Back to Journals » Journal of Pain Research » Volume 18
Interlaminar Endoscopic Treatment of Adjacent Segment Disease After Posterior Instrumented Lumbar Fusion
Authors Liang M, Shao X , Zhu R, Li K , Shi L, Wang Y
Received 30 September 2024
Accepted for publication 10 February 2025
Published 27 February 2025 Volume 2025:18 Pages 1011—1019
DOI https://doi.org/10.2147/JPR.S498800
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Alaa Abd-Elsayed
Min Liang,1 Xinyang Shao,1 RenHan Zhu,1 Kun Li,1 Liangchen Shi,2 Yansong Wang1
1Department of Spinal Surgery, The First Affiliated Hospital of Harbin Medical University, Harbin, People’s Republic of China; 2Orthopedics Department, People’s Hospital of Fengxian County, Xuzhou, People’s Republic of China
Correspondence: Liangchen Shi, Email [email protected]; Yansong Wang, Email [email protected]
Purpose: This study aimed to investigate the feasibility of interlaminar endoscopic surgery for the treatment of adjacent segment disease (ASD) after posterior instrumented lumbar fusion.
Materials and Methods: Between January 2019 and March 2023, the data of 22 patients with ASD who underwent revision interlaminar technique (R-ILT) endoscopic surgery after posterior instrumented lumbar fusion were retrospectively analyzed. For comparison, the data of 30 patients with single segment lumbar spinal stenosis who underwent primary interlaminar technique (P-ILT) endoscopic surgery were collected. The patient demographics and perioperative indicators were recorded and the clinical outcomes were analyzed with relevant evaluation scales. The surgical satisfaction was assessed using the modified MacNab criteria, lumbar stability was evaluated using the change in dynamic position radiographs of the spine at the final follow-up.
Results: There were no statistical differences in patient demographics. The operation time, blood loss, fluoroscopy time, and the incidence of dural sac tears were higher in R-ILT group (p < 0.05). Both groups had significant relief in their lower back and leg pain symptoms, but the relief of the low back pain in R-ILT group was not as good as that in P-ILT group. Regarding recovery of lower limb function, the results of both groups were similar, according to the modified MacNab criteria, the good-to-excellent rate was 81.82% in R-ILT group and 86.66% in P-ILT group. The change in dynamic position X-ray of the spine proved that ILT would not destroy the stability of the spine.
Conclusion: Interlaminar endoscopic surgery is a feasible option for treating ASD. However, due to the impact of the initial operation, the difficulty and risk of reoperation have increased; therefore, surgical indications must be strictly controlled, and superb surgical skills are required.
Keywords: adjacent segment disease, interlaminar technique, lumbar spinal stenosis, primary surgery, revision surgery
Introduction
Posterior instrumented lumbar fusion surgery is widely used to treat degenerative lumbar diseases, as it provides sufficient decompression and immediate segmental stabilization. However, it also increases the biomechanical loading and abnormal motion of the adjacent segments, which may contribute to the occurrence of adjacent segment disease (ASD).1,2 ASD refers to symptomatic adjacent segment degeneration following spinal fusion,3 and its progression can lead to corresponding symptoms such as low back pain, unilateral or bilateral lower limb radiation pain, and intermittent claudication, ultimately affecting the surgical outcome. According to different reports, the incidence of ASD after lumbar spine surgery ranges from 5.2% to 31%.4,5
When treating ASD, if the symptoms are not serious, conservative treatment can be performed, whereas surgical treatment is required when obvious spinal cord compression and nerve root symptoms occur. Surgical treatments aim to relieve spinal canal stenosis, restore vertebral stability, and improve clinical symptoms.6,7 Traditionally, posterior revision decompression and extension of fusion surgery have been the main surgical methods.6 However, considering the economic pressure and significant trauma, it is difficult for most people to accept open surgery again, especially for the elderly and patients with chronic diseases.
