Back to Journals » Drug Design, Development and Therapy » Volume 19
Investigating Remedial Strategies for Missed or Delayed Dose of Sertraline in Chinese Adolescent Patients with Depressive Disorders via Population Pharmacokinetics Modeling and Simulation Approaches
Authors Xia H, Deng G, Liang F, Zhang Z, Huang W , Guo Z , Song Q, Wen Y, Shang D, Tan Y
Received 14 November 2024
Accepted for publication 11 April 2025
Published 17 April 2025 Volume 2025:19 Pages 3001—3016
DOI https://doi.org/10.2147/DDDT.S504521
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Georgios Panos
Hui Xia,1,2,* Guowei Deng,1,2,* Fengtao Liang,3 Zi Zhang,1 Wanting Huang,1 Zhihao Guo,1 Qi Song,4 Yuguan Wen,1,2 Dewei Shang,1,2 Yaqian Tan1,2
1Department of Pharmacy, The Affiliated Brain Hospital, Guangzhou Medical University, Guangzhou, People’s Republic of China; 2Key Laboratory of Neurogenetics and Channelopathies of Guangdong Province and the Ministry of Education of China, Guangzhou Medical University, Guangzhou, People’s Republic of China; 3Department of Obstetrics, Panyu Hexian Memorial Hospital of Guangzhou, Guangzhou, People’s Republic of China; 4Department of Pharmacy, Guangzhou Institute of Cancer Research, The Affiliated Cancer Hospital, Guangzhou Medical University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yaqian Tan, Department of Pharmacy, The Affiliated Brain Hospital, Guangzhou Medical University, Mingxin Road 36, Liwan District, Guangzhou, 510370, People’s Republic of China, Email [email protected] Dewei Shang, Department of Pharmacy, The Affiliated Brain Hospital, Guangzhou Medical University, Mingxin Road 36, Liwan District, Guangzhou, 510370, People’s Republic of China, Email [email protected]
Background: Sertraline is a commonly used medication for adolescent patients with depression, and missed or delayed dose has been frequently observed during long-term treatment. However, little is known about the remedial strategies for missed or delayed dose of sertraline. Hence, we designed the remedial strategies of different missed dosage scenarios based on population pharmacokinetics (PPK) model simulation, aiming to provide medication guidance to Chinese adolescent depressive patients.
Methods: A total of 221 sertraline concentration monitoring data were collected from 103 sertraline-treated adolescent patients with depression. Data analysis was performed using nonlinear mixed-effects model, and situations of different missed doses were simulated in the sertraline PPK model, including one single missed dose and double/triple missed doses under different therapeutic dosages.
Results: A one-compartment model without covariates was established to characterize the PPK of sertraline in adolescent patients with depression. Population typical values of sertraline clearance and apparent distribution volume were 65.8 L/h and 1570 L, respectively. Our simulation results revealed that when adolescent patients missed or delayed sertraline administration, the scheduled dose should be administered immediately. Additionally, our results suggested that the dose of the next remedial time should be adjusted according to the duration of the delay and the frequency of missed doses.
Conclusion: In this study, we developed remedy strategies for missed or delayed dosing of sertraline in Chinese adolescents with depression based on our PPK model simulations. These findings may provide valuable clinical guidance for sertraline treatment in this population.
Keywords: sertraline, adolescents, missed or delayed dose, population pharmacokinetics, modeling and simulation
Graphical Abstract:

Introduction
Depression is a severe recurrent disease with persistent mood disorders and strong self-harm or suicidal tendencies.1,2 With the dramatic increase in the incidence of adolescent depression in the past decade, the treatment of adolescent depression has caused heavy medical burden worldwide.3–5 The treatment of depression mainly includes drug therapy, psychotherapy, and cognitive behavioral therapy.6,7 At present, drug therapy remains the predominant method of treatment for depression. As a selective serotonin re-uptake inhibitor, sertraline has been widely used in the treatment of psychiatric diseases, including depression.8,9 The pharmacokinetic characteristics of sertraline mainly include long half-life, high plasma clearance, and high plasma protein affinity.10,11 The common daily dose of sertraline is 25–200 mg, and the therapeutic reference concentration range is defined based on the trough concentration.12
Among adolescents with depression, poor adherence is considered to be a major barrier during treatment.13 Of all the medication adherence problems, missed or delayed dosing is the most common clinically and may lead to problems such as therapeutic overdose or therapeutic underdose.14,15 Therapeutic overdose may lead to concentration-dependent adverse drug reactions, such as tachycardia, drowsiness, nausea and vomiting, while insufficient remedial doses may reduce therapeutic efficacy.16–19 Therefore, considering the wide use of sertraline in adolescent depression, a rational remedial medication strategy is required to reduce the risk of adverse reactions while maintaining clinical efficacy.
