Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
Introducing S.C.O.P.E.™ (Schizophrenia Clinical Outcome Scenarios and Patient–Provider Engagement), an Interactive Digital Platform to Educate Healthcare Professionals on Schizophrenia Care
Authors Correll CU , Rubio JM, Citrome L , Mychaskiw MA, Thompson S, Franzenburg KR, Suett M , Kotak S, Kane JM
Received 8 June 2024
Accepted for publication 21 September 2024
Published 19 October 2024 Volume 2024:20 Pages 1995—2010
DOI https://doi.org/10.2147/NDT.S477674
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Christoph U Correll,1– 4,* Jose M Rubio,1– 3,* Leslie Citrome,5,* Marko A Mychaskiw,6 Stephen Thompson,6 Kelli R Franzenburg,7 Mark Suett,8 Sameer Kotak,9 John M Kane1– 3,*
1Department of Psychiatry, The Zucker Hillside Hospital, Northwell Health, Glen Oaks, NY, USA; 2Department of Psychiatry, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, NY, USA; 3Institute of Behavioral Science, Feinstein Institutes for Medical Research, Manhasset, NY, USA; 4Department of Child and Adolescent Psychiatry, Charité – Universitätsmedizin Berlin, Berlin, Germany; 5Department of Psychiatry and Behavioral Sciences, New York Medical College, Valhalla, NY, USA; 6Global Health Economics and Outcomes Research, Teva Branded Pharmaceutical Products R&D, Inc., West Chester, PA, USA; 7Global Medical Affairs, Teva Branded Pharmaceutical Products R&D, Inc., West Chester, PA, USA; 8Global Medical Affairs, Teva UK Limited, Harlow, United Kingdom; 9Yorker Health Corp., Glen Rock, NJ, USA
*These authors contributed equally to this work
Correspondence: Christoph U Correll, Email [email protected]
Abstract: Despite evidence of benefits beyond those of oral antipsychotics, long-acting injectable antipsychotics (LAIs) are underused in schizophrenia treatment. Underuse may be partially a result of misconceptions held by some healthcare professionals (HCPs) pertaining to LAIs. A panel of four experts convened between January 2022 and May 2022 to identify these misconceptions, and example cases or scenarios were created to illustrate common clinical situations relevant to these beliefs. Ultimately, an online platform and heuristic tool, Schizophrenia Clinical Outcome Scenarios and Patient-Provider Engagement (S.C.O.P.E.™), was developed to help prescribing clinicians and other HCPs better understand common clinical dilemmas, as well as the place for LAIs in schizophrenia treatment. Three main misconceptions related to the use of LAIs to treat schizophrenia were identified and included “physicians/providers know when patients are nonadherent”, “patients do not accept/want LAI treatment”, and “LAIs are only appropriate for patients who have demonstrated nonadherence”. All misconceptions are refuted by current evidence and were used to develop clinical scenarios with questions to consider when patients present to various sites of care for treatment. These cases are presented on the S.C.O.P.E. educational platform. The platform also includes videos designed to help non-prescribing HCPs and mental health professionals address patient/caregiver concerns and to communicate LAI benefits. In addition, S.C.O.P.E. provides a section with information about each LAI that is currently FDA approved in the United States for the treatment of schizophrenia, to help familiarize HCPs with characteristics of LAIs. S.C.O.P.E. is an educational tool designed for HCPs to help improve their understanding of how to manage common clinical dilemmas in the treatment of people with schizophrenia, to clarify the role of LAIs in medication management, and to increase understanding of the characteristics of available LAIs. S.C.O.P.E. also aims to improve care in schizophrenia by facilitating increased awareness to patients and caregivers.
Plain Language Summary: Schizophrenia is a serious, lifelong mental health disorder that affects about 2.8 million adults in the United States and many more worldwide. Symptoms can include hallucinations (ie, hearing “voices”), delusions (ie, convinced something is true when it is not), poor attention, lack of motivation and interest, and cognitive problems. Schizophrenia can have considerable impact on people with the disorder as well as their families, friends, and communities. There are several treatment options available for healthcare professionals (HCPs), patients, and caregivers to consider, with antipsychotic medicines being the cornerstone of the treatment for schizophrenia. Long-acting injectable antipsychotics (LAIs) have shown benefits over antipsychotics taken orally (by mouth), but are underused, and this is likely due to some common misconceptions.
Four experts in schizophrenia treatment met repeatedly online to identify some of these misconceptions and created a tool to help HCPs learn about misconceptions, using example cases of patients with schizophrenia who have different types of clinical situations and concerns. On the Schizophrenia Clinical Outcome Scenarios and Patient-Provider Engagement (S.C.O.P.E.™) interactive digital platform, HCPs can choose in which type of case they are interested in and see details of the case, information they should obtain about the case, and appropriate considerations for LAI use. The tool also provides videos about communicating with patients and their families about LAIs, and information about the different LAIs currently available.
The goal of providing this tool to HCPs is to improve understanding of how to treat patients with schizophrenia and the role that LAIs can play.