In the past few years, spinal endoscopy has developed rapidly, and many degenerative diseases of the lumbar spine can be performed under endoscopy, even including spinal instability and the management of tumors and infections,8 which can offer the potential benefit of symptom alleviation without incurring the risks and complications of open surgery;9–11 thus, spinal endoscopy may play a role in the treatment of ASD.
As one of the main techniques used for spinal endoscopy, the interlaminar technique (ILT) has been widely used, and related studies have demonstrated its safety and effectiveness in the treatment of spinal disorders.12–14 In this study, we retrospectively analyzed the application of ILT in treating ASD, which is associated with lumbar spinal stenosis (LSS), and compare it with primary ILT surgery, with the aim of providing a reference for clinical practice.
Materials and Methods
Patients
Between January 2019 and March 2023, the data of 22 consecutive patients with LSS secondary to posterior instrumented lumbar fusions who underwent revision ILT were collected and compared with the data of 30 patients with single-segment LSS who underwent primary ILT surgery, patients in both groups underwent single-level unilateral endoscopic decompression. Based on the different treatment processes, patients were divided into primary ILT (P-ILT) and revision ILT (R-ILT) groups.
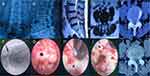
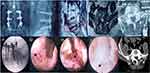
The inclusion criteria were: 1. in the R-ILT group, there should be a minimum 12-month pain-free period after lumbar fusion surgery; 2. neurogenic claudication and/or radicular pain and failure of conservative treatment for at least 3 months; 3. in both groups, LSS was mainly caused by facet joint hyperplasia, ligamentum flavum and disc herniation, and it was confirmed by imaging findings (Figures 1A–E and 2A–E), which corresponded to the patient’s symptoms; in the R-ILT group, the lesion segment was adjacent to the previous fusion surgery location; and 4. age 60 years or older. 5. the minimum follow-up time in this study was 24 months.
The exclusion criteria were: 1. segmental instability or spondylolisthesis on dynamic radiographs; and 2. low back pain being the main symptom; 3. accompanied by other pathological spinal conditions, including trauma, tumor, or infection; and 4. lost to follow-up.
Surgical Procedure
Patients in both groups underwent single-level unilateral endoscopic decompression, in the ILT endoscopic approach, the patient was placed in the prone position. A G-arm X-ray machine was used to confirm the responsible segment (Figure 1F and 2F), and the entry point was approximately 2 cm from the midline. After injecting local anesthesia, a transverse incision, approximately 15 mm long, was made, the working channel was placed, and a 10 mm large channel endoscope (Spinendos GmbH, München, Germany) was inserted. The interlaminar space was enlarged by removing part of the surrounding tissues, and the thickened ligamentum flavum and hyperplastic facet joint were carefully resected (Figure 1G, H and 2G, H). To maintain spinal stability, the resection range did not exceed the outer edge of the nerve root and the attachment point of the ligamentum flavum; after the epidural space was exposed (Figure 1I and 2I), the working channel was rotated, the nerve root was pushed gently away from the channel, and the herniated discs were removed.
Outcome Assessment
The clinical efficacy was analyzed with relevant evaluation scales, the visual analog scale (VAS)15 was used to assess low back and leg pain, Oswestry Disability Index (ODI)16 and Japanese Orthopedic Association (JOA) scale17 were used to evaluate the recovery of lower limb function, and the results were recorded preoperatively and at 1 week, 6 months, 12 months, and 24 months postoperatively. Surgical satisfaction was assessed using the modified MacNab criteria18 at the final follow-up. Lumbar segmental stability was evaluated using dynamic position radiography of the spine at the final follow-up, and we selected a commonly used definition: a change in intervertebral angulation greater than 10° or vertebral translation greater than 4 mm on the dynamic position radiograph of the spine was considered segmental instability (Figure 3A–D).19,20
 |
Figure 3 The changes in angulation (A and B) and translation distance (C and D) on the lateral flexion and extension were used to reflect the spine’s stability. |
Statistical Analysis
Another researcher who was blinded to the group allocation independently collected and assessed the data. The categorical variables were compared using the chi-square test and independent continuous variables were compared using the Student’s t-test; p < 0.05 was considered significant. Statistical analyses were performed using the IBM SPSS software (version 23.0; IBM Corp).