The approach of population pharmacokinetics (PPK) has been widely applied in individual pharmacokinetic studies by employing therapeutic drug monitoring and precise use of known pharmacokinetic models.20 Classical pharmacokinetic models rely on dense data points to obtain accurate parameters, whereas the PPK models are able to reflect typical population characteristics by fitting parameters of multiple sparse samples.16,21 Due to the mandatory irregular dosing trials violated morality and ethics, previous clinical trials were unable to conduct studies with missed or delayed dosing.22–24 The methodology of PPK allows us to quantitatively estimate the impact of missed or delayed dose on patients, simulate appropriate remedial dose, and provide remedial plans. Thus, there are no relevant studies on remedial measure for missed or delayed sertraline administration in Chinese adolescent population. Therefore, in this study, we developed remedial strategies under different missed doses situations via the approaches of PPK modeling and simulations. Our findings would provide valuable clinical guidance for the sertraline treatment in adolescents with depression.
Materials and Methods
Patients and Data Sources
All data were sourced from the therapeutic drug monitoring (TDM) and hospital information system of the Affiliated Brain Hospital of Guangzhou Medical University. The time range of data collection was between 1st January 2019 and 31st December 2023. The inclusion criteria for recruitment were as follows: (1) took sertraline for treatment, (2) had at least two sertraline concentration data per patient, (3) aged between 13 and 17 years, (4) with complete pharmacotherapeutic records. Patients were excluded if: (1) patients were non-Chinese, (2) serum concentration of sertraline was zero, or below the lower limit/above the upper limit, of quantification, (3) two data points of sertraline concentration were not within the same hospitalization period.
Data collected from each patient included demographic information such as gender, age, height, and body weight. Missing values for height and body weight were replaced by median values in each gender. The clinical metrics included sertraline serum concentration, co-administered drugs, and detailed information of sertraline administration, such as dosage and timing. All hospitalized patients were administered sertraline at prescribed time points, and sertraline blood samples were collected at 6–7 a.m. before subsequent medication. This study was approved by the Institutional Review Board (IRB) of the Affiliated Brain Hospital of Guangzhou Medical University (approval number: 2021027). All patients had signed informed consent for off-label medication before sertraline administration.
Determination of Sertraline Concentration
Serum sertraline concentration was determined by HPLC-MS/MS (Shimadzu, Japan). Acetonitrile was used to extract analytes in protein precipitation, and sertraline-d3 was employed as an internal standard. Separation was performed on Agilent Eclipse XDB-C18 column (4.6 × 50 mm, 1.8 µm) at 35°C, and A: B (1: 1, v: v) constituted the mobile phase for analysis with a separation flow rate of 0.6 mL/min. Mobile phase A was a methanol-water mixture including 5 mmol/L ammonium formate and B was pure methanol. The applied linearity range of sertraline was 5–500 ng/mL, and the relative standard error (RSE) of intra-day and inter-day precision was below 15%. The therapeutic reference range and laboratory alert level for sertraline were 10–150 ng/mL and 300 ng/mL, respectively. Since there was no therapeutic reference range for adolescents, we used the therapeutic reference range for adults as a measure. All statistical analysis was performed using Microsoft Excel 2019, and all demographic characteristics were presented as median and range.
The PPK Model
Model Development
In this study, a nonlinear mixed effects model (NONMEM, version 7.3.0) was used for the analysis of PPK, the design and validation of the model was achieved by Pirana (version 2.9.0). The model parameters were estimated by a one-compartment model including the first-order conditional estimation method, which can be used for inter-individual and intra-individual variability interactions.
Most of the blood samples collected were in the elimination phase stage with no significant change in concentration. The absorption phase cannot be evaluated as there is no serum concentration data for the absorption phase. Therefore, the absorption rate constant (Ka) from related studies was adopted, and Ka was fixed at 0.5.25 The inter-individual variation (IIV) was described by an exponential model (Equation 1), and the associative error (Equation 2) was used to fit the intra-individual variation.
In Equation 1, Pij is the individual parameter values, Ptv,j is the typical values of population parameters, and ηi denotes the random effect of the ith individual, which obeys a normal distribution with mean 0 and variance ω.2 In Equation 2, Y and F are the observed and predicted value of sertraline concentrations, respectively. ε1 and ε2 represent the proportional error and summed error, respectively. ε1 and ε2 conform to a normal distribution with a mean of 0 and variances of σ12 and σ22, respectively.
Covariate Filtering
The final model of sertraline was constructed by screening covariates using the forward inclusion method. If a covariate reduced the objective function value by more than 6.63 (P < 0.01), the covariate was then considered statistically significant and retained in the model. Continuous type variables such as height, body weight, and age were evaluated using Equation 3, whereas categorical variables such as gender and concomitant medication were assessed using Equation 4.
