Back to Journals » Infection and Drug Resistance » Volume 18
Investigating the Therapeutic Mechanisms of Honeysuckle (China) in Sepsis Through Network Pharmacology and Experimental Validation
Authors Liu P, Zeng L, Fu H, Li F
Received 12 November 2024
Accepted for publication 27 February 2025
Published 3 July 2025 Volume 2025:18 Pages 3257—3277
DOI https://doi.org/10.2147/IDR.S499975
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sandip Patil
Pingping Liu,1 Linna Zeng,2 Hongyun Fu,3 Fuzhu Li4
1Emergency Medicine Center, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, Hunan, 421000, People’s Republic of China; 2Department of Endocrinology, The Third People’s Hospital of Yongzhou, Yongzhou, Hunan, 425000, People’s Republic of China; 3Department of Laboratory Medicine, The Affiliated Nanhua Hospital, Hengyang Medical School, University of South China, Hengyang, Hunan, 421000, People’s Republic of China; 4Department of Neurosurgery Intensive Care Unit, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, Hunan, 421000, People’s Republic of China
Correspondence: Fuzhu Li, Department of Neurosurgery Intensive Care Unit, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, Hunan, 421000, People’s Republic of China, Tel +86 15773435682, Email [email protected]
Background: Sepsis is a critical condition characterized by an atypical immune response to infection, resulting in systemic inflammatory response syndrome. Honeysuckle, a traditional Chinese medicinal plant with anti-inflammatory, anti-tumor, and antioxidant characteristics, shows promise in mitigating sepsis-related inflammatory responses. The precise mechanism by which honeysuckle confers protection against sepsis remains uncertain. This research utilized network pharmacology to explore the mechanisms through which honeysuckle mitigates sepsis.
Methods: Bioactive compounds of honeysuckle were sourced from the Traditional Chinese Medicine System Pharmacology database, and their corresponding targets were identified. Sepsis-associated genes were gathered from the GeneCards and Online Mendelian Inheritance in Man databases. The regulatory network depicting “active ingredients-target” relationships was constructed utilizing Cytoscape 3.9.1 software. Target genes affected by honeysuckle in sepsis were analyzed utilizing the String database to create a protein-protein interaction network. Kyoto Encyclopedia of Genes and Genomes and Gene Ontology analyses were performed through the Database for Annotation, Visualization, and Integrated Discovery platform, aiming to identify pathways associated with these pivotal targets. Subsequently, molecular docking confirmed the binding capacity of these bioactive ingredients to pivotal targets. Subsequent in vitro experiments were conducted for additional validation. Network pharmacology analysis employed R software for extensive data processing. A Student’s T-test compared the mean values of the two groups in the in vitro experimental data. Statistical analysis for multiple group comparisons was carried out utilizing one-way ANOVA, with Tukey’s post hoc test applied for further analysis.
Results: The screening process identified 23 active ingredients in honeysuckle, which are linked to 291 targets. Molecular docking demonstrated strong binding affinities of kaempferol, luteolin, and quercetin to the target proteins TP53, TLR4, and MYD88. In vitro experiments demonstrated that honeysuckle and its active components mitigate sepsis by inhibiting the TLR4/MYD88 signaling pathways, thereby reducing proinflammatory cytokine production.
Conclusion: These findings indicate that honeysuckle may be an effective treatment for sepsis by modulating key proteins such as TP53, TLR4, and MYD88.
Keywords: network pharmacology, sepsis, molecular docking, traditional Chinese medicine, inflammatory response syndrome
Introduction
Sepsis, a critical systemic inflammatory reaction to microbial invasion, is notably triggered by lipopolysaccharide (LPS).1 It is a systemic inflammatory response syndrome that may progress to septic shock, marked by significant mortality, morbidity, and treatment expenses.2 When the infection triggers widespread systemic symptoms like fever, increased heart rate, or elevated respiratory rate, it is identified as sepsis. If the systemic response escalates and leads to organ dysfunction remote from the infection site, the condition is classified as severe sepsis. Septic shock, on the other hand, is characterized by severe organ dysfunction accompanied by persistent hypotension or hypoperfusion that does not improve with initial fluid resuscitation.3,4 Sepsis is a prevalent, life-threatening condition in clinical practice and a leading cause of death in ICU patients. Annually, sepsis impacts approximately 31.5 million individuals worldwide, resulting in 5.3 million deaths.5 In China, sepsis patients have a mortality rate of 35.5%, with severe cases experiencing rates over 50%.6 The management of sepsis primarily involves fluid resuscitation, antibiotics administration, anticoagulation therapy, glucocorticoid usage, and renal replacement therapy;3,7–9 however, recurrent infections and multi-drug resistance pose significant challenges in clinical treatment.10–12 Recently, the utilization of Chinese herbal medicine in the management of sepsis has progressively emerged as a pivotal and efficacious therapeutic approach, significantly reducing sepsis-related mortality rates.13–15 Sepsis falls within the domain of heat disorders in traditional Chinese medicine, where the Inner Canon advocates for “treating heat with coldness”, thus predominantly emphasizing the selection of drugs that possess heat-clearing and detoxifying properties.16,17
Honeysuckle (Lonicera japonica Thunb)., a widely recognized traditional Chinese medicinal herb, has a rich history of therapeutic use. The first recorded mention appears in the Miscellaneous Records of Famous Physicians (Ming Yi Bie Lu). Ethnobotanically, honeysuckle is valued for its ability to clear heat, detoxify, expel wind, and eliminate dampness.18,19 According to the Chinese Pharmacopoeia (2020 edition), honeysuckle is described as sweet in taste and cooling properties, primarily acting on the stomach and lung meridians.20,21 Its clinical applications include the treatment of febrile diseases, sore throats, dysentery, heat toxicity, and wind-heat colds. Morphologically, honeysuckle is a perennial, twining vine with opposite leaves and fragrant, tubular flowers that transition from white to yellow as they age. It is distributed across East Asia, including China, Japan, and Korea, thriving in temperate and subtropical climates. Recent pharmacological research indicates that honeysuckle exhibits anti-inflammatory, antiviral, antioxidative, antibacterial, and hepatoprotective properties.22–24 Recent research indicates that honeysuckle improves the prognosis and complications of sepsis by exerting anti-inflammatory, improving endothelial function, improving renal injury, improving myocardial injury, and regulating gastrointestinal function.25–27 However, the key components and specific mechanisms of honeysuckle in sepsis therapy are still unclear.
