Back to Journals » Infection and Drug Resistance » Volume 17
Intestinal Helminth Infections and Their Association with QuantiFERON-TB Gold Plus Test Performance in an Endemic Setting, Northwest Ethiopia
Authors Zenebe Y , Abebe M, Munshea A, Yismaw G, Zewde M, Alemayehu M , Lang R, Habtamu M
Received 15 July 2024
Accepted for publication 19 September 2024
Published 17 October 2024 Volume 2024:17 Pages 4487—4500
DOI https://doi.org/10.2147/IDR.S476492
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yohannes Zenebe,1– 3 Markos Abebe,3 Abaineh Munshea,2,4 Gizachew Yismaw,5 Meaza Zewde,3 Mekdelawit Alemayehu,3 Roland Lang,6 Meseret Habtamu3
1Department of Medical Laboratory Science, College of Medicine and Health Sciences, Bahir Dar University, Bahir Dar, Ethiopia; 2Health Biotechnology Division, Institute of Biotechnology, Bahir Dar University, Bahir Dar, Ethiopia; 3Mycobacteria and Other Bacterial Diseases Research Division, Armauer Hansen Research Institute, Addis Ababa, Ethiopia; 4Department of Biology, Science College, Bahir Dar University, Bahir Dar, Ethiopia; 5Department of Microbiology, Amhara Public Health Institute, Bahir Dar, Ethiopia; 6Institute for Clinical Microbiology, Immunology and Hygiene, University Hospital of Erlangen, Friedrich-Alexander Universität Erlangen-Nürnberg, Erlangen, Germany
Correspondence: Yohannes Zenebe, Email [email protected]
Background: Timely detection and treatment of latent TB infection (LTBI) is part of WHO’s strategy against tuberculosis (TB). Helminth infections can modulate immune responses, potentially impacting the performance of interferon-gamma release assays (IGRAs) such as the QuantiFERON-TB Gold Plus (QFT-Plus). This study evaluated the association between helminth infections and QFT-Plus results among participants from a TB-endemic region.
Methods: A cross-sectional study was conducted from October 2022 to March 2023 in Bahir Dar, Ethiopia. Stool samples of 314 potential participants were examined for helminths using wet mount and Kato-Katz techniques. LTBI was assessed by QFT-Plus from a total of 100 gender-matched helminth-positive and -negative participants. The association between helminth infection status, egg count, and QFT-Plus positivity was analyzed, and p values < 0.05 were considered significant.
Results: Overall, 53 of 314 screened participants were infected with helminths (16.9%), with A. lumbricoides (47.2%) and hookworm (30.2%) as most prevalent species. The overall QFT-Plus positivity rate was 30.0%, with similar rates observed between helminth-positive and helminth-negative participants. Although QFT-Plus positivity was slightly lower in hookworm carriers (25%) compared to those with A. lumbricoides (32%), a higher-than-median hookworm egg burden was significantly associated with reduced QFT-Plus positivity (P = 0.029). QFT-Plus positivity was significantly higher among male participants than females (P = 0.032).
Conclusion: While overall helminth infection status did not significantly affect QFT-Plus positivity, higher hookworm burden was associated with reduced QFT-Plus reactivity. These findings suggest that the type of helminth and infection intensity, rather than its mere presence, may influence IGRA performance. Further studies with larger sample sizes are warranted to understand the species-specific effect of helminth infection on immune modulation of the host.
Keywords: intestinal helminths, LTBI, QFT-Plus, MTB, Ethiopia
Introduction
Tuberculosis (TB) remains a major public health threat worldwide.1 Exposure of a person with Mycobacterium tuberculosis (M.tb) can result in one of the three outcomes: the host’s immune system controls and clears the pathogen immediately; or M.tb may proliferate, causing primary TB disease; or the host’s immune system may contain the bacilli controlling proliferation, resulting in latent tuberculosis infection (LTBI). A person with LTBI does not have any symptoms but can potentially develop active TB disease later in life depending on the individual’s immune status.2
One-third of the world population is estimated to be infected with M.tb and more than 2 billion people are also infected with helminths globally.3 The two debilitating infectious agents co-exist, mainly in middle- and low-income countries,3 where Ethiopia is part of the region. Intestinal parasitic infection is the second leading cause of outpatient morbidity in Ethiopia with more than one-third of children infected with soil-transmitted helminth (STH) (4, 5).4,5 Studies found that Ascaris (A). lumbricoides is the most prevalent STH in Ethiopia, with one-third of the population being infected with this parasite.6,7 The study conducted from Arba-Minch, Ethiopia, revealed 26.3% co-infection of helminth and TB among adult participants, with the highest co-infection rate of A. lumbricoides followed by hookworms and T. trichiura.8
M.tb and helminth infection mount different host immune responses. Type 1 immune response with robust production of interferon-gamma (IFN-γ) is the primary immunity type required for controlling M.tb infection.9 IFN-γ is essential for macrophage activation, resulting in the stimulation of nitric oxide (NO) synthase and NO production, vitamin D-dependent autophagy, phagolysosome fusion, and bacterial cell death.10–12 In contrast, helminth infections are characterized by strong Type 2 immunity with higher anti-inflammatory response characterized by IL-4 and IL-13 production, which can impair the development of type-1 immunity and then dampen protective immune responses to M.tb.13 Therefore, helminth infection may promote the progression of LTBI to active TB disease and affect the diagnostic performance of IGRA tests.14,15 In reverse, a Th1-mediated immunity to M.tb infection may also favor an immune escape of helminths by down-regulation of the Th2 immune response.16
Early diagnosis and treatment of LTBI is a promising approach for the TB eradication program. Tuberculin skin test (TST) and IGRAs are the frequently used LTBI screening tools.17 The TST is commonly used and inexpensive but low in its specificity.17 Interferon gamma-release assays have higher specificity,18 and are recommended by the Centers for Disease Control and Prevention (CDC) and Infectious Disease Society of America (IDSA) as a method for latent tuberculosis screening.19,20 QFT-Plus is the latest generation LTBI screening IGRA test. The previous generations tested only IFN-γ production by the CD4 cells. However, the new QFT-Plus test now looks at not only the CD4 response (peptides from RD1-restricted protein antigens called ESAT-6 and CFP-10 in TB1) but also contains additional peptides (TB2) triggering M.tb-specific CD8 T cells.21–23
Concomitant intestinal helminth infection in patients with TB can skew the cytokine profile of the host toward a Th2 immune response, which may affect the screening performance of IGRA tests. Studies showed that purified protein derivative (PPD)-specific IFN-γ production and T-cell proliferation were improved with albendazole treatment of helminth-positive individuals.24,25 However, there is a lack of strong evidence for the impact of helminth infection on QFT-Plus test performance. Hence, the main objective of this study was to determine the effect of helminth infection on the performance of the QFT-Plus test for LTBI detection.