Keywords: long-acting injectable antipsychotic, schizophrenia, treatment, education, patient outcomes
Introduction
Schizophrenia is a chronic mental health disorder that is estimated to affect 2.8 million adults in the United States (US) and more than 21 million people worldwide.1 The broad range of symptoms associated with the disorder include positive symptoms (eg, hallucinations, delusions), negative symptoms (eg, amotivation, anhedonia, asociality), and cognitive impairment (eg, poor attention, memory problems); these symptoms can interfere with education, work, interpersonal relationships, and self-care.2
Following treatment of acute episodes of psychosis, maintenance therapy with antipsychotics has been shown to significantly reduce the likelihood of relapse.3–5 However, nonadherence or poor adherence to oral antipsychotics has been consistently reported, accounting for the most common and most impactful risk factor for relapse and hospitalization in schizophrenia.6 Studies have found that the use of long-acting injectable antipsychotics (LAIs) helps to control positive symptoms and decreases the risk of the patient experiencing hospitalization, relapse, or premature death.7–12 Evidence from a small number of studies and a meta-analysis suggest LAIs may also improve adherence.7,8,10 However, this evidence is limited and lacks direct assessments of adherence. LAIs may be preferred by patients, if appropriately given the choice of receiving them.13,14 In addition, a survey of caregivers of patients with schizophrenia in the United States found that those caring for patients receiving LAI antipsychotic treatments generally had fewer barriers (eg, less strain on their emotional health, less inability to have a satisfying personal life) than those caring for patients not receiving such treatments.15
Although both clinical data and published treatment guidelines have supported the utility of LAIs in schizophrenia management, LAIs remain underused.13,14,16–20 Commercial claims and survey data suggest that many fewer patients receive LAIs than oral antipsychotics, and that treatment with LAIs is reserved for later in the disease course, more severe disease, and/or patients who have clearly demonstrated adherence problems.21–23 US-based claims data showed only ≈5% of patients with newly-diagnosed schizophrenia received treatment with an LAI, and more than half (118 of 202 [58%]) of patients received ≥2 different oral antipsychotics before initiating an LAI.13,22 This delayed use is common, despite the evidence that earlier use of LAIs in the treatment algorithm has been shown to positively affect outcomes in people with schizophrenia.24–28
The specific reasons for the underuse of LAIs are unclear. Challenges in treating patients with schizophrenia, such as multiple comorbidities, substance abuse, lack of patient illness insight, or response to oral antipsychotics for which an equivalent LAI formulation is not available, may complicate optimal patient management and cause uncertainty regarding use of LAIs.1,14 Furthermore, mental health professionals may hold misconceptions regarding LAIs and their role in the treatment of patients with schizophrenia.14,29,30
Educational initiatives targeting the most common dilemmas or issues faced by healthcare professionals (HCPs) could potentially alleviate knowledge gaps and clarify the role that LAIs could play in treating patients with schizophrenia. To address unmet educational needs regarding the potential benefits of LAIs for patients and caregivers, a panel of experts identified misconceptions surrounding their use and provided both expert opinions based on their clinical experience and supportive evidence to help dispel these misinformed beliefs. This process informed the development of a case-based learning tool to help clinicians better understand how to manage common clinical dilemmas and where to place LAIs in the treatment regimen of people with schizophrenia.
Methods
During multiple online meetings held between January 2022 and May 2022, a panel of four experts collated common myths and misconceptions on the use of LAIs in the treatment of schizophrenia based on their clinical experience and knowledge of the relevant medical literature. Taking the identified misconceptions into consideration, the panel then identified key clinical dilemmas (scenarios) in the treatment management of people with schizophrenia in which LAI treatment could be useful based on empirical evidence, often in contrast to common misconceptions. The experts developed a heuristic tool of considerations that both prescribing and non-prescribing HCPs may use when facing these dilemmas in the emergency department (ED), inpatient units, and outpatient clinics. Patient scenarios were based on real-life situations consistently seen in the clinical setting by the expert panel. Evidence supporting the use of LAIs in these scenarios is presented as found in the medical literature, based on research reports and reviews published by the expert panel, and others as cited in this paper.
The Schizophrenia Clinical Outcome Scenarios and Patient-Provider Engagement tool (S.C.O.P.E.™; https://www.scopeengage.com/) is an interactive digital platform that is designed to educate HCPs on schizophrenia treatment. S.C.O.P.E. does not replace clinical judgment, guidelines, or continuing medical education and is not a platform for recording patient-level data, nor is it intended for payer negotiations or access-related questions by HCPs and non-prescribing mental health professionals. No data are captured from users. S.C.O.P.E. allows exploration of the potential usefulness of LAIs across clinical scenarios and settings. Upon entering the S.C.O.P.E. website, an introductory page reviews the purpose of the platform and provides videos of the expert panel discussing its use. The platform consists of three parts:
- Clinical Dilemmas: The key clinical dilemmas (scenarios) identified by the panel are compiled in the S.C.O.P.E. interactive resource. These include scenarios observed in the ED, inpatient units, and outpatient clinics or office settings. The potential usefulness of LAIs in these cases is also explored.
- Clinical Education Videos: Select clinical dilemmas were further illustrated with clinical education videos, which provided directional information aimed to address patients’ concerns and help HCPs, including non-prescribing mental health professionals, answer any questions related to LAIs.
- Selected LAIs: Characteristics are provided for each available LAI currently approved by the Food and Drug Administration for the treatment of schizophrenia in the US, based on prescribing information for each product (current as of May 2024).31–45
Results
Identification of Myths and Misconceptions
The expert panel identified the three main and most common myths or misconceptions relating to the use of LAIs to treat people with schizophrenia that are refuted by current evidence (Table 1).6,8,18,19,23,30,46–49
 |
Table 1 Myths and Misconceptions Surrounding the Use of Long-Acting Injectable Antipsychotics for Treatment of Schizophrenia |
Myth #1: Physicians/Providers Know When Patients Are Nonadherent
Many clinicians are confident in their ability to assess adherence in their patients. However, several lines of evidence have demonstrated that clinicians are generally inaccurate when determining the medication adherence status of their patients.50,51 Studies using the Medication Event Monitoring System™ (a cap that fits on standard medicine bottles and records the time and date each time the bottle is opened and closed) have found nonadherence rates with oral antipsychotics of 42% to 57% in patients with schizophrenia or schizoaffective disorder, while prescribers, caregivers, and patients estimated nonadherence rates of 0% to 7%.52–54 Likewise, a comparison of clinician assessment versus antipsychotic plasma levels found that nearly 20% (10 of 53) of patients thought to be adherent had undetectable plasma drug levels.55 Conversely, some patients thought to be nonadherent had detectable plasma drug levels.55
The assumption that a patient is adherent to medication can lead to their misclassification as treatment-resistant. One study of 36 patients who were clinically identified as having treatment-resistant schizophrenia found almost half of these patients (n=16) showed evidence of poor adherence based on undetectable or subtherapeutic drug concentrations.50 Hence, these patients may have been “pseudo-resistant”, or failing to show clinical benefits of treatment due to lack of adherence rather than lack of response to the medication.51 This misclassification is highly consequential, as it could involve recommending clozapine, a drug with severe and potentially lethal side effects and strict monitoring requirements,56 to patients who could respond to other antipsychotics not associated with such risks or monitoring. To rule out pseudo-resistance due to inadequate treatment adherence, one set of guidelines on treatment-resistant schizophrenia proposes an optimal definition of treatment resistance that includes ≥1 failed trial with an LAI given for ≥6 weeks after it has achieved steady-state (generally, ≥4 months from commencing treatment).51
Myth #2: Patients Do Not Accept/Want LAI Treatment
While many clinicians believe LAIs are underused, they also report that only a minority of their patients would be open to accepting LAI use.57 This is an incorrect assumption in the opinion of the experts and based on the available literature. Perceived barriers to LAI treatment acceptance by patients include negative attitudes, stigma and loss of autonomy/control related to LAIs, and fear of needles.14,57 However, a cross-sectional study using semi-structured interviews to assess patient and HCP perceptions of LAIs (164 patients, 63 physicians/nurses) found that clinicians overestimated patient fears of pain, embarrassment, reduced autonomy, feelings of being controlled, and stigma.29 A cluster-randomized clinical trial found that when clinical trial site staff were educated on the benefits of LAIs and trained in communication strategies and how to overcome logistical barriers, there was a strong acceptance by patients of possible LAI use (86%).30,48 Furthermore, US-based commercial claims databases showed that once initiated on an LAI, 86% of patients stayed on that LAI until successful implementation (defined as treatment for ≥90 consecutive days with ≤7 day gaps).22 These data support that while LAIs may be underused, assumptions regarding patient concerns or willingness to use them may not be accurate, and education on overcoming barriers can help clinicians to successfully implement the use of LAIs.