Sepsis pathogenesis involves complex genetic and molecular changes, making it challenging for single-target therapies to achieve optimal outcomes.28–30 Network pharmacology is extensively applied in traditional Chinese medicine research to clarify drug molecular mechanisms and aid in predicting individual drugs or compounds.31,32 It can examine the overarching relationship between drugs and diseases. This study employs molecular docking and network pharmacology to identify the bioactive ingredients, relevant targets, and signaling pathways of honeysuckle in sepsis therapy, aiming to elucidate its mechanism of action and offer insights for clinical application and research.
Methods
Identification of Bioactive Compounds in Honeysuckle
The active ingredients of honeysuckle were screened through the use of the Traditional Chinese Medicine System Pharmacology (TCMSP) database and analysis platform (https://old.tcmsp-e.com/tcmsp.php). The TCMSP platform is an integrated database designed to analyze the pharmacokinetic properties of compounds in Chinese herbal medicine. It facilitates the identification of bioactive components and their potential targets through criteria such as drug-likeness (DL) and oral bioavailability (OB). To identify the active ingredients, the term “honeysuckle” was searched within the TCMSP database. OB and DL were chosen as evaluation indicators due to their roles in the human body’s metabolism, distribution, absorption, and excretion processes. OB denotes the proportion of a drug’s effective concentration that reaches systemic circulation following oral administration. A higher OB indicates that the compound can be efficiently absorbed through the gastrointestinal tract and retain significant concentrations even after first-pass metabolism in the liver. Compounds with OB ≥ 30% generally ensure sufficient biological efficacy without requiring high doses, which is crucial for reducing toxicity and improving patient compliance. If OB is insufficient, alternative administration methods like injection or structural modifications might be required, leading to increased development costs and time. This assessment is grounded in the chemical properties of approved medications to determine if a compound adheres to druggability criteria, such as Lipinski’s Rule of Five and Veber’s Rule. Statistical analyses suggest that compounds with a DL value ≥ 0.18 are more structurally and chemically similar to known drugs. Conversely, compounds with a DL below 0.18 may fall outside the chemical space of drugs and require extensive optimization to meet drug development requirements. Selecting compounds with DL ≥ 0.18 enhances the accuracy of druggability predictions, avoiding excessive resource allocation to potential failures during early screening. Compounds with OB ≥ 30% and DL ≥ 0.18 are likely to possess favorable ADME properties and align with the chemical space criteria for drug development.
Screening Targets of Honeysuckle
The TCMSP was utilized to predict the targets of honeysuckle, which were then uploaded to the UniProt database for search, selecting Homo sapiens as the target species. The 2D structures of these active components saved in SDF format were obtained from a PubChem search for honeysuckle. The PharmMapper platform was employed to predict honeysuckle-related targets through reverse pharmacophore matching, which was also uploaded to the UniProt database for screening against human targets. Finally, the BATMAN-TCM database was used to predict high-relevance targets of honeysuckle by uploading its PubChem ID. The integration of these three methods facilitated the elimination of redundant, non-human, and unqualified targets, thereby identifying honeysuckle-related targets.
Identification of Sepsis-Related Targets
The databases GeneCards (https://www.genecards.org) and OMIM (https://www.omim.org) were searched using the keyword “Sepsis” to find target genes related to sepsis. Then, Excel software was used to summarize and analyze the relevant target genes corresponding to the disease, and duplicate genes were deleted to build the target database of sepsis. To determine the crucial intersection between honeysuckle and sepsis, the Venn website (http://bioinformatics.psb.ugent.be/webtools/Venn/) was employed.
Construction of Active Ingredient-Target Network
To identify common targets between honeysuckle and sepsis, we employed the online Venn diagram tool for visualization. The study identified common targets between sepsis and honeysuckle’s active ingredient by intersecting their respective targets and visualizing the overlap using Venn diagrams. These overlapping genes indicate potential targets of honeysuckle in the therapy of sepsis. Additionally, we predicted the target genes related to active components and sepsis and standardized their nomenclature into gene names. To build a network diagram of active components and targets, sepsis-related targets were brought into Cytoscape 3.9.1.
Construction of the Protein-Protein Interaction (PPI) Network
To establish the PPI network, we first identified the intersection of targets related to honeysuckle and sepsis. These targets were submitted to the STRING database (version 12.0), with the species restricted to “Homo sapiens” to ensure the results are specific to human biology. The STRING database calculates interactions based on experimental data, curated databases, and computational predictions, assigning confidence scores to indicate interaction reliability. To improve data reliability, we employed “Homo sapiens” PPI data with a minimum confidence score of 0.7, prioritizing the largest connected component in the PPI network. The network created by STRING was brought into Cytoscape 3.9.1 for additional analysis. Using the CytoHubba plugin in Cytoscape, we applied topological algorithms (Betweenness, Degree, and Closeness) to analyze the network’s structure and identify key targets. These algorithms evaluate node significance in a network by: 1) Degree, which counts a node’s direct connections to indicate centrality; 2) Betweenness, which gauges a node’s presence on shortest paths between others to reflect its connectivity role; and 3) Closeness, which measures a node’s communication efficiency with all other nodes. The value of these parameters indicates the importance of a node among all targets, with higher values indicating greater significance. Targets surpassing average parameters were chosen, and the CytoHubba plugin was employed to determine the leading 10 nodes according to their ranking, as previously outlined. This systematic approach ensures the identification of significant targets that are most likely to influence the pathophysiology of sepsis and the therapeutic effects of honeysuckle.