COVmed denotes the median value of the continuous variable, θj is the variable used to adjust the jth PK parameter. For gender covariates, a COV of 1 represents females and 0 represents males. For concomitant medication, a COV of 1 represents co-administration and 0 represents no co-administration.
Model Evaluation
To evaluate the stability and predictability of the final model, the goodness-of-fit (GOF) plots, normalized prediction distribution error (NPDE), and bootstrap were used for validation. The GOF plots were created by GraphPad Prism (version 10.1.2), and NPDE package in R (version 4.3. 2) was applied to generate NPDE plots. The dataset was sampled repeatedly for 1000 times using the bootstrap. The accuracy of pharmacokinetics parameters was evaluated by comparing the median and 95% confidence intervals of the original data and the results.
Model Simulation
Based on our established PPK model, the typical values of the population were collected, and the simulation design was carried out. The common clinical dosages of sertraline were 50 mg, 100 mg, and 200 mg, and the common dosing regimen was once-daily (QD) administration at 8:30 a.m.
We assumed that an adolescent patient needed long-term sertraline and was required to take sertraline at 8:30 a.m. each day. Simulations were performed using population typical values from the final model. Supposing that a typical adolescent patient had a single missed dose (day 33), double missed doses (days 33 and 34), and triple missed doses (days 33, 34, and 35) during the sertraline administration, we simulated the following three scenarios.
Scenario 1: Skip the missed dose and take the regular dosing regimen at the next dosing time.
Scenario 2: Take the missed dose immediately and then simulate whether a remedial dose is required to ensure rapid recovery of the sertraline steady-state concentration.
Scenario 3: Skip the missed dose and quickly restore the sertraline steady-state concentration by increasing the remedial dose.
The dosage form of sertraline used in this study was 50 mg tablets, and the minimum remedial dose selected was 12.5 mg (one quarter of the 50 mg tablet).
Results
Demographic Characteristics
A total of 221 sertraline concentration monitoring data during 2019–2023 were collected from 103 sertraline-treated Chinese adolescent patients with depression. All data were sourced from 30 males and 73 females. The median age was 15 years (range 13–17). The median weight was 57.5 kg (range 39.9–89) and 50 kg (range 34–91) for male and female, respectively. The demographic characteristics and drug information were listed in Tables 1 and S1.
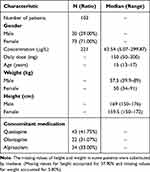 |
Table 1 Demographic Data and Characteristics of Patients |
Model Establishment
Covariates were added independently by the stepwise forward inclusion method, and no significant covariates were screened. As shown in Table 2, one-compartment PPK model of primary absorption was established for the description of sertraline data, and population typical values of CL/F and V/F were 65.8 L/h and 1570 L, respectively. The RSE values were below 30%, indicating a reasonable range of the parameters in the final model.
 |
Table 2 Population Pharmacokinetic Parameters and Bootstrap Results for Sertraline |
Model Evaluation
Figure 1 displayed the GOF plot for the final model, including population predicted concentrations versus observed concentrations (Figure 1A), individual predicted concentrations versus observed concentrations (Figure 1B), conditional weighted residual errors versus individual predicted concentrations (Figure 1C), and conditional weighted residual errors versus time after last dose (Figure 1D). From Figure 1, there was a good linear relationship between the observed concentration and the predicted concentration, indicating that the overall fitting effect was good.
NPDE was used to evaluate the pharmacokinetic parameters of sertraline, and the NPDE results were illustrated in Figure 2. As shown in the distribution histogram (Figure 2A) and quantile–quantile plot (Figure 2B), the variance and mean were 1.14 and 0.152, respectively. No significant trend was found for NPDE versus time (Figure 2C) or NPDE versus the predicted concentrations (Figure 2D). Table 2 listed the results of the bootstrap method. The method succeeded 928 out of 1000 validations, with a success rate of 92.8%. Together, these results indicated good stability and predictability of the final model for sertraline.
Model Simulation
In this study, we selected the trough serum concentration as the reference concentration for choosing the optimal remedial regimen. Figure 3 demonstrated the serum drug concentration fluctuations without taking any remedial plan. Specifically, Figure 3A–C exhibited the serum drug concentration fluctuations of single missed dose of 50 mg, 100 mg, and 200 mg, respectively. Regarding the scenario of double missed doses, the serum drug concentration fluctuations of 50 mg, 100 mg, and 200 mg were exhibited in Figure 3D–F. In Figure 3G–I, the serum drug concentration fluctuations of triple missed doses of 50 mg, 100 mg, and 200 mg were presented accordingly. The data of simulation indicated that the steady-state trough concentration of patients without missed or delayed doses was 22.34 μg/L under the 50 mg regimen, 44.69 μg/L under the 100 mg regimen, and 89.37 μg/L under the 200 mg regimen. These data suggested that all concentrations were within the therapeutic window. The trough serum concentration was collected at 7:00 a.m. prior to administration, and the peak serum concentration was collected at 4.5 hours post-administration. There was an increased likelihood of a patient’s serum concentration falling below the treatment threshold following a missed dose. For all simulation scenarios, if the delay time was within 14 hours, it was appropriate to take the delayed dose immediately. The remedial dose at the next scheduled dosing time depended on the number of missed doses and the length of the delay.