Myth #3: LAIs are Only Appropriate for Patients Who Have Demonstrated Nonadherence
LAIs are often thought of as a last resort treatment option to manage nonadherence in chronically ill individuals. However, when used proactively in early-phase schizophrenia, LAIs can prolong time to first hospitalization and reduce the rate of relapse. In a cluster-randomized trial including 489 patients, the mean time to first hospitalization was significantly longer for early-phase illness patients treated with an LAI versus those who received the clinician’s choice of medication.24 In another study of 83 patients, early-phase schizophrenia patients were randomly assigned to a once-every-2-weeks dosage of the LAI formulation of risperidone or once-daily oral risperidone found that treatment with the LAI formulation was associated with significantly fewer relapses and longer mean time to relapse.8 Conversely, the randomized, open-label EULAST trial found no substantial advantage of LAIs versus oral antipsychotics when comparing time to all-cause discontinuation in patients with early-phase schizophrenia.58 However, several notable limitations of the study require consideration.59 First, patients were directly randomized to an LAI or an oral formulation of aripiprazole or paliperidone, which is not consistent with clinical practice, where patients who respond to and tolerate a particular oral antipsychotic are usually either continued on the same oral medication or switched to the LAI formulation. This design feature biased the results toward all-cause treatment failure in the oral and the LAI arms, which is also reflected by relatively high overall discontinuation rates of 64% on LAIs and 71% on oral antipsychotics at 19 months. Second, upfront randomization to one of four treatment arms does not consider the importance of patient preference in the treatment of schizophrenia.59–61 Finally, the primary outcome of “all-cause discontinuation” included patients who continued their assigned treatment but who may have received doses outside of the approved dose range and/or an added second antipsychotic, which is not uncommon in clinical care, including in patients receiving LAIs.62 Together, these points diminish the desired “pragmatic” nature of the EULAST study.
In a nationwide cohort involving 2588 patients hospitalized for the first time with schizophrenia, LAIs were associated with a significantly lower risk of rehospitalization than oral formulations of the same antipsychotics (adjusted hazard ratio, 0.36; 95% CI: 0.17–0.75; P=0.007).7 Additionally, in a US database study, the preventive use of LAIs before a first ED visit, first hospitalization, or signs of nonadherence had the best outcome for patients with schizophrenia and was incrementally superior compared with waiting for hospitalizations and/or signs of nonadherence.26
Expert consensus supports the use of LAIs regardless of adherence status in patients with poor insight into illness and need for treatment, those who are experiencing homelessness or have unstable housing situations, those with substance use disorders, those with prominent psychotic symptoms or cognitive impairment, those who are aged 18 to 25 years, and those with a history of multiple hospitalizations for psychotic relapses, violence to others, or a suicide attempt.46 Moreover, American Psychiatric Association guidelines recommend that patients receive treatment with an LAI if they prefer such treatment.19 Notably, even in patients with first episode schizophrenia, the Florida Best Practice Guidelines recommend initial treatment with monotherapy with a second-generation antipsychotic other than clozapine, followed by the same antipsychotic given either orally or as an LAI (ie, without waiting for signs of nonadherence or relapse).18 Given this discordance between recommendations and real-world usage, education is needed around recommendations for wider use of LAIs, and not as a “last resort treatment.”
The S.C.O.P.E.™ Platform
Clinical Dilemmas
Considering the three myths regarding LAI use identified above, 11 specific scenarios were developed with questions to consider when patients present to various sites of care for treatment. The clinical dilemmas presented on S.C.O.P.E. can be filtered based on clinical setting, current treatment, patient status, and clinical concerns (Figure 1). This approach allows users a “hands-on” interactive experience, and reference citations throughout the tool linking the scenario to available supporting evidence facilitate self-directed learning.
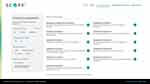 |
Figure 1 Clinical dilemmas on the S.C.O.P.E. platform. Abbreviations: S.C.O.P.E, Schizophrenia Clinical Outcome Scenarios and Patient–Provider Engagement. |
In the Clinical Dilemmas module, one ED scenario, five inpatient scenarios, and five outpatient scenarios are available, each describing patients with various treatment histories (eg, untreated or unknown, oral antipsychotic, LAI), clinical statuses (eg, stable, residual symptoms, acute symptoms in first episode or relapse), and clinical concerns (eg, nonadherence, substance use). Each scenario includes a detailed description and a specific set of questions and supporting information to aid in the learning process. An “items to consider” checklist includes tests and assessments of the patient that may be needed. Each chosen scenario concludes with an exploration of the role of LAIs; an “LAI considerations” section highlights how LAIs may be useful in the scenario.