Functional Enrichment Analysis
The intersection of honeysuckle- and sepsis-related key targets were input into the DAVID database (http://david.ncifcrf.gov/), with the parameters set to human (Homo sapiens). An analysis was conducted on key targets to determine their enrichment in biological processes from Gene Ontology (GO) and pathways from KEGG. The GO biological enrichment analysis included molecular function (MF), cellular component (CC), and biological process (BP). Genes that had a P-value under 0.05 were identified, with the leading 10 from GO analysis and the leading 20 from KEGG pathway analysis chosen for more detailed exploration.
Molecular Docking of Active Ingredients with Primary Targets
A 3D structure of the active ingredient was created from its 2D structure, which was sourced from PubChem, utilizing Chem3D software version 14.0. The target protein’s crystal structure was retrieved from the PDB database (https://www.rcsb.org/) and processed in PyMOL software (version 2.5) to delete water molecules. Subsequently, AutoDock Tools software was utilized to process the target protein by adding hydrogens, charges, etc. The 2D structure of honeysuckle’s active compounds was imported into ChemBio3D software and saved in mol2 format to obtain its 3D structure. Both the target protein and original ligand were set in pdbqt format recognized by AutoDock Tools software with the original ligand’s position serving as an active pocket on the target protein. Molecular docking analysis between honeysuckle’s active ingredients and the target proteins was conducted to evaluate their affinity. The binding energy serves as a crucial indicator for assessing effective binding between ligand and receptor molecules; lower values indicate better binding performance.
Cell Culture and Reagents
The key active compounds of honeysuckle (Kaempferol, Quercetin, and Luteolin) were investigated in this research. Kaempferol (CAS No. 520–18-3), Luteolin (CAS No. 491–70-3), and Quercetin (CAS No.117–39-5) were acquired from Shanghai Haoyuan Medchemexpress Co., Ltd, China. Sigma-Aldrich in St. Louis, MO, USA, supplied LPS (L2880). The RAW264.7 murine macrophages were sourced from the Stem Cell Bank of the Chinese Academy of Sciences in Shanghai, China, and obtained from ATCC. The culture medium for the cells was DMEM with 100 μg/mL streptomycin, 2 mM L-glutamine, 50 U/mL penicillin, and 10% FBS (GIBCO), and incubated at 37°C with 5% CO2. The EZ-PCR Mycoplasma Test Kit (Beijing Novo Biotechnology Co., LTD) confirmed the absence of mycoplasma contamination. Cells were pretreated with PBS or a 160 μM solution of kaempferol, luteolin, and quercetin for 2 hours, then incubated with 100 ng/mL LPS for 6 hours. Cells and supernatants were subsequently collected for enzyme-linked immunosorbent assay (ELISA) and quantitative real-time PCR (qRT-PCR).
ELISA
An ELISA Kit from ExCell Bio in Shanghai, China, was used to measure IL-1β and IL-6 concentrations in the supernatant, following the instructions provided by the manufacturer.
qRT-PCR
The isolation of total RNA from RAW264.7 cells was carried out using TRIzol reagent (Solarbio, China) following the manufacturer’s instructions. RNA purity and concentration were assessed using ultraviolet spectrophotometry. Following extraction, the RNA was reverse-transcribed to generate complementary DNA. An RT-qPCR detection system and SYBR Premix Ex Taq II were employed for RT-qPCR assays. Normalization of mRNA expression levels was performed with β-actin as the internal control, and the 2−ΔΔCt method was used to determine relative expression levels. Table 1 presents the RT-qPCR primer sequences for IL-6, IL-1β, TP53, TLR4, and MYD88.
 |
Table 1 qRT-PCR Primer Sequences |
MTT Assay
MTT (CAS 298–93-1) from Sigma-Aldrich was dissolved in PBS at pH 7.2 to create a stock solution with a concentration of 5 mg/mL. A final concentration of 0.5 mg/mL was obtained by diluting the stock solution with RPMI 1640 medium, creating the MTT working solution. Samples were applied at different concentrations to RAW264.7 cells (5 × 10^4) seeded in 96-well plates for a duration of 6.5 hours. Following treatment, wells were rinsed with PBS and then 100 μL of MTT solution was added. To dissolve the formazan crystals, 100 µL of dimethyl sulfoxide was added to each well after a 4-hour incubation at 37 °C. Next, the plates underwent incubation for a further 10 minutes at 37 °C. To assess cell viability, absorbance was measured at 580 nm utilizing a Tecan Infinite® M1000 Microplate Reader (Tecan, Männedorf, Switzerland). To calculate cell viability percentage, the formula was applied: (%) = [100 × (sample absorbance) / (control absorbance)].
Statistical Analyses
Network pharmacology analysis involved comprehensive data processing using R software. In vitro experimental data were statistically analyzed with the help of GraphPad Prism 8.0.1 (GraphPad Software Inc., San Diego, CA, USA). A Student’s T-test compared mean values between two groups, and One-way ANOVA with Tukey’s post hoc test enabled multiple comparisons. Statistical significance is represented by a p-value of less than 0.05.