The remedial results of single missed dose of sertraline were shown in Figure 4. For the 50 mg dosing regimen, if administration was delayed within 7 hours, taking the delayed dose immediately followed by the regular dose at the next scheduled time (8:30) could quickly restore the serum drug concentration to a steady-state level (Figure 4A). When the dose delay was between 7 and 14 hours, it was appropriate to take three-quarters of the delayed dose at the next scheduled time (Figure 4B). When the delay time exceeded 14 hours and reached the time point of the next dosing, a remedial plan of 1.5 times of missed dose would be appropriate (Figure 4C). For the 100 mg dosing regimen, if administration was delayed within 5 hours, taking the delayed dose immediately followed by the regular dose at the next scheduled time (8:30) could quickly restore the serum drug concentration to a steady-state level (Figure 4D). If the delay time was between 5 and 14 hours, three-quarters of the delayed dose should be taken at the next scheduled time (Figure 4E). When the delay time exceeded 14 hours, a remedial plan of 1.5 times of missed dose would be appropriate (Figure 4F). When the 200 mg dose regimen was delayed within 4 hours, take the delayed dose immediately could quickly restore the serum drug concentration to a steady-state level (Figure 4G). If the delay time was between 4 and 9 hours, it was appropriate to take the delayed dose immediately and 175 mg at the next scheduled time (Figure 4H). Three-quarters of the delayed dose were appropriate at the next scheduled time for the delay time between 9 and 14 hours (Figure 4I). Notably, when the delay time was between 14 and 24 hours, skip the missed dose and take the regular dose at the next scheduled time could quickly restore the serum drug concentration to a steady-state level (Figure 4J).
Figure 5 exhibited the concentration–time curves of sertraline for double missed doses of 50 mg, 100 mg, or 200 mg QD and the corresponding remedial strategies. For the 50 mg dosing regimen, when the delay time was within 14 hours, taking 1.25 times of the regular dose at the next scheduled time could quickly restore the serum drug concentration to a steady-state level (Figure 5A). When the delay time was between 14 and 24 hours, it was appropriate to take 1.75 times of the regular dose at the next scheduled time (Figure 5B). For the 100 mg dosing regimen, when the delay time was within 14 hours (Figures 5C) or between 14 and 24 hours (Figures 5D), the remedial plans were the same as the 50 mg dosing regimen. For the 200 mg dosing regimen, when the delay time was within 2 hours, it was appropriate to take 225 mg dosage at the next scheduled time (Figure 5E). When the delay time exceeded 2 hours, skipping the missed dose and taking 300 mg at the next scheduled time could quickly restore steady-state serum drug concentration (Figure 5F).
The remedial results of triple missed sertraline dose of 50 mg, 100 mg and 200 mg were presented in Figure 6. For the 50 mg dosing regimen, Figure 6A exhibited the concentration–time curves for taking the missed dose when the delay time was within 6 hours and taking 1.5 times of the missed dose at the next scheduled time. The concentration–time curves for taking 2 times of the missed dose when the delay time was between 6 and 24 hours were shown in Figure 6B. For the 100 mg dosing regimen, when the delay time was within 6 hours (Figure 6C) or between 6 and 24 hours (Figure 6D), the remedial plans were the same as the 50 mg dosing regimen. For the 200 mg dosing regimen, Figure 6E displayed the concentration–time curves for taking the missed dose when the delay time was within 14 hours and taking 1.25 times of the missed dose at the next scheduled time. The concentration–time curves for taking 1.5 times of the missed dose when the delay time was between 14 and 24 hours were exhibited in Figure 6F. The serum concentration of sertraline rose rapidly in most remedial scenarios, which might increase the risk of adverse reactions. Furthermore, we found that the steady-state concentration could restore slowly after a single missed dose of sertraline over approximately five days. When the patient missed triple doses, the sertraline serum concentration was below the lower limit of the effective therapeutic. In order to restore the steady-state concentration, it was necessary to increase the remedial dose, but at the same time, this would increase the risk of adverse reactions. Thus, it would be appropriate to skip the missed dose and take the regular dose at the next scheduled time (8:30 a.m.) to restart a new course of treatment.
The theoretical remedial doses of sertraline in different missed scenarios were listed in Table S2. Noteworthy, the maximum daily dose of 200 mg could be easily exceeded if a remedial strategy was adopted for the dosage of 200 mg, thereby increasing the risk of adverse reactions. Thus, if the single remedial dose exceeded 200 mg, we did not recommend remedial measures for patients.
