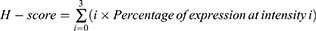Back to Journals » International Journal of Nanomedicine » Volume 20
Investigating the Therapeutic Potential of Sericin Nanofibers and Rice-Encapsulated Nanosericin for Psoriasis: Mechanistic Insights from a 3D Skin Model
Authors Sukphopetch P, Aramwit P, Reamtong O, Thiangtrongjit T, Kanjanapruthipong T, Muangkaew W, Kengkoom K, Fongsodsri K, Ampawong S
Received 27 November 2024
Accepted for publication 18 March 2025
Published 7 April 2025 Volume 2025:20 Pages 4257—4284
DOI https://doi.org/10.2147/IJN.S508995
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lijie Grace Zhang
Passanesh Sukphopetch,1 Pornanong Aramwit,2– 4 Onrapak Reamtong,5 Tipparat Thiangtrongjit,5 Tapanee Kanjanapruthipong,6 Watcharamat Muangkaew,1 Kanchana Kengkoom,7 Kamonpan Fongsodsri,6 Sumate Ampawong6
1Department of Microbiology and Immunology, Faculty of Tropical Medicine, Mahidol University, Ratchathewi, Bangkok, 10400, Thailand; 2Department of Pharmacy Practice, Faculty of Pharmaceutical Sciences and Center of Excellence in Bioactive Resources for Innovative Clinical Applications, Chulalongkorn University, Pathum Wan, Bangkok, 10330, Thailand; 3The Academy of Science, The Royal Society of Thailand, Dusit, Bangkok, 10330, Thailand; 4Faculty of Pharmacy, Silpakorn University, Nakhon Pathom, 73000, Thailand; 5Department of Molecular Tropical Medicine and Genetics, Faculty of Tropical Medicine, Mahidol University, Ratchathewi, Bangkok, 10400, Thailand; 6Department of Tropical Pathology, Faculty of Tropical Medicine, Mahidol University, Ratchathewi, Bangkok, 10400, Thailand; 7Independent Researcher, Thaweewatthana, Bangkok, 10170, Thailand
Correspondence: Sumate Ampawong, Department of Tropical Pathology, Faculty of Tropical Medicine, Mahidol University, 420/6 Ratchawithi Road, Ratchathewi, Bangkok, 10400, Thailand, Tel + 662 3549100, Fax + 662 6447938, Email [email protected]
Purpose: Psoriasis, a chronic inflammatory skin disorder affecting 2– 3% of the global population, presents significant treatment challenges, including high recurrence, adverse effects, and socioeconomic burdens. This study explores the therapeutic potential of sericin-based nanofibers and rice-encapsulated nanosericin (ReS) as innovative treatments, aiming to address current limitations by enhancing drug delivery, stability, and efficacy, and providing a targeted approach to managing this complex condition.
Methods: This study investigates the efficacy of sericin nanofibers and derivatives, including ReS, for psoriasis treatment using a 3D artificial human skin model. Comprehensive evaluations were conducted through histopathological, immunohistochemical, molecular, and proteomic analyses.
Results: Results showed that desolvation with glutaraldehyde crosslinking produced stable nanofibrils, while desolvation without crosslinking yielded nanogranules; nanoforms demonstrated high biocompatibility and safety. Treatments with sericin, rice extract, nanosericin, and ReS alleviated psoriasis-induced histopathology, with downregulation of IL-1β, WNT, and β-defensin particularly in the ReS and rice extract groups, suggesting an immunomodulatory effect. Caspase-3 reduction was more pronounced in the sericin and nanosericin groups. Proteomic analysis revealed notable exosomal protein involvement, with sericin modulating cell death through the PAK-2p34 pathway and proteasome activity, while nanosericin enhanced glycolysis and gluconeogenesis via exosomal proteins. Both ReS and nanosericin activated antioxidant pathways, mediated by upregulation of TGF-β and Nrf-2, respectively, especially sericin-based treatment through the selenoamino acid metabolism pathway. ReS further reduced keratinocyte differentiation by targeting cornified envelope proteins, correlating with reduced WNT expression. Gene expression analysis confirmed anti-inflammatory effects and skin barrier restoration, as evidenced by decreased S100-family proteins and increased filaggrin, caspase-14, and involucrin.
Conclusion: ReS and nanosericin show significant therapeutic potential for psoriasis by targeting immunomodulatory pathways, modulating keratinocyte activity, reducing oxidative stress, and enhancing skin barrier restoration. Future research should optimize scalability, assess long-term safety, and explore synergistic effects with existing therapies, while further investigating molecular mechanisms for targeted treatment advancements.
Keywords: psoriasis, sericin, nanotherapeutics, rice extract, 3D skin model, proteomics
Graphical Abstract:

Introduction
Psoriasis is a chronic skin disease caused by immune system dysfunction, involving both innate and adaptive immune responses, which leads to abnormal growth of epidermal cells, particularly keratinocytes.1 The prevalence of psoriasis varies significantly across different regions, with estimates as high as 8.5% among adults and 2.1% among children globally. In certain Northern European countries, prevalence rates may reach up to 11%.2–5 In Thailand, the incidence is estimated at about 1–2% of the population, with psoriasis vulgaris or plaque psoriasis being the most commonly observed type compared to other forms.6–8 Despite available treatments—including topical agents, phototherapy, and systemic therapies—effective management remains challenging due to the chronic and recurrent nature of the disease, compounded by treatment-related complications, adverse effects, and substantial socioeconomic and lifestyle burdens for patients. Natural extracts present a promising alternative, potentially reducing adverse effects, enhancing therapeutic efficacy, and improving long-term outcomes.