Materials and Methods
Study Population, Area and Design
An institution-based cross-sectional study was conducted from October 2022 to March 2023 at Shimbit Health Center, Bahir Dar city administration to determine the association between helminth infection and QFT-Plus test results. Bahir Dar is the capital city of Amhara National Regional State, located 565 km driving distance from the capital city of Ethiopia, Addis Ababa. Gender-matched study participants from outpatient setting were conveniently selected and screened for their helminth status with both wet mount and Kato-Katz methods at Shimbit Health Center. In addition, we identified a comparable number of individuals who tested negative for helminth infection, with no parasite eggs or larvae detected in their stool samples.
Inclusion/Exclusion Criteria
Participants seropositive for HIV, HBV or with evidence of clinical anemia, overt signs of acute infection, history of TB disease during the last 2 years, evidence of immunosuppressive disease conditions, on immunosuppressive treatment or pregnant women who were positive for hCG were excluded from the study. Hence, from the initial screening of eligible participants (n = 314), we identified 53 helminth-infected and 47 helminth non-infected individuals, and grouped them as cases or controls based on their helminth infection status for further analysis (Figure 1).
Parasitological Examination
From each study participant, about 3gm of stool sample was collected with a clean screwed plastic stool cup. Within 30 minutes of stool sample collection, duplicates of slides were prepared for each of the Kato-Katz and wet mount examinations. The slides were examined by two experienced laboratory professionals independently. When there were discordant results between the two examiners, the third laboratory expert examined the slides to settle the discordant result. The wet mount preparations were examined within 30 minutes and Kato-Katz slides were examined within 30–60 minutes as per the WHO recommendation.26 For identification of S. haematobium, urine sample was also examined. The study participants were considered as helminth-positive, when at least one helminth ova and/or larva with at least one of the screening methods was detected, and the total egg count per gram of stool (EPG) was determined by the Kato-Katz slides and averaged from the two examiners.
QuantiFERON-TB Gold Plus Test
QuantiFERON-TB Gold Plus is the latest LTBI screening test with a compartment of four tubes (Nil, TB1, TB2, and Mitogen). The Nil and Mitogens are negative and positive controls, respectively, whereas TB1 and TB2 are M.tb antigens. The QFT-Plus test employs a peptide cocktail simulating culture filtrate protein-10 (CFP-10) and early secretory antigenic target-6 (ESAT-6) proteins to stimulate cells in heparinized whole blood. ESAT-6 and CFP-10 are the major immunodominant antigens of M.tb.27 Both are encoded by region of difference 1 (RD1), which is present in all M.tb strains, but not in the vaccine strain M. bovis BCG.
About 5 mL of venous blood sample was collected from each study participant with lithium-heparin tube. Blood samples were transported to Amhara Public Health Institute (APHI) within three hours of blood collection. After mixing the collected blood samples, one mL of blood was transferred to each of the four QFT-Plus tubes and mixed gently ten times by inverting up and down to ensure the appropriate contact of blood with antigens or mitogen. Subsequently, the tubes were incubated at 37°C for 16 to 24 hours. After incubation, plasma was harvested following centrifugation at 3000 rpm for 15 minutes at room temperature and stored at −80°C. The stored plasma samples were then transported to the Armauer Hansen Research Institute (AHRI) with dry ice for the ELISA experiment, which was performed according to the QFT-Plus kit protocol. The optical densities (ODs) values were measured using Emax® plus microplate reader, and the IFN-γ concentration in the sample was calculated based on the curve generated from the standards using QFT-Plus Analysis Software. The concentration of IFN-γ was used for the interpretation of quantitative QFT-Plus results, and qualitative results were defined based on the manufacturer’s algorithm.
