, the diagnosis of migraines depended on participants’ self-reported medical histories, which may introduce misclassification bias due to underreporting of milder cases or conflation with tension-type headaches. The absence of clinician-confirmed diagnoses precludes definitive phenotyping.
Future research should prioritize prospective cohort studies and more extensive datasets to address these limitations, elucidating the mechanisms linking muscle mass and migraines, and informing appropriate care options for migraine sufferers. RCT are needed to evaluate whether increasing muscle mass through resistance training, aerobic exercise or anaerobic exercise reduces migraine incidence or severity. Additionally, studies should establish specific ALM thresholds to guide migraine prevention strategies. Mechanistic investigations are also essential to clarify the role of inflammatory biomarkers in mediating the muscle-migraine relationship.
Conclusion
In summary, our study indicates that elevated muscle mass is associated with a reduced risk of migraines, with systemic inflammation partially mediating this relationship. These findings highlight an effective non-pharmacological management strategy, emphasizing the importance of maintaining muscle mass through vigorous physical activity.
Abbreviations
BMR, Basal Metabolic Rate; ALM, Appendicular Lean Mass; ALM/BMI, Appendicular Lean Mass Normalized to Body Mass Index; NHANES, National Health and Nutrition Examination Survey; MR, Mendelian Randomization; CGRP, Calcitonin Gene-Related Peptide; SO, Sarcopenic obesity; NCHS, National Center for Health Statistics; IRB, Institutional Review Board; HHS, Health and Human Services; MEC, Mobile Examination Center; DXA, Dual-energy X-ray Absorptiometry; AMPP, American Migraine Prevalence and Prevention; BMI, Body Mass Index; NIH, National Institutes of Health; FNIH, Foundation for the National Institutes of Health; CRP, C-reactive protein; WBC, White Blood Cell; NEU, Neutrophil; MONO, Monocyte; CHD, Coronary Heart Disease; FFM, Fat-Free Mass; SNP, Single Nucleotide Polymorphism; IV, Instrumental Variable; GWAS, Genome-Wide Association Studies; IEU, Integrative Epidemiology Unit; LD, Linkage Disequilibrium; IVW, Inverse Variance Weighted; UVMR, Univariable Mendelian Randomization; MVMR, Multivariable Mendelian Randomization; MRC, Medical Research Council; OR, Odds Ratio; CI, Confidence Interval; RCS, Restricted Cubic Splines; IL, Interleukin; TNF-α, Tumor Necrosis Factor α; RCT, Randomized Control Trial.
Data Sharing Statement
This research examined publically accessible datasets. For additional queries, feel free to reach out to the corresponding author.
Ethics Approval and Consent to Participate
The Qingdao University Affiliated Hospital’s Ethics Committee carries out its independent ethical assessment duties by abiding by the Helsinki Declaration and international ethical standards for human health research. This study employs publically accessible data that has been lawfully acquired and satisfies the criteria for exemption from review as outlined in the ethical review protocols for life sciences and medical research involving human subjects.
Acknowledgments
We express our gratitude to all the researchers involved in the National Health and Nutrition Examination Survey and the IEU Open GWAS project database, appreciating their contributions and participation in providing the genome-wide association study data. During the process of preparing this work, the authors utilized ChatGPT as a tool to enhance the clarity, fluency, and overall quality of the language.
Funding
This study was supported under Grant Number 4568 from The Affiliated Hospital of Qingdao University (Qingdao, China) to C.J. Also, this study was supported by project 23-2-1-142-zyyd-jcha supported by Qingdao Natural Science Foundation to L.L.
Disclosure
We declare that we have no commercial or associative interests that could be considered a conflict of interest in relation to the work submitted.