To explore novel therapeutic options, we evaluated the efficacy of sericin and rice extract (Oryza sativa L.: SRNC05053-6-2) in psoriasis treatment using both in vivo and in vitro models. Our results indicate that sericin and rice extract creams significantly alleviate psoriatic skin histopathological changes. Sericin demonstrated anti-inflammatory effects by inhibiting C-C motif chemokine 20 (CCL20) and pro-inflammatory cytokines derived from Th17 cells through modulation of the Janus Kinase-Signal Transducer and Activator of Transcription (JAK-STAT) signaling pathway.9 Additionally, sericin was found to modulate immune responses by upregulating galectin-3, downregulating sphingosine-1-phosphate lyase 1 (Sgpl1), and regulating keratinocyte proliferation through nucleoside diphosphate kinase B (Nme2). Rice extract reduced psoriasis severity by preventing epidermal cell apoptosis through caspase-3 modulation, increasing anti-inflammatory cytokines (IL-10, TGF-β), and decreasing pro-inflammatory cytokines (IL-6, IL-8, IL-20, IL-22, TNF-α), chemokines (CCL-20), and antimicrobial peptides (psoriasin, β-defensin).6 It also promoted the expression of genes critical to psoriatic healing, including caspase-14, involucrin, filaggrin, psoriasin, β-defensin, and koebnerisin isoforms. To further optimize treatment suitability, we developed sericin-based formulations such as thin polymeric films using photolithography10 and hydrogel sheets,11 which exhibited comparable efficacy by modulating the mTOR pathway and Th17 differentiation signaling. These formulations acted through mechanisms involving proliferative proteins, antimicrobial peptides, apoptotic markers, chemokines, pro-inflammatory cytokines, inflammatory modulators, and keratinization-associated proteins. Our findings indicate that each therapeutic formulation provides a multifaceted approach to psoriasis treatment. Each material demonstrates distinct therapeutic properties through various mechanisms, including anti-inflammatory effects, modulation of immune responses, restoration of the skin barrier, and promotion of skin repair. These advancements in formulation development represent promising alternative strategies that could complement existing psoriasis therapies, while addressing the limitations inherent in current treatment modalities.
Sericin’s versatility as a natural, biocompatible polymer with excellent crosslinking properties has been well-documented in various biomedical applications, including tumor inhibition, antimicrobial activity, antioxidation, and wound healing. Efforts to enhance its stability, functionalization, and drug delivery capacity have led to the development of sericin-based nano-medicines, such as nanoparticles, micelles, films, hydrogels, and sericin hybrids.12 The process of creating silk-based nanoparticles is straightforward and can be achieved through methods such as desolvation, self-assembly, and salt-out. However, each method has its own advantages and disadvantages. Several studies have been conducted with the main objective of exploring the versatility of sericin-based nano-formulations and their potential applications in various biomedical fields. Currently, sericin is being developed into nano-formulations for preclinical studies in various forms, such as sericin-chitosan used as drug carriers for anticancer agents like doxorubicin, which increases anticancer efficacy while reducing toxicity, especially to the heart.13 Poly-L-lysine-coated sericin nanoparticles improve cellular adhesion and proliferation,14 while sericin-poly(ethylcyanoacrylate) nanospheres enhance gastrointestinal absorption and reduce lipid levels.15 Sericin-folate nanoparticles optimize anticancer drug delivery with enhanced hemocompatibility,14 and sericin-silver nanoparticles exhibit improved antimicrobial efficacy with reduced toxicity.16 Moreover, sericin nanoparticles facilitate tumor inhibition and the targeted delivery of active compounds such as turmeric extract.17,18
Despite extensive research on nanosericin, its application in psoriasis treatment remains limited, and no prior studies have explored the combination of rice extract with nanosericin. This study hypothesizes that sericin-based nanofibers, including rice-encapsulated nanosericin (ReS), could provide a targeted and effective approach to alleviating psoriasis by modulating immune pathways, keratinocyte activity, and restoring the skin barrier. Leveraging 3D cell culture technology, which offers a more accurate representation of human skin compared to traditional two-dimensional models,19–21 this approach enables in-depth studies of disease pathophysiology and treatment evaluation by replicating the skin’s complex architecture and cellular interactions.
Using a full-thickness 3D reconstituted human skin model, the study developed and evaluated novel sericin nanofibers and ReS formulations through histopathological, immunohistochemical, molecular, and proteomic analyses. These findings underscore the potential of these innovative formulations as adjuncts to existing therapies, aiming to enhance patient outcomes and reduce psoriasis’s disease burden. This research offers a novel, evidence-based framework for psoriasis management, presenting promising alternative treatments for both patients and clinicians.
Materials and Methods
Preparation of Rice Extract Encapsulated with Sericin Nanoform
Rice (Oryza sativa L.: SRNC05053-6-2) Extraction
Rice (Oryza sativa L.: SRNC05053-6-2), grown through organic practices, incompliance with the standards of the Ministry of Agriculture and Cooperatives, Thailand, was used for this study. This study utilized the rice extraction method previously established and validated in our earlier research.6 Briefly, the rice was husked once, immersed in ethanol and then subjected to extraction using a high-speed herb extractor (Model HX-50) provided by Thai-China Flavors and Fragrances Industry Co., Ltd. (TCFF). The extract was evaluated for key active components including anthocyanin, vitamin E, vitamin B, and antioxidant activity, to ensure the research materials met quality standard. These analyses were conducted at Central Laboratory (Thailand) Co., Ltd., and the Institute of Nutrition, Mahidol University, Thailand.
Sericin Extraction
Sericin was extracted following the standard protocol routinely practiced in our laboratory, using silk cocoons from the Bombyx mori species, supplied by Chul Thai Silk Co., Ltd.10,11,22–24 The process involved cleaning the cocoons, cutting them into small pieces, and then immersing them in distilled water at a 1:50 (w/v) ratio. The mixture was autoclaved at 120°C for 60 minutes under 15 psi pressure. Following this, the solution was filtered through Whatman filter paper No. 42 to eliminate impurities and fibers, and then stored in a desiccator at ambient temperature. To ensure quality control, each batch of sericin extract was analyzed for amino acid composition, including Serine, Aspartic acid, and Glycine, at the Central Laboratory (Thailand) Co., Ltd., maintaining a 3:2:1 ratio.