Results
Identification of Active Compounds in Honeysuckle and their Primary Target Genes
Active compounds in honeysuckle were identified using the TCMSP database. Applying the criteria of DL ≥ 0.18 and OB ≥ 30%, 23 bioactive compounds were identified (Table 2). The TCMSP database was then utilized to identify the targets linked to these active compounds, resulting in 291 unique targets after deduplication and summarization.
 |
Table 2 Active Compounds in Honeysuckle |
Acquisition of Target Genes Related to Sepsis
A total of 947 potential target genes associated with sepsis were identified by screening and removing redundant entries using the GeneCards, OMIM, DrugBank, and TTD databases. The intersection between these 947 sepsis-related targets and the 291 active ingredients-related targets yielded 78 key targets as depicted in Figure 1.
 |
Figure 1 Venn diagram of honeysuckle’s active ingredients and sepsis-related target genes. |
Construction of the PPI Networks
The STRINC11.0 database received 78 potential target genes, with Homo sapiens set as the species, for the purpose of constructing a PPI network. The network was subsequently analyzed using Cytoscape 3.9.1 software. The network analysis employed degree, betweenness, and closeness as primary criteria for node selection. Nodes were selected if their degree exceeded twice the network nodes’ median degree, their betweenness surpassed the median degree, and their closeness was greater than the median degree. The results were visualized using Cytoscape 3.9.1, as depicted in Figure 2A. There are 867 nodes and 893 edges in the network, which has an average node degree of 2.06 and a local clustering coefficient of 0.980. Using Cytoscape 3.9.1 and the NetworkAnalyzer plug-in, a PPI network diagram (Figure 2B) was created from the imported network data, focusing on node density values. A node’s size and color intensity increase with its degree. The higher the interaction score between proteins, the thicker the line connecting the nodes, and the deeper the color. The proteins TP53, TLR4, EP300, MYD88, IL1-β, FN1, CTNN1, JUN, AKT1, and CXCR4 ranked highest in degree centrality measures, including Degree, Betweenness, and Closeness (Figure 2C). TP53, TLR4, and MYD88 were pinpointed at the intersection of targets identified by the three methods used (Figure 3 and Table 3). These target proteins are likely to be an important part of the pivotal targets for honeysuckle in sepsis therapy.
 |
Table 3 Information on the Top 10 Key Targets for Honeysuckle in the Treatment of Sepsis |
 |
Figure 3 Selection of key target genes. Key targets identified by intersecting centrality measures (Degree, Closeness, and Betweenness). |
Results of GO Biological Enrichment Analysis
Using the DAVID database for GO enrichment analysis, the key targets of honeysuckle were primarily associated with 1600 biological processes (BP), 183 cellular components (CC), and 280 molecular functions (MF). Figure 4 illustrates the leading 10 biological processes (BP), cellular components (CC), and molecular functions (MF) identified by a screening criterion of P<0.05, ranked by target numbers. The BP included inflammatory and innate immune responses, cellular response to lipopolysaccharide, positive modulation of gene expression and angiogenesis, response to hypoxia, and production of tumor necrosis factor and interleukin-8. The CC included the extracellular space and region, extracellular exosome, plasma membrane’s external side, cell surface, blood microparticle, endoplasmic reticulum lumen, macromolecular complex, and platelet alpha granule lumen. The MF included receptor binding, cytokine and hormone activity, identical protein, integrin, enzyme, and protease binding, transmembrane signaling receptor activity, and protein kinase binding. The results of the GO enrichment analysis indicate that these crucial targets are mainly associated with the progression of sepsis, particularly in regulating the inflammatory response, though further experimental validation is required.
 |
Figure 4 Analysis of GO enrichment. |
Results of KEGG Pathway Enrichment Analysis
Through KEGG pathway enrichment analysis utilizing the DAVID database, 172 signaling pathways were found. We chose and displayed the top 20 signaling pathways by using a screening criterion of P < 0.05, focusing on target numbers (Figure 5). In the visualization, each bubble’s size and darkness indicate the significance and abundance of enriched key targets within the pathway. The top 20 signaling pathways encompass those associated with cancer, atherosclerosis, lipid metabolism, PI3K-Akt, cytokine-cytokine receptor interaction, NF-kappa B, neutrophil extracellular trap formation, TNF, tuberculosis, human papillomavirus, NOD-like receptor, Kaposi sarcoma-associated herpesvirus, JAK-STAT, neurodegeneration, human cytomegalovirus, Epstein-Barr virus, neuroactive ligand-receptor interaction, MAPK, influenza A, Herpes simplex virus 1, and Toll-like receptor signaling. Using Cytoscape software, a “target-pathway” network diagram was generated to visually represent the primary pathways and their associated targets (Figure 6). The development of sepsis heavily relies on the TLR signaling pathway, which is also significantly related to inflammatory infiltration. Figure 7 illustrates that TLR signaling pathways, influenced by honeysuckle’s active ingredient targets, play a role in sepsis development, as evidenced by data integration from KEGG and the Genome Encyclopedia. The Pi3k-Akt and NF-kappa B pathways play a role in creating an inflammatory microenvironment and are implicated in sepsis development. Overall, the findings indicate that honeysuckle primarily influences mechanisms related to the inflammatory response, offering potential treatment for sepsis.