The tool also provides a set of queries/questions to consider when patients present to the ED and a framework to inform clinical dilemmas and scenarios observed in inpatient care and outpatient clinics. The checklist includes questions such as, “Were the ED notes reviewed/available?” and buttons for “Yes”, “No”, and “N/A”. Only one option can be chosen for each question. For example, the ED scenario involves an acutely psychotic patient with a history of oral antipsychotic treatment who reports stopping her medication the previous month (Figure 2). After a checklist regarding the review of existing hospital records and patient history, another checklist on adherence assessment includes information on the reliability of various methods, such as self-report and plasma level, based on available evidence.6,52,55 A reminder is included to assess factors that may affect adherence, such as substance use and social determinants.19,63,64 Finally, as mentioned above, an LAI considerations section is provided, including patient education on risk of relapse following the discontinuation of maintenance treatment and the advantages of LAIs for maintenance treatment, documentation of the discussion on LAIs for handoff/follow-up, and guidance on considering restarting on an oral antipsychotic that has an LAI formulation to allow for an easy transition.7,10,15,19,24,48
Figure 2 Continued. Figure 2 Continued.

In one of the inpatient scenarios, a patient with no previous diagnosis of schizophrenia presents with his first episode (Supplemental Figure 1). Again, an “items to consider” checklist covers routine assessments and patient history. The LAI considerations section then discusses points relating to patients with first-episode schizophrenia, such as increased risk for nonadherence, importance of communicating the benefits of LAIs for relapse prevention, and possible adverse consequences of repeated relapses.7,24,48,65–68
In an outpatient scenario, a patient currently on an oral antipsychotic reports substance use (Supplemental Figure 2). The “items to consider” checklist on adherence assessment is again suggested, as well as evaluations of adverse events and substance use.51,64,69 The LAI considerations section suggests discussing an LAI formulation to help ensure continuity and mitigate psychotogenic effects of substances of abuse.7,68,70,71
Clinical Education Videos
S.C.O.P.E. also includes clinical education videos designed to help prepare non-prescribing HCPs and mental health professionals (eg, social workers, case managers) to address any concerns patients and their families may have and to communicate the potential benefits of LAI treatment. The videos contain useful information that can help to address concerns of patients (ie, clients) and answer questions related to LAIs. Featuring computer-generated actors, each video presents an example case from a patient’s or caregiver’s perspective and shows considerations for using LAIs from the point of view of a case manager or social worker (Figure 3 and Supplemental Figures 3 and 4).
The clinical education videos provide real-world narratives of the rationale and consequences of nonadherence to antipsychotic treatments and include testimonials from patients, families, case managers, and social workers. In one video, a patient named Pam who had an acute episode following a discontinuation from her oral antipsychotic treatment describes the events surrounding the episode.
Pam also describes a discussion with her case manager regarding the use of LAIs:
Fiona, told me about getting my meds with a shot and about how the medicine will stay in my body longer and help reduce the chance of me getting sick again; but, to be honest, I don’t like the idea of shots or needles at all really. They’re painful and I just don’t want to do it, but I know I need to do something, so we’ll see how things go.
The case manager acknowledges the patient’s apprehensions and mentions,
She actually told me her uncle has diabetes and takes a shot every day, so I think deep down she understands that this isn’t so bad. Also, I thought it was important to relay the other potential benefits from long acting treatment. You get a lot of confidence when you know no one is checking to see if you’ve taken your medication (Figure 3).
In another video, a parent of Pam also describes the acute episode and states,
And that’s the first time we learned about long-acting treatment. It would be life-changing to not worry about her taking her meds so much.
A social worker who works on Pam’s case states,
So, when I first heard about long-acting injectables, I was very skeptical…Over time though, a lot of my beliefs have changed. I think long-acting injectable treatment offers the patients and families I see more autonomy. That autonomy comes from being well and having freedom from some of that worry (Supplemental Figure 3).
Selected LAIs
S.C.O.P.E. compiles data from prescribing information from each current US Food and Drug Administration-approved LAI to help acquaint HCPs with the different characteristics or features of LAIs available in the United States for the treatment of people with schizophrenia (current as of May 2024).31–45 S.C.O.P.E. offers a selector that filters LAIs by approved indication(s), initiation regimen, reconstitution requirements, dosing strengths and frequency, injection volumes and routes, and supply and storage information based on approved product labels (Table 2, Figure 4). Differences in amenities of care (eg, how and where the injection is given, the volume injected, injection frequency, reconstitution requirements [if any], need for refrigeration) play an important role in formulation selection, given the limited array of molecules available.72 The LAI selector does not provide LAI safety and efficacy data; thus, HCPs are directed to visit individual product websites for this information or consult product information leaflets and/or labels. As noted above, this tool is not intended for use as a substitute for clinical judgment as to the appropriateness of any product for individual patients, but rather to allow HCPs to filter LAI products based solely on characteristics other than safety and efficacy.
 |
Table 2 LAI Filter Properties and Selection Options |
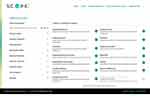 |
Figure 4 Selected LAIs and filter properties. Abbreviations: LAI, long-acting injectable antipsychotic; S.C.O.P.E, Schizophrenia Clinical Outcome Scenarios and Patient–Provider Engagement. |
Discussion
Nonadherence to medications for any chronic illnesses is problematic, and schizophrenia is no exception.73,74 Clinicians often overestimate antipsychotic medication adherence and underestimate nonadherence.6,50 Clinicians may also overestimate patient fears regarding LAI antipsychotics, and underestimate patients’ willingness to try an LAI treatment.14,29
LAIs have been shown to prolong time to hospitalization and reduce relapse rates,10 including in early-phase schizophrenia,7,8,24 supporting their use beyond demonstrated or emerging nonadherence. However, data suggest that LAIs continue to be reserved for later in the course of disease, particularly after demonstrating nonadherence.21,22 To counteract this delay or underuse, education is needed to dispel false ideas and clarify the potential role of LAIs in order for clinicians to make, together with their patients, appropriate, evidence-based decisions regarding treatment.30,74,75
Clinical cases are widely used in medical education and may be associated with deeper learning (ie, the enhanced ability to apply lessons to practice).76 Similarly, a heuristic approach enables clinicians to discover or learn for themselves. Therefore, the S.C.O.P.E. interactive digital platform was developed as a freely available resource providing evidence-based educational materials for clinicians, non-prescribing HCPs, and mental health professionals involved in the continuum of care for patients with schizophrenia. A variety of common clinical scenarios, along with checklists and questions, serve as heuristic learning tools for shared decision-making, monitoring of adverse effects, and other aspects of care. Along with standard psychiatric evaluations in inpatient and outpatient settings, S.C.O.P.E. can be used to assist clinicians to better understand how to manage common clinical dilemmas and explore the potential of LAIs in the treatment algorithm.20 The hope is that educating on the appropriate use of LAIs will provide important benefits to patients, their caregivers, clinicians, and society at large, including reduced relapses, decreased hospitalizations, reduced family and caregiver burden, and improvements in health economics outcomes.