Nanosericin Formation
Desolvation with Glutaraldehyde Crosslinking
Nanosericin formation and its derivative was adapted from the study by Bhuyan et al,17 where 6% sericin was desolvated with 80% ethanol (v/v) in a 1:4 ratio. 80% ethanol was gradually added during stirring at 700–800 rpm for 1 hour. The mixture was then crosslinked by adding a glutaraldehyde solution to achieve a final concentration of 2% and stirred at 200 rpm overnight at room temperature. Following this, the mixture was centrifuged at 10,000 rpm for 10 minutes to collect the nanosericin particles. The glutaraldehyde was detoxified with a 5 mg/mL glycine solution, performing this process at least 3–5 times with shaking. The precipitate was washed with sterile distilled water at least 3–5 times with shaking. Finally, the nanosericin particles were preserved in 80% ethanol until needed for further studies.
One-Step Desolvation
Desolvation of 1% sericin with 80% ethanol (v/v) was performed in a 1:4 ratio by gradually adding 80% ethanol during stirring at 700–800 rpm for 1 hour. The mixture was then centrifuged at 1000 rpm for 10 minutes to remove larger particles. The supernatant was collected and centrifuged at 10,000 rpm for 10 minutes to precipitate the sericin nanoparticles. The nanoparticle sediment was washed with sterile distilled water at least 3–5 times with shaking and then stored in 80% ethanol to preserve its structure until further studies.
Physical Characterization of Sericin Nanoform
To confirm that sericin from both methods was in nanoform with proper dispersion, its physical characteristics, including shape and size, were evaluated using scanning electron microscopy (SEM). Nano-sediment from two different preparation methods was treated with 2.5% glutaraldehyde in 0.1 M sucrose phosphate buffer (SPB) at pH 7.4 for 1 hour. The fixed samples were then spread on glass coverslips and dried using a critical point drying machine (Leica, EM CPD300, Germany). Following drying, a 10 nm gold–palladium film was applied using a coating device (Quorum, Q150R-S Plus, UK). These samples were then analyzed for nano-formation using an SEM (Jeol, JSM-6610LV, Japan). The preparation method’s suitability was also assessed to ensure it produced a sufficient quantity of sericin nanoparticles for the entire research project.
Rice Extract Encapsulated with Sericin Nanofibers (ReS)
To determine the appropriate release dose of rice extract-encapsulated sericin nanofibers (ReS) for antipsoriatic studies, as described in Chemical Characterization of ReS, the selected sericin nanoform was washed 3–5 times with sterile distilled water under constant shaking. The nanosericin, at concentrations of 5, 2.5, and 1.25 mg/mL, were then soaked in rice extract solutions at 15%, 7.5%, and 3.75% (w/v), respectively, and shaken overnight at 4°C. Following this, the samples were centrifuged at 10,000 rpm for 10 minutes. The ReS were stored at 4°C and used in subsequent steps within 6 hours. All procedures were performed under sterile conditions.
Chemical Characterization of ReS
To estimate the amount of rice extract released after soaking with different concentrations of ReS, as described in Rice Extract Encapsulated With Sericin Nanofibers (ReS), colorimetric spectrophotometry was applied, following the method reviewed by Tena, Martín, and Asuero (2020).25 The ReS were resuspended in sterile distilled water. Measurements were taken at various time intervals—5, 10, 15, 20, 30, 45, 60, 120 minutes, and 24 hours—using a microplate reader (Biotek synergy H1, USA) at 540 nm. A standard curve of rice extract concentration and optical density levels was created for calculation.
Cytotoxicity Assay for ReS
To assess the safety and determine the optimal concentrations for further studies of the prepared substances—nanosericin, ReS, sericin, and rice extract—a cytotoxicity assay was performed using the HaCaT cell line (Cat. No.: 300493, CLS Cell Lines Service, Germany). HaCaT cells are spontaneously transformed keratinocytes derived from histologically normal human skin and serve as a key target cell in psoriasis. In summary, the extracts, in at least five different concentrations, were tested for cellular viability using the MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Sigma, USA) to assess their toxicity at the mitochondrial and plasma membrane levels. Concentrations that maintained approximately 95% (high viability), 80% (moderate viability), and 60% (low viability) keratinocyte cell viability were selected for subsequent experiments. These criteria were adopted from Wu et al, 2024,26 and Mosmann, 1983.27 The selected dose ranges were based on their relevance to: (i) the dose-response relationship, enabling clear characterization of cellular changes after treatment; (ii) therapeutic window identification, allowing the determination of the threshold at which a compound transitions from therapeutic to toxic effects; and (iii) robust statistical evaluation, providing distinct and measurable endpoints.
Bacterial and Fungal Contaminations in ReS
To examine the sterility of nanosericin and ReS concerning fungal and bacterial contamination, a microbiological study was conducted by cultivating HaCaT cells with the mentioned substances at concentrations that allowed 80% cell viability. The cells were incubated for 3 and 7 days, and during these intervals, the supernatants from the cultures were collected and plated on specialized fungal and bacterial media to detect any potential contamination. For bacterial testing, small aliquots of each sample were aseptically inoculated onto Luria Agar (LA) for broad range of bacterial contaminants. The inoculated plates were incubated at 37°C for 24–48 hours under aerobic conditions and observed for any bacterial growth.28 For fungal contamination, the samples were inoculated onto Potato Dextrose Agar (PDA), a medium specifically designed to support the growth of fungi. The PDA plates were incubated at 25–28°C for 5–7 days, during which they were monitored daily for fungal colony formation. All inoculations and incubations were conducted under sterile conditions to prevent environmental contamination.29,30
Antipsoriatic Property of ReS
3D Reconstituted Human Psoriasis Skin Cultures
To investigate the antipsoriatic properties of ReS (40, 80, and 160 µg/mL) in comparison with nanosericin (150, 300, and 600 µg/mL), sericin (50 µg/mL), rice extract (400 µg/mL), standard treatment (Xamiol, containing calcitriol 1 µg/g, vitamin D3 50 µg/g, and betamethasone 0.5 µg/g),6 and control groups (untreated psoriatic skin and normal skin), a full-thickness 3D reconstituted human skin model of psoriasis (SOR-300-FT) and its control (SOR-300-FT-CTR) (MatTek Corporation, USA) were used. The experiment was performed in triplicate for each group. Three-dimensional (3D) cell cultures, arranged as a hanging membrane, were maintained in a 24-well plate under conditions recommended by the manufacturer and in accordance with established protocols from our previous studies.6 Briefly, prior to the experiment, the cells were stabilized at 37°C with 5% CO2 for at least 24 to 48 hours. During the experiment, the media (SOR-300-FT-MM and SOR-300-FT-CTR-MM for psoriatic and normal skin, respectively), containing different extract forms, were replaced every 3 days. After 7 days, samples were collected. Each full-thickness artificial skin sample was divided into three parts: one was preserved in 10% neutral buffer formalin (NBF) for histopathological and immunohistochemical analyses, another in RNAlater (Thermo Scientific, USA) at −20°C for gene expression evaluation, and the remainder stored at −80°C for proteomics studies.