The graphical representations of recommended remedial strategies were demonstrated in Figure 7, and the delay time was defined as the duration of the missed medication. If the missed dose was not included in our remedial strategies, the remedial dose for patients should be administered under the evaluation of physicians.
 |
Figure 7 Graphical representation of recommended remedial strategies for delayed or missed dose of sertraline. |
Discussion
To our knowledge, this is the first study investigating remedial strategies for missed or delayed dose of sertraline in Chinese adolescent patients with depression using PPK model and simulation approaches. Depression has seriously affected the social performance of adolescents, and one of the major problems in medication adherence is missed or delayed dosing, which is closely related to factors such as age, working conditions, and educational level.26–29 Drug omission may result in serum concentrations below the effective therapeutic range, thus leading to depression aggravated.30 Therefore, our current study established a PPK model of sertraline in adolescent patients with depression and demonstrated the stability and predictive performance of the model through model validations. Based on this PPK model, we further simulated and designed different missed or delayed dose scenarios and provided remedial plans.
Our data of demographic characteristics indicated that no covariates significantly affecting the pharmacokinetic parameters of sertraline. Previous studies have shown that factors including age, gender, gene polymorphism, and liver function can affect pharmacokinetic parameters of sertraline.31–33 Our very recent study indicated that age had a significant effect on the clearance of sertraline, and the serum concentrations in adolescents were lower than those in adults due to a higher clearance of adolescents.34 In this study, we did not detect a significant effect of age on sertraline clearance as the age of adolescent patients was only distributed within a small range of 13–17. Besides, body weight was also believed to have a significant effect on sertraline clearance and apparent distribution volume. For every 10 kg increase in body weight, sertraline clearance and apparent volume of distribution increased by 4.9% and 16.1% accordingly.25 In this study, missing values for height and weight were replaced by median values for males or females, and our results suggested no significant impact of body weight on clearance rate or apparent distribution. This finding might be attributed to the relatively small weight difference between adolescent patients in this study. Other studies also reported no significant effect of body weight on sertraline clearance, which is consistent with our results.34,35 Regarding the effect of liver function, previous findings had suggested that sertraline clearance was significantly affected by the serum concentration of its metabolite, N-desmethyl sertraline.36 In this study, we were unable to analyze the effect of N-desmethyl sertraline due to the lack of relevant data.
Extensive studies have revealed that sertraline is metabolized by Cytochrome P450 (CYP) enzyme system, and the main metabolic enzymes are CYP2B6 and CYP2C19.25,37,38 Among them, CYP2C19 is a significant factor affecting the clearance rate of sertraline, especially in East Asian population. The Clinical Pharmacogenetics Implementation Consortium (CPIC) showed that 13% of East Asian individuals are poor metabolizers, 46% are intermediate metabolizers, and only 38% are normal metabolizers.28 Unfortunately, the CYP2C19 genotype was not tested in patients in this study, and therefore the effect of CYP2C19 genotyping on our PPK model could not be assessed. Nonetheless, we collected sufficient data that allowed us to monitor serum drug concentrations without genetic information. In this study, sertraline was adjusted to the appropriate dose and stabilized within the therapeutic range before patients were discharged from the hospital. After discharge, patients returned to the hospital for regular monitoring of serum drug concentrations. In addition, although genotype information can explain metabolic differences between patients to some extent, further validation of serum drug concentration is still necessary.39 On the other hand, TDM can provide guidance for dose adjustment in the absence of genotyping data. However, it is important to note that individual variability factors, such as genetic polymorphisms and underlying health conditions, may potentially affect the metabolism of sertraline. Therefore, the combined approach of genotyping and TDM would be optimal to provide individualized medication guidance for patients. Taken together, despite the lack of CYP2C19 genotyping, based on the therapeutic window and alert concentration, our data could still preliminarily evaluate patient medication safety, and the model fitting and its results were not affected. Moreover, we examined the influence of concomitant medication on CYP2C19 enzyme metabolism. Our study primarily investigated the effects of other psychiatric drugs on sertraline clearance. However, due to the low frequency of concomitant use in this study, no drugs affecting sertraline clearance were identified in our PPK model. Similarly, in another sertraline PPK study, no significant effects of psychiatric drugs, such as lamotrigine, quetiapine, and venlafaxine on the clearance rate or volume of distribution of sertraline were found.34 Nevertheless, further research with more diverse concomitant medications would be helpful to comprehensively evaluate potential drug–drug interactions in this model.
The rate of drug elimination in the body is generally described by the elimination rate constant, expressed as CL/V.40 In this study, the CL/F and V/F of sertraline were 65.8 L/h and 1570 L. However, we found the CL/V value was 0.042/h, which was inconsistent with the CL/V values in previous PPK studies.25,34 Such difference in CL/V value might be attributed to racial and age differences in these study populations, resulting in differences in drug elimination rates.