 |
Figure 5 KEGG pathway enrichment analysis. |
 |
Figure 6 Figure 8 Diagram depicting the network connections between targets and signaling pathways. |
 |
Figure 7 TLR signaling pathways by which honeysuckle protects against sepsis. |
Results of Molecular Docking
The protein targets of honeysuckle’s active compounds were connected to their respective genes using the UniProt protein database. Cytoscape 3.9.1 was used to create a network diagram depicting the interactions between honeysuckle, its active components, and target genes. As illustrated in Figure 8, this network comprises a total of 315 nodes (including 1 Traditional Chinese Medicine node, 23 nodes representing active ingredients, and 291 nodes representing corresponding target proteins). Notably, kaempferol, luteolin, and quercetin showed the highest degree of centrality and stronger binding to TP53, TLR4, and MYD88 (Figure 9). The main active compounds in honeysuckle demonstrated significant binding energies with the targets TLR4 (−4.39, −4.59, and −4.81 kcal/mol), TP53 (−6.31, −4.59, and −5.05 kcal/mol), and MYD88 (−5.6, −4.84, and −4.69 kcal/mol). These results indicate robust binding interactions between honeysuckle’s main active ingredients and their target proteins. In molecular docking analyses (Figures 10–12), TP53 established hydrogen bonds with kaempferol through GLU-1575, GLU-1573, and PHE-1553 residues, whereas TLR4 interacted via the ALA-287 residue. MYD88 engaged with kaempferol through GLN-181, TRP-286, THR-287, and LYS-282 residues, forming multiple hydrogen bonding sites. Similarly, luteolin utilized GLN-181 and TYR-276 residues for binding to MYD88, while it binds to TP53 via MET1584, LEU-1547, and PHE-1553 residues, and binds to TLR4 by GLY-291, LYS-288, SER-286 residues. Quercetin interacted with TLR4 via ASN-260, LYS-288, and THR-259 residues, TP53 by GLU-1575 and SER-1554, and MYD88 through ASP-162, ARG-160, LEU-191, VAL-1, and CYS-203. These findings underscore the significant binding affinity between honeysuckle’s active components and their respective protein targets.
 |
Figure 9 Heat map displaying binding affinities between active ingredients and selected key targets based on molecular docking results. |
 |
Figure 10 Molecular docking analysis of active compounds with TP53. (A) kaempferol-TP53; (B) luteolin-TP53; (C) quercetin-TP53. |
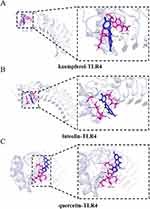 |
Figure 11 Molecular docking analysis of active compounds with TLR4. (A) kaempferol-TLR4; (B) luteolin-TLR4; (C) quercetin-TLR4. |
 |
Figure 12 Molecular docking analysis of active compounds with MYD88. (A) kaempferol-MYD88; (B) luteolin-MYD88; (C) quercetin-MYD88. |
Honeysuckle Mitigates LPS-Induced Sepsis in Macrophages by Decreasing Proinflammatory Cytokine Production
Excessive proinflammatory cytokine production during infections can trigger a cytokine storm, which significantly increases mortality in sepsis syndrome.33,34 Macrophages are essential to innate immunity, initiating inflammatory responses against diverse pathogens. To investigate the impact of honeysuckle and its active constituents on sepsis, RAW 264.7 cells were subjected to different concentrations of honeysuckle and its active components, kaempferol, luteolin, and quercetin, to assess their effects on sepsis. The MTT assay indicated that 160 μM concentrations of honeysuckle, kaempferol, luteolin, and quercetin were non-cytotoxic to RAW 264.7 cells (Figure 13A). Therefore, subsequent experiments were conducted using a concentration of 160 μM. We investigated the impact of honeysuckle and its active components on LPS-induced inflammation in RAW264.7 macrophages by assessing mRNA levels of proinflammatory cytokines. Our results demonstrated that honeysuckle and its active components significantly lowered the mRNA expression of IL-1β and IL-6 induced by LPS in RAW264.7 cells (Figure 13B–E). ELISA assays demonstrated that honeysuckle and its active components reduced LPS-induced IL-1β and IL-6 protein production in macrophages (Figure 13F–I). The results indicate that honeysuckle reduces LPS-induced inflammation by suppressing inflammatory cytokine production in both macrophages and mice.
Active Components of Honeysuckle Suppress LPS-Induced Activation of the TLR4/MYD88 Signaling Pathways
Molecular docking and network pharmacology experiments revealed a strong binding affinity between honeysuckle’s active ingredient and the key target genes TLR4, TP53, and MYD88. The planar structures of kaempferol, luteolin, and quercetin in silico active compounds are shown in Figure 14A. Prior research has emphasized the crucial involvement of TLR4, TP53, and MYD88 in sepsis development.35–37 LPS, acting as a TLR4 agonist, primarily induces proinflammatory responses via the TLR4/MYD88 signaling pathway. To validate the impact of honeysuckle’s active components on these core targets, qRT-PCR assays were conducted. The results indicated significant reductions in TLR4, TP53, and MYD88 expression levels following treatment with honeysuckle, kaempferol, luteolin, and quercetin (Figure 14B–D). The study indicates that honeysuckle’s active components alleviate LPS-induced sepsis by inhibiting the TLR4/MYD88 signaling pathways, supporting conclusions from molecular docking and network pharmacology analyses.
Discussion
Honeysuckle, a traditional tonic medicine, demonstrates anti-inflammatory, antiviral, antioxidative, and antibacterial properties.38,39 This research employed molecular docking and network pharmacology to explore the mechanisms by which honeysuckle combats sepsis. Our findings suggest that kaempferol, luteolin, and quercetin are the principal bioactive compounds responsible for honeysuckle’s therapeutic effects, particularly through their interaction with key proteins TP53, TLR4, and MYD88. These compounds play crucial roles in modulating inflammation-related pathways, as confirmed by both computational docking and experimental validation.
