Back to Journals » Cancer Management and Research » Volume 17
Inflammatory and Nutritional Biomarker in Different Subtypes of Early Stage Diffuse Large B-Cell Lymphoma: Towards a Diagnostic Model
Authors Oehadian A , Gunadi SV, Mersiana L, Susandi E , Wijaya I , Martha JW, Supriyadi R, Prihatni D
Received 12 March 2025
Accepted for publication 29 June 2025
Published 12 July 2025 Volume 2025:17 Pages 1361—1367
DOI https://doi.org/10.2147/CMAR.S527855
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Amaylia Oehadian,1,* Stephanie Victoria Gunadi,2,* Lusi Mersiana,1 Evan Susandi,2 Indra Wijaya,1 Januar Wibawa Martha,3 Rudi Supriyadi,4 Delita Prihatni5
1Division of Hematology and Oncology Medic, Department of Internal Medicine Faculty of Medicine, Universitas Padjadjaran/Dr. Hasan Sadikin General Hospital, Bandung, Indonesia; 2Department of Internal Medicine Faculty of Medicine, Universitas Padjadjaran/Dr. Hasan Sadikin General Hospital, Bandung, Indonesia; 3Department of Cardiology and Vascular Medicine Faculty of Medicine, Padjadjaran University, Bandung, West Java, Indonesia; 4Division of Nephrology and Hypertension, Department of Internal Medicine Faculty of Medicine, Padjadjaran University/Hasan Sadikin General Hospital, Bandung, West Java, Indonesia; 5Department of Clinical Pathology Faculty of Medicine, Universitas Padjadjaran/Dr. Hasan Sadikin General Hospital, Bandung, Indonesia
*These authors contributed equally to this work
Correspondence: Delita Prihatni, Department of Clinical Pathology, Padjadjaran University/ Dr. Hasan Sadikin General Hospital, Jl. Pasteur No. 38, Pasteur, Bandung, West Java, 40161, Indonesia, Email [email protected]
Background: Diffuse Large B-Cell Lymphoma (DLBCL) is a heterogeneous malignancy with distinct Germinal Center B-Cell Like (GCB) and non-GCB subtypes. Accurate subtyping is crucial due to differences in prognosis and treatment response. While gene expression profiling is the gold standard, it is often unavailable in low-resource settings. Inflammatory and nutritional biomarkers such as Systemic Immune-Inflammation Index (SII), Prognostic Nutritional Index (PNI), and Advanced Lung Cancer Inflammation Index (ALI) offer a practical alternative. This study aims to evaluate their diagnostic potential in early-stage DLBCL subtypes.
Methods: A cross-sectional analysis was conducted on 60 early stage DLBCL patients (30 GCB, 30 non-GCB) at Dr Hasan Sadikin General Hospital Bandung. Clinical characteristics, hematological parameters, and inflammation-based indices (SII, PNI, and ALI) were evaluated. Differences between subtypes were analyzed using the Mann–Whitney U-test, and Receiver Operating Characteristic (ROC) analysis was used to determine diagnostic performance.
Results: The median SII was significantly higher in non-GCB compared with GCB (982,575; IQR: 609,112– 2,239,917 vs 575,598; IQR: 454,578– 886,426, p = 0.014). Conversely, PNI was higher in GCB compared to non-GCB group (49.18; IQR: 46.38– 56.38 vs 45.96; IQR 40.05– 52.28, p = 0.011). ALI values were also higher in the GCB than non-GCB (44.14; IQR: 27.69– 67.18 vs 24.51; IQR: 14.34– 42.47,p=0.003). ROC analysis revealed that ALI had the highest diagnostic accuracy (AUC = 0.724; 95% CI: 0.593– 0.831), followed by SII (AUC = 0.685, 95% CI: 0.552– 0.799) and PNI (AUC = 0.691 95% CI: 0.558– 0.804). Optimal cut-off values were ≤ 1,234,133 for SII, > 43.27 for PNI, and > 27.41 for ALI. ALI demonstrated the best balance between sensitivity (76.7%) and specificity (63.3%), making it the most reliable marker for distinguishing DLBCL subtypes.