Results
Preoperative Demographic Characteristics
As shown in Table 1, a total of 52 patients were included. There were 22 in R-ILT group, (14 males, 8 females), and 30 in P-ILT group (14 males, 16 females). The mean age was 73.59 ± 5.84 years in R-ILT group and 72.37 ± 5.56 years in P-ILT group; the average symptom duration was 8.09 ± 3.18 months in R-ILT group and 9.30 ± 3.37 months in P-ILT group. We found no statistical differences in age, sex, disease duration, or co-morbidities between the two groups (p>0.05). In R-ILT group, the interval time between the reoperation and the primary operation was 26.27 ± 8.88 months, 18 cases had lesions on the cephalic side and 4 cases on the caudal side of the fused segment. The distribution of lesion segments of the two groups showed no statistically significant differences (p>0.05) (Table 1).
 |
Table 1 Demographics of Study Patients |
Perioperative Indicators and Complications
As shown in Table 2, the average operation time in R-ILT group and P-ILT group was 99.36 ± 10.77 and 91.63 ± 8.16 minutes, respectively, with a P value = 0.005, the average blood loss in R-ILT group and P-ILT group was 27.59 ± 7.08 and 21.57 ± 5.49 mL, respectively, with a P value = 0.002, and the average fluoroscopy time in R-ILT group and P-ILT group was 23.05 ± 4.81 and 18.53 ± 4.07 seconds, respectively. With a P value = 0.001, the operation time, blood loss, and fluoroscopy time of the R-ILT group were significantly higher than those of the P-ILT group (p < 0.05). There was no statistical differences in the number of transient dysesthesia, infection, and reoperation (p>0.05), but the incidence of dural sac tears in R-ILT group was higher than that in P-ILT group (p = 0.039).
 |
Table 2 Perioperative Indicators and Complications of the Two Groups |
Clinical Results
In this study, the mean follow-up time of all patients was 24.94 ± 0.89 months. As shown in Figure 4A and B, there was no significant difference in the preoperative back and leg-related VAS scores between the two groups. Post-surgery, the back and leg-related VAS scores of the two groups showed a clear downward trend. The average 12-month postoperative back-related VAS scores in R-ILT group and P-ILT group was 3.36 ± 0.85 and 2.83 ± 0.95, respectively, with a P value = 0.043, in the 24-month follow, the average back-related VAS scores in R-ILT group and P-ILT group was 3.18 ± 0.73 and 2.50 ± 1.01, respectively, with a P value = 0.007, the back-related VAS scores of the R-ILT group were higher at 12 and 24 months (p < 0.05), which indicated that the relief of back pain symptoms in P-ILT group was more significant.
 |
Figure 4 Effects of clinical outcome over time. (A and B) The VAS scores of back and leg pain in different groups. (C and D) The ODI and JOA scores in different groups. * p < 0.05. |
As shown in Figure 4C and D, during the entire follow-up period, there was a significant improvement in functions as measured with ODI and JOA scores in both groups, in addition, the modified MacNab criteria results at the final follow-up showed that the good-to-excellent rate was 81.82% in R-ILT group and 86.66% in P-ILT group, respectively (Figure 5A and B).
 |
Figure 5 (A) Outcome of the modified MacNab criteria in R-ILT group. (B) Outcome of the modified MacNab criteria in P-ILT group. |
Stability of the Lumbar Spine
The postoperative computed tomography (CT) imaging in this study showed that, after surgical treatment, the volume of the spinal canal has significantly expanded in both groups [Figure 1J and 2J], according to the record of dynamic position radiography of the spine before and after surgery (Table 3), the postoperative change in intervertebral angle and vertebral translation increased compared with those before the operation, and there was a statistically significant difference in postoperative change in intervertebral angle between the two groups. However, the change in angulation was still less than 10°, and the translation distance was still within 4 mm in both groups after the operation; therefore, the surgical segments remained stable.
 |
Table 3 Comparison of Lumbar Stability of the Two Groups |
Discussion
In this study, we can see ILT is a feasible option for treating ASD that occurs after posterior lumbar decompression surgery with instruments.