Interpretation of QFT-Plus Result
The QFT-Plus assay was interpreted as follows: The Nil control baseline IFN-γ level should be low (must be <8 IU/mL) to make sure the test interpretable, usually near 0 IU/mL. Mitogen positive control stimulates all T cells to produce IFN-γ. The Mitogen minus Nil value should be greater than 0.5 IU/mL to allow for proper test interpretation. The TB1 Antigen exposes CD4 (helper cells) to peptides from 2 proteins (CFP-10 and ESAT-6) only found in TB and sees if the immune system recognizes them and secretes IFN-γ. When the value of TB1 minus Nil and/or TB2 minus Nil was greater than 0.35 IU/mL, the result was interpreted as positive. TB2 Antigen stimulates not only CD4 cells but also CD8 (cytotoxic T cells) protein epitopes only found in TB (22). The IFN-γ concentration greater than 0.35 IU/mL with at least one of the TB1 or TB2 was considered a positive result for LTBI.
Statistical Analysis
Data was double-entered, cleaned, and verified into the project-specific REDCap database. The data analysis was executed using SPSS version 20 statistical packages, and Graph pad prism version 6 was also used for graphical presentations. Binary logistic regression and chi-square test were employed to determine the association of QFT-Plus positivity with helminth infection and associated risk factors (age, gender, BMI, residence, occupation, and marital status). Descriptive analysis including, mean, standard deviation, frequency, and percentages were used. Odds Ratio (OR), Chi-square, P value, and 95% Confidence Intervals (CI) were calculated, and the result was considered statistically significant at P value <0.05.
Results
Socio-Demographic Characteristic
Initially, a total of 314 eligible participants were screened for their helminth status. Among those, 53 were infected with one or more helminths (16.87%) (Figure 1). For further QFT-Plus experiment and subsequent data analysis, we selected 100 gender-matched study participants (53 helminth-positives and 47 helminth-negatives). The mean age and mean BMI of the study participants were 28.51 (SD = 9.76) and 21.0 (SD = 2.73), respectively. Majority of the study participants were urban dwellers (95.0% (95/100)). The detailed socio-demographic and other related variables of the study participants are described in Table 1.
 |
Table 1 Socio-Demographic Profile of the Study Participants, Northwest Ethiopia, 2023 (n = 100) |
Helminth Infection and Associated Factors
Seven intestinal helminths-species were identified from 53 helminth-positive participants. Of 53, four of them had mixed infection. Specifically, A. lumbricoides was the most common detected, intestinal helminth (47.2% (25/53)), followed by hookworm accounting 30.2% (16/53) (Figure 2). Helminth infection was higher among female (64% (32/50)) participants than male (42% (21/50)), and the difference was statistically significant (P = 0.028) (Figure 3A). The proportion of helminth infection was highest among participants in the age group of 14–26 years, 56.6% (30/53) (Table 2, Figure 3B). Majority of the study participants (79.0% (79/100)) had normal BMI range (Table 1). However, among those with a BMI <18.5, 85.7% (12/14)) were infected with one or more helminths, and the difference was statistically significant (P = 0.018) (Figure 3C).
 |
Table 2 Association of Variables to Helminths Infection Among Study Participants, Northwest Ethiopia, 2023 (n = 100) |
QFT-Plus Positivity Rate and Determinant Factors
The QFT-Plus results showed IFN-γ levels above the manufacturer-recommended cut-off value of 0.35 IU/mL (in response to TB1 or TB2 stimulation) in 30 out of 100 participants. As individuals with signs and symptoms of TB disease were excluded, QFT-Plus positivity indicated LTBI in 30% of our study population. Among QFT-Plus positive participants 23.3% (7/30) had discordant results for stimulation with TB1 and TB2, of which 10% (3/30) were TB1+/TB2- and 13.3% (4/30) were TB1-/TB2+. Based on the cut-off value, the overall correlation between TB1 and TB2 was high (R2 = 0.965; P < 0.001) (Figure 4A). Moreover, the correlations between TB1 and TB2 among helminth positive (R2=0.970; P < 0.001) (Figure 4B) and helminths-negative (R2 = 0.953; P < 0.001) (Figure 4C) participants were quite similar.
A borderline range (0.20 to 0.70 IU/mL) around the cut-off level (0.35 IU/mL) was assessed, which is suggested for the earlier QFT reversion or conversion.28,29 From QFT-Plus results of the 100 participants in the present study, 8% (8/100) were borderline negative (0.20 to 0.34 IU/mL), 6% (6/100) were borderline positive (0.35 to 0.7 IU/mL), 24% (24/100) were positive (≥7.0 IU/mL), and 2% (2/100) had indeterminate result.
The proportion of QFT positivity was higher among male participants (40% (20/50)) than female participants (20% (10/50)), and the difference was statistically significant (OR = 2.93; 95% CI: 1.07, 8.00; P = 0.032) (Table 3, Figure 5A). QFT-Plus positivity was also significantly higher in the 40–52 age group compared to the 14–26 age group (OR = 5.32, 95% CI: 1.34, 21.12; P = 0.018). Additionally, there was an increasing trend in QFT-plus positivity with age (Figure 5B). Conversely, QFT positivity was lower among participants with a BMI <18.5, (21.4% (3/14)) compared to those with a BMI ≥18.5, (31.4% (27/86)), though, the difference was not statistically significant (Figure 5C).
