Back to Journals » Therapeutics and Clinical Risk Management » Volume 21
Inflammatory Burden Index Associated with Recurrence of Atrial Fibrillation After Radiofrequency Catheter Ablation
Authors Peng S, Li F, Jin M, Zhang Y, Li H, Yin J
Received 21 January 2025
Accepted for publication 12 May 2025
Published 15 May 2025 Volume 2025:21 Pages 681—689
DOI https://doi.org/10.2147/TCRM.S518620
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor De Yun Wang
Siliang Peng, Feng Li, Mengchao Jin, You Zhang, Hui Li, Jiayu Yin
Department of Cardiology, The Second Affiliated Hospital of Soochow University, Suzhou, Jiangsu, 215004, People’s Republic of China
Correspondence: Jiayu Yin, Department of Cardiology, The Second Affiliated Hospital of Soochow University, Suzhou, Jiangsu, 215004, People’s Republic of China, Email [email protected]
Background: Recurrence rates of atrial fibrillation (AF) remain high after radiofrequency catheter ablation (RFCA), and inflammation plays an important role in the process. Inflammatory burden index (IBI) as a new inflammatory marker has been found to be associated with worse prognosis in cardiovascular disease. But there are no studies on its role in predicting AF recurrence. The aim of this study was to assess the value of IBI in predicting recurrence of AF after RFCA.
Methods: This was a single-center retrospective observational study. Consecutive enrolment of PersAF who underwent first-time radiofrequency ablation between January 2021 and June 2024. Inflammatory Burden Index (IBI) was calculated as C-reactive protein (CRP) × neutrophil/lymphocyte (NLR).
Results: A total of 142 (27.2%) patients experienced recurrence after RFCA. Multivariate analysis showed that PersAF (OR = 1.599; 95% CI: 1.028 ~ 2.486, p = 0.018), CHA2DS2-VASc score≥ 2 (OR = 1.769; 95% CI: 1.142 ~ 2.741, p = 0.011), LAD (OR = 1.098; 95% CI: 1.054 ~ 1.145, p < 0.001) and IBI (OR = 1.028; 95% CI: 1.007 ~ 1.050, p = 0.009), were independent predictors of recurrence. ROC analysis shows superiority of IBI (AUC=0.695, 95% CI: 0.647 ~ 0.743, p < 0.001) over CRP and NLR in predicting AF recurrence. When IBI was integrated into the traditional model (including PersAF, LAD and CHA2DS2-VASc Score), the discrimination and reclassification accuracy for the recurrence were significantly improved.
Conclusion: Inflammatory load index associated with the recurrence of AF after RFCA. Integration of IBI can improve the model about the recurrence of AF after RFCA.
Keywords: atrial fibrillation, radiofrequency catheter ablation, recurrence, inflammation, inflammatory burden index
Introduction
The incidence of atrial fibrillation (AF) increases with age and has been shown to cause or aggravate heart failure, stroke, myocardial infarction and vascular dementia.1–4 Although catheter ablation currently has a better success rate in restoring sinus rhythm, 20–30% of patients still experience recurrence after ablation.5,6 How to identify risk factors for recurrence by non-invasive means has become an important clinical issue.
Inflammation and endothelial dysfunction are the strongest predictors of AF and actually promote the development of AF at almost all stages. Coronary artery disease is one of the strongest risk factors for AF, which is closely related to inflammation.7–9 Previous studies have identified induction of atrial remodelling by inflammatory factors and alteration of membrane potential fluctuations as important factors in the development and maintenance of AF.10,11 Current clinical indicators of inflammation, including high sensitivity C-reactive protein (hs-CRP), Lymphocyte, Neutrophil and ST2, have been shown to be associated with AF recurrence after Radiofrequency ablation (RFCA).11,12 As the most widely used inflammatory factor, hs-CRP has been extensively studied and basic research has shown that it can promote the progression of AF through the complement activation pathway.13 However, compared to the use of a single inflammatory factor, composite inflammatory markers calculated from several haematological markers, such as SII, NLR, SIRI, etc., have a better clinical value in predicting recurrence.14,15 It is now being studied extensively because it is more readily available and can provide better information about immune activity.
