Back to Journals » Cancer Management and Research » Volume 17
Integrated Traditional Chinese and Western Medicine in Lung Adenocarcinoma with Rare EGFR Mutation and Nephrotic Syndrome: A Case Report
Authors Zhang L , Cai L, Ruan L, Li J
Received 14 March 2025
Accepted for publication 24 June 2025
Published 2 July 2025 Volume 2025:17 Pages 1293—1299
DOI https://doi.org/10.2147/CMAR.S526571
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Lirong Zhang,1 Liye Cai,1 Luwei Ruan,1 Jie Li2
1Department of Oncology, Affiliated Sanming Integrated Medicine Hospital of Fujian University of Traditional Chinese Medicine, Sanming, Fujian, 365001, People’s Republic of China; 2Department of Oncology, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, 100053, People’s Republic of China
Correspondence: Lirong Zhang, Department of Oncology, Affiliated Sanming Integrated Medicine Hospital of Fujian University of Traditional Chinese Medicine, Sanming, Fujian, 365000, People’s Republic of China, Email [email protected]
Abstract: This case report presents a 67-year-old male diagnosed with stage IV lung adenocarcinoma harboring rare EGFR exon 18/20 mutations (Gly719Cys/Ser768Ile) and a concurrent TP53 mutation, complicated by nephrotic syndrome. The scarcity of approved EGFR-TKIs targeting rare EGFR mutations in NSCLC, coupled with nephrotic syndrome-induced renal impairment, hypoalbuminemia, and massive pleural effusion refractory to conventional management, prompted the development of a personalized multimodal approach.A multimodal therapeutic regimen incorporating albumin-bound paclitaxel, intrathoracic perfusion of Endostar (recombinant human endostatin), and traditional Chinese medicine (TCM) was implemented, achieving effective disease control. Notably, the treatment resulted in significant tumor shrinkage (reduction rate: 48.1% by RECIST 1.1), complete resolution of malignant pleural effusion, and marked improvement in nephrotic syndrome parameters. The synergistic effects of targeted chemotherapy, anti-angiogenic therapy, and TCM-based symptom modulation highlight the potential of integrative approaches in managing advanced malignancies with complex molecular profiles and multisystem complications.
Keywords: nephrotic syndrome, lung adenocarcinoma, albumin-bound paclitaxel, endostar, EGFR mutation, malignant pleural effusion
Introduction
Lung adenocarcinoma, the most common histological subtype of lung cancer, exhibits close correlation with epidermal growth factor receptor (EGFR) gene mutations in its pathogenesis.1 Although targeted therapies have achieved breakthrough advancements in the treatment of EGFR-mutated lung adenocarcinoma, patients harboring rare mutations (eg, exon 18/20 mutations) or complicated by severe comorbidities (such as nephrotic syndrome) still face significant therapeutic challenges. Rare EGFR mutations collectively constitute approximately 15% of all EGFR mutations in NSCLC, among rare EGFR mutations in NSCLC, G719X substitutions (including G719S, G719A, G719C, and G719D variants) account for approximately 1.5–3% of all EGFR mutations in NSCLC.2 The co-occurrence of these mutations with other atypical EGFR variants (eg, S768I) and TP53 frameshift mutations further complicates therapeutic decision-making. Moreover, nephrotic syndrome significantly impacts treatment tolerance due to hypoalbuminemia-induced drug toxicity and renal insufficiency. Although afatinib remains the sole EGFR-TKI approved for advanced NSCLC with rare EGFR mutations, real-world evidence regarding its efficacy in cases with concurrent TP53 mutations remains sparse, particularly concerning resistance mechanisms. This study presents a clinically significant case of pulmonary adenocarcinoma with atypical EGFR mutations (Gly719Cys/Ser768Ile) and TP53 frameshift mutation complicated by nephrotic syndrome. The case demonstrated primary resistance to conventional tyrosine kinase inhibitors (TKIs) along with complex clinical manifestations including malignant pleural effusion, renal insufficiency, and hypoalbuminemia. Notably, an innovative therapeutic regimen combining albumin-bound paclitaxel, recombinant human endostatin (Endostar), prednisone, and traditional Chinese medicine (TCM) achieved remarkable clinical efficacy. This case provides novel insights and a theoretical foundation for the clinical management of similar complex cases, particularly those involving EGFR atypical mutations with concurrent systemic complications.