In this study, we collected 23 chemical components from the TCMSP database, with kaempferol, luteolin, and quercetin identified as the principal ones. These compounds target multiple nodes within the network alongside other active components. Kaempferol, luteolin, and quercetin, among the identified active ingredients, have demonstrated significant therapeutic efficacy against sepsis. A functional enrichment analysis involving 78 core targets was executed with the help of the GO and KEGG databases. Protein binding was highlighted as the most enriched GO term by the results, suggesting that honeysuckle could modulate sepsis progression by influencing protein-protein interactions. Furthermore, analysis of 172 KEGG pathways revealed enrichment of sepsis-related genes primarily within inflammatory pathways, indicating that honeysuckle’s intervention in sepsis could involve modulation of inflammation-associated signaling pathways.
We explored the molecular mechanisms by which honeysuckle may treat sepsis using network pharmacology, focusing on active ingredient and target screening within the PPI network. We identified three primary bioactive ingredients and their corresponding representative targets. Specifically, kaempferol, luteolin, and quercetin were molecularly docked with TP53, TLR4, and MYD88 proteins to validate our network pharmacology predictions. The docking results confirmed that these ingredients bind effectively to the proteins. Additionally, our findings demonstrated that honeysuckle mitigates LPS-induced sepsis in macrophages and mice by reducing proinflammatory cytokine production. Kaempferol, luteolin, and quercetin, the active components of honeysuckle, inhibit LPS-induced activation of the TLR4/MYD88 signaling pathways. TP53, a well-documented tumor suppressor protein, has been increasingly recognized for its role in regulating immune responses. Recent studies have highlighted TP53’s involvement in modulating immune response and ferroptosis, which are critical during sepsis.40 For example, p53 is closely associated with poor outcomes in sepsis and exerts an immunosuppressive effect by promoting the expansion of functional Tregs during the development of sepsis.41 Similarly, TLR4 and MYD88, as pivotal components of innate immunity, initiate macrophage proinflammatory cascades and regulate macrophage polarization during sepsis.42,43 Suppression of these targets has been shown to alleviate sepsis-related organ dysfunction, consistent with the findings of our study. Kaempferol, luteolin, and quercetin exhibited high docking affinities with TP53, TLR4, and MYD88, suggesting their potential to disrupt the progression of sepsis by modulating these proteins. Comparatively, prior studies have shown that traditional Chinese medicines mitigate sepsis-related multiple organ dysfunction in rats by modulating the inflammatory response and macrophage activation via the TLR4/MYD88 signaling pathways. For instance, Xijiao Dihuang decoction was reported to reduce sepsis-induced cardiac inflammation by inhibiting TLR4/NF-κB signaling and modulating cytokine expression.44 Similarly, Wang et al demonstrated that puerarin attenuates sepsis-induced multiple-organ injury and Inflammatory response via regulation of TLR and MAPK signaling.45 In line with these studies, our findings revealed that honeysuckle’s active components inhibit the TLR4/MYD88 pathway, a key mediator of proinflammatory cytokine release in sepsis. This consistency reinforces the role of honeysuckle as an anti-sepsis agent targeting inflammatory signaling.
This study has certain limitations. Our research on honeysuckle’s effects on sepsis employed modern bioinformatics methods, such as network pharmacology and molecular docking. The existing network information technology necessitates further enhancement, and the precision and promptness of database information require scientific verification. Our analysis may exclude compounds or targets that are unconfirmed or unrecorded. While luteolin, kaempferol, and quercetin were identified as the main bioactive ingredients of honeysuckle for sepsis treatment, they do not fully encapsulate the plant’s therapeutic potential. Therefore, additional pharmacodynamic and molecular biology experiments are necessary to expand our current findings. Furthermore, some of our results have limited precedent in previous studies. The absence of prior explanations and validations regarding the effects and mechanisms of these potential active ingredients on sepsis presents substantial opportunities for future research.
Conclusion
The research utilized network pharmacology to determine the active compounds and crucial targets of honeysuckle in addressing sepsis. Molecular docking analysis demonstrated a high binding affinity between honeysuckle’s active compounds and key targets, including TP53, TLR4, and MYD88. In vitro experiment results indicated that honeysuckle has a protective effect against sepsis by reducing proinflammatory cytokine production. Additionally, the active compounds in honeysuckle (kaempferol, luteolin, and quercetin) exhibit therapeutic potential by inhibiting the TLR4/MYD88 signaling pathways. These findings highlight the significance of understanding honeysuckle’s mechanisms, providing valuable insights for developing new therapeutic strategies for sepsis management.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Li R, Li X, Zhao J, et al. Mitochondrial STAT3 exacerbates LPS-induced sepsis by driving CPT1a-mediated fatty acid oxidation. Theranostics. 2022;12(2):976–998.
2. Meyhoff TS, Hjortrup PB, Wetterslev J, et al. Restriction of intravenous fluid in ICU patients with septic shock. N Engl J Med. 2022;386(26):2459–2470.
3. Vincent JL. Current sepsis therapeutics. EBioMedicine. 2022;86:104318.
4. Barichello T, Generoso JS, Singer M, Dal-Pizzol F. Biomarkers for sepsis: more than just fever and leukocytosis-a narrative review. Crit Care. 2022;26(1):14.
5. Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–272.
6. Xie J, Wang H, Kang Y, et al. The epidemiology of sepsis in Chinese ICUs: a national cross-sectional survey. Crit Care Med. 2020;48(3):e209–e218.