Histopathological and Immunohistochemical Studies
To assess the therapeutic efficacy of ReS in mitigating psoriasis relative to other referenced substances, a histopathological analysis was performed following the established protocols previously employed by our research team in prior investigations.6,10,11 This focused on the pathological changes between treated and untreated psoriatic artificial skin samples, with an emphasis on the occurrence of acanthosis with elongation of rete ridges, indicated by epidermal thickening. Additionally, the expression levels of specific immunological markers were measured using immunohistochemistry staining.
Artificial skin samples were fixed in 10% NBF for 24 hours. The fixed samples then underwent standard tissue processing, which included dehydration in a graded ethanol series, infiltration with graded paraffin in xylene, and embedding in pure paraffin. The paraffin-embedded samples were sectioned at a thickness of 5 µm and stained with hematoxylin and eosin (H&E). Stained sections were examined under a light microscope (BX51, Olympus, Japan) equipped with a digital camera system (DP70, Olympus, Japan). Epidermal thickness, defined as the distance from the stratum basale to the stratum corneum, was measured using Image J software (version 1.5J8, NIH). Measurements were performed on five randomly selected images per sample using a 40× objective lens.
To elucidate the mechanisms underlying the immunomodulatory effects of ReS in psoriasis, an immunohistochemical analysis was conducted. Rabbit polyclonal antibodies from MyBioSource Inc., USA, were used as primary antibodies to assess (i) inflammatory markers, including β-defensin (MBS1490249), tumor necrosis factor (TNF)-α (MBS820357), interleukins (IL)-1β (MBS127158), IL-6 (MBS2001862), IL-8 (MBS9607651), IL-17 (MBS2032548), IL-21 (MBS8247452), IL-22 (MBS8305903), and CC chemokine ligand (CCL)-20 (MBS631195); (ii) anti-inflammatory responses, using transforming growth factor (TGF)-β (MBS8242488); (iii) antioxidative effects, as indicated by nuclear factor erythroid 2-related factor (Nrf)-2 (MBS9608128); (iv) cellular degeneration and apoptosis, via caspase-3 (MBS9700614); and (v) cellular proliferation, using wingless-related integration site (WNT) (MBS1497402). Tissue processing and immunostaining protocols were conducted in accordance with the methodologies outlined in our recent studies.6,10,11,31 Briefly, artificial skin paraffin sections from each group were first deparaffinized with xylene, then underwent antigen retrieval using a heat-induced method in citrate buffer (pH 6). Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol, and non-specific binding was prevented with a protein block serum-free solution (Dako, Denmark). The artificial skin paraffin sections were incubated with primary antibodies at 1:100 dilution in PBS with 1% normal goat serum and further incubated with a labeled polymer HRP anti-mouse/rabbit EnVision kit (Dako, Denmark). Visualization was achieved using diaminobenzidine (DAB) (Dako, Denmark), and the tissue was counterstained with hematoxylin and permanently mounted with DPX (Electron Microscopy Sciences; EMS, USA).
The expression levels of proteins in the epidermal area were semi-quantified using the H-score, which ranges from 0 to 300, where 300 represents strong expression and 0 indicates no expression, as described in our previous studies.6,10,11,23,24,32 The H-score combines both the percentage of expression and the intensity of staining to provide a comprehensive assessment of protein immunolabeling. Epidermal areas in each sample group were measured by capturing at least five images per group at a resolution of 640×480 pixels (400x magnification) using a light microscope (BX51: Olympus, Japan) equipped with a digital camera system (DP70: Olympus, Japan). A morphometric analysis was performed to quantify the percentage of protein expression per field using ImageJ software (version 1.5J8, NIH). In brief, the color images were converted to 8-bit grayscale using the “image-type-8 bit” mode, and the positively stained areas were highlighted as black pixels. The number of black pixels was then calculated in “analyze-measure” mode as the area fraction limited to the threshold, providing the percentage of expression. Additionally, the intensity of immunolabeling in each skin sample was assessed using a grading score with four levels: (i) 0 = no staining, (ii) 1 = low expression, (iii) 2 = medium expression, and (iv) 3 = high expression. The H-score, which was used to compare differences between groups, is calculated using the formula;
Where i is the intensity score (0–3).