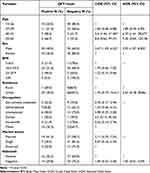 |
Table 3 Association of Determinant Factors with QFT Positivity Among Study Participants, Northwest Ethiopia, 2023 (n = 100) |
Effect of Helminth Species and Parasite Load on QFT-Plus Test Outcome
The proportions of QFT-Plus positivity among the helminth positive and negative participants were 30.2% (16/53) and 29.8% (14/47), respectively (P = 0.965) (Table 3, Figure 6A). Since soil-transmitted helminths (STH) are most common in Ethiopia, we tried to assess the QFT-Plus positivity rate among STH positive and negative participants. From the 53 helminth positive individuals, 44 of them were infected with STH. Of the 44 STH infected individuals the proportion of QFT-Plus positivity rate was 29.5% (13/44), which was also comparable to helminth-negative participants (29.8% (14/47). Considering the two predominantly identified helminth species, the proportion of QFT-Plus positivity rate was lower in participants infected with hookworm compared to A. lumbricoides (Figure 6B). However, the difference was not statistically significant.
Following WHO classification, we categorized hookworm infected participants into light (<1, 999 EPG), moderate (2000–3999 EPG) and heavy (≥4,000 EPG) infection groups.30 However, all but two participants (one with moderate and one with heavy infection) were classified under the light infection category. Similarly, for A. lumbricoides, we identified 10 participants with light infection (1–4999 EPG), 14 with moderate infection (5000–49,999 EPG), and one with heavy infection (≥50,000 EPG). Due to the small number of participants in these categories, we used median egg count for statistical analysis. Using the median egg count, the QFT-Plus positivity rate was significantly lower in participants with hookworm egg counts above the median (P = 0.029) (Figure 6C). However, this trend was not observed in participants with A. lumbricoides infection (P = 0.471) (Figure 6D).
Discussion
Helminth and M. tb infections are common in third-world countries, including Ethiopia.8,31,32 The outcome of the co-infection mainly depends on different immunological responses including cytokine/chemokine dynamics.33 It is well established that intestinal helminth infection leads to the expansion of a Th2-dominated immune response and Th3 immune-regulatory response33–35 that can mitigate the Th1 and Th17 type of immunity,36 which is essential for defense against TB, and cytokine-based IGRA tests for the diagnosis of LTBI.
In the present study, the overall prevalence of helminth infection was 16.87%, in which A. lumbricoides and hookworm were the two predominantly identified helminth species. Likewise, the two most prevalent species were reported by a recently published systematic review5 and individual studies conducted in Amhara and Oromia regions, Ethiopia.37,38 Though S. mansoni is highly prevalent in Bahir Dar and surroundings39,40 only a few participants were positive in our study (Figure 2). The possible reason might be that the majority of studies conducted among school-age children (6 to 12 years). Children in this age group are most vulnerable to S. mansoni infection due to certain play habits such as swimming in infested water bodies.38,41 However, our study was conducted largely on adult participants, mostly from an urban community.
Low BMI was significantly associated with helminth infections (P = 0.018) (Figure 3C). A similar finding has been reported by Alemu et al.8 Helminths are parasitic worms that depend on a living host for food and shelter while causing poor nutrient absorption that compromises the nourishment, and subsequently an immune response of the host.42 Studies revealed reduced numbers of T cells and their function among malnourished individuals.43,44 Thus, parasite infection could be considered as one of the predisposing factors for low BMI, resulting in reduced IFN-γ production and the progression of LTBI to active TB.
In the current study, the result of QFT-Plus indicated that about one-third (30.0%) of the study participants were LTB-infected. This finding is lower than the results of other studies conducted in Ethiopia.45,46 The possible reason could be a different age distribution of study participants; for example in Tilahun et al study, >60% were older than 30 years, whereas in our case 50% were younger than 27 years (Table 1). Nevertheless, our result is similar to the general population as noted by the WHO, which estimates around 30%.47 On the other hand, our finding is higher compared to the study conducted among Nepal migrants in the United Kingdom (UK) (15.8%).48 The lower prevalence of LTBI among Nepal migrants might be due to population difference and the lower age group of study participants (median age was 19.3 years (IQR 18.7–20.0 years)). From the total QFT-Plus result, 14% (14/100) were borderline (0.20 to 0.7 IU/mL) and the proportion of the borderline result was higher among helminth positive participants compared to negatives (16.9% vs 10.6%), but the difference was not significant (P = 0.195). The borderline results, which are considered a zone of uncertainty, could have a high conversion or reversion rate.49–51
In this study, there was a gender-based difference in QFT-Plus positivity where being male was 2.93 times more likely to be QFT-Plus positive compared to female participants (P = 0.032). This finding is in line with the studies done in Ethiopia and Latin America,46,52 while other studies showed no gender-based association.53,54 The possible reason might be; first, culturally males are more exposed to outdoor social activities in the study area, which may surge the probably of M. tb exposure; secondly, in the current study the number of helminth positivity, particularly hookworm (56.3% (9/16)) infection, was higher among females than males that could induce a Th2 response, which led to an inverse association with QFT positivity in our study (Figure 6C).
Though the difference was not statistically significant, QFT positivity was lower among participants with a BMI <18.5 compared to those with a BMI ≥18.5 (Figure 5C). In the current study, helminth positivity was higher among participants with a BMI <18.5, while QFT positivity was lower (Figure 3C, Figure 5C). This might compromise the M.tb-specific immune response (primarily IFN-γ production) of the host.