7. Fleiss N, Schwabenbauer K, Randis TM, Polin RA. What’s new in the management of neonatal early-onset sepsis? Arch Dis Child Fetal Neonatal Ed. 2023;108(1):10–14. doi:10.1136/archdischild-2021-323532
8. Kheterpal S, Singh K, Topol EJ. Digitising the prediction and management of sepsis. Lancet. 2022;399(10334):1459. doi:10.1016/S0140-6736(22)00658-4
9. Mushtaq A, Kazi F. Updates in sepsis management. Lancet Infect Dis. 2022;22(1):24. doi:10.1016/S1473-3099(21)00773-8
10. Sands K, Carvalho MJ, Portal E, et al. Characterization of antimicrobial-resistant gram-negative bacteria that cause neonatal sepsis in seven low- and middle-income countries. Nat Microbiol. 2021;6(4):512–523. doi:10.1038/s41564-021-00870-7
11. Thomson KM, Dyer C, Liu F, et al. Effects of antibiotic resistance, drug target attainment, bacterial pathogenicity and virulence, and antibiotic access and affordability on outcomes in neonatal sepsis: an international microbiology and drug evaluation prospective substudy (BARNARDS. Lancet Infect Dis. 2021;21(12):1677–1688.
12. Solomon S, Akeju O, Odumade OA, et al. Prevalence and risk factors for antimicrobial resistance among newborns with gram-negative sepsis. PLoS One. 2021;16(8):e0255410.
13. Wang XH, Xu DQ, Chen YY, et al. Traditional Chinese medicine: a promising strategy to regulate inflammation, intestinal disorders and impaired immune function due to sepsis. Front Pharmacol. 2022;13:952938.
14. Wu X, He C, Liu C, et al. Mechanisms of JinHong formula on treating sepsis explored by randomized controlled trial combined with network pharmacology. J Ethnopharmacol. 2023;305:116040.
15. Song Y, Lin W, Zhu W. Traditional Chinese medicine for treatment of sepsis and related multi-organ injury. Front Pharmacol. 2023;14:1003658.
16. Fan TT, Cheng BL, Fang XM, Chen YC, Su F. Application of Chinese medicine in the management of critical conditions: a review on sepsis. Am J Chin Med. 2020;48(6):1315–1330. doi:10.1142/S0192415X20500640
17. Lim CL. Heat sepsis precedes heat toxicity in the pathophysiology of heat stroke-a new paradigm on an ancient disease. Antioxidants. 2018;7(11):149.
18. Shang X, Pan H, Li M, Miao X, Ding H. Lonicera japonica Thunb.: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J Ethnopharmacol. 2011;138(1):1–21. doi:10.1016/j.jep.2011.08.016
19. Li W, Zhang L, He P, et al. Traditional uses, botany, phytochemistry, and pharmacology of Lonicerae japonicae flos and Lonicerae flos: a systematic comparative review. J Ethnopharmacol. 2024;322:117278. doi:10.1016/j.jep.2023.117278
20. Feng YF, Qin GZ, Jing ZX, Wang YH, Zhou YY. Anaphylaxis effect and substance basis of honeysuckle extract. Chin Herb Med. 2021;13(3):403–409. doi:10.1016/j.chmed.2021.04.013
21. Xu X, Xu H, Shang Y, et al. Development of the general chapters of the Chinese Pharmacopoeia 2020 edition: a review. J Pharm Anal. 2021;11(4):398–404. doi:10.1016/j.jpha.2021.05.001
22. Guo L, Qiao J, Zhang L, et al. Critical review on anthocyanins in blue honeysuckle (Lonicera caerulea L.) and their function. Plant Physiol Biochem. 2023;204:108090. doi:10.1016/j.plaphy.2023.108090
23. Du XQ, Shi LP, Cao WF, Chen ZW, Zuo B, Hu JY. Add-on effect of honeysuckle in the treatment of coronavirus disease 2019: a systematic review and meta-analysis. Front Pharmacol. 2021;12:708636. doi:10.3389/fphar.2021.708636
24. Wang H, Tian L, Han Y, Ma X, Hou Y, Bai G. Mechanism assay of honeysuckle for heat-clearing based on metabolites and metabolomics. Metabolites. 2022;12(2);121.