Conclusions
S.C.O.P.E. is an educational tool for HCPs to improve their understanding of how to manage common clinical dilemmas when treating patients with schizophrenia. S.C.O.P.E. aims to clarify the potential role of LAIs in the treatment of people with schizophrenia, as well as enable clinicians to understand the characteristics of the currently available LAIs. The S.C.O.P.E. framework includes considerations for shared decision-making, monitoring of adverse effects, and the use of alternative antipsychotic formulations when needed, including the use of LAIs, with the goals of improving care and outcomes for patients with schizophrenia.
Acknowledgments
Medical writing support was provided by Mark Skopin, PhD, CMPP, and Jennifer Steeber, PhD, CMPP, and editorial support by Kelsey Gribbon, MS, all of Ashfield MedComms, an Inizio company, and was funded by Teva Branded Pharmaceutical Products R&D, Inc.
This paper was presented at the 35th Annual Psych Congress; September 17–20, 2022; New Orleans, Louisiana as a poster presentation with interim findings. The abstract has not been published in a journal.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Teva Branded Pharmaceutical Products R&D, Inc.
Disclosure
CUC has been a consultant for or has received honoraria from AbbVie, Acadia Pharmaceuticals, Adock Ingram, Alkermes, Allergan, Angelini Pharma, Aristo Pharma, Biogen, Boehringer Ingelheim, Cardio Diagnostics, Cerevel Therapeutics, CNX Therapeutics, COMPASS Pathways, Darnitsa, Denovo Biopharma, Gedeon Richter, Hikma, Holmusk, Intra-Cellular Therapies, Janssen, Johnson & Johnson, Karuna Therapeutics, LB Pharmaceuticals, Laboratorios Farmacéuticos ROVI, Lundbeck, MedAvante-ProPhase, MedinCell, Merck, Mindpax, Mitsubishi Tanabe Pharma, Maplight, Mylan, Neumora Therapeutics, Neurocrine Biosciences, Neurelis, Newron Pharmaceuticals, Noven, Novo Nordisk, Otsuka, Pharmabrain, PPD Biotech, Recordati, Relmada, Reviva, Sunovion, Supernus Pharmaceuticals, Takeda, Teva Pharmaceuticals, and Viatris; has provided expert testimony for Janssen and Otsuka and served on data safety monitoring boards or advisory boards for COMPASS Pathways, Denovo Biopharma, Laboratorios Farmacéuticos ROVI, Lundbeck, Relmada, Reviva, Sage Therapeutics, Supernus Pharmaceuticals, Tolmar, and Teva Pharmaceuticals; has received grant support from Janssen and Takeda and received royalties from UpToDate; serves on the board of directors for the American Society of Clinical Psychopharmacology; and is a stock option shareholder of Cardio Diagnostics, Mindpax, LB Pharmaceuticals, PsiloSterics, and Quantic Group. JMR has been a consultant for and has received support for attending meetings/travel from Teva Pharmaceuticals; has received honoraria from Lundbeck; has received grants from Alkermes, Janssen, Karuna, and the National Institute of Mental Health (NIMH); has received royalties/licensing fees from UpToDate; and owns stock/stock options in Doximity. LC is a consultant for AbbVie/Allergan, Acadia, Adamas, Alkermes, Angelini, Astellas, Avanir, Axsome, BioXcel, Boehringer Ingelheim, Cadent Therapeutics, Cerevel, Clinilabs, COMPASS, Delpor, Eisai, Enteris BioPharma, HLS Therapeutics, Idorsia, INmune Bio, Impel, Intra-Cellular Therapies, Janssen, Karuna, Lundbeck, Lyndra, Medavante-ProPhase, Marvin, Merck, Mitsubishi-Tanabe Pharma, Neurocrine, Neurelis, Noema, Novartis, Noven, Otsuka, Ovid, Praxis, Recordati, Relmada, Reviva, Sage, Sunovion, Supernus, Teva, and University of Arizona, Vanda, Wells Fargo, and provides one-off ad hoc consulting for individuals/entities conducting marketing, commercial, or scientifi c scoping research; is a speaker for AbbVie/Allergan, Acadia, Alkermes, Angelini, Axsome, BioXcel, Eisai, Idorsia, Intra-Cellular Therapies, Janssen, Lundbeck, Neurocrine, Noven, Otsuka, Recordati, Sage, Sunovion, Takeda, Teva, and CME activities organized by medical education companies, such as Medscape, NACCME, NEI, Vindico, and universities and professional organizations/societies; holds stocks (small number of shares of common stock) in Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, and Pfizer, purchased over 10 years ago, with stock options in Reviva; receives royalties/publishing income from Taylor & Francis (Editor-in-Chief, Current Medical Research and Opinion, 2022–present), Wiley (Editor-in-Chief, International Journal of Clinical Practice, through the end of 2019), UpToDate (reviewer), Springer Healthcare (book), and Elsevier (Topic Editor, Psychiatry, Clinical Therapeutics). MAM, ST, KRF, and MS are employees or stockholders, or both, of Teva Pharmaceuticals. SK is an employee of Yorker Health, which has received payments from Teva Pharmaceuticals in relation to this study. JMK has been a consultant for or received honoraria from Alkermes, Boehringer Ingelheim, Cerevel, Click Therapeutics, Dainippon Sumitomo, HealthRhythms, HLS Therapeutics, Eli Lilly, EnVivo Pharmaceuticals (Forum), Forest (Allergan), Genentech, Indivior, Intra-Cellular Therapies, Janssen, Johnson & Johnson, Karuna Therapeutics, LB Pharmaceuticals, Lundbeck, Lyndra Therapeutics, Mapi, Maplight, Merck, Neurocrine Biosciences, Minerva, Neurocrine, Newron, Novartis, NW PharmaTech, Otsuka, Pierre Fabre, Reviva Pharmaceuticals, Roche, Saladax Biomedical, Sunovion, Takeda, Terran, and Teva Pharmaceuticals; has received grant support from Otsuka, Lundbeck, and Janssen; and is a shareholder of Cerevel, North Shore Therapeutics, LB Pharmaceuticals and Vanguard Research Group; has received royalties from UpToDate.