Case Presentation
A 67-year-old patient presented with recurrent dyspnea over 2 years, exacerbated in the past 2 months. Initial evaluation at the Department of Respiratory and Critical Care Medicine on November 4, 2024, revealed the following CT findings: a space-occupying lesion in the right hilar region suggestive of malignancy, accompanied by mediastinal lymphadenopathy; bilateral pulmonary inflammatory changes with suspected metastatic lesions; and massive right-sided pleural effusion with minimal left-sided effusion. Thoracentesis was performed to drain the substantial pleural fluid. Cytopathological analysis using cell block preparation techniques was conducted on the aspirated effusion. Hematoxylin-eosin (H&E) staining and immunohistochemical (IHC) analysis of the cell block specimens confirmed the presence of lung adenocarcinoma cells (Figures 1–3). Subsequent next-generation sequencing (NGS) of the pleural effusion on November 21, 2024, identified an EGFR exon 18 missense mutation (Ser78Ile), an EGFR exon 20 missense mutation (Gly719Cys), and a TP53 frameshift mutation (Gly105fs), with variant allele frequencies of 43.47%, 44.38%, and 22.35%, respectively (Table 1). The patient initially received antimicrobial therapy for pneumonia, achieving symptomatic relief before discharge. However, due to persistent dyspnea and recurrent pleural effusion, the patient was readmitted on November 28, 2024, for further management.
 |
Table 1 Results of Genetic Testing |
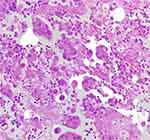 |
Figure 1 Tumor cells in the cell block of pleural effusion. |
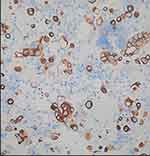 |
Figure 2 Demonstrates positive cytokeratin 7 (CK7) immunoreactivity in tumor cells. |
 |
Figure 3 Tumor cells were TTF-1 positive. |
Treatment
A patient presenting with elevated serum creatinine, increased urinary protein, and progressive hypoalbuminemia initiated comprehensive treatment on November 30, 2024. Following nephrology consultation, immunosuppressive therapy with prednisone (60 mg/day, 1 mg/kg, orally once daily) was administered. Thoracentesis was performed for symptomatic management of pleural effusion. Systemic chemotherapy was subsequently initiated on December 17, 2024, utilizing an albumin-bound paclitaxel regimen (100 mg/m² administered intravenously on days 1, 8, and 15 of a 21-day cycle). Concurrently, intrapleural perfusion therapy with Endostar (recombinant human endostatin, 45 mg administered on days 1 and 4) was implemented for malignant pleural effusion control. Adjunctive traditional Chinese herbal medication was incorporated into the therapeutic regimen to mitigate chemotherapy-associated adverse effects and enhance systemic homeostasis.
Outcomes
Following two treatment cycles, follow-up CT imaging revealed significant reductions in tumor volume and pleural effusion. Concurrent decreases in serum tumor marker levels were observed alongside patient-reported subjective improvement in respiratory function. Notably, nephrotic syndrome-related manifestations, including peripheral edema and proteinuria, demonstrated marked alleviation, suggesting improved renal function. The multimodal therapeutic regimen exhibited favorable tolerability with no severe adverse events documented during treatment (Table 2).