ASD is a broad concept,21 for ASD caused by different reasons, we need to administer corresponding treatments according to the specific etiology. In this study, we mainly studied adjacent segment spinal canal stenosis after lumbar fusion, with the primary goal of relieving spinal canal stenosis and alleviating the corresponding symptoms.
Currently, many studies have shown that spinal endoscopy has significant advantages in treating degenerative spinal disorders.22–24 However, whether these advantages still exist in the treatment of ASD after lumbar fusion, and how the postoperative effect is, still, need further exploration, which led to this study.
Lumbar spine endoscopy is mainly divided into transforaminal and interlaminar approaches. Compared with the interlaminar approach, the transforaminal approach can avoid the influence of initial postoperative scars and internal fixation,25,26 which may affect the placement of the working channel and increase the incidence of complications such as dural sac tear and nerve damage. However, previous research found that, compared with the transforaminal approach, the interlaminar approach can more effectively relieve lumbar spinal stenosis caused by disc fragments, ligamentum flavum hypertrophy, facet hyperplasia, and osteophytes.13 Therefore, for better therapeutic effect, ILT was selected to treat ASD in this study.
During the surgery, we found that in P-ILT group, the working channel was mainly surrounded by muscle tissue, with less bleeding, and the anatomical structure was easy to identify; however, in R-ILT group, the working channel was mainly surrounded by scar tissue, and there was interference from internal fixation, making hemostasis difficult, and the anatomical structure was unclear to identify; therefore, more X-ray fluoroscopy was needed in R-ILT group to ensure the correct position of the working channel. After reaching the epidural space, more epidural adhesions can be found in R-ILT group, we believe that it is caused by the expansion of intraspinal adhesions to adjacent segments after the initial operation; therefore, the R-ILT group had higher blood loss and longer operation time, and the incidence of epidural tears was higher in the R-ILT group, when the scope of dural sac tear was small, the problem can be solved by tight deep fascia suture, when the scope of tear was large, open surgery was needed to suture the dural sac.
On the basis of relevant clinical literature, percutaneous endoscopic surgery could achieve satisfactory clinical effects in ASD treatment after lumbar fusion.21 According to the postoperative follow-up results of this study, both groups of patients showed significant relief in their lower back and leg pain symptoms, perhaps because of the influence of the initial operation, the relief of lower back pain in the R-ILT group was not as good as that in the P-ILT group. At the same time, the lower limb function of the two groups was also restored, and related literature has reported that, after ILT, the area of the dural tube can be increased up to 408.0% (range: 211–774%),11,27,28 the postoperative computed tomography (CT) imaging in this study showed that, after surgical treatment, the volume of the spinal canal has significantly expanded in both groups, which should be the key to the relief of related symptoms and the recovery of lower limb function.
According to the record of dynamic position radiography of the spine, during the follow-up period, there was no lumbar instability in either group, but the postoperative change in intervertebral angle and vertebral translation increased compared with that before surgery. Whether this situation will continue to progress, and whether lumbar instability will occur in the future, needs further observation.
Limitations
The present study has some limitations, including a relatively small number of observed cases and a short follow-up period. The indicators related to the operation process, the effect of the operation, and the incidence of complications is all closely related to the operation of the surgeon. As is well known, ASD is a broad concept that refers to a variety of complications,21 therefore, to facilitate the research, we only selected the cases of lumbar spinal stenosis for study. Finally, this was a retrospective clinical study, and higher quality clinical studies are needed to verify our findings.