Effect of ReS on the mRNA Expressions
To evaluate the efficacy of the extract in alleviating psoriasis, the expression levels of specific gene markers associated with disease severity, such as Filaggrin (FLG), Caspase-14, Involucrin, Psoriasin-hS100A7 (S100A7), Koeberisin 15S-hS100A15S (S100A15S), and Koebnerisin 15L-hS100A15L (S100A15L), were measured using qRT-PCR, following to our previous studies.6,10,23,24 In this study, mRNA was extracted from artificial skin treated with different test substances using the RNeasy Mini Kit (Qiagen, Canada). The samples were homogenized in 600 μL of lysis buffer, followed by centrifugation. The supernatant was transferred to a spin column where the RNA was bound, washed several times with buffer, and eluted in 35 μL of RNase-free water. RNA concentrations were measured with a NanoDrop™ 2000/2000c spectrophotometer (Thermo Scientific, USA). RT-qPCR was then conducted using iTaq™ Universal SYBR Green Supermix (BIO‐RAD, USA) with primers listed in Table 1. All samples were analyzed with the CFX96 Touch™ Real-time PCR detector, and gene expression was quantified using the 2−∆∆Ct method. Finally, gene expression levels were compared across the different groups.
 |
Table 1 Primer Sequences for qRT-PCR |
Proteomics Study
This study employed proteomics techniques to discover the relationship between different forms of sericin—traditional, nanoform, and nanoform designed to absorb rice extract—and their therapeutic effects on psoriasis in a full-thickness 3D reconstituted human skin model. A label-free proteomic approach was conducted, as described in our previous studies.24
3D Skin Protein Extraction
Artificial skin samples from each group were solubilized in 500 μL of lysis buffer containing 1% SDS, 1% Triton-X, and 0.5% sodium chloride, followed by ultrasonication on ice for 2 minutes. After centrifugation at 10,000 × g for 10 minutes at 4°C, the supernatant was collected, and the total protein concentration was measured using the Bradford protein assay (Bio-Rad, USA).
Label-Free Proteomic Analysis
The proteins were precipitated in ice-cold acetone (1:5 v/v) by centrifugation and then reconstituted in 0.25% RapidGest SF (Waters, USA) in 15 mM ammonium bicarbonate (Sigma-Aldrich, USA). For each group, 60 µg of protein was prepared for gel-free digestion. Sulfhydryl bond reduction was carried out with 5 mM DTT (Sigma-Aldrich, USA) in 15 mM ammonium bicarbonate at 72°C for 1 hour, followed by sulfhydryl alkylation with IAA (Sigma-Aldrich, USA) in 15 mM ammonium bicarbonate at room temperature for 30 minutes in the dark. The solution was then desalted using a Zeba Spin Desalting Column (Thermo Scientific, USA), digested with trypsin (Promega, USA), and incubated at 37°C for 3 hours. After digestion, the samples were dried and reconstituted in 0.1% formic acid for analysis by LC–MS/MS. The experiment was conducted in three biological replicates.
LC–MS/MS data were acquired in positive mode using an HF-X Hybrid Quadrupole-Orbitrap™ Mass Spectrometer coupled with an EASY-nLC1000 nano-LC system fitted with a nano-C18 column. The mobile phase consisted of 0.1% formic acid (Phase A) and 90% acetonitrile with 0.1% formic acid (Phase B). Samples were loaded onto an analytical C18 column for analysis.
Protein Identification and Quantification
Raw mass spectra (raw files) were analyzed with MaxQuant v2.4.2.0, using the UniProt human database (www.uniprot.org) for protein identification. The analysis parameters included a 20 ppm MS tolerance, 0.05 Da MS/MS tolerance, trypsin as the digestion enzyme, cysteine carbamidomethylation as a fixed modification, and methionine oxidation as a variable modification. A false discovery rate of 1% was applied for peptide and protein identification. Proteins with significant changes (p < 0.05) and fold changes ≥2.0 for up-regulation or ≤0.9 for down-regulation were categorized by their biological functions using the UniProt database and PubMed. Protein interactions were further explored using STRING v12.0, developed by the Global Biodata Coalition and ELIXIR Core Data Resources (www.string-db.org).
Statistical Analysis
Statistical analyses were conducted using SPSS Statistics software version 18. A power analysis was performed to ensure adequate sample size, with a minimum power of 80% to detect significant differences. Data are expressed as mean ± standard error of the mean (SEM). Statistical comparisons were made using parametric tests, including independent t-tests and one-way analysis of variance (ANOVA) for normally distributed data, and non-parametric tests, such as the Mann–Whitney U-test and Kruskal–Wallis test, for data that did not meet normality assumptions. Significance was set at p < 0.05.
Results
Physical Property of Sericin Nanofibers
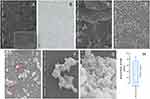
This study utilized two methods for preparing nanosericin particles: desolvation with glutaraldehyde cross-linking and one-step desolvation. Experimental results indicated that the first method yielded a higher amount of nanosericin, approximately 4–5 grams, compared to only 50–60 milligrams obtained from the second method. This suggests that the first method should be selected for future nanosericin preparation in this research project to ensure sufficient quantity. Physical properties in terms of shape, size, and dispersion were examined to confirm the nanosericin characteristics. Under SEM, the rice extract showed a spherical shape with sizes ranging from 10 to 200 µm, with relatively uniform distribution (Figure 1A). In contrast, the sericin extract displayed an irregular, sawdust-like sheet shape with a length range of 2–200 µm and an uneven distribution (Figure 1B and C).
Nanosericin prepared using the one-step desolvation method yielded small, spherical particles with five size distributions: 50, 100, 125, 250, and 600 nm, along with uniform dispersion (Figure 1D and E). Meanwhile, preparing nanosericin from sericin extract via desolvation with glutaraldehyde cross-linking produced nanosericin with a nanofibril structure, forming a mesh-like network with a sponge-like aggregate and consistent distribution. The nanofibrils had fiber diameters of 60–90 nm, and mesh pore sizes ranged from 50 to 150 nm (Figure 1F–H).
Chemical, Cytotoxicity, and Sterility Properties of Nanosericin and ReS
Apart from the main amino acid contents in sericin, the active components of ReS, as detailed in Table 2, confirm its antioxidant properties and key chemical constituents, including anthocyanins, biotin, cobalamin, pantothenic acid, iron, zinc, potassium and calcium. Following the encapsulation of rice extract within nanosericin particles, this study employed colorimetric spectrophotometry to quantitatively assess the release profile of rice extract over various time intervals: 5, 10, 15, 20, 30, 45, 60, and 120 minutes, extending up to 24 hours. The results demonstrated that nanosericin particles-initiated rice extract release within the first 5 minutes of dissolution in the solvent, with an initial concentration measured at 1000 µg/mL. Subsequently, the concentration increased incrementally, reaching a maximum of approximately 1500 µg/mL by 30 minutes, and maintained a stable concentration up to the 24-hour mark (Figure 2A and B). These findings indicate that the nanosericin particles prepared in this study exhibit a rapid initial release within 5 minutes, followed by sustained concentration stability over a 24-hour period.