QFT positivity was significantly higher among the age group 40–52 years compared to the age group 14–26 years (P = 0.03) and marginally showed increasing trends with the age group of study participants, which is in keeping with other data.46,52,54 One possible reason might be the higher probability of cumulative exposure to M.tb with increasing age. On the other hand, helminth infection showed a decreasing trend in the higher age group, while QFT positivity inversely increased in the current study (Figure 5B). Thus, helminth infection, specifically hookworm infection, could be responsible for the lower QFT-Plus positivity. Even though aging is another possible factor for immunodeficiency, this is usually common in elderly people (≥65 years).55 In our study, the age range was 14–65 years, and the majorities (85.0%) of participants were below the age group of 40 years. Hence, the role of old age-related immunodeficiency in affecting QFT-Plus positivity could be less likely.
In our study, the QFT-Plus positivity rate was comparable among helminth positive and negative participants. The majority of participants resided in urban areas, which along with other idiopathic host-related factors may have contributed to the observed low association between helminth infections and QFT-Plus test performance. We used egg counts in stool samples as a surrogate measure of the helminth parasite density determines the infection’s intensity. While the relationship between parasite density and QFT positivity is not well established, some studies suggest that helminth infections are associated with increased levels of regulatory T cells and IL-10, which may suppress the Th1 response.
Although the overall helminth infection did not show an association with QFT-Plus positivity in this study, we sought to determine whether parasite density could impact QFT results for the two predominant parasites identified. We found that QFT positivity rates were significantly lower in hookworm-infected individuals with egg counts above the median value (P = 0.029) (Figure 6C). A study from Uganda showed that HIV-positive participants with hookworm infection had lower average CD4+ cell counts than participants infected with other helminths.56 Another study detected a lower frequency of activated CD4+ and CD8+ T cells among TB-hookworm co-infected group as compared to healthy controls and the non-co-infected pTB patients.57 Moreover, the study done by George et al demonstrated a significant inhibitory effect of coincident hookworm infection on protective cell-mediated immune cells (Th1 and Th17) response in LTBI.58 In contrast, QFT positivity was slightly increased while the parasite load of A. lumbricoides increased, which is an inverse finding compared to hookworm, but the difference was not statistically significant (Figure 6D). A. lumbricoides is known to be a benign infection that may not have strong effect on IFN-γ production.25 A study conducted in Venezuela reported that A. lumbricoides-modulated Th2 and IL-10 levels can increase the result of a positive TST after M. tb exposure.36 Another study in Brazil showed that A. lumbricoides infection had no impact on Th1, Th2 and Th17 responses.59 Further studies about immune modulation of helminth species and parasite intensity with large sample sizes are suggested.
Though this study delivers valuable information about helminth infection and the new IGRA test called QFT-Plus, it has some limitations. The first limitation is the limited sample size, which may affect the statistical modeling and analysis. Moreover, we did not use molecular tests, known for improved sensitivity for helminth diagnosis, which may affect the accurate categorization of the study participants as it is not the gold standard.
Conclusion and Recommendation
There was no difference in QFT-Plus test positivity between helminths positive and negative participants. However, the QFT Plus positivity rate was significantly lower when a parasitic load of hookworm became higher than the median egg count. Hence, hookworm infection could have a significant effect on the performance of IGRA tests compared to other helminth species. The lower QFT-Plus positivity among female participants might be due to the higher helminth infection in females than in males. This may be due to the Th2 bias that affects the performance of the QFT-Plus test. More studies with larger sample sizes and sensitive molecular diagnostic methods for helminth infection are suggested to clarify the species-specific effect of helminths on the immune response of the host and the performance of the QFT-Plus test.
Abbreviations
AHRI, Armauer Hansen Research Institute; APHI, Amhara Public Health Institute; BMI, Body Mass Index; CDC, Centers for Disease Control and Prevention; CFP-10, Culture Filtrate Protein-10; ELISA, Enzyme Linked Immunosorbent Assay; ESAT-6, Early Secretory Antigenic Target-6; hCG, Human Chorionic Gonadotropin; IDSA, Infectious Disease Society of America; IFN-γ, Interferon Gamma; IGRA, Interferon Gamma Releasing Assay; IL, Interleukin: LTBI, Latent TB Infection; M.tb, Mycobacterium tuberculosis; OD, Optical Densities; PPD, Purified Protein Derivative; QFT-Plus, QuantiFERON-TB Gold Plus; SPSS, Statistical Package for the Social Sciences; STH, Soil Transmitted Helminth; TB, Tuberculosis; TST, Tuberculin Skin Test; WHO, World Health Organization.
Data Sharing Statement
All data pertaining to this study are contained and presented in this document.
Ethical Consideration
Ethical approval was obtained from Armauer Hansen Research Institute (AHRI) and Institute of Biotechnology (IoB), Bahir Dar University with a reference number of PRCSVD/167/2023. Written consent was obtained from the study participants and for those who were under 18 years, from their parents/guardian. They were adequately informed about the aims, methods, possible conflicts of interest, institutional affiliations of the researcher, the anticipated benefits, potential risks and the discomfort it may have during sample collection. They have been also informed of the right to refuse to participate in the study or to withdraw consent to participate at any time without reprisal. The blood samples were collected by trained, authorized, and experienced laboratory professionals following standard aseptic procedures. When the study participants tested positive for intestinal helminth, they were treated with the appropriate drug as per the treatment guidelines. All study documents bearing participant information and test results were kept confidential. In general, our study complies with the declaration of Helsinki.