References
1. Goldstone LW. Unmet medical needs and other challenges in the treatment of patients with schizophrenia. Am J Manag Care. 2020;26(3 Suppl):S48–54.
2. Patel KR, Cherian J, Gohil K, Atkinson D. Schizophrenia: overview and treatment options. P T. 2014;39(9):638–645.
3. Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063–2071. doi:10.1016/S0140-6736(12)60239-6
4. Ostuzzi G, Bertolini F, Tedeschi F, et al. Oral and long-acting antipsychotics for relapse prevention in schizophrenia-spectrum disorders: a network meta-analysis of 92 randomized trials including 22,645 participants. World Psychiatry. 2022;21(2):295–307. doi:10.1002/wps.20972
5. Ostuzzi G, Bertolini F, Del Giovane C, et al. Maintenance treatment with long-acting injectable antipsychotics for people with nonaffective psychoses: a network meta-analysis. Am J Psychiatry. 2021;178(5):424–436. doi:10.1176/appi.ajp.2020.20071120
6. Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216–226. doi:10.1002/wps.20060
7. Tiihonen J, Haukka J, Taylor M, Haddad PM, Patel MX, Korhonen P. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168(6):603–609. doi:10.1176/appi.ajp.2011.10081224
8. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72(8):822–829. doi:10.1001/jamapsychiatry.2015.0270
9. Rubio JM, Malhotra AK, Kane JM. Towards a framework to develop neuroimaging biomarkers of relapse in schizophrenia. Behav Brain Res. 2021;402:113099. doi:10.1016/j.bbr.2020.113099
10. Kishimoto T, Hagi K, Kurokawa S, Kane JM, Correll CU. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021;8(5):387–404. doi:10.1016/S2215-0366(21)00039-0
11. Correll CU, Solmi M, Croatto G, et al. Mortality in people with schizophrenia: a systematic review and meta-analysis of relative risk and aggravating or attenuating factors. World Psychiatry. 2022;21(2):248–271. doi:10.1002/wps.20994
12. Correll CU, Bitter I, Hoti F, et al. Factors and their weight in reducing life expectancy in schizophrenia. Schizophr Res. 2022;250:67–75. doi:10.1016/j.schres.2022.10.019
13. American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Treatment of Patients with Schizophrenia.
14. Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1–24. doi:10.4088/JCP.15032su1
15. Citrome L, Belcher E, Stacy S, Suett M, Mychaskiw M, Salinas GD. Perceived burdens and educational needs of caregivers of people with schizophrenia: results of a national survey study. Patient Prefer Adherence. 2022;16:159–168. doi:10.2147/PPA.S326290
16. Arango C, Baeza I, Bernardo M, et al. Long-acting injectable antipsychotics for the treatment of schizophrenia in Spain. Rev Psiquiatr Salud Ment (Engl Ed). 2019;12(2):92–105. doi:10.1016/j.rpsm.2018.03.006
17. Bareis N, Olfson M, Wall M, Stroup TS. Variation in psychotropic medication prescription for adults with schizophrenia in the United States. Psychiatr Serv. 2022;73(5):492–500. doi:10.1176/appi.ps.202000932
18. Florida Medicaid Drug Therapy Management Program. 2019–2020 Florida best practice psychotherapeutic medication guidelines for adults. In: Florida Agency for Health Care Administration. The University of South Florida; 2020. Available from: https://floridabhcenter.cbcs.usf.edu/wp-content/uploads/2021/04/2019-Psychotherapeutic-Medication-Guidelines-for-Adults-with-References_06-04-20.pdf.