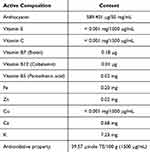 |
Table 2 Active Composition of Materials Using for the Preparation of Rice (Oryza sativa L.: SRNC05053-6-2) Extract Encapsulated With Sericin Nanofibers |
To evaluate the safety of various extract forms—specifically, rice extract, sericin, nanosericin, and ReS—this study determined concentration levels suitable for assessing each extract’s efficacy and specific mechanisms in mitigating psoriasis using an artificial human skin model. The findings showed that concentrations maintaining at least 80% cellular viability in keratinocyte cells were as follows (Figure 2C–F): sericin at 60–65 µg/mL, nanosericin at 300 µg/mL, rice extract at 370–400 µg/mL, and ReS at 80–100 µg/mL. These results indicate that nanoscale modification of sericin markedly reduces its cytotoxicity, enhancing cell compatibility by up to fivefold.
To assess bacterial and fungal contamination in various test substances, samples were dissolved in cell culture media and co-incubated with keratinocyte cells. After 3 and 7 days of incubation—aligned with the media change schedule and investigation of specific effects and mechanisms related to psoriasis mitigation in the artificial human skin model—the cell culture media were evaluated for fungal and bacterial contamination. The results indicated that rice extract, sericin, nanosericin particles, and ReS showed no evidence of fungal or bacterial contamination at any testing stage.
Nanosericin and ReS Effects on Epidermal Thickening in Psoriatic Skin
The treated artificial skin from each experimental group was subjected to pathological examination, with a focus on identifying acanthosis with elongation of rete ridges and hyperkeratosis with parakeratosis, characterized by increased epidermal thickness, as detailed in Figure 3A–L. The findings demonstrated that all test substances—excluding ReS at a concentration of 160 µg/mL—including the reference treatment (calcitriol), rice extract, sericin, ReS at concentrations of 40 and 80 µg/mL, and nanosericin at concentrations of 150, 300, and 600 µg/mL, significantly attenuated pathological changes in epidermal thickness associated with psoriasis, as compared to untreated skin, which exhibited markedly increased epidermal depth. Notably, when compared to normal skin, only the skins treated with calcitriol, rice extract, ReS at 40 µg/mL, and nanosericin at 150 µg/mL achieved a reduction in epidermal thickness that was statistically indistinguishable from normal skin.
Evaluation results of epidermal thickness across test substances in their nanoparticle forms indicated that ReS-treated groups at all concentrations exhibited significantly greater thickness than the rice extract group; however, at the 40 µg/mL concentration, no significant difference was observed compared to the calcitriol treatment. Additionally, while no significant differences in epidermal thickness were observed between sericin and nanosericin treatments at any concentration, both exhibited significantly greater thickness compared to the rice extract group. Notably, epidermal thickness in the nanosericin-treated skin at 150 µg/mL remained statistically comparable to that of the standard treatment group.
Antipsoriatic Effects of Nanosericin and ReS on the Expressions of β-Defensin, TNF-α, IL-1β, Caspase-3, and WNT
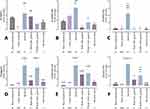
To investigate the mechanisms underlying the therapeutic effects of various test substances on psoriasis in a more complex manner, this study employed immunohistochemistry techniques to indicate the expression levels of proteins associated with the pathogenesis of psoriasis both in the epidermis and dermis. This includes examining the induction of cell death, the efficacy of antioxidant activity, the ability to reduce inflammatory levels, and mechanisms related to the stimulation of abnormal cell proliferation. The study found that all test substances significantly reduced β-defensin expression levels compared to the untreated group, reaching levels similar to or indistinguishable from normal skin, except for the group treated with nanosericin at concentrations of 150 and 300 µg/mL. Notably, in groups treated with rice extract and ReS at all three concentrations, as well as with nanosericin at 300 µg/mL, the expression level of β-defensin was not significantly different from that in the calcitriol-treated group, although there was a slight upward trend (Figure 4Ai-xii). However, the expression level of TNF-α did not differ significantly among the test groups in this study, despite a trend indicating higher expression levels in the untreated group and the group treated with ReS at 40 µg/mL compared to others (Figure 4Bi-xii).
Additionally, the results showed that IL-1β expression levels were significantly reduced in all experimental groups compared to the untreated group (Figure 4Ci-xii). Meanwhile, IL-6 expression levels in the groups treated with nanosericin at concentrations of 150 and 300 µg/mL were significantly lower compared to the untreated group, as well as those treated with calcitriol, rice extract, sericin, ReS at all concentrations, and 600 µg/mL of nanosericin. This expression was also not different from that observed in normal skin. It is also noteworthy that IL-6 expression in the ReS groups at 40 and 80 µg/mL was significantly higher than that observed in the rice extract group (Figure 4Di-xii). The expression level of caspase-3 in the groups treated with sericin, ReS at 40 µg/mL, and nanosericin at 150 and 300 µg/mL was significantly lower than in the untreated group, reaching levels close to those found in normal skin. Additionally, it was observed that caspase-3 expression increased in the groups treated with higher concentrations of ReS and nanosericin (Figure 5Ai-xii).
The groups treated with nanosericin at concentrations of 300 and 600 µg/mL showed significantly higher Nrf-2 expression compared to the untreated group, with levels trending higher than those in other treatment groups, particularly rice extract, sericin, and ReS at 40 µg/mL (Figure 5Bi-xii). For TGF-β expression, the results indicate that the ReS group at 40 µg/mL had slightly higher levels than the untreated group, but expression decreased at higher concentrations. In contrast, higher concentrations of nanosericin tended to induce increased TGF-β expression, especially at 600 µg/mL (Figure 5Ci-xii). Additionally, WNT expression was significantly reduced in all treatment groups compared to the untreated group (Figure 5Di-xii).