Inflammatory Burden Index (IBI), calculated from C-reactive protein (CRP) × neutrophil/lymphocyte (NLR), is now widely used in tumour-associated diseases as a novel indicator of inflammation.16,17 A recent study suggests that IBI is associated with adverse cardiovascular outcomes,18 but there are no studies on its role in predicting AF recurrence. The aim of this study was to assess the value of IBI in predicting recurrence of AF after RFCA.
Methods
Study Population
This is a single-centre retrospective observational clinical study. We consecutively enrolled 621 patients who underwent first-time RFCA for AF between January 2021 and June 2024. Exclusion criteria were: 1) History of rheumatic valvular disease, moderate to severe valvular stenosis or dysfunction; 2) Severe hepatic and renal insufficiency, thyroid dysfunction, respiratory disease and history of malignant tumour; 3) Previous catheter ablation for AF; 4) Hematological diseases, malignant tumors, autoimmune diseases, infections, and systemic inflammation; 5) Acute infection during or before hospitalisation. This study complies with the Declaration of Helsinki. The study protocol was reviewed and approved by the Ethics Committee of Second Affiliated Hospital of Soochow University (No. JD-LK2024034-IR01). As this was a retrospective study with no risk to patients, the requirement for written informed consent was waived. A total of 523 patients were included. (Figure 1)
 |
Figure 1 Patients flowchart. Abbreviations: AF, atrial fibrillation; RFCA, radiofrequency catheter ablation. |
According to the 2020 ESC Guidelines for the diagnosis and management of AF, PersAF was defined as AF that is continuously sustained beyond 7 days.19
Data Collection
Demographic information, comorbidities, admission clinical characteristics, laboratory and echocardiographic data were collected through the electronic medical record system. Venous blood was drawn within 24 hours of admission for measurement of biochemical parameters including serum total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), albumin, white blood cells (WBCs), neutrophils, and other biomarkers. Neutrophil-to-lymphocyte ratio (NLR) is calculated as neutrophil count divided by lymphocyte count. Inflammatory Burden Index (IBI) was calculated as C-reactive protein (CRP) × NLR. All patients underwent transthoracic echocardiography within 12 hours of admission to measure left ventricular ejection fraction (LVEF) and left atrial (LA) diameter.
Pulmonary Vein Isolation Procedures
All patients underwent pre-procedure transesophageal echocardiography or computed tomography to exclude left atrial thrombus. After observing the left atrial geometry, a complete electroanatomical model of the left atrium was constructed using intravenous fentanyl under conscious sedation and access to the left atrium through the interatrial septum with an ablation catheter guided by the Ensite Precision calibration system. The right and left pulmonary veins were isolated point-by-point using an TCQ or TCSE cold saline-filled ablation catheter. Radiofrequency (RF) energy was 40–45 watts (saline infusion 17–25mL/min), Lesion Size index(LSI) of 4.5–4.8 for atrial wall, roof and bottom ablation and 3.8–4.0 for posterior wall ablation, and 5–20 g was considered the optimal contact force to deliver RF energy to each site. The distance between each two ablation sites is 3–4 mm. Cardioversion for patients who do not return to sinus rhythm, after which additional ablation was performed at the discretion of the operator.