19. Keepers GA, Fochtmann LJ, Anzia JM, et al. The American psychiatric association practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. 2020;177(9):868–872. doi:10.1176/appi.ajp.2020.177901
20. Correll CU, Martin A, Patel C, et al. Systematic literature review of schizophrenia clinical practice guidelines on acute and maintenance management with antipsychotics. Schizophrenia (Heidelb). 2022;8(1):5. doi:10.1038/s41537-021-00192-x
21. Fitzgerald HM, Shepherd J, Bailey H, Berry M, Wright J, Chen M. Treatment goals in schizophrenia: a real-world survey of patients, psychiatrists, and caregivers in the United States, with an analysis of current treatment (long-acting injectable vs oral antipsychotics) and goal selection. Neuropsychiatr Dis Treat. 2021;17:3215–3228. doi:10.2147/NDT.S330936
22. Kane JM, Mychaskiw MA, Lim S, et al. Treatment journey from diagnosis to the successful implementation of a long-acting injectable antipsychotic agent in patients in early adulthood with schizophrenia. J Clin Psychiatry. 2023;84(3):22m14544. doi:10.4088/JCP.22m14544
23. Rubio JM, Mychaskiw MA, Lim S, et al. Predictors for initiation of atypical long-acting injectable antipsychotic agents in a commercial claims cohort of individuals with early-phase schizophrenia. J Clin Psychiatry. 2023;84(2):22m14604. doi:10.4088/JCP.22m14604
24. Kane JM, Schooler NR, Marcy P, et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77(12):1217–1224. doi:10.1001/jamapsychiatry.2020.2076
25. Kane JM, Chen A, Lim S, et al. Early versus late administration of long-acting injectable antipsychotic agents among patients with newly diagnosed schizophrenia: an analysis of a commercial claims database. Int Clin Psychopharmacol. 2023;38(4):240–248. doi:10.1097/YIC.0000000000000452
26. Correll CU, Benson C, Emond B, et al. Comparison of clinical outcomes in patients with schizophrenia following different long-acting injectable event-driven initiation strategies. Schizophrenia. 2023;9(1):9. doi:10.1038/s41537-023-00334-3
27. Kim S, Kim S, Koh M, et al. Effects of long-acting injectable paliperidone palmitate on clinical and functional outcomes in patients with schizophrenia based on illness duration. J Clin Psychiatry. 2021;82(1):20m13446. doi:10.4088/JCP.20m13446
28. Wei Y, Yan VKC, Kang W, et al. Association of long-acting injectable antipsychotics and oral antipsychotics with disease relapse, health care use, and adverse events among people with schizophrenia. JAMA Netw Open. 2022;5(7):e2224163. doi:10.1001/jamanetworkopen.2022.24163
29. Cahling L, Berntsson A, Bröms G, Öhrmalm L. Perceptions and knowledge of antipsychotics among mental health professionals and patients. BJPsych Bull. 2017;41(5):254–259. doi:10.1192/pb.bp.116.055483
30. Kane J, McEvoy JP, Correll CU, Llorca PM. Controversies surrounding the use of long-acting injectable antipsychotic medications for the treatment of patients with schizophrenia. CNS Drugs. 2021;35(11):1189–1205. doi:10.1007/s40263-021-00861-6
31. Otsuka Pharmaceutical Co. Ltd. Abilify Maintena [Internet]; 2020 June [cited January 24, 2024]. Podcast. Available from: https://www.otsuka-us.com/sites/g/files/qhldwo7866/files/media/static/Abilify-M-PI.pdf.
32. Otsuka Pharmaceutical Co. Ltd. Abilify Asimtufii [Internet]. Deerfield, IL; 2023 February [cited January 24, 2024]. Podcast. Available from: https://otsuka-us.com/sites/g/files/qhldwo7866/files/media/static/Abilify-Asimtufii-PI.pdf.
33. Alkermes, Inc. Aristada [Internet]; 2020. Podcast. Available from: https://www.aristadahcp.com/downloads/ARISTADA-PI.pdf.
34. Alkermes, Inc. Aristada Initio [Internet]; 2020. Podcast. Available from: https://www.aristadahcp.com/downloads/ARISTADA-INITIO-PI.pdf.
35. Janssen Pharmaceutical Companies. Invega Trinza [Internet]; 2019 [cited June 17, 2020]. Podcast.
36. Janssen Pharmaceutical Companies. Invega Sustenna [Internet]; 2021. Podcast. Available from: https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/INVEGA+SUSTENNA-pi.pdf.
37. Janssen Pharmaceutical Companies. Invega Hafyera [Internet]; 2021. Podcast. Available from: https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/INVEGA+HAFYERA-pi.pdf.
38. Lilly USA, LLC. Zyprexa Relprevv [Internet]; 2021. Podcast. Available from: http://pi.lilly.com/us/zyprexa_relprevv.pdf.
39. Janssen Pharmaceutical Companies. Risperdal Consta [Internet]. Titusville, NJ; 2021 [cited January 24, 2024]. Podcast. Available from: https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/RISPERDAL+CONSTA-pi.pdf.
40. Indivior Inc. Perseris [Internet]. North Chesterfield, VA; 2021. Podcast.
41. Teva Neuroscience, Inc. Uzedy [Internet]. Parsippany, NJ; 2023 May. Podcast. Available from: https://www.uzedy.com/globalassets/uzedy/prescribing-information.pdf.
42. Shandong Luye Pharmaceutical Co. Ltd. Rykindo [Internet]. Yantai, Shandong, China; 2023 [cited January 24, 2024]. Podcast. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/212849s000lbl.pdf.
43. Janssen Pharmaceutical Companies. HALDOL [Internet]. Titusville, NJ; 2020. Podcast.
44. APP Pharmaceutical. Fluphenazine decanoate [Internet]; 2010 [cited March 22, 2022]. Podcast. Available from: https://www.parpharm.com/pdfs/catalog/sterile/Fluphenazine_PI_20200609.pdf.
45. Hojlund M, Correll CU. Switching to long-acting injectable antipsychotics: pharmacological considerations and practical approaches. Expert Opin Pharmacother. 2023;24(13):1463–1489. doi:10.1080/14656566.2023.2228686
46. Sajatovic M, Ross R, Legacy SN, et al. Identifying patients and clinical scenarios for use of long-acting injectable antipsychotics - expert consensus survey part 1. Neuropsychiatr Dis Treat. 2018;14:1463–1474. doi:10.2147/NDT.S167394
47. Grover S, Sahoo S, Mehra A. Perceptions of psychiatrists toward the use of long-acting injectable antipsychotics: an online survey study from India. J Clin Psychopharmacol. 2019;39(6):611–619. doi:10.1097/JCP.0000000000001109
48. Kane JM, Schooler NR, Marcy P, Achtyes ED, Correll CU, Robinson DG. Patients with early-phase schizophrenia will accept treatment with sustained-release medication (long-acting injectable antipsychotics): results from the recruitment phase of the PRELAPSE trial. J Clin Psychiatry. 2019;80(3):18m12546. doi:10.4088/JCP.18m12546
49. Parks J Clinical strategies to promote medication adherence. 2020; Available from: www.thenationalcouncil.org/wp-content/uploads/2020/04/Clinical-Strategies-to-Promote-Medication-Adherence-6.20.18.pdf?daf=375ateTbd56.