Conclusion
For patients with ASD after posterior instrumented lumbar fusion, ILT surgery is a feasible option, especially for the elderly, similar to the primary ILT surgery, as long as decompression is sufficient, good clinical results can be obtained without causing spinal instability, but the difficulty and risk of ILT surgery will increase; therefore, surgical indications must be strictly controlled, and superb surgical skills are required.
Abbreviations
ASD, adjacent segment disease; LSS, lumbar spinal stenosis; R-ILT, revision interlaminar technique; P-ILT, primary interlaminar technique; VAS, visual analog scale; ODI, Oswestry disability index; JOA, Japanese orthopedic association; CT, computed tomography.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics Approval and Informed Consent
This study was approved by the institutional review board of the First Affiliated Hospital of Harbin Medical University, and written informed consent was obtained from participants prior to data collection. This study was conducted in accordance with the Declaration of Helsinki.
Funding
There is no financial support for this study.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Früh A, Leißa P, Tkatschenko D. et al. Decompression with or without fusion in degenerative adjacent segment stenosis after lumbar fusions. NEUROSURG REV. 2022;45(6):3739–3748. doi:10.1007/s10143-022-01875-4
2. Lee JC, Kim Y, Soh JW, Shin BJ. Risk factors of adjacent segment disease requiring surgery after lumbar spinal fusion: comparison of posterior lumbar interbody fusion and posterolateral fusion. SPINE. 2014;39(5):e339–45. doi:10.1097/BRS.0000000000000164
3. Virk SS, Niedermeier S, Yu E, Khan SN. Adjacent Segment Disease. Orthopedics. 2014;37(8):547–555. doi:10.3928/01477447-20140728-08
4. Makino T, Honda H, Fujiwara H, Yoshikawa H, Yonenobu K, Kaito T. Low incidence of adjacent segment disease after posterior lumbar interbody fusion with minimum disc distraction: a preliminary report. Medicine. 2018;97(2):e9631. doi:10.1097/MD.0000000000009631
5. Bagheri SR, Alimohammadi E, Zamani Froushani A, Abdi A. Adjacent segment disease after posterior lumbar instrumentation surgery for degenerative disease: incidence and risk factors. J Orthop Surg. 2019;27(2). doi:10.1177/2309499019842378
6. Dantas F, Caires A, Caires AC, et al. Adjacent segment degeneration after posterolateral lumbar fusion: results and complications of posterior revision surgery. Journal of Neurosurgical Sciences. 2021;67(4):446–453. doi:10.23736/S0390-5616.21.05315-7
7. Huang X, Cai Y, Chen K, et al. Risk factors and treatment strategies for adjacent segment disease following spinal fusion (Review). MOL MED REP. 2024;31(2). doi:10.3892/mmr.2024.13398.
8. Chung AS, Mcknight B, Wang JC. Scientific View on Endoscopic Spine Surgery: can Spinal Endoscopy Become a Mainstream Surgical Tool? World Neurosurgery. 2020;145:708–711. doi:10.1016/j.wneu.2020.05.238
9. Shin DA, Kim KN, Shin HC, Yoon DH. The efficacy of microendoscopic discectomy in reducing iatrogenic muscle injury. J Neurosurg Spine. 2008;8(1):39–43. doi:10.3171/SPI-08/01/039
10. Huang TJ, Hsu RW, Li YY, Cheng -C-C. Cheng CC. Less systemic cytokine response in patients following microendoscopic versus open lumbar discectomy. J Orthop Res. 2005;23(2):406–411. doi:10.1016/j.orthres.2004.08.010
11. Yadav YR, Parihar V, Kher Y, Bhatele P. Bhatele PR. Endoscopic inter laminar management of lumbar disease. Asian Journal of Neurosurgery. 2016;11(1):1–7. doi:10.4103/1793-5482.145377
12. Guan Y, Huang T, Gang, et al. Percutaneous Endoscopic Interlaminar Lumbar Discectomy with Local Anesthesia for L5-S1 Disc Herniation: a Feasibility Study. Pain Physician. 2019;22(6):e649–654.