The US Food and Drug Administration (FDA) instructions recommended that if a dose is missed, the missed dose should be taken immediately, unless the time is close to the time of the next dose.41 However, the FDA instructions did not provide a specific remedial time frame or remedial dose for missed doses, nor do they provide specific remedy recommendations to patients. Our model suggested that it took at least five days for sertraline steady-state serum concentrations to restore if patient did not take a remedy after a missed dose. This suggested that the effective treatment of the patient in this scenario could be seriously influenced. Thus, a two-time remedy was recommended. The dosage forms of sertraline tablet commonly used clinically are 25 mg, 50 mg, and 100 mg. In our simulation model, 50 mg tablet was used for remediation with the minimum remedial dose of 12.5 mg. The patients can use a drug cutter for doses below 50 mg to ensure operability and convenience of the remedial regimen. Furthermore, we simplified the model of compliance, and only simulated the theoretical treatment dose before 22:30 pm (14 hours delay). Our results suggested that a delay of 14 hours would push the remedial time point close to the next dosing time point. Therefore, it is recommended that the patients proceed directly at the next time point. Together, these findings indicated that remedial regimens should be adjusted based on the duration of delay and the frequency of missed doses.
Since 200 mg sertraline was likely to exceed the maximum daily dose and increased the risk of adverse reactions, we also explored the regimen of 200 mg missed dose. Our simulation results suggested that when patient took the 200 mg dosing regimen and one missed dose was delayed within 14 hours, the patient can take a remedial dose within 200 mg at the next schedule time. When double or triple doses of 200 mg were missed, the patient should take the missed dose immediately, and the remedial dose at the next schedule time was 225–300 mg. If the remedial dose exceeded 200 mg, we suggested that the patient skip the missed dose and take the regular dose at the next schedule time. Taken together, our results suggested that remedial plans can be adopted in the case of single and double missed doses when the dosing regimens were 50 mg and 100 mg. When a patient took 200 mg of sertraline, remedial measures should be taken with reference to the remedial plan. Importantly, if the missed dose was not included in our remedial regimen, the remedial dose for patients should be administered under the evaluation of physicians. It is important to emphasize that, since our findings primarily apply to adolescent patients aged 13–17 years, the administration of remedial doses in younger patient populations, such as pediatric patients (<12 years), should be carefully evaluated and monitored by physicians. Hence, we plan to include younger patient populations in future studies to enhance the generalizability and robustness of our findings.
In conclusion, we established a PPK model of sertraline in Chinese adolescent patients with depression. Our model showed good stability and predictive performance that can be applied to predict serum concentration of sertraline. We further developed remedial plans for missed or delayed dose in different scenarios based on our PPK model simulations. The results of this study may provide clinical guidance and recommendations for the individualized treatment of adolescent depression.
Limitations
Our current study has several limitations that should be noted. First, this study was retrospective in nature and some incomplete demographic data existed, such as height and body weight. Second, we only focused on the remedial regimen of QD in this study, and it would be of great value for further studies to explore remedial plans in other regimen, such as twice daily and three times daily. Lastly, our study lacked genotype data of CYP2C19, which limited in the examination of influencing factors. Hence, the inclusion of genotype data in future studies would help to further optimize our model.
Data Sharing Statement
All data sets are available upon request from the corresponding authors.
Ethics Approval and Informed Consent
This study was approved by the Institutional Review Board of the Affiliated Brain Hospital of Guangzhou Medical University (approval number: 2021027). This study was a retrospective study, and the informed consent was waived by the IRB. We confirm that this study was conducted in accordance with the Declaration of Helsinki. All participants’ related data were deidentified to ensure privacy and confidentiality.