Conclusion: SII, PNI, and ALI differ significantly between DLBCL subtypes. These findings suggest that integrating these indices into a diagnostic model could enhance risk stratification and guide therapeutic decision-making in DLBCL. Further studies with larger cohorts are warranted to validate these findings.
Keywords: acute lung inflammation index, ALI, germinal center B-cell like, GCB, non-germinal center B-cell like, Non-GCB, prognostic nutritional index, PNI, systemic immune, inflammation index, SII
Introduction
Diffuse Large B-Cell Lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL), accounting for approximately 30% of adult NHL cases worldwide. Although morphologically similar, DLBCL represents a biologically heterogeneous group of malignancies with significant differences in pathogenesis, molecular profile, and clinical outcome. Based on cell of origin and gene expression profiling, DLBCL can be subdivided into germinal center B-cell-like (GCB) and non-GCB subtypes based on cell of origin and gene expression profiles.1–4 This classification is clinically relevant, as the non-GCB subtype is typically associated with higher systemic inflammation and poorer prognosis compared to the GCB subtype.5,6
Accurate subtyping of DLBCL is essential for guiding treatment decisions and predicting outcomes. However, genomic sequencing, as the gold standard for molecular classification, remains limited in many clinical settings due to high costs, technical requirements, and limited accessibility.7 As a result, there is an increasing need to identify practical, affordable surrogate markers that can support subtype classification in routine clinical practice.
Emerging evidence has highlighted the crucial role of systemic inflammation, immune response and nutritional status in the pathogenesis, progression, and prognosis of DLBCL, further underscoring the value of biologically informed subtyping.8,9 Several inflammation-based and nutritional indices, including the Systemic Immune-Inflammation Index (SII), Prognostic Nutritional Index (PNI), and Advanced Lung Cancer Inflammation Index (ALI), have emerged as potential biomarkers for assessing systemic inflammation, immune response, and nutritional status in malignancies, including hematological cancers.10–15 These indices are calculated from routinely available clinical parameters such as neutrophil, lymphocyte, and platelet counts, serum albumin, and body mass index, making them feasible, cost-effective, and accessible even in rural healthcare settings.
Despite increasing evidence of their prognostic value, the role of inflammatory and nutritional biomarkers in distinguishing between molecular subtypes of early-stage DLBCL remains underexplored. Given the challenges of molecular classification in resource-limited settings, these indices offer a promising and accessible alternative for subtype differentiation. This study aims to analyze differences in SII, PNI, and ALI between GCB and non-GCB subtypes in early stage DLBCL and evaluate their potential utility as accessible diagnostic markers for subtype classification.
Materials and Methods
This study was a retrospective comparative cross-sectional analysis utilizing secondary data from the Lymphoma registry of Dr Hasan Sadikin General Hospital from January 2020 to August 2024. The registry contains routinely collected clinical, pathological, and laboratory data from newly diagnosed lymphoma patients. A total of 60 subjects with early stages DLBCL (Ann Arbor stage I or II) were included and classified into GCB (n = 30) and non-GCB (n = 30) groups based on immunohistochemical profiling using the Hans algorithm, which incorporates CD10, BCL-6, and MUM1 markers.7
Subjects included in the study were aged 18 years or older, had a histologically confirmed diagnosis of DLBCL, subtyping confirmed (GCB or non-GCB) using Hans algorithm, and were chemotherapy-naïve at the time of data collection to ensure consistency in biomarker timing. Subjects with concurrent malignancies, a history of prior chemotherapy or immunosuppressive therapy, infection (HIV or hepatitis), or end-stage kidney disease were excluded because these conditions could independently affect inflammatory and nutritional biomarkers and their baseline values would not reflect the true diagnostic potential.