Data Analysis
Numerical variables are expressed as mean ± standard deviation. Normal distribution of numerical data was tested using the Kolmogorov–Smirnov test. For normally distributed data, two-group comparisons were made using the Student’s t-test; for non-parametric distributions, the Mann-Whitney U-test was used. Categorical variables were expressed as percentages and compared using the chi-squared test or Fisher’s exact test. Univariate logistic regression analysis was used to compare risk factors between patients with and without recurrence. Forward stepwise multivariate logistic regression analyses were used to detect any independent significant predictors (expressed as odds ratio [OR] and 95% confidence interval). Variables that were statistically significant (p < 0.05) or near significant (p < 0.1) in univariate analyses were included in the multivariate logistic regression risk model. Restricted cubic splines (RCS) were used to explore the dose-response relationship between IBI and recurrence of AF. The area under the curve (AUC) was determined using receiver operating characteristic (ROC) curve analysis and calculated for optimal sensitivity and specificity. All statistical analyses were performed using SPSS version 23.0 (IBM, Armonk, NY, USA). p values < 0.05 were considered statistically significant. A power analysis was conducted to confirm the adequacy of the sample size for detecting meaningful differences. All statistical analyses were performed with SPSS version 23.0 (IBM, Armonk, NY, USA).
Results
Baseline Characteristics of the Study Population
From 523 patients who underwent RF ablation, 142 (27.2%) patients experienced recurrence AF. Compared with the non-recurrent group, the recurrent group was on average older, had a larger left atrial diameter (LAD) and more people with persistent AF. In addition, The proportion of patients with CHA2DS2-VASc score≥2 was higher in the recurrence group. In inflammatory factors, CRP, Neutrophil, Lymphocyte, IBI, NLR were different in both groups (p<0.05) (Table 1).
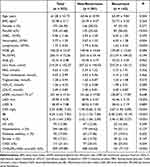 |
Table 1 Baseline Characteristic |
Logistic Regression Analysis for Recurrence of AF
Univariate analysis showed that Age, PersAF, LAD, IBI and CHA2DS2-VASc score≥2 associated with recurrence (p<0.05). Multivariate regression analysis was then performed on all indicators with p-value < 0.05. Multivariate analysis showed that PersAF (OR = 1.599; 95% CI: 1.028 ~ 2.486, p = 0.018), CHA2DS2-VASc score≥2 (OR = 1.769; 95% CI: 1.142 ~ 2.741, p = 0.011), LAD (OR = 1.098; 95% CI: 1.054 ~ 1.145, p < 0.001) and IBI (OR = 1.028; 95% CI: 1.007 ~ 1.050, p = 0.009), were independent predictors of termination (Table 2). RCS results indicated a non-linear dose-response relationship between IBI and recurrence of AF, both before and after adjustment, suggesting that higher IBI may increase the risk of recurrence of AF (Figure 2).
 |
Table 2 Association of Patient Characteristics with Recurrence: Univariate and Multivariate Regression Analysis |
Value of IBI in Predicting Recurrence of AF
The ROC was used to analyze the variables as the critical value for predicting the AF recurrence after RFCA. The cut-off value of IBI was, the sensitivity was 82.4%, and the specificity was 50.7% (AUC=0.695, 95% CI: 0.647 ~ 0.743, p < 0.001). Based on the results of the multivariate regression analysis, a traditional model including PersAF, LAD and CHA2DS2-VASc score was developed. ROC analysis of the traditional model showed the sensitivity was 65.5%, and the specificity was 65.4% (AUC=0.708, 95% CI: 0.660 ~ 0.756, p<0.001). Then, a new model was developed after integrating IBI, ROC analysis showed the the sensitivity was 76.1%, and the specificity was 60.9% (AUC=0.730, 95% CI:0.684–0.777, p<0.001) (Figures 3,4 and Supplementary Table 1).