Acknowledgments
We are grateful to all participants for their willingness to participate in this study. We thank International Science Editing for editing this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (82104223 and 82204372), Guangdong Basic and Applied Basic Research Foundation (2020A1515110008), Science and Technology Program of Guangzhou (202102021022, 2023A04J0601, 2024A04J10001, 2024A03J0304, and 2025A03J3308), Guangzhou Municipal Science and Technology Project for Medicine and Healthcare (20211A011044), the clinical characteristic technology project of Guangzhou Region (2023C-TS22), and Guangzhou Municipal Key Discipline in Medicine (2025-2027).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Zwolińska W, Dmitrzak-Węglarz M, Słopień A. Biomarkers in child and adolescent depression. Child Psychiatry Hum Dev. 2023;54(1):266–281. doi:10.1007/s10578-021-01246-y
2. Poleszak E, Wośko S, Sławińska K, et al. Influence of the endocannabinoid system on the antidepressant activity of bupropion and moclobemide in the behavioural tests in mice. Pharmacol Rep. 2020;72(6):1562–1572. doi:10.1007/s43440-020-00088-0
3. Thapar A, Eyre O, Patel V, Brent D. Depression in young people. Lancet. 2022;400(10352):617–631. doi:10.1016/s0140-6736(22)01012-1
4. Shorey S, Ng ED, Wong CHJ. Global prevalence of depression and elevated depressive symptoms among adolescents: a systematic review and meta-analysis. Br J Clin Psychol. 2022;61(2):287–305. doi:10.1111/bjc.12333
5. Hetrick SE, McKenzie JE, Bailey AP, et al. New generation antidepressants for depression in children and adolescents: a network meta-analysis. Cochrane Database Syst Rev. 2021;5(5). doi:10.1002/14651858.CD013674.pub2
6. Zhou X, Teng T, Zhang Y, et al. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7(7):581–601. doi:10.1016/s2215-0366(20)30137-1
7. Dubovsky SL, Ghosh BM, Serotte JC, Cranwell V. Psychotic depression: diagnosis, differential diagnosis, and treatment. Psychother Psychosom. 2021;90(3):160–177. doi:10.1159/000511348
8. Kamo T, Maeda M, Oe M, et al. Dosage, effectiveness, and safety of sertraline treatment for posttraumatic stress disorder in a Japanese clinical setting: a retrospective study. BMC Psychiatry. 2016;16(1):434. doi:10.1186/s12888-016-1138-5
9. Holck A, Wolkowitz OM, Mellon SH, et al. Plasma serotonin levels are associated with antidepressant response to SSRIs. J Affect Disord. 2019;250:65–70. doi:10.1016/j.jad.2019.02.063
10. Heinonen E, Blennow M, Blomdahl-Wetterholm M, et al. Sertraline concentrations in pregnant women are steady and the drug transfer to their infants is low. Eur J Clin Pharmacol. 2021;77(9):1323–1331. doi:10.1007/s00228-021-03122-z
11. Alhadab AA, Brundage RC. Population pharmacokinetics of sertraline in healthy subjects: a model-based meta-analysis. Aaps J. 2020;22(4):73. doi:10.1208/s12248-020-00455-y
12. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1–02):9–62. doi:10.1055/s-0043-116492
13. Solmi M, Miola A, Croatto G, et al. How can we improve antidepressant adherence in the management of depression? A targeted review and 10 clinical recommendations. Braz J Psychiatry. 2021;43(2):189–202. doi:10.1590/1516-4446-2020-0935
14. Clark ED, Lawley SD. Should patients skip late doses of medication? A pharmacokinetic perspective. J Pharmacokinet Pharmacodyn. 2022;49(4):429–444. doi:10.1007/s10928-022-09812-0
15. Bigos KL, Bies RR, Pollock BG. Population pharmacokinetics in geriatric psychiatry. Am J Geriatr Psychiatry. 2006;14(12):993–1003. doi:10.1097/01.JGP.0000224330.73063.6c
16. Xiao T, Wang Z, Li G, et al. What to do about missed doses? A retrospective study of olanzapine in the elderly. Drug Des Devel Ther. 2021;15:3411–3423. doi:10.2147/dddt.S316110
17. Wang Z, Li H, Kang Y, Liu Y, Shan L, Wang F. Risks of digestive system side-effects of selective serotonin reuptake inhibitors in patients with depression: a network meta-analysis. Ther Clin Risk Manag. 2022;18:799–812. doi:10.2147/tcrm.S363404
18. Sepúlveda-Lizcano L, Arenas-Villamizar VV, Jaimes-Duarte EB, et al. Metabolic adverse effects of psychotropic drug therapy: a systematic review. Eur J Investig Health Psychol Educ. 2023;13(8):1505–1520. doi:10.3390/ejihpe13080110
19. Luo X, Zhu D, Li J, et al. Selection of the optimal dose of sertraline for depression: a dose-response meta-analysis of randomized controlled trials. Psychiatry Res. 2023;327:115391. doi:10.1016/j.psychres.2023.115391