The sample size was calculated using the formula for an unpaired numerical comparative study, which indicated a requirement of 30 subjects per group, resulting in a total sample size of 60 subjects. However, due to limitations in the number of eligible subjects available during the study period, the researchers were unable to include more subjects.
Demographic and clinical data were retrieved from the registry from January 2020 to August 2024. Biomarker values (SII, PNI, and ALI) were collected from laboratory results taken at the time of diagnosis before initiation of any treatment. Definitions of biomarkers:10–15
- SII: platelet count × neutrophil–lymphocyte ratio (NLR). SII reflects systemic inflammatory burden, incorporating thrombocytosis, neutrophilia, and lymphopenia as indicators of tumor-related immune dysregulation.
- PNI: 10 × serum albumin (g/dL) + 0.005 × total lymphocyte count/mm.3 PNI is an indicator of nutritional and immune status, reflecting protein reserves and adaptive immune function.
- ALI: body mass index (BMI) × blood albumin level (g/dL) ÷ NLR. ALI integrates nutritional status and systemic inflammation and has been used as a composite marker of host condition in malignancies.
Statistical analysis was performed using SPSS 27.0 and GraphPad Prism 9.2.0. The categorical variables were presented as frequencies and percentages. Continuous variables were first tested for normality using the Shapiro–Wilk test. Normally distributed data were expressed as mean ± standard deviation (SD), whereas non-normally distributed data were presented as median, interquartile range (IQR). In this study, the SII, PNI and ALI values were not normally distributed. Therefore, group comparisons between GCB and non-GCB subtypes were performed using the non-parametric Mann–Whitney U-test. Receiver Operating Characteristics (ROC) curve analysis was used to assess the diagnostic performance of SII, PNI and ALI in distinguishing GCB from non-GCB subtypes. The area under the ROC curve (AUC), optimal cut-off values, sensitivity, and specificity were calculated for each biomarker. A p-value <0.05 was considered statistically significant.
The study received ethical approval from Health Research Ethics Committee of the Faculty of Medicine, Padjadjaran University - Dr. Hasan Sadikin General Hospital Bandung, under approval number DP.04.03/D.XIV.6.5/430/2024. Ethical conduct of the study was ensured in compliance with the Declaration of Helsinki.
Results
Clinical Characteristics
A total of 60 DLBCL patients were included in this study, stratified into 30 GCB and 30 non-GCB subtypes. The baseline characteristics, including age, sex distribution, BMI, disease stage, and laboratory parameters, were analyzed to assess their association with inflammatory and nutritional indices as presented in Table 1.
 |
Table 1 Clinical Characteristics |
There was no significant difference in the mean age between GCB and non-GCB group (56 ± 13 vs 51 ± 14 years, p 0.115). Similarly, there was no significant difference in gender distribution between the two groups. A significant difference was found in BMI, GCB group had higher BMI than the non-GCB (24.4 ± 4.1 vs 21.8 ± 3.1kg/m², p 0.008). The lymphocyte count in GCB was higher than non-GCB [2,147 (1,334–2,785) vs 1,321 (834–2,287) /µL, p 0.030]. The Neutrophil-to-Lymphocyte Ratio (NLR) was significantly lower in GCB compared to non-GCB subtype [2.26 (1.48–3.05) vs 3.09 (1.95–6.25), p 0.014].
Comparative Analysis of SII, PNI, and ALI Between GCB and Non-GCB Subtypes
Table 2 shows that the median of Systemic Immune-Inflammation Index (SII) was significantly higher in non-GCB compared to GCB. In contrast, the Prognostic Nutritional Index (PNI) and Advanced Lung Cancer Inflammation Index (ALI) was higher in GCB than non-GCB.
 |
Table 2 Differences in SII, PNI, and ALI Values Between GCB and Non-GCB |
Diagnostic Performance of SII, PNI, and ALI
Receiver Operating Characteristic (ROC) curve analysis demonstrated that among the three indices evaluated, ALI exhibited the highest diagnostic accuracy, with an Area Under Curve (AUC) of 0.724 (95% CI: 0.593–0.831). This suggests that ALI is the most reliable indicator for differentiating between GCB and non-GCB subtypes of DLBCL. In comparison, the SII demonstrated a slightly lower diagnostic performance, with an AUC of 0.685 (95% CI: 0.552–0.799), while the PNI showed moderated accuracy, with an AUC of 0.691 (95% CI: 0.558–0.804) as seen in Figures 1–3.