Comparison Between Different Models
The results of the DeLong test showed that the AUC of IBI for AF recurrence was significantly higher than that of NLR (Z = 2.733, p=0.006) and CRP (Z = 3.612, p<0.001). After integrating IBI, the AUC of the new model for AF recurrence was significantly higher than that of the traditional model (Z = 3.050, p = 0.002) (Supplementary Table 2). Next, NRI and IDI were calculated and compared between the traditional and new models. The NRI was 0.276 (95% CI: 0.092 ~ 0.461), p=0.003, and the IDI was 0.018 (95% CI: 0.005 ~ 0.031), p=0.008. These results indicate that integration of IBI could significantly improve the ability to predict the recurrence of AF after RFCA (Table 3).
 |
Table 3 Incremental Value of IBI for AF Recurrence After RFCA |
Discussion
To our knowledge, this is the first study to investigate IBI association with AF recurrence after RFCA. The main findings of this study were: 1) IBI is an independent factor for AF recurrence after RFCA and has better predictive value than CRP and NLR; 2) Integration of IBI with PersAF, LAD and CHA2DS2-VASc score significantly improves the models about the AF recurrence after RFCA.
The incidence of AF, one of the most common clinical arrhythmias, has increased in recent years.6 Excessively fast and disturbed heart rate can induce and promote the development of heart failure, stroke, vascular dementia and other diseases.4,20,21 RFCA has become the first-line clinical treatment because it can effectively restore and maintain sinus rhythm.19 However, some patients still experience recurrence after ablation.5 Risk factors for AF recurrence include atrial enlargement, obesity, smoking and Obstructive sleep apnea hypopnea syndrome (OSAHS).22–24 Identifying and controlling risk factors plays an important role in the postoperative management of patients with AF.
In recent years, more and more studies have demonstrated the importance of inflammation in cardiovascular diseases.14,25,26 IBI, a novel inflammation indicator, is calculated from CRP/NLR. Recent studies have shown that IBI has a good value in predicting the prognosis of tumour-related diseases and is also important in predicting the prognosis of cardiovascular diseases.14,17,18 In this study, IBI was found to be more effective in predicting the recurrence of atrial fibrillation than CRP and NLR. This suggests that IBI is a better indicator of the inflammatory burden in AF patients. Previous studies have indicated that CRP is independently associated with worse clinical outcomes largely attributable to the excess inflammation.27–29 Current basic research suggests that the release of inflammatory factors and oxidative stress, leading to atrial fibrosis and ion channel alterations, promotes atrial remodelling leading to the development and maintenance of AF.30
Excessive accumulation of adipocytes, especially epicardial fat, leads to a chronic low-grade systemic inflammatory state, which is important for the recurrence of AF.31,32 Existing studies have shown that the main immune cells infiltrating the atrial myocardium in patients with AF are lymphomonocytes.33 Lymphocytes secrete inflammatory mediators such as IL-6, transforming growth factor (TGF)-βand tumour necrosis factor (TNF)-α, which contribute to atrial remodelling and are involved in the development and maintenance of AF.33,34 Complex inflammatory factors obtained from a simple complete blood count have been shown to be more predictive of AF recurrence than single inflammatory factors.25 Gibson et al demonstrated that elevated NLR, both pre- and post-operatively, is associated with the development of post-operative AF.35 In addition, a number of chronic inflammatory diseases, such as inflammatory bowel disease, autoimmune diseases and chronic obstructive pulmonary disease, have been shown to increase the risk of AF.36–38 Therefore, IBI, as a non-invasive assessment tool that can reflect the systemic inflammatory burden, is important in the postoperative management of patients with AF.
Consistent with previous studies, we also found that persistent AF, enlarged atria and higher CHA2DS2-VASc score were also independent risk factors for recurrence of AF. The integration of IBI significantly improved the model of AF recurrence after RFCA. Therefore, our study provides important information for AF recurrence risk stratification. Our results suggest that IBI may be another valid reference for these patients. Patients with a high IBI may benefit from receiving enhanced follow-up or anti-inflammatory intervention measures.
Limitation
This study has several limitations that need to be highlighted. First, this is a single-centre, retrospective study and the sample size is small. There may be some potential confounding factors in this study, such as medication use, lifestyle factors, or genetic predispositions. A multicenter and a prospective study design would be needed to strengthen generalizability and reduce potential biases. Secondly, loss to follow-up and exclusion of asymptomatic recurrences due to the lack of continuous rhythm monitoring again comes as a limitation. Perhaps an implantable loop recorders or extended Holter monitoring would have allowed to capture all recurrences.