Acknowledgments
We would like to thank Deutsche Forschungsgemeinschaft (DFG) for financial assistance (project LA 1262/8-1). The staffs of Shimbit Health Center deserve our special thanks for their support in participant recruitment and data collection. We are also grateful to the Amhara Public Health Institute for their support to do part of our laboratory work in the institute. Finally, we would like to thank the study participants who participated in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure Statement
Dr Roland Lang reports grants from Deutsche Forschungsgemeinschaft (DFG), during the conduct of the study. The authors declare no competing interests.
Funding
This study was supported by funding from Deutsche Forschungsgemeinschaft (DFG) (grant number LA 1262/8–1).
References
1. Zumla A, George A, Sharma V, Herbert N. Baroness Masham of I. WHO’s 2013 global report on tuberculosis: successes, threats, and opportunities. Lancet. 2013;382(9907):1765–1767. doi:10.1016/S0140-6736(13)62078-4
2. Muñoz L, Stagg HR, Abubakar I. Diagnosis and management of latent tuberculosis infection. Cold Spring Harb Perspect Med. 2015;5(11):a017830. doi:10.1101/cshperspect.a017830
3. Babu S, Nutman TB. Helminth-tuberculosis co-infection: an immunologic perspective. Trends Immunol. 2016;37(9):597–607. doi:10.1016/j.it.2016.07.005
4. Tesfa-Yohannes TM, Kloos H. Intestinal parasitism. In: Zein AZ, Kloos H, editors. Ecology of Health and Disease in Ethiopia. Ethiopia: Ministry of Health, Addis Ababa; 1988:214–230.
5. Hailegebriel T, Nibret E, Munshea A. Prevalence of soil-transmitted helminth infection among school-aged children of Ethiopia: a systematic review and meta-analysis. Infect Dis. 2020;13:1178633720962812. doi:10.1177/1178633720962812
6. Deribe K, Meribo K, Gebre T, et al. The burden of neglected tropical diseases in Ethiopia, and opportunities for integrated control and elimination. Parasit Vectors. 2012;5:240. doi:10.1186/1756-3305-5-240
7. Mama M, Alemu G. Prevalence and factors associated with intestinal parasitic infections among food handlers of Southern Ethiopia: cross sectional study. BMC Public Health. 2016;16:105. doi:10.1186/s12889-016-2790-x
8. Alemu G, Mama M. Intestinal helminth co-infection and associated factors among tuberculosis patients in Arba Minch, Ethiopia. BMC Infect Dis. 2017;17(1):68. doi:10.1186/s12879-017-2195-1
9. Romero-Adrian TB, Leal-Montiel J, Fernández G. Valecillo A. Role of cytokines and other factors involved in the Mycobacterium tuberculosis infection. World J Immunol. 2015;5(1):16–50. doi:10.5411/wji.v5.i1.16
10. Mattila JT, Ojo OO, Kepka-Lenhart D, et al. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J Immunol. 2013;191(2):773–784. doi:10.4049/jimmunol.1300113
11. Duque-Correa MA, Kühl AA, Rodriguez PC, et al. Macrophage arginase-1 controls bacterial growth and pathology in hypoxic tuberculosis granulomas. Proc Natl Acad Sci U S A. 2014;111(38):E4024–32. doi:10.1073/pnas.1408839111
12. Fabri M, Stenger S, Shin DM, et al. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci transl med. 2011;3(104):104ra2. doi:10.1126/scitranslmed.3003045
13. Resende Co T, Hirsch CS, Toossi Z, Dietze R, Ribeiro-Rodrigues R. Intestinal helminth co-infection has a negative impact on both anti-Mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clin Exp Immunol. 2007;147(1):45–52. doi:10.1111/j.1365-2249.2006.03247.x
14. Malhotra I, Mungai P, Wamachi A, et al. Helminth- and Bacillus Calmette-Guérin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J Immunolo. 1999;162(11):6843–6848. doi:10.4049/jimmunol.162.11.6843
15. Guadalupe I, Mitre E, Benitez S, Chico ME, Nutman TB, Cooper PJ. Evidence for in utero sensitization to Ascaris lumbricoides in newborns of mothers with ascariasis. J Infect Dis. 2009;199(12):1846–1850. doi:10.1086/599214
16. Fishman MA, Perelson AS. Th1/Th2 cross regulation. J Theor Biol. 1994;170(1):25–56. doi:10.1006/jtbi.1994.1166
17. Getahun H, Matteelli A, Chaisson RE, Raviglione M. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015;372(22):2127–2135. doi:10.1056/NEJMra1405427
18. Zellweger JP, Sotgiu G, Corradi M, Durando P. The diagnosis of latent tuberculosis infection (LTBI): currently available tests, future developments, and perspectives to eliminate tuberculosis (TB). La Medicina del lavoro. 2020;111(3):170–183. doi:10.23749/mdl.v111i3.9983
19. Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for disease control and prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):e1–e33. doi:10.1093/cid/ciw778
20. Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection - United States, 2010: morbidity and mortality weekly report recommendations and reports. MMWR Recomm Rep. 2010;59(Rr–5):1–25.