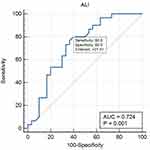 |
Figure 1 ROC Analysis and AUC for ALI Sensitivity and Specificity. |
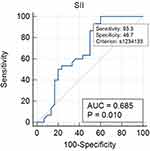 |
Figure 2 ROC Analysis and AUC for SII Sensitivity and Specificity. |
 |
Figure 3 ROC Analysis and AUC for PNI Sensitivity and Specificity. |
To establish the most effective threshold for distinguishing between GCB and non-GCB subtypes, optimal cut-off values were identified for each index. The cut-off value for SII was ≤1,234,133, indicating that values above this threshold may be more associated with non-GCB cases. Meanwhile, the optimal cut-off for PNI was >43.27, suggesting that patients with higher PNI values are more likely to belong to the GCB subtype. Lastly, the threshold for ALI was >27.41, reinforcing its superior diagnostic potential in subclassifying DLBCL patients.
Discussion
Significant differences were observed in the median SII, PNI and ALI values between the GCB and non-GCB group as seen in Table 2. SII values significantly higher in the non-GCB group than in the GCB group, suggesting a greater inflammatory burden and worse prognosis. This finding aligns with the study by Bo Wu et al, who also reported higher SII levels in non-GCB DLBCL.16 Guan et al further demonstrated that non-GCB DLBCL secretes IL-16 through active caspase-3 cleavage at a higher rate than GCB subtypes, stimulating CXCL-8 (IL-8) expression, which promotes neutrophil recruitment and systemic inflammation.17,18 Additionally, increased IL-10 expression in non-GCB DLBCL contributes to an immunosuppressive tumor microenvironment, leading to reduced lymphocyte activity and adaptive immune suppression through NF-κB pathway activation.18,19
Similarly, PNI and ALI values were significantly lower in the non-GCB group, supporting previous research indicating that lower PNI and ALI correlate with worse prognosis and heightened inflammation.20,21 PNI is calculated using albumin and lymphocyte count, both of which were significantly lower in the non-GCB group. The lower albumin levels in non-GCB DLBCL have been linked to NF- κB pathway activation, which enhances pro-inflammatory cytokine production and ubiquitin-proteasome activity, leading to muscle protein degradation and cachexia.18,22,23 ALI, which incorporates BMI, albumin and the Neutrophil-to-Lymphocyte Ratio (NLR), was significantly lower in non-GCB DLBLC.
ROC curve analysis (Figures 1–3), demonstrated that ALI had the highest diagnostic accuracy (AUC = 0.724, 95% CI: 0.593–0.831), followed by PNI (AUC = 0.691, 95% CI: 0.558–0.804) and SII (AUC = 0.685, 95% CI: 0.552–0.799). This result differed from that reported by Liu et al, who reported no significant differences in SII, PNI, and ALI between DLBCL subtypes.21 However, the disparity in results may be attributed to differences in patient selection, as our study specifically focused on early-stage DLBCL, potentially capturing variations in inflammatory and nutritional markers that were not evident in previous studies.
Among the three indices, ALI exhibited the best balance between sensitivity (80%) and specificity (60%), making it a more reliable standalone diagnostic marker. While SII and PNI showed higher sensitivity (93.3%), their lower specificity (46.7% and 40%, respectively) limits their standalone diagnostic utility. These findings support the use of a multi-marker approach, where combining SII, PNI, and ALI may enhance risk stratification and improve clinical decision-making in DLBCL. Future research should focus on validating these findings in larger cohorts, assessing their longitudinal prognostic value, and exploring their potential role in treatment response monitoring.