Conclusion
Inflammatory load index associated with the recurrence of AF after RFCA. Integration of IBI can improve the model about the recurrence of AF after RFCA.
Data Sharing Statement
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants but are available from the correspondence upon reasonable request.
Statement of Ethics
This study complies with the Declaration of Helsinki. The study protocol was reviewed and approved by the Ethics Committee of Second Affiliated Hospital of Soochow University (No. JD-LK2024034-IR01). As this was a retrospective study with no risk to patients, the requirement for written informed consent was waived.
Funding
This work was financially supported by the National Natural Science Foundation of China (82170831) and Discipline Construction Support Project of the Second Affiliated Hospital of Soochow University (XKTJ-RC202403).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Oltman CG, Kim TP, Lee JWY, et al. Prevalence, management, and comorbidities of adults with atrial fibrillation in the United States, 2019 to 2023. JACC Adv. 2024;3(11):101330. doi:10.1016/j.jacadv.2024.101330
2. Teppo K, Airaksinen KEJ, Halminen O, et al. Temporal trends of ischemic stroke risk in patients with incident atrial fibrillation before anticoagulation. JACC Clin Electrophysiol. 2024;11(3):583–592. doi:10.1016/j.jacep.2024.10.029
3. McManus DD, Hsu G, Sung SH, et al. Atrial fibrillation and outcomes in heart failure with preserved versus reduced left ventricular ejection fraction. J Am Heart Assoc. 2013;2(1):e005694. doi:10.1161/JAHA.112.005694
4. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circul. 2019;139(10):e56–e528. doi:10.1161/CIR.0000000000000659
5. Poole JE, Bahnson TD, Monahan KH, et al. Recurrence of atrial fibrillation after catheter ablation or antiarrhythmic drug therapy in the CABANA trial. J Am Coll Cardiol. 2020;75(25):3105–3118. doi:10.1016/j.jacc.2020.04.065
6. Virk SA, Chieng D, Segan L, et al. Incidence, characteristics and prognostic significance of early recurrences following different ablation approaches for persistent atrial fibrillation. Heart Rhythm. 2024. doi:10.1016/j.hrthm.2024.12.031
7. Batta A, Hatwal J, Sharma YP. Assessment of coronary artery disease in non-valvular atrial fibrillation: is this light at the end of the tunnel? Vasc Health Risk Manag. 2024;20:493–499.
8. Mekhael M, Marrouche N, El Hajjar AH, et al. The relationship between atrial fibrillation and coronary artery disease: understanding common denominators. Trends Cardiovasc Med. 2024;34(2):91–98.
9. Batta A, Hatwal J, Batta A, et al. Atrial fibrillation and coronary artery disease: an integrative review focusing on therapeutic implications of this relationship. World J Cardiol. 2023;15(5):229–243. doi:10.4330/wjc.v15.i5.229
10. Chen Y, Zhou B, Peng C, et al. Prognostic implications of system inflammation response index in atrial fibrillation patients with type 2 diabetes mellitus. Sci Rep. 2025;15(1):1025. doi:10.1038/s41598-024-84666-9
11. Shitole SG, Heckbert SR, Marcus GM, et al. Assessment of inflammatory biomarkers and incident atrial fibrillation in older adults. J Am Heart Assoc. 2024;13(24):e035710. doi:10.1161/JAHA.124.035710
12. Chen L, Chen W, Shao Y, et al. Association of soluble suppression of tumorigenicity 2 with new-onset atrial fibrillation in acute myocardial infarction. Cardiol. 2022;147(4):381–388. doi:10.1159/000524765
13. Engelmann MDM, Svendsen JH. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur Heart J. 2005;26(20):2083–2092. doi:10.1093/eurheartj/ehi350
14. Wang F, Sun Y, Lu Y, et al. Predictive value of platelet-to-albumin ratio combined with the C (2) HEST score for new-onset atrial fibrillation in elderly patients with acute ST-segment elevation myocardial infarction. BMC Cardiovasc Disord. 2024;24(1):521. doi:10.1186/s12872-024-04200-7