 |
Table 2 Laboratory Findings |
The patient presenting with elevated serum creatinine, increased urinary protein excretion, and progressive hypoalbuminemia initiated prednisone therapy on November 30, 2024. Thoracentesis was performed for therapeutic drainage of symptomatic pleural effusion. Systemic chemotherapy commenced on December 17, 2024, achieving tumor reduction with subsequent radiographic evaluation confirming partial response (PR) per RECIST criteria (Figure 4).
 |
Figure 4 The patient’s right hilar mass decreased from 6.6×5.2cm to 3.8×2.3cm after 2 cycles of treatment. PR was evaluated for efficacy. |
Discussion
This case represents a rare clinical scenario involving a patient with concurrent EGFR atypical mutations (Gly719Cys/Ser768Ile), nephrotic syndrome (NS), and massive malignant pleural effusion. The management of such complex presentations necessitates multidisciplinary collaboration to elucidate disease mechanisms and alleviate symptoms.
A critical diagnostic advancement in this case was the application of cell block technology to pleural effusion specimens. Through centrifugation-induced cellular concentration, formalin fixation, paraffin embedding, and sectioning, this technique enables histomorphological evaluation comparable to conventional tissue biopsies. The preserved tumor cellularity in cell blocks significantly enhances diagnostic accuracy for serous effusions, facilitating subsequent immunohistochemical and molecular analyses—a methodological approach strongly endorsed by the International Academy of Cytology guidelines.
Emerging evidence suggests paraneoplastic associations between NS and malignancies, where tumor-directed therapy may induce renal remission.3 Documented cases include:1.NS secondary to membranous nephropathy (MN) in non-small cell lung cancer (NSCLC), resolved after iodine-125 brachytherapy without immunosuppressants.4 2.EGFR-positive adenocarcinoma with MN-associated NS achieving complete remission following erlotinib therapy.5 The detection of compound EGFR mutations (Gly719Cys/Ser768Ile) presents therapeutic challenges, as these variants demonstrate reduced sensitivity to first-generation tyrosine kinase inhibitors (TKIs).6 Pooled analysis from LUX-Lung trials (2/3/6) revealed afatinib’s clinical activity in rare EGFR mutations (G719X, S768I, L861Q), with an objective response rate (ORR) of 77.8% and median progression-free survival (PFS) of 13.8 months across 14 patients.7 These findings underpinned the FDA’s 2018 expansion of afatinib indications to include G719X-mutant NSCLC. Emerging evidence suggests that EGFR S768I mutations co-occurring with TP53 aberrations demonstrate heightened propensity for acquired resistance mechanisms and inferior prognostic outcomes.1
Given the patient’s renal impairment (serum creatinine elevation), the therapeutic strategy prioritized nephroprotective agents: Albumin-bound paclitaxel: Hepatically metabolized taxane with favorable renal safety profiles. Endostar: Recombinant human endostatin for angiogenesis inhibition in malignant effusions. Traditional Chinese Medicine (TCM): A formulated decoction containing: Astragalus membranaceus (Huangqi): Immunomodulatory and anti-proteinuric effects via TGF-β1 suppression. Ligustrum lucidum (Nüzhenzi) and Eclipta prostrata (Mohanlian): Antioxidant nephroprotection. Poria cocos (Fuling) and Alisma plantago-aquatica (Zexie): Diuretic modulation of aquaporin-2. Dictamnus dasycarpus (Baixianpi) and Cryptotympana pustulata (Chanyi): Anti-inflammatory cytokine regulation.
Mechanistic studies suggest TCM may ameliorate NS through gut microbiota modulation, endotoxin reduction, and intestinal barrier stabilization.8 Post two-cycle treatment, radiographic and biochemical improvements (effusion reduction, proteinuria decline, creatinine normalization) validated this multimodal approach.
Notably, despite detectable EGFR/TP53 co-mutations, targeted therapy was deferred due to compromised renal clearance capacity. The observed clinical synergy between chemotherapy, anti-angiogenesis agents, and TCM underscores the imperative for personalized therapeutic integration in genomically complex malignancies.