Study Limitations
This study is subject to several limitations. Firstly, the relatively small sample size may have introduced bias in the findings, emphasizing the need for larger, multi-center studies to enhance the validity of the results. Secondly, as the research was conducted at a single institution, selection bias may have influenced the outcomes, thereby limiting the generalizability of the findings to wider populations. Lastly, the retrospective nature of the study presents potential biases, including missing data and inconsistencies in clinical documentation. A prospective study design would offer more robust and reliable evidence.
Conclusion
This study highlights the significant differences in inflammatory and nutritional indices between GCB and non-GCB subtypes of DLBCL, with ALI emerging as the most accurate diagnostic marker. The findings support the integration of SII, PNI, and ALI into routine clinical practice, providing a cost-effective and widely available tool for subtype differentiation, risk assessment, and therapeutic decision-making. Future studies with larger, multicenter populations are warranted to further validate these findings and refine their clinical application in DLBCL management.
Ethics Statement
This study was approved by the ethics committees of the Hasan Sadikin General Hospital and was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declared no conflicts of interest in this work.
References
1. Jamil A, Mukkamalla SKR. Lymphoma. Treasure Island (FL): StatPearls Publishing; 2023.
2. Singh R, Shaik S, Negi BS, et al. Non-Hodgkin’s lymphoma: a review. J Family Med Prim Care. 2020;9(4):1834. doi:10.4103/jfmpc.jfmpc_1037_19
3. Susanibar-Adaniya S, Barta SK. 2021 update on diffuse large B cell lymphoma: a review of current data and potential applications on risk stratification and management. Am J Hematol. 2021;96(5):617–629. doi:10.1002/ajh.26151
4. Shimkus G, Nonaka T. Molecular classification and therapeutics in diffuse large B-cell lymphoma. Front Mol Biosci. 2023;10:1124360. doi:10.3389/fmolb.2023.1124360
5. Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2(1):1–9.
6. Liang X, Hu R, Li Q, Wang C, Liu Y. Prognostic factors for diffuse large B-cell lymphoma: clinical and biological factors in the rituximab era. Exp Hematol. 2023;122:1–9. doi:10.1016/j.exphem.2023.03.003
7. Wn WAW, Azlan H, Faezahtul AH. Classifying DLBCL according cell of origin using Hans algorithm and its association with clinicopathological parameters: a single centre experience. Med J Malaysia. 2020;75(2):98–102.
8. Shain KH, Dalton WS, Tao J. The tumor microenvironment shapes hallmarks of mature B-cell malignancies. Oncogene. 2015;34(36):4673–4682. doi:10.1038/onc.2014.403
9. Tamma R, Ranieri G, Ingravallo G, et al. Inflammatory cells in diffuse large B cell lymphoma. J Clin Med. 2020;9(8):2418. doi:10.3390/jcm9082418
10. Shen Z, Wang F, He C, et al. The value of prognostic nutritional index (PNI) on newly diagnosed diffuse large B-cell lymphoma patients: a multicenter retrospective study of HHLWG based on propensity score matched analysis. J Inflamm Res. 2021;14:5513–5522. doi:10.2147/JIR.S340822
11. Rodriguez RMDJP, Beltran BE, Vilela L, et al. Prognostic significance of the neutrophil/lymphocyte ratio in diffuse large B-cell lymphoma: a systematic review and meta-analysis. Blood. 2023;142:6303. doi:10.1182/blood-2023-185194