In this study, IL-8 and IL-21 expression levels were low across all groups, with no significant differences between them (Figure 6Ai-xii and Bi-xii). Conversely, although IL-17, IL-22, and CCL-20 exhibited more noticeable expression levels, the values varied widely, preventing clear distinctions among test groups regarding their effectiveness in alleviating psoriasis (Figure 6Ci-xii – Ei-xii). However, there was a trend suggesting that higher concentrations of nanosericin may induce increased expression of IL-17, IL-22, and CCL-20.
Proteomics Studies
A comparative analysis was conducted on the differential expression of proteins involved in psoriasis treatment across test groups that showed promising results in prior experiments. Specifically, comparisons were made between (i) 50 µg/mL of sericin and 150 µg/mL of nanosericin, (ii) 40 µg/mL of ReS and 150 µg/mL of nanosericin, and (iii) 40 µg/mL of ReS and the untreated control, to elucidate the distinct molecular mechanisms of different extract formulations in alleviating psoriasis. The study identified a total of 418 proteins in the group treated with 50 µg/mL of sericin compared to 150 µg/mL of nanosericin, with 125 proteins exhibiting statistically significant differential expression. Further analysis based on fold-change thresholds (≥2-fold upregulation and ≤0.9-fold downregulation) yielded 67 upregulated and 34 downregulated proteins (Table 3).
Similarly, a total of 418 proteins were identified in the group treated with 40 µg/mL of ReS compared to 150 µg/mL of nanosericin, with only 69 proteins showing significant differential expression. Based on fold-change thresholds for upregulation and downregulation, the number of differentially expressed proteins was further refined to 18 upregulated and 29 downregulated proteins (Table 4). Additionally, a total of 461 proteins were identified in the group treated with 40 µg/mL of ReS compared to the untreated group, with only 48 proteins showing significant differential expression. Based on fold-change thresholds for upregulation and downregulation, the number of differentially expressed proteins was refined to 8 upregulated and 31 downregulated proteins (Table 5).
Proteomic analysis revealed that the fold-change values indicating changes in protein expression levels for each comparison group showed an increase in protein expression in the groups of (i) 50 µg/mL of sericin compared to 150 µg/mL of nanosericin, (ii) 40 µg/mL of ReS compared to 150 µg/mL of nanosericin, and (iii) 40 µg/mL of ReS compared to the untreated group, with ranges of 2.02–7.75, 2.01–22.8, and 2.07–7.7, respectively. Meanwhile, proteins with decreased expression levels were in the ranges of 0.02–0.9, 0.08–0.89, and 0.88–0.09, respectively, indicating substantial levels of change. Considering the protein datasets with both upregulated and downregulated expression across each comparison pair, the overall analysis reveals that the downregulated protein set had a higher proportion meeting the thresholds for % coverage (≥ 10%) and protein score (≥ 25) than the upregulated set, with rates of 71–91% and 12–38%, respectively. Here, % coverage indicates the proportion of the protein detected and identified in the analysis, while protein score reflects the confidence level of the results—a higher score indicates greater reliability or likelihood that the identified protein matches the specified type. Additionally, the protein dataset identified several proteins potentially involved in the pathogenesis of psoriasis, such as S100-A6, S100-A7, S100-A8, S100-A11, involucrin, caspase-14, filaggrin, cornifin-A, and various large ribosomal subunit proteins.
Protein Expression Analysis in 50 µg/mL Sericin Versus 150 µg/mL Nanosericin Treatment Groups
The analysis of protein-to-protein interactions revealed that, when comparing protein expression levels in skin treated with 50 µg/mL of sericin versus 150 µg/mL of nanosericin, the upregulated proteins were predominantly those functioning as extracellular exosomes. Additionally, proteins involved in innate immune response were also notably increased. An interesting finding is the identification of two main clusters of proteins with high interaction levels: one consisting of proteasome-related proteins and the other of cytoplasmic ribosome proteins. Both clusters are closely associated with key mechanisms relevant to the pathogenesis of psoriasis, specifically: (i) regulation of activated PAK-2p34, which is linked to cell death, proliferation, survival, and cellular stress responses, involving proteins such as PSMA2, PSMA7, PSMA8, PSMB2, PSMB3, PSMC5, and PSMD3; and (ii) selenocysteine synthesis, related to antioxidant mechanisms, comprising proteins like RPL23, RPL13A, RPL19, RPLP1, RPS25, and RPLP0 (Figure 7A).
The set of proteins with decreased expression primarily consisted of those functioning as extracellular exosomes, followed by proteins involved in the innate immune response, showing a distribution pattern similar to that of the upregulated proteins mentioned above. However, in this group, only one main cluster of proteins with high interaction was identified, closely related to the mechanisms of glycolysis and gluconeogenesis. This cluster includes ENO1, LDHA, PGAM1, PGAM2, PGK1, P4HB, TPI1, and GAPDH. Additionally, the analysis revealed a smaller set of proteins with lower interaction levels but significant relevance to psoriasis, specifically involved in keratinocyte growth in the epidermis through mechanisms associated with the function of the cornified envelope and serpin conserved site. These proteins include KRT6B, FLG2, IVL, CASP14, DSP, and SERPINB3 (Figure 7B).
Protein Expression Analysis in 40 µg/mL ReS Versus 150 µg/mL Nanosericin Treatment Groups
When comparing protein expression levels in skin treated with 40 µg/mL ReS versus 150 µg/mL nanosericin, the upregulated proteins, though few in number, predominantly consisted of those functioning as extracellular exosomes. This was followed by proteins involved in the innate immune response, as before. Additionally, a small cluster of proteins with limited interactions was identified, including RARS1, RPS11, RPS26, and RPLP0. This group is closely associated with the mechanism of selenoamino acid metabolism, which functions in antioxidant activity (Figure 8A).