References
1. Munjal S, Singh P, Reed ML, et al. Most bothersome symptom in persons with migraine: results from the Migraine in America Symptoms and Treatment (MAST) study. Headache. 2020;60(3):416–429. doi:10.1111/head.13708
2. Ashina M, Buse DC, Ashina H, et al. Migraine: integrated approaches to clinical management and emerging treatments. Lancet. 2021;397(10283):1505–1518. doi:10.1016/S0140-6736(20)32342-4
3. Charles A. The pathophysiology of migraine: implications for clinical management. Lancet Neurol. 2018;17(2):174–182. doi:10.1016/S1474-4422(17)30435-0
4. Goadsby PJ, Holland PR, Martins-Oliveira M, et al. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97(2):553–622. doi:10.1152/physrev.00034.2015
5. Steiner TJ, Stovner LJ. Global epidemiology of migraine and its implications for public health and health policy. Nat Rev Neurol. 2023;19(2):109–117. doi:10.1038/s41582-022-00763-1
6. Ashina M, Terwindt GM, Al-Karagholi MA, et al. Migraine: disease characterisation, biomarkers, and precision medicine. Lancet. 2021;397(10283):1496–1504. doi:10.1016/S0140-6736(20)32162-0
7. Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci. 2015;35(17):6619–6629. doi:10.1523/JNEUROSCI.0373-15.2015
8. Gross EC, Lisicki M, Fischer D, et al. The metabolic face of migraine—from pathophysiology to treatment. Nat Rev Neurol. 2019;15(10):627–643. doi:10.1038/s41582-019-0255-4
9. Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55(1):533–552. doi:10.1146/annurev-pharmtox-010814-124701
10. Burstein R, Jakubowski M, Garcia-Nicas E, et al. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol. 2010;68(1):81–91. doi:10.1002/ana.21994
11. Schulte LH, Allers A, May A. Hypothalamus as a mediator of chronic migraine: evidence from high-resolution fMRI. Neurology. 2017;88(21):2011–2016. doi:10.1212/WNL.0000000000003963
12. Coppola G, Di Renzo A, Tinelli E, et al. Thalamo-cortical network activity during spontaneous migraine attacks. Neurology. 2016;87(20):2154–2160. doi:10.1212/WNL.0000000000003327
13. Sparaco M, Feleppa M, Lipton RB, et al. Mitochondrial dysfunction and migraine: evidence and hypotheses. Cephalalgia. 2006;26(4):361–372. doi:10.1111/j.1468-2982.2005.01059.x
14. Kursun O, Yemisci M, van den Maagdenberg A, et al. Migraine and neuroinflammation: the inflammasome perspective. J Headache Pain. 2021;22(1):55. doi:10.1186/s10194-021-01271-1
15. Haghdoost F, Togha M. Migraine management: non-pharmacological points for patients and health care professionals. Open Med. 2022;17(1):1869–1882. doi:10.1515/med-2022-0598
16. Najib U, Moore M, Watson D. Unique considerations for special populations in episodic migraine: the underserved. Curr Pain Headache Rep. 2019;23(2):9. doi:10.1007/s11916-019-0749-1
17. Han X, Yu S. Non-pharmacological treatment for chronic migraine. Curr Pain Headache Rep. 2023;27(7):663–672. doi:10.1007/s11916-023-01162-x
18. Agbetou M, Adoukonou T. Lifestyle modifications for migraine management. Front Neurol. 2022;13:719467. doi:10.3389/fneur.2022.719467
19. Verrotti A, Di Fonzo A, Penta L, et al. Obesity and headache/migraine: the importance of weight reduction through lifestyle modifications. Biomed Res Int. 2014;2014:420858. doi:10.1155/2014/420858
20. Bond DS, Roth J, Nash JM, et al. Migraine and obesity: epidemiology, possible mechanisms and the potential role of weight loss treatment. Obes Rev. 2011;12(5):e362–e371. doi:10.1111/j.1467-789X.2010.00791.x
21. Donini LM, Busetto L, Bischoff SC, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Obes Facts. 2022;15(3):321–335. doi:10.1159/000521241