12. Ma S, Zhang B, Lu T, et al. Value of the prognostic nutritional index (PNI) in patients with newly diagnosed, CD5-positive diffuse large B-cell lymphoma: a multicenter retrospective study of the Huaihai Lymphoma Working Group. Cancer. 2022;128(19):3487–3494. doi:10.1002/cncr.34405
13. Wang Z, Zhang J, Luo S, Zhao X. Prognostic significance of systemic immune-inflammation index in patients with diffuse large B-cell lymphoma. Front Oncol. 2021;11:655259. doi:10.3389/fonc.2021.655259
14. Li S, Xia Z, Cao J, et al. Proposed new prognostic model using the systemic immune-inflammation index for primary central nervous system lymphoma: a prospective-retrospective multicohort analysis. Front Immunol. 2022;13:1039862. doi:10.3389/fimmu.2022.1039862
15. Zhao Y, Shi Y, Shen H, et al. The prognostic value of platelet-lymphocyte ratio and neutrophil-lymphocyte ratio in the treatment response and survival of patients with peripheral T-cell lymphoma. Leuk Lymphoma. 2020;61(3):623–630. doi:10.1080/10428194.2019.1700244
16. Wu XB, Hou SL, Liu H. Systemic immune inflammation index, ratio of lymphocytes to monocytes, lactate dehydrogenase and prognosis of diffuse large B-cell lymphoma patients. World J Clin Cases. 2021;9(32):9825. doi:10.12998/wjcc.v9.i32.9825
17. Smith S, Wu PW, Seo JJ, et al. IL-16/miR-125a axis controls neutrophil recruitment in pristane-induced lung inflammation. JCI Insight. 2018;3(15). doi:10.1172/jci.insight.120798
18. Guan X, Fang T, Wang Y, Liu W, Hao M, Qiu L. High level of IL-16 secreted by non-GCB DLBCL facilitates tumor growth though the tumor-promoting and immunosuppressive tumor microenvironment. Blood. 2023;142:6080. doi:10.1182/blood-2023-180840
19. Foureau D, Druhan LJ, Steuerwald NM, et al. Cytokine profiling of ABC-subtype and GCB-subtype diffuse large B cell lymphoma: systemic Nfkb activation and impact on myeloid-derived suppressor cells distribution. Blood. 2015;126(23):2666. doi:10.1182/blood.V126.23.2666.2666
20. He J, Yin H, Xia Y, et al. Prognostic nutritional index, a novel biomarker which predicts worse prognosis in diffuse large B cell lymphoma. Leuk Res. 2021;110:106664. doi:10.1016/j.leukres.2021.106664
21. Liu T, Ye F, Li Y, Liu A. Comparison and exploration of the prognostic value of the advanced lung cancer inflammation index, prognostic nutritional index, and systemic immune-inflammation index in newly diagnosed diffuse large B-cell lymphoma. Ann Palliat Med. 2021;10(9):9650–9659. doi:10.21037/apm-21-2067
22. Kim M, Suh C, Jaejoon KIM, Hong JY. Difference of clinical parameters between GCB and non-GCB subtype DLBCL. Blood. 2017;130:5231.
23. Han B, Kim S, Koh J, et al. Immunophenotypic landscape and prognosis of diffuse large B-cell lymphoma with MYC/BCL2 double expression: an analysis of a prospectively immunoprofiled cohort. Cancers. 2020;12(11):3305. doi:10.3390/cancers12113305
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
The Clinical Value of the Combined Detection of Systemic Immune-Inflammation Index (SII), Systemic Inflammation Response Index (SIRI), and Prognostic Nutritional Index (PNI) in Early Diagnosis of Gastric Cancer
Zheng J, Zheng L, Wang X, Mao X, Wang Q, Yang Y, Mo D
Journal of Inflammation Research 2025, 18:813-826
Published Date: 18 January 2025
Combined Systemic Immune-Inflammation Index-Prognostic Nutritional Index Score in Evaluating the Prognosis of Patients with Severe Community-Acquired Pneumonia
Chen X, Hao L, Zhou Y, Zhang H, Wang H, Yu W
Journal of Inflammation Research 2025, 18:7105-7114
Published Date: 31 May 2025