25. Feng J, Liu Z, Chen H, et al. Protective effect of cynaroside on sepsis-induced multiple organ injury through Nrf2/HO-1-dependent macrophage polarization. Eur J Pharmacol. 2021;911:174522. doi:10.1016/j.ejphar.2021.174522
26. Palíková I, Valentová K, Oborná I, Ulrichová J. Protectivity of blue honeysuckle extract against oxidative human endothelial cells and rat hepatocyte damage. J Agric Food Chem. 2009;57(15):6584–6589. doi:10.1021/jf9003994
27. Ma J, Miao Y, Li J, et al. Incorporation of blue honeysuckle juice into fermented goat milk: physicochemical, sensory and antioxidant characteristics and in vitro gastrointestinal digestion. Foods. 2022;11(19):3065. doi:10.3390/foods11193065
28. van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17(7):407–420. doi:10.1038/nri.2017.36
29. Huang M, Cai S, Su J. The pathogenesis of sepsis and potential therapeutic targets. Int J Mol Sci. 2019;20(21):5376. doi:10.3390/ijms20215376
30. Zhang YY, Ning BT. Signaling pathways and intervention therapies in sepsis. Signal Transduct Target Ther. 2021;6(1):407. doi:10.1038/s41392-021-00816-9
31. Nogales C, Mamdouh ZM, List M, Kiel C, Casas AI, Schmidt H. Network pharmacology: curing causal mechanisms instead of treating symptoms. Trends Pharmacol Sci. 2022;43(2):136–150. doi:10.1016/j.tips.2021.11.004
32. Zhao L, Zhang H, Li N, et al. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J Ethnopharmacol. 2023;309:116306. doi:10.1016/j.jep.2023.116306
33. Athale J, Busch LM, O&apos NP. Grady, cytokine release syndrome and sepsis: analogous clinical syndromes with distinct causes and challenges in management. Infect Dis Clin North Am. 2022;36(4):735–748. doi:10.1016/j.idc.2022.07.001
34. Li XY, Liu M, Fu YJ, Jiang YJ, Zhang ZN. Alterations in levels of cytokine following treatment to predict outcome of sepsis: a meta-analysis. Cytokine. 2023;161:156056. doi:10.1016/j.cyto.2022.156056
35. Ye W, Liu X, Bai Y, et al. Sepsis activates the TLR4/MyD88 pathway in Schwann cells to promote infiltration of macrophages, thereby impeding neuromuscular function. Shock. 2021;55(1):90–99. doi:10.1097/SHK.0000000000001557
36. Yang Q, Wang Y, Cao G, Li X, Zhao T. Anti-sepsis effect of Xiaochaihu decoction based on the TLR4/MyD88/NF-κB signalling pathway. Heliyon. 2024;10(5):e26712. doi:10.1016/j.heliyon.2024.e26712
37. Sun M, Li J, Mao L, et al. p53 deacetylation alleviates sepsis-induced acute kidney injury by promoting autophagy. Front Immunol. 2021;12:685523. doi:10.3389/fimmu.2021.685523
38. Jurikova T, Rop O, Mlcek J, et al. Phenolic profile of edible honeysuckle berries (Genus Lonicera) and their biological effects. Molecules. 2011;17(1):61–79. doi:10.3390/molecules17010061
39. Golubev D, Zemskaya N, Shevchenko O, et al. Honeysuckle extract (Lonicera pallasii L.) exerts antioxidant properties and extends the lifespan and healthspan of Drosophila melanogaster. Biogerontology. 2022;23(2):215–235. doi:10.1007/s10522-022-09954-1
40. Gao N, Tang AL, Liu XY, Chen J, Zhang GQ. p53-Dependent ferroptosis pathways in sepsis. Int Immunopharmacol. 2023;118:110083. doi:10.1016/j.intimp.2023.110083
41. Zhang H, Wu T, Ren C, Dong N, Wu Y, Yao Y. p53 promotes the expansion of regulatory T cells via DNMT3a- and TET2- mediated Foxp3 expression in sepsis. Burns Trauma. 2023;11:tkad021. doi:10.1093/burnst/tkad021
42. Hering M, Madi A, Sandhoff R, et al. Sphinganine recruits TLR4 adaptors in macrophages and promotes inflammation in murine models of sepsis and melanoma. Nat Commun. 2024;15(1):6067. doi:10.1038/s41467-024-50341-w
43. Li X, Li X, Huang P, et al. Acetylation of TIR domains in the TLR4-Mal-MyD88 complex regulates immune responses in sepsis. EMBO j. 2024;43(21):4954–4983. doi:10.1038/s44318-024-00237-8
44. Li W, Lin M, Li J, et al. Xijiao Dihuang decoction protects against murine sepsis-induced cardiac inflammation and apoptosis via suppressing TLR4/NF-κB and activating PI3K/AKT pathway. J Inflamm Res. 2024;17:853–863. doi:10.2147/JIR.S428305
45. Wang L, Liang Q, Lin A, et al. Puerarin increases survival and protects against organ injury by suppressing NF-κB/JNK signaling in experimental sepsis. Front Pharmacol. 2020;11:560. doi:10.3389/fphar.2020.00560
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Anti-Inflammatory Effects and Molecular Mechanisms of Shenmai Injection in Treating Acute Pancreatitis: Network Pharmacology Analysis and Experimental Verification
He Y, Hu C, Liu S, Xu M, Liang G, Du D, Liu T, Cai F, Chen Z, Tan Q, Deng L, Xia Q
Drug Design, Development and Therapy 2022, 16:2479-2495
Published Date: 2 August 2022
Metabolomics Combined with Network Pharmacology-Based Strategy to Reveal the Underlying Mechanism of Zhenhuang Submicron Emulsion in Treating Oropharyngeal Mucositis Complications of Radiation Therapy for Head and Neck Cancer
Chen W, Li C, Jin D, Shi Y, Zhang M, Bo M, Qian D, Wang M, Li G
Drug Design, Development and Therapy 2022, 16:3169-3182
Published Date: 17 September 2022
A Novel Approach Based on Gut Microbiota Analysis and Network Pharmacology to Explain the Mechanisms of Action of Cichorium intybus L. Formula in the Improvement of Hyperuricemic Nephropathy in Rats

Amatjan M, Li N, He P, Zhang B, Mai X, Jiang Q, Xie H, Shao X
Drug Design, Development and Therapy 2023, 17:107-128
Published Date: 20 January 2023
Network Pharmacology and Experimental Validation to Explore That Celastrol Targeting PTEN is the Potential Mechanism of Tripterygium wilfordii (Lév.) Hutch Against IgA Nephropathy

Zhao J, Liu H, Xia M, Chen Q, Wan L, Leng B, Tang C, Chen G, Liu Y, Zhang L, Liu H
Drug Design, Development and Therapy 2023, 17:887-900
Published Date: 23 March 2023
Integration of Network Pharmacology, Transcriptomics, and Metabolomics Strategies to Uncover the Mechanism of Chaihuang Qingfu Pill in Treating Sepsis-Induced Liver Injury
Zhang C, Chen F, Jiang Y, Deng J, Yan X, Yin X, Su B, Liu W
Drug Design, Development and Therapy 2025, 19:4665-4688
Published Date: 2 June 2025