50. McCutcheon R, Beck K, Bloomfield MA, Marques TR, Rogdaki M, Howes OD. Treatment resistant or resistant to treatment? Antipsychotic plasma levels in patients with poorly controlled psychotic symptoms. J Psychopharmacol. 2015;29(8):892–897. doi:10.1177/0269881115576688
51. Howes OD, McCutcheon R, Agid O, et al. Treatment-resistant schizophrenia: treatment response and resistance in psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry. 2017;174(3):216–229. doi:10.1176/appi.ajp.2016.16050503
52. Byerly M, Fisher R, Whatley K, et al. A comparison of electronic monitoring vs. clinician rating of antipsychotic adherence in outpatients with schizophrenia. Psychiatry Res. 2005;133(2–3):129–133. doi:10.1016/j.psychres.2004.11.002
53. Byerly MJ, Thompson A, Carmody T, et al. Validity of electronically monitored medication adherence and conventional adherence measures in schizophrenia. Psychiatr Serv. 2007;58(6):844–847. doi:10.1176/ps.2007.58.6.844
54. Acosta FJ, Bosch E, Sarmiento G, Juanes N, Caballero-Hidalgo A, Mayans T. Evaluation of noncompliance in schizophrenia patients using electronic monitoring (MEMS) and its relationship to sociodemographic, clinical and psychopathological variables. Schizophr Res. 2009;107(2–3):213–217. doi:10.1016/j.schres.2008.09.007
55. Lopez LV, Shaikh A, Merson J, Greenberg J, Suckow RF, Kane JM. Accuracy of clinician assessments of medication status in the emergency setting: a comparison of clinician assessment of antipsychotic usage and plasma level determination. J Clin Psychopharmacol. 2017;37(3):310–314. doi:10.1097/JCP.0000000000000697
56. Correll CU, Agid O, Crespo-Facorro B, et al. A guideline and checklist for initiating and managing clozapine treatment in patients with treatment-resistant schizophrenia. CNS Drugs. 2022;36(7):659–679. doi:10.1007/s40263-022-00932-2
57. Schwartz S, Carilli C, Mian T, Ruekert L, Kumar A. Attitudes and perceptions about the use of long-acting injectable antipsychotics among behavioral health practitioners. Ment Health Clin. 2022;12(4):232–240. doi:10.9740/mhc.2022.08.232
58. Winter-van Rossum I, Weiser M, Galderisi S, et al. Efficacy of oral versus long-acting antipsychotic treatment in patients with early-phase schizophrenia in Europe and Israel: a large-scale, open-label, randomised trial (EULAST). Lancet Psychiatry. 2023;10(3):197–208 . doi:10.1016/S2215-0366(23)00005-6; Erratum in: Lancet Psychiatry. 2023;10(4):e10.
59. Kane JM, Kishimoto T, Achtyes E, Rubio J, Correll C. The use of long-acting injectables in early-phase schizophrenia. Lancet Psychiatry. 2023;10(7):480–481. doi:10.1016/S2215-0366(23)00069-X
60. Price MZ, Price RL. The use of long-acting injectables in early-phase schizophrenia. Lancet Psychiatry. 2023;10(7):480. doi:10.1016/S2215-0366(23)00141-4
61. Tiihonen J. The use of long-acting injectables in early-phase schizophrenia. Lancet Psychiatry. 2023;10(7):481–482. doi:10.1016/S2215-0366(23)00180-3
62. Gallego JA, Bonetti J, Zhang J, Kane JM, Correll CU. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res. 2012;138(1):18–28. doi:10.1016/j.schres.2012.03.018
63. Velligan DI, Weiden PJ, Sajatovic M, et al. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(Suppl 4):1–46. doi:10.4088/JCP.7090su1cj
64. Schoeler T, Petros N, Di Forti M, et al. Poor medication adherence and risk of relapse associated with continued cannabis use in patients with first-episode psychosis: a prospective analysis. Lancet Psychiatry. 2017;4(8):627–633. doi:10.1016/S2215-0366(17)30233-X
65. Kane JM, Garcia-Ribera C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry Suppl. 2009;52:S63–67.
66. Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036–1042. doi:10.1038/s41386-018-0278-3
67. Lin D, Joshi K, Keenan A, et al. Associations between relapses and psychosocial outcomes in patients with schizophrenia in real-world settings in the United States. Front Psychiatry. 2021;12:695672. doi:10.3389/fpsyt.2021.695672
68. Rubio JM, Taipale H, Tanskanen A, Correll CU, Kane JM, Tiihonen J. Long-term continuity of antipsychotic treatment for schizophrenia: a nationwide study. Schizophr Bull. 2021;47(6):1611–1620. doi:10.1093/schbul/sbab063
69. Ernest D, Vuksic O, Shepard-Smith A, Webb E. Schizophrenia: An Information Guide. 2017.
70. Misawa F, Kishimoto T, Hagi K, Kane JM, Correll CU. Safety and tolerability of long-acting injectable versus oral antipsychotics: a meta-analysis of randomized controlled studies comparing the same antipsychotics. Schizophr Res. 2016;176(2–3):220–230. doi:10.1016/j.schres.2016.07.018
71. Gopal S, Xu H, McQuarrie K, et al. Caregiver burden in schizophrenia following paliperidone palmitate long acting injectables treatment: pooled analysis of two double-blind randomized phase three studies. NPJ Schizophr. 2017;3(1):23. doi:10.1038/s41537-017-0025-5
72. Citrome L. Long-acting injectable antipsychotics: what, when, and how. CNS Spectr. 2021;26(2):118–129. doi:10.1017/S1092852921000249
73. Buckley PF, Foster AE, Patel NC, Wermert A. Adherence to Mental Health Treatment. New York, NY: Oxford University Press; 2009.
74. Faden J, Ramirez C, Martinez V, Citrome L. An overview of the currently available and emerging long-acting formulations of risperidone for schizophrenia and bipolar disorder. Expert Rev Neurother. 2024;24(8):761–771. doi:10.1080/14737175.2024.2370349
75. Citrome L, Belcher E, Stacy S, Suett M, Mychaskiw M, Salinas GD. Management of schizophrenia with long-acting injectable antipsychotic medications: an assessment of the educational needs of clinicians. Neuropsychiatr Dis Treat. 2022;18:111–123. doi:10.2147/NDT.S326299
76. McLean SF. Case-based learning and its application in medical and health-care fields: a review of worldwide literature. J Med Educ Curric Dev. 2016;3:
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.