21. Rozot V, Vigano S, Mazza-Stalder J, et al. Mycobacterium tuberculosis-specific CD8+ T cells are functionally and phenotypically different between latent infection and active disease. Eur J Immunol. 2013;43(6):1568–1577. doi:10.1002/eji.201243262
22. QuantiFERON®-TB Gold Plus (QFT®-Plus) ELISA package insert: the whole blood IFN-γ test measuring responses to ESAT-6 and CFP-10 peptide antigens: QIAGEN; 2023. Available from: https://www.quantiferon.com/wpcontent/uploads/2017/04/English_QFTPlus_ELISA_R04_022016.pdf.
23. Caccamo N, Guggino G, Meraviglia S, et al. Analysis of Mycobacterium tuberculosis-specific CD8 T-cells in patients with active tuberculosis and in individuals with latent infection. PLoS One. 2009;4(5):e5528. doi:10.1371/journal.pone.0005528
24. Elias D, Wolday D, Akuffo H, Petros B, Bronner U, Britton S. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guérin (BCG) vaccination. Clin Exp Immunol. 2001;123(2):219–225. doi:10.1046/j.1365-2249.2001.01446.x
25. Kiflie A, Bewket G, Tajebe F, Abate E, Schӧn T, Blomgran R. Helminth species-specific effects on IFN-γ producing T cells during active and latent tuberculosis. PLoS Negl Trop Dis. 2023;17(1):e0011094. doi:10.1371/journal.pntd.0011094
26. WHO. Bench Aids for the Diagnosis of Intestinal Parasites.
27. Gonzalo-Asensio J, Marinova D, Martin C, Aguilo N. MTBVAC: attenuating the human pathogen of tuberculosis (TB) toward a promising vaccine against the TB epidemic. Front Immunol. 2017;8:1803. doi:10.3389/fimmu.2017.01803
28. Ringshausen FC, Schablon A, Nienhaus A. Interferon-gamma release assays for the tuberculosis serial testing of health care workers: a systematic review. J Occup Med Toxicol. 2012;7(1):6. doi:10.1186/1745-6673-7-6
29. Nemes E, Rozot V, Geldenhuys H, et al. Optimization and interpretation of serial QuantiFERON testing to measure acquisition of Mycobacterium tuberculosis infection. Am J Respir Crit Care Med. 2017;196(5):638–648. doi:10.1164/rccm.201704-0817OC
30. Montresor A, Crompton DWT, Hall A, et al. Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level: a guide for managers of control programmes. World Health Organization; 1998. https://iris.who.int/handle/10665/63821.
31. Mhimbira F, Hella J, Said K, et al. Prevalence and clinical relevance of helminth co-infections among tuberculosis patients in urban Tanzania. PLoS negl trop dis. 2017;11(2):e0005342. doi:10.1371/journal.pntd.0005342
32. Zenebe Y, Habtamu M, Abebe M, et al. Intestinal helminth co-infection and associated factors among pulmonary tuberculosis patients in Africa and Asia: a systematic review and meta-analysis. BMC Infect Dis. 2023;23(1):739. doi:10.1186/s12879-023-08716-9
33. O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi:10.1146/annurev-immunol-032712-095939
34. Chatterjee S, Nutman TB. Helminth-induced immune regulation: implications for immune responses to tuberculosis. PLoS Pathog. 2015;11(1):e1004582. doi:10.1371/journal.ppat.1004582
35. Gebreegziabiher D, Desta K, Desalegn G, Howe R, Abebe M. The effect of maternal helminth infection on maternal and neonatal immune function and immunity to tuberculosis. PLoS One. 2014;9(4):e93429. doi:10.1371/journal.pone.0093429
36. Verhagen LM, Hermans PW, Warris A, et al. Helminths and skewed cytokine profiles increase tuberculin skin test positivity in Warao Amerindians. Tuberculosis. 2012;92(6):505–512. doi:10.1016/j.tube.2012.07.004
37. Jemaneh L. The epidemiology of schistosomiasis mansoni and soil-transmitted helminths in elementary school children from the South Gondar Zone of the Amhara National Regional State, Ethiopia. Ethiop Med J. 2000;38(2):105–118.
38. Molla E, Mamo H. Soil-transmitted helminth infections, anemia and undernutrition among schoolchildren in Yirgacheffee, South Ethiopia. BMC Res Notes. 2018;11(1):585. doi:10.1186/s13104-018-3679-9
39. Workineh L, Yimer M, Gelaye W, Muleta D. The magnitude of Schistosoma mansoni and its associated risk factors among Sebatamit primary school children, rural Bahir Dar, Northwest Ethiopia: a cross-sectional study. BMC Res Notes. 2019;12(1):447. doi:10.1186/s13104-019-4498-3
40. Afework Bitew A, Abera B, Seyoum W, et al. Soil-transmitted Helminths and Schistosoma mansoni infections in Ethiopian orthodox church students around Lake Tana, Northwest Ethiopia. PLoS One. 2016;11(5):e0155915. doi:10.1371/journal.pone.0155915
41. Abere A, Aschale Y, Tegegne B, Eshetu T. Derso AA. 10-Year Trend Analysis of Intestinal Parasitic Infections at Grarigy Health Center, Northwest Ethiopia: implication for Epidemic Preparedness. Infect Drug Resist. 2023;16:3183–3190. doi:10.2147/IDR.S413078
42. Sweeny AR, Clerc M, Pontifes PA, Venkatesan S, Babayan SA, Pedersen AB. Supplemented nutrition decreases helminth burden and increases drug efficacy in a natural host-helminth system. Proc Biol Sci. 2021;288(1943):20202722. doi:10.1098/rspb.2020.2722
43. Chandra RK. Numerical and functional deficiency in T helper cells in protein energy malnutrition. Clin Exp Immunol. 1983;51(1):126–132. PMID: 6219837.