13. Liang M, Wang Y, Jiang Y, et al. Clinical Efficacy of Interlaminar and Transforaminal Spinal Endoscopy in the Treatment of Lumbar Spinal Stenosis. Clin Interv Aging. 2023;18:881–890. doi:10.2147/CIA.S406566
14. Li R, An G, Guan Y, et al. Minimally Invasive Interlaminar Decompression with a 10-mm Endoscope and Microscope in Cases of Adult Degenerative Scoliosis. Pain Physician. 2021;24(6):e867–875.
15. Mc Cormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med. 1988;18(4):1007–1019. doi:10.1017/S0033291700009934
16. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271–273.
17. Fukui M, Chiba K, Kawakami M, et al. Japanese Orthopaedic Association Back Pain Evaluation Questionnaire. Part 2. Verification of its reliability : The Subcommittee on Low Back Pain and Cervical Myelopathy Evaluation of the Clinical Outcome Committee of the Japanese Orthopaedic Associat. Journal of orthopaedic science: official journal of the Japanese Orthopaedic Association. 2007;12(6):526–532. doi:10.1007/s00776-007-1168-4
18. Macnab I. Negative Disc Exploration: an Analysis Of The Causes Of Nerve-root Involvement In Sixty-eight Patients. J Bone Joint Surg Am. 1971;53:1.
19. Kitab SA, Wakefield AE, Benzel EC. Postlaminectomy lumbopelvic sagittal changes in patients with developmental lumbar spinal stenosis grouped into Roussouly lumbopelvic sagittal profiles: 2- to 10-year prospective follow-up. J NEUROSURG-SPINE. 2021;36(5):695–703. doi:10.3171/2021.8.SPINE21797
20. Pearson AM, Spratt KF, Genuario J. Genuario J,et al.Precision of Lumbar Intervertebral Measurements. Spine. 2011;36(7):572–580. doi:10.1097/BRS.0b013e3181e11c13
21. Wang N, Xie Y, Liu X, et al. Safety and clinical efficacy of endoscopic procedures for the treatment of adjacent segmental disease after lumbar fusion: a systematic review and meta-analysis. PLoS One. 2023;18(2):e0280135. doi:10.1371/journal.pone.0280135
22. Lu HG, Pan XK, Hu MJ, et al. Percutaneous transforaminal endoscopic decompression for lumbar lateral recess stenosis. Front Surg. 2021;8. doi:10.3389/fsurg.2021.631419
23. Mattei TA, Fassett DR. Endoscope-assisted spinal decompression surgery for lumbar spinal stenosis: technical note. J Neurosurg Spine. 2013;19(6):644–671. doi:10.3171/2013.5.SPINE13474
24. Komp M, Hahn P, Oezdemir S, et al. Bilateral spinal decompression of lumbar central stenosis with the full-endoscopic interlaminar versus microsurgical laminotomy technique: a prospective, randomized, controlled study. Pain Physician. 2015;18(1):61–70. doi:10.36076/ppj/2015.18.61
25. Ahn Y, Park HB. Transforaminal Endoscopic Lumbar Foraminotomy for Juxta-Fusional Foraminal Stenosis. J Clin Med. 2023;12(17):5745. doi:10.3390/jcm12175745
26. Wu JJ, Chen HZ, Zheng C. Transforaminal Percutaneous Endoscopic Discectomy and Foraminoplasty after Lumbar Spinal Fusion Surgery. Pain Physician. 2017;20(5):e647–E651.
27. Wada K, Sairyo K, Sakai T, Yasui N. Minimally invasive endoscopic bilateral decompression with a unilateral approach (endo-BiDUA) for elderly patients with lumbar spinal canal stenosis. Minim Invasive Neurosurg. 2010;53(02):65–68. doi:10.1055/s-0030-1247559
28. Sairyo K, Sakai T, Higashino K, Inoue M, Yasui N, Dezawa A. Complications of endoscopic lumbar decompression surgery. Minim Invasive Neurosurg. 2010;53(04):175–178. doi:10.1055/s-0030-1262814
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.