20. Goutelle S, Woillard JB, Buclin T, et al. Parametric and nonparametric methods in population pharmacokinetics: experts’ discussion on use, strengths, and limitations. J Clin Pharmacol. 2022;62(2):158–170. doi:10.1002/jcph.1993
21. Bigos KL, Chew ML, Bies RR. Pharmacokinetics in geriatric psychiatry. Curr Psychiatry Rep. 2008;10(1):30–36. doi:10.1007/s11920-008-0007-4
22. Brittain ST, Wheless JW. Pharmacokinetic simulations of topiramate plasma concentrations following dosing irregularities with extended-release vs. immediate-release formulations. Epilepsy Behav. 2015;52(Pt A):31–36. doi:10.1016/j.yebeh.2015.08.029
23. Gu JQ, Guo YP, Jiao Z, Ding JJ, Li GF. How to handle delayed or missed doses: a population pharmacokinetic perspective. Eur J Drug Metab Pharmacokinet. 2020;45(2):163–172. doi:10.1007/s13318-019-00598-0
24. Millum J, Grady C. The ethics of placebo-controlled trials: methodological justifications. Contemp Clin Trials. 2013;36(2):510–514. doi:10.1016/j.cct.2013.09.003
25. Poweleit EA, Taylor ZL, Mizuno T, et al. Escitalopram and sertraline population pharmacokinetic analysis in pediatric patients. Clin Pharmacokinet. 2023;62(11):1621–1637. doi:10.1007/s40262-023-01294-8
26. Wang L, Jiang S. Class climate, adolescent financial and academic strain, and depressive symptoms. J Affect Disord. 2023;324:270–278. doi:10.1016/j.jad.2022.12.081
27. Albassam A, Hughes DA. What should patients do if they miss a dose? A systematic review of patient information leaflets and summaries of product characteristics. Eur J Clin Pharmacol. 2021;77(2):251–260. doi:10.1007/s00228-020-03003-x
28. Grossberg A, Rice T. Depression and Suicidal Behavior in Adolescents. Med Clin North Am. 2023;107(1):169–182. doi:10.1016/j.mcna.2022.04.005
29. Sajatovic M, Levin J, Fuentes-Casiano E, Cassidy KA, Tatsuoka C, Jenkins JH. Illness experience and reasons for nonadherence among individuals with bipolar disorder who are poorly adherent with medication. Compr Psychiatry. 2011;52(3):280–287. doi:10.1016/j.comppsych.2010.07.002
30. Osterberg LG, Urquhart J, Blaschke TF. Understanding forgiveness: minding and mining the gaps between pharmacokinetics and therapeutics. Clin Pharmacol Ther. 2010;88(4):457–459. doi:10.1038/clpt.2010.171
31. Saiz-Rodríguez M, Belmonte C, Román M, et al. Effect of polymorphisms on the pharmacokinetics, pharmacodynamics and safety of sertraline in healthy volunteers. Basic Clin Pharmacol Toxicol. 2018;122(5):501–511. doi:10.1111/bcpt.12938
32. Yuce-Artun N, Baskak B, Ozel-Kizil ET, et al. Influence of CYP2B6 and CYP2C19 polymorphisms on sertraline metabolism in major depression patients. Int J Clin Pharm. 2016;38(2):388–394. doi:10.1007/s11096-016-0259-8
33. Wang JH, Liu ZQ, Wang W, et al. Pharmacokinetics of sertraline in relation to genetic polymorphism of CYP2C19. Clin Pharmacol Ther. 2001;70(1):42–47. doi:10.1067/mcp.2001.116513
34. Zhang Z, Guo Z, Tan Y, et al. Population pharmacokinetic approach to guide personalized sertraline treatment in Chinese patients. Heliyon. 2024;10(3):e25231. doi:10.1016/j.heliyon.2024.e25231
35. Castillo CEC, Garibay SEM, Segovia R, et al. Population pharmacokinetics of sertraline in psychiatric and substance use disorders. J Clin Pharmacol. 2024;64(10):1267–1277. doi:10.1002/jcph.2457
36. Stoiljkovic M, Nikolic VN, Ilic N, et al. Population pharmacokinetic modeling to inform sertraline dosing optimization in patients with depression. Pharmacology. 2023;108(4):409–415. doi:10.1159/000530084
37. Bousman CA, Stevenson JM, Ramsey LB, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A genotypes and serotonin reuptake inhibitor antidepressants. Clin Pharmacol Ther. 2023;114(1):51–68. doi:10.1002/cpt.2903
38. Brown JT, Gregornik DB, Jorgenson A, Watson D, Roiko SA, Bishop JR. Sertraline dosing trends in children and adolescents stratified by CYP2C19 genotype. Pharmacogenomics. 2022;23(4):247–253. doi:10.2217/pgs-2021-0135
39. Spina E, de Leon J. Clinical applications of CYP genotyping in psychiatry. J Neural Transm. 2015;122(1):5–28. doi:10.1007/s00702-014-1300-5
40. Martinez MN, Jelliffe RW, Proost JH. Expert discussion of the role of rate constant versus clearance approaches to define drug pharmacokinetics: theoretical and clinical considerations. Aaps j. 2020;22(2):25. doi:10.1208/s12248-019-0407-x
41. Li ZR, Wang CY, Lin WW, Chen YT, Liu XQ, Jiao Z. Handling delayed or missed dose of antiseizure medications: a model-informed individual remedial dosing. Neurology. 2023;100(9):e921–e931. doi:10.1212/wnl.0000000000201604
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.