44. Haque R, Mondal D, Shu J, et al. Correlation of interferon-gamma production by peripheral blood mononuclear cells with childhood malnutrition and susceptibility to amebiasis. Am J Trop Med Hyg. 2007;76(2):340–344. PMID: 17297046. doi:10.4269/ajtmh.2007.76.340
45. Tilahun M, Shibabaw A, Kiflie A, Bewket G, Abate E, Gelaw B. Latent tuberculosis infection and associated risk factors among people living with HIV and apparently healthy blood donors at the University of Gondar referral hospital, Northwest Ethiopia. BMC Res Notes. 2019;12(1):515. doi:10.1186/s13104-019-4548-x
46. Chekesa B, Gumi B, Chanyalew M, Zewude A, Ameni G. Prevalence of latent tuberculosis infection and associated risk factors in prison in East Wollega Zone of western Ethiopia. PLoS One. 2020;15(5):e0233314. doi:10.1371/journal.pone.0233314
47. WHO. WHO global tuberculosis report 2017; 2017. Available from: https://www.who.int/tb/publications/global_report/gtbr2017_main_text.pdf.
48. O’Shea MK, Fletcher TE, Muller J, et al. Human Hookworm infection enhances Mycobacterial growth inhibition and associates with reduced risk of tuberculosis infection. Front Immunol. 2018;9:2893. doi:10.3389/fimmu.2018.02893
49. Schablon A, Nienhaus A, Ringshausen FC, Preisser AM, Peters C. Occupational screening for tuberculosis and the use of a borderline zone for interpretation of the IGRA in German healthcare workers. PLoS One. 2014;9(12):e115322. doi:10.1371/journal.pone.0115322
50. Ringshausen FC, Nienhaus A, Schablon A, Schlösser S, Schultze-Werninghaus G, Rohde G. Predictors of persistently positive Mycobacterium-tuberculosis-specific interferon-gamma responses in the serial testing of health care workers. BMC Infect Dis. 2010;10:220. doi:10.1186/1471-2334-10-220
51. Anzaku AA, Osa-Afiana C, Ayorinde O, Jean OP, Abimiku A, Okpokoro E Conversion and reversion rate amongst health care workers with latent tuberculosis infection in North Central Nigeria. The Global Health Network Collections; 2023. https://doiorg/1021428/3d48c34a8c4f1e5e.
52. Bustamante-Rengifo JAG-SL, Osorio-Certuche N, Bejarano-Lozano Y, Tovar Cuevas JR, Astudillo-Hernández M. Prevalence of and risk factors associated with latent tuberculosis infection in a Latin American region. PeerJ. 2020;8:e9429. doi:10.7717/peerj9429.2020
53. Barry M. Prevalence of latent tuberculosis infection in the Middle East and North Africa: a systematic review. Pulm Med. 2021;2021:6680651. doi:10.1155/2021/6680651
54. Teklu T, Legesse M, Medhin G, et al. Latent tuberculosis infection and associated risk indicators in pastoral communities in southern Ethiopia: a community based cross-sectional study. BMC Public Health. 2018;18(1):266. doi:10.1186/s12889-018-5149-7
55. Lee SB, Oh JH, Park JH, Choi SP, Wee JH. Differences in youngest-old, middle-old, and oldest-old patients who visit the emergency department. Clin Exp Emerg Med. 2018;5(4):249–255. doi:10.15441/ceem.17.261
56. Morawski BM, Yunus M, Kerukadho E, et al. Hookworm infection is associated with decreased CD4+ T cell counts in HIV-infected adult Ugandans. PLoS Negl Tropl Dis. 2017;11(5):e0005634. doi:10.1371/journal.pntd.0005634
57. Li XX, Chen JX, Wang LX, et al. Profiling B and T cell immune responses to co-infection of Mycobacterium tuberculosis and hookworm in humans. Infect Dis Poverty. 2015;4:20. doi:10.1186/s40249-015-0046-0
58. George PJ, Anuradha R, Kumaran PP, Chandrasekaran V, Nutman TB, Babu S. Modulation of mycobacterial-specific Th1 and Th17 cells in latent tuberculosis by coincident hookworm infection. J Immunol. 2013;190(10):5161–5168. doi:10.4049/jimmunol.1203311
59. Santos JHA, Bührer-Sékula S, Melo GC, et al. Ascaris lumbricoides coinfection reduces tissue damage by decreasing IL-6 levels without altering clinical evolution of pulmonary tuberculosis or Th1/Th2/Th17 cytokine profile. Rev Soc Bras Med Trop. 2019;52:e20190315. doi:10.1590/0037-8682-0315-2019
 © 2024 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a
Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the
original author and source are credited.
© 2024 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a
Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the
original author and source are credited.