15. Hu J, Huang L, Zhao X, et al. Strong positive correlations between the levels of systemic inflammation markers and the occurrence of persistent atrial fibrillation. Int Heart J. 2024;65(6):1004–1011. doi:10.1536/ihj.23-665
16. Aoyama T, Maezawa Y, Hashimoto I, et al. Inflammatory burden index is an independent prognostic factor for esophageal cancer patients who receive curative treatment. Vivo. 2024;38(6):2928–2934. doi:10.21873/invivo.13775
17. Zhai J, Yuan B, Liu T, et al. Association between the inflammatory burden index and rheumatoid arthritis and its all-cause mortality: data from NHANES 1999-2018. Front Med. 2024;11:1421497. doi:10.3389/fmed.2024.1421497
18. Wen ZG, Long JJ, Wang Y. Association between inflammatory burden index and coronary slow flow phenomenon in patients with chest pain and no obstructive coronary arteries. BMC Cardiovasc Disord. 2024;24(1):595. doi:10.1186/s12872-024-04281-4
19. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi:10.1093/eurheartj/ehaa612
20. Rivard L, Friberg L, Conen D, et al. Atrial fibrillation and dementia: a report from the AF-SCREEN international collaboration. Circul. 2022;145(5):392–409. doi:10.1161/CIRCULATIONAHA.121.055018
21. Parkash R, Wells G, Rouleau J, et al. A randomized ablation-based atrial fibrillation rhythm control versus rate control trial in patients with heart failure and high burden atrial fibrillation: the RAFT-AF trial rationale and design. Am Heart J. 2021;234:90–100. doi:10.1016/j.ahj.2021.01.012
22. Gaita F, Scaglione M, Battaglia A, et al. Very long-term outcome following transcatheter ablation of atrial fibrillation. are results maintained after 10 years of follow up? Europace. 2018;20(3):443–450. doi:10.1093/europace/eux008
23. Patel SV, Gill H, Shahi D, et al. High risk for obstructive sleep apnea hypopnea syndrome predicts new onset atrial fibrillation after cardiac surgery: a retrospective analysis. Sleep Breath. 2018;22(4):1117–1124. doi:10.1007/s11325-018-1645-3
24. Vermeer JR, van den Broek J, Dekker LRC. Impact of lifestyle risk factors on atrial fibrillation: mechanisms and prevention approaches - A narrative review. Int J Cardiol Cardiovasc Risk Prev. 2024;23:200344. doi:10.1016/j.ijcrp.2024.200344
25. Peng L, Liu L, Chai M, et al. Predictive value of neutrophil to lymphocyte ratio for clinical outcome in patients with atrial fibrillation: a systematic review and meta-analysis. Front Cardiovasc Med. 2024;11:1461923. doi:10.3389/fcvm.2024.1461923
26. Naser A, Sayilan S, Güven O, et al. Inflammation burden and atrial fibrillation burden: a bidirectional relationship. Arq Bras Cardiol. 2024;121(6):e20230680. doi:10.36660/abc.20230680
27. Batta A, Hatwal J, Panda P, et al. Impact of initial high sensitivity C-reactive protein on outcomes in nonvalvular atrial fibrillation: an observational study. Future Cardiol. 2024;20(5–6):295–303. doi:10.1080/14796678.2024.2354110
28. Zhang S, Xu W, Xu J, et al. Association of C-reactive protein level with adverse outcomes in patients with atrial fibrillation: a meta-analysis. Am J Med Sci. 2024;367(1):41–48. doi:10.1016/j.amjms.2023.11.009
29. Meyre PB, Sticherling C, Spies F, et al. C-reactive protein for prediction of atrial fibrillation recurrence after catheter ablation. BMC Cardiovasc Disord. 2020;20(1):427. doi:10.1186/s12872-020-01711-x
30. Nso N, Bookani KR, Metzl M, et al. Role of inflammation in atrial fibrillation: a comprehensive review of current knowledge. J Arrhythmia. 2020;37(1):1–10. doi:10.1002/joa3.12473
31. Wong CX, Ganesan AN, Selvanayagam JB. Epicardial fat and atrial fibrillation: current evidence, potential mechanisms, clinical implications, and future directions. Eur Heart J. 2017;38(17):1294–1302. doi:10.1093/eurheartj/ehw045