22. Caponnetto V, Deodato M, Robotti M, et al. Comorbidities of primary headache disorders: a literature review with meta-analysis. J Headache Pain. 2021;22(1):71. doi:10.1186/s10194-021-01281-1
23. Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi:10.1038/nrn2297
24. Yang J, Jiang F, Yang M, et al. Sarcopenia and nervous system disorders. J Neurol. 2022;269(11):5787–5797. doi:10.1007/s00415-022-11268-8
25. Mirzai S, Carbone S, Batsis JA, et al. Sarcopenic obesity and cardiovascular disease: an overlooked but high-risk syndrome. Curr Obes Rep. 2024;13(3):532–544. doi:10.1007/s13679-024-00571-2
26. Lemmens J, De Pauw J, Van Soom T, et al. The effect of aerobic exercise on the number of migraine days, duration and pain intensity in migraine: a systematic literature review and meta-analysis. J Headache Pain. 2019;20(1):16. doi:10.1186/s10194-019-0961-8
27. Deodato M, Granato A, Buoite Stella A, et al. Efficacy of a dual task protocol on neurophysiological and clinical outcomes in migraine: a randomized control trial. Neurol Sci. 2024;45(8):4015–4026. doi:10.1007/s10072-024-07611-8
28. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88(4):1379–1406. doi:10.1152/physrev.90100.2007
29. Pedersen BK. The diseasome of physical inactivity—and the role of myokines in muscle-fat cross talk. J Physiol. 2009;587(23):5559–5568. doi:10.1113/jphysiol.2009.179515
30. National Center for Health Statistics. NHANES survey methods and analytic guidelines. 2025. Available from: https://wwwn.cdc.gov/nchs/nhanes/AnalyticGuidelines.aspx.
31. van Delden JJ, van der Graaf R. Revised CIOMS international ethical guidelines for health-related research involving humans. JAMA. 2017;317(2):135–136. doi:10.1001/jama.2016.18977
32. Buse DC, Loder EW, Gorman JA, et al. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53(8):1278–1299. doi:10.1111/head.12150
33. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69(5):547–558. doi:10.1093/gerona/glu010
34. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89–R98. doi:10.1093/hmg/ddu328
35. Dönertaş HM, Fabian DK, Valenzuela MF, et al. Common genetic associations between age-related diseases. Nat Aging. 2021;1(4):400–412. doi:10.1038/s43587-021-00051-5
36. Loh PR, Kichaev G, Gazal S, et al. Mixed-model association for biobank-scale datasets. Nat Genet. 2018;50(7):906–908. doi:10.1038/s41588-018-0144-6
37. Pei YF, Liu YZ, Yang XL, et al. The genetic architecture of appendicular lean mass characterized by association analysis in the UK biobank study. Commun Biol. 2020;3(1):608. doi:10.1038/s42003-020-01334-0
38. Lyon MS, Andrews SJ, Elsworth B, et al. The variant call format provides efficient and robust storage of GWAS summary statistics. Genome Biol. 2021;22(1):32. doi:10.1186/s13059-020-02248-0
39. Ojha P, Malhotra V. Implication of high body fat percentage on migraine chronification in premenopausal females. Neurol Res Int. 2022;2022:8219254. doi:10.1155/2022/8219254
40. Kristoffersen ES, Børte S, Hagen K, et al. Migraine, obesity and body fat distribution—a population-based study. J Headache Pain. 2020;21(1):97. doi:10.1186/s10194-020-01163-w
41. Ornello R, Ripa P, Pistoia F, et al. Migraine and body mass index categories: a systematic review and meta-analysis of observational studies. J Headache Pain. 2015;16(1):27. doi:10.1186/s10194-015-0510-z
42. Razeghi Jahromi S, Ghorbani Z, Martelletti P, et al. Association of diet and headache. J Headache Pain. 2019;20(1):106. doi:10.1186/s10194-019-1057-1
43. Tam V, Patel N, Turcotte M, et al. Benefits and limitations of genome-wide association studies. Nat Rev Genet. 2019;20(8):467–484. doi:10.1038/s41576-019-0127-1
44. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393(10191):2636–2646. doi:10.1016/S0140-6736(19)31138-9
45. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(4):601. doi:10.1093/ageing/afz046
46. Bellanti F, Lo Buglio A, Vendemiale G. Mitochondrial impairment in sarcopenia. Biology. 2021;10(1):31. doi:10.3390/biology10010031
47. Jimenez-Gutierrez GE, Martínez-Gómez LE, Martínez-Armenta C, et al. Molecular mechanisms of inflammation in sarcopenia: diagnosis and therapeutic update. Cells. 2022;11(15):2359. doi:10.3390/cells11152359
48. Grech O, Mollan SP, Wakerley BR, et al. The role of metabolism in migraine pathophysiology and susceptibility. Life. 2021;11(5):415. doi:10.3390/life11050415
49. Jensen GL. Inflammation: roles in aging and sarcopenia. JPEN J Parenter Enteral Nutr. 2008;32(6):656–659. doi:10.1177/0148607108324585
50. Bian AL, Hu HY, Rong YD, et al. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur J Med Res. 2017;22(1):25. doi:10.1186/s40001-017-0266-9
51. Gül ZB, Çelik RGG, Selçuk B, et al. New indicator of inflammation in migraine: red blood cell distribution. Haydarpasa Numune Med J. 2021;61(2):166.
52. Welch KM, Brandes AW, Salerno L, et al. C-reactive protein may be increased in migraine patients who present with complex clinical features. Headache. 2006;46(1):197–199. doi:10.1111/j.1526-4610.2006.00330.x
53. Edvinsson L, Haanes KA, Warfvinge K. Does inflammation have a role in migraine? Nat Rev Neurol. 2019;15(8):483–490. doi:10.1038/s41582-019-0216-y
54. Erdener ŞE, Kaya Z, Dalkara T. Parenchymal neuroinflammatory signaling and dural neurogenic inflammation in migraine. J Headache Pain. 2021;22(1):138. doi:10.1186/s10194-021-01353-0
55. Wang Y, Wang Y, Yue G, et al. Energy metabolism disturbance in migraine: from a mitochondrial point of view. Front Physiol. 2023;14:1133528. doi:10.3389/fphys.2023.1133528
56. Johnstone AM, Murison SD, Duncan JS, et al. Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am J Clin Nutr. 2005;82(5):941–948. doi:10.1093/ajcn/82.5.941
57. Goran MI. Energy metabolism and obesity. Med Clin North Am. 2000;84(2):347–362. doi:10.1016/S0025-7125(05)70225-X
58. Wang Z, Heshka S, Wang J, et al. Metabolically active portion of fat-free mass: a cellular body composition level modeling analysis. Am J Physiol Endocrinol Metab. 2007;292(1):E49–E53. doi:10.1152/ajpendo.00485.2006
59. Gargus JJ. Genetic calcium signaling abnormalities in the central nervous system: seizures, migraine, and autism. Ann N Y Acad Sci. 2009;1151(1):133–156. doi:10.1111/j.1749-6632.2008.03572.x
60. Gaul C, Diener HC, Danesch U. Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: a randomized, placebo-controlled, double-blind, multicenter trial. J Headache Pain. 2015;16(1):516. doi:10.1186/s10194-015-0516-0
61. Fila M, Chojnacki C, Chojnacki J, et al. Nutrients to improve mitochondrial function to reduce brain energy deficit and oxidative stress in migraine. Nutrients. 2021;13(12):433. doi:10.3390/nu13124433
62. Almuraikhy S, Sellami M, Al-Amri HS, et al. Impact of moderate physical activity on inflammatory markers and telomere length in sedentary and moderately active individuals with varied insulin sensitivity. J Inflamm Res. 2023;16:5427–5438. doi:10.2147/JIR.S429899
63. Henson J, Yates T, Edwardson CL, et al. Sedentary time and markers of chronic low-grade inflammation in a high risk population. PLoS One. 2013;8(10):e78350. doi:10.1371/journal.pone.0078350
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