This study has limitations that require acknowledgment. The single-case design inherently limits generalizability and prevents definitive causal attribution. The absence of long-term follow-up precludes evaluation of outcome durability or delayed adverse events. Chinese medicine interventions introduce potential confounding due to herbal formulation variability and personalized treatment protocols, particularly given the lack of standardized biomarkers for traditional diagnostic classifications. These limitations emphasize the necessity for prospective multicenter studies incorporating extended follow-up periods, quantitative outcome metrics, and rigorous control of therapeutic variables to validate these observations.
Conclusion
This case report demonstrates the successful management of a lung adenocarcinoma patient with rare EGFR mutations (Gly719Cys/Ser768Ile) complicated by nephrotic syndrome and massive pleural effusion through a multimodal therapeutic regimen combining albumin-bound paclitaxel, Endostar, and traditional Chinese medicine. The integrated treatment approach achieved remarkable tumor regression, substantial reduction of pleural effusion, and clinical improvement in nephrotic syndrome-related manifestations, thereby highlighting the critical role of comprehensive treatment strategies in managing complex oncological cases. Further investigations are warranted to elucidate the potential synergistic mechanisms among these therapeutic modalities in similar clinical scenarios.
Ethical Statement
Institutional approval for publishing this case was obtained from Sanming Integrated Medicine Hospital, Fujian Province. Written informed consent was obtained from the patient for publication of this case report and any accompanying clinical details, imaging data, and laboratory results. All personally identifiable information has been anonymized to protect patient privacy.
Acknowledgments
We extend our sincere gratitude to the patient and their family for their trust and cooperation throughout this treatment process. We acknowledge the dedicated support from the following departments at Sanming Integrated Medicine Hospital, Fujian Province: Department of Oncology for therapeutic strategy implementation; Department of Pathology for molecular diagnostics and mutation analysis; Department of Nephrology for collaborative management of nephrotic syndrome. This work was conducted with the collective effort of multidisciplinary teams to ensure comprehensive patient care.
Funding
This work was supported by the Natural Science Foundation of Fujian Province (Grant No. 2024J011507).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhu H, Tang H, Peng H, Ding W. Concurrent TP53 mutations facilitate resistance evolution in EGFR exon 20 S768I mutant lung adenocarcinoma: a case report and review of the literature. Case Rep Oncol. 2025;18(1):220–230. doi:10.1159/000543453
2. Harrison PT, Vyse S, Huang PH. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin Cancer Biol. 2020;61:167–179. doi:10.1016/j.semcancer.2019.09.015
3. Gu D, Liao W, Su Q, Gu J, Chen Y. Primary lung cancer firstly presents as nephrotic syndrome: one case report and literature review. Transl Cancer Res. 2019;8(8):2933–2935. doi:10.21037/tcr.2019.11.18
4. Yu X, Fan Z, Chen W, Wang Z. Lung cancer with nephrotic syndrome as a paraneoplastic syndrome: a case report. Mol Clin Oncol. 2020;13(6):86. doi:10.3892/mco.2020.2156
5. Liu X, Bai Y, Zhou X, Gu X, Zhao L. Complete remission of membranous nephropathy in a patient with lung adenocarcinoma treated with erlotinib. J Clin Pharm Ther. 2020;45(2):388–393. PMID: 31730733. doi:10.1111/jcpt.13078
6. Sun Y, Ma L, Zhang X, Wang Z. Advances in the treatment of rare mutations in non-small cell lung cancer. Onco Targets Ther. 2024;17:1095–1115. doi:10.2147/OTT.S487870
7. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of Afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830–838. doi:10.1016/S1470-2045(15)00026-1
8. Li J, Xu Y, Sun T, et al. Exploration of the pathogenesis of nephrotic syndrome and traditional Chinese medicine intervention based on gut microbiota. Front Immunol. 2024;15:1430356. doi:10.3389/fimmu.2024.1430356
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