32. Iacobellis G. Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol. 2022;19(9):593–606.
33. Conte M, Petraglia L, Cabaro S, et al. Epicardial adipose tissue and cardiac arrhythmias: focus on atrial fibrillation. Front Cardiovasc Med. 2022;9:932262.
34. Vyas V, Hunter RJ, Longhi MP, et al. Inflammation and adiposity: new frontiers in atrial fibrillation. Europace. 2020;22(11):1609–1618. doi:10.1093/europace/euaa214
35. Gibson PH, Cuthbertson BH, Croal BL, et al. Usefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2010;105(2):186–191.
36. Kotlyarov S, Lyubavin A. Early detection of atrial fibrillation in chronic obstructive pulmonary disease patients. Medicina. 2024;60(3):352.
37. Rujirachun P, Wattanachayakul P, Taveeamornrat S, et al. Atrial fibrillation recurrence risk after catheter ablation in patients with rheumatoid arthritis: a systematic review and meta-analysis. Clin Cardiol. 2025;48(1):e70021. doi:10.1002/clc.70021
38. Goyal A, Jain H, Maheshwari S, et al. Association between inflammatory bowel disease and atrial fibrillation: a systematic review and meta-analysis. Int J Cardiol Heart Vasc. 2024;53:101456.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Development and Validation of a Risk Nomogram Model for Predicting Recurrence in Patients with Atrial Fibrillation After Radiofrequency Catheter Ablation
Zhao Z, Zhang F, Ma R, Bo L, Zhang Z, Zhang C, Wang Z, Li C, Yang Y
Clinical Interventions in Aging 2022, 17:1405-1421
Published Date: 25 September 2022
Platelet-to-Lymphocyte Ratio Improves the Predictive Ability of the Risk Score for Atrial Fibrillation Recurrence After Radiofrequency Ablation
Huang W, Sun H, Tang Y, Luo Y, Liu H
Journal of Inflammation Research 2023, 16:6023-6038
Published Date: 11 December 2023
Predictive Value of Serum microRNA-29b-3p in Recurrence of Atrial Fibrillation After Radiofrequency Catheter Ablation
Zhan J, Peng C, Liu Y, Bi Z, Lu G, Hao S, Tong Y, Zhang G
Clinical Interventions in Aging 2024, 19:715-725
Published Date: 3 May 2024
Neutrophil-to-Lymphocyte Ratio, Lymphocyte-to-Monocyte Ratio and Platelet-to-Lymphocyte Ratio as Predictors of Short- and Long-Term Outcomes in Ischemic Stroke Patients with Atrial Fibrillation
Guo J, Wang D, Jia J, Zhang J, Liu Y, Lu J, Zhao X, Yan J
Journal of Inflammation Research 2024, 17:6661-6672
Published Date: 23 September 2024
New Insights into the Role of Inflammatory Pathways and Immune Cell Infiltration in Sleep Deprivation-Induced Atrial Fibrillation: An Integrated Bioinformatics and Experimental Study
Liang J, Tang B, Shen J, Rejiepu M, Guo Y, Wang X, Shao S, Guo F, Wang Q, Zhang L
Journal of Inflammation Research 2025, 18:791-812
Published Date: 18 January 2025