In this comparison set, downregulated proteins, extracellular exosome and innate immune response proteins were still observed, showing an overall pattern similar to the previously mentioned groups. Additionally, an extra group of proteins related to the cornified envelope was identified, including KRT6A, KRT6C, KRT5, KRT14, KRT16, KRT17, IVL, and DSP. These proteins were found to form a single cluster with high interaction, closely associated with the mechanism of keratinocyte differentiation. Furthermore, the experiment also revealed another group of proteins with moderately high interactions, consisting of proteins functioning as extracellular exosomes and those involved in the innate immune response (Figure 8B).
Protein Expression Analysis in Artificial Skin Treated with and without 40 µg/mL ReS
When comparing protein expression levels in skin treated with 40 µg/mL ReS versus the untreated group, it was found that the upregulated proteins were very few, and these proteins showed minimal interaction. Meanwhile, the downregulated proteins were mostly extracellular exosome proteins and those related to the function of the cornified envelope. The proteins functioning as extracellular exosomes exhibited moderate interaction, while the proteins associated with the cornified envelope, including KRT5, KRT6A, KRT8, KRT17, KRT16, KRT80, KRT14, IVL, and KLK7, showed high interaction levels and were closely associated with the mechanism of keratinocyte differentiation. This pattern was similar to that observed in the comparison between skin treated with 40 µg/mL ReS and 150 µg/mL nanosericin (Figure 8C).
Nanosericin and ReS Effect on Gene-Related Psoriasis
To evaluate the expression levels of genes indicating psoriasis severity, such as Filaggrin (FLG), Caspase-14, Involucrin, Psoriasin-hS100A7 (S100A7), Koebnerisin 15S-hS100A15S (S100A15S), and Koebnerisin 15L-hS100A15L (S100A15L), this research measured the mRNA levels of these genes using qRT-PCR. The analysis results showed that the mRNA expression of the S100A7 gene in skin treated with rice extract was significantly lower than in the group treated with 50 µg/mL sericin and 40 µg/mL ReS. When compared with the proteomics study results, although there was no significant difference in gene expression between the 50 µg/mL sericin and 150 µg/mL nanosericin groups, the experiment showed a tendency for skin treated with 50 µg/mL sericin to have higher S100A7 gene expression than the group treated with 150 µg/mL nanosericin (Figure 9A). Additionally, the expression levels of S100A15S and S100A15L in the skin treated with 50 µg/mL sericin were significantly higher than in the other groups (Figure 9B and C).
The expression patterns of the Filaggrin, Caspase-14, and Involucrin genes (Figure 9D–F) were similar, showing that skin treated with 50 µg/mL sericin and 150 µg/mL nanosericin generally had higher expression levels compared to skin treated with other test substances. Additionally, when considering groups with the inclusion of the rice extract in nano-formulation, there was a tendency for an increase in the expression levels of these three genes, though not as much as in the skin treated with 50 µg/mL sericin and 150 µg/mL nanosericin.
Discussion
The present study aimed to develop a novel sericin-based nanoformulation combined with a natural anti-psoriatic agent, specifically crude rice extract (Oryza sativa L.: SRNC05053-6-2), to alleviate psoriasis symptoms through a targeted therapeutic approach tailored to address the complex pathophysiology of the disease. Our findings demonstrated that desolvation of sericin with glutaraldehyde crosslinking resulted in the formation of nanofibrils, while non-crosslinked desolvation produced nanogranules under sterile conditions, free from microbial contamination. These observations underscore the critical role of glutaraldehyde in stabilizing sericin molecules via covalent bonding, leading to the creation of a more ordered, fibrillar structure, whereas the absence of crosslinking produced weaker, spherical nanogranules. These results align with prior studies that highlight the influence of crosslinking agents like glutaraldehyde on the morphology of protein-based nanoparticles, facilitating the formation of nanofibrils.33 By contrast, self-assembled silk sericin under non-crosslinked conditions often yields spherical forms.34 This distinction emphasizes the versatility and precise structural control achievable through crosslinking.
Our findings reveal that nanosericin particles exhibit a release profile characterized by a rapid initial burst followed by sustained concentration stability over 24 hours. This “burst release” is driven by rice extract molecules loosely bound to or near the particle surface, which diffuse readily upon dissolution, providing an immediate therapeutic effect.12,18 The subsequent sustained release results from the gradual diffusion of encapsulated rice extract through the crosslinked sericin matrix, which acts as a diffusion barrier. This controlled release is influenced by factors such as the degree of crosslinking, particle size, and matrix porosity, ensuring a steady and prolonged therapeutic effect.13,15 Consistent with previous studies, the crosslinking not only enhances the structural stability of the nanoparticles but also minimizes swelling, making nanosericin an ideal candidate for psoriasis treatment by delivering continuous therapeutic benefits with reduced dosing frequency.33,34
In addition, the present study also observed that nanosericin exhibits greater safety for HaCaT cells compared to its traditional format, suggesting enhanced biocompatibility and reduced cytotoxic effects. The improved safety profile may result from its smaller size and increased surface area, facilitating better cellular uptake and interaction. Unlike traditional formulations, which can aggregate and create localized high concentrations leading to cytotoxicity, nanosericin allows for more uniform distribution and controlled release. Previous studies support these findings, indicating that nanoparticle formulations enhance biocompatibility and reduce toxicity to skin cells.35–37 This trend highlights the potential of nanosericin as a safer alternative for dermatological applications.
To evaluate the efficacy of these formulations on psoriasis treatment, we utilized a full-thickness 3D reconstituted human skin model of psoriasis (SOR-300-FT) alongside its control (SOR-300-FT-CTR), offering a robust platform for assessing skin response to treatment. Histopathological studies revealed that psoriatic skin treated with calcitriol, 400 µg/mL of rice extract, 40 µg/mL of ReS, and 150 µg/mL of nanosericin exhibited a significant reduction in epidermal thickness, comparable to that of normal skin. This suggests the efficacy of these formulations in normalizing the hyperproliferation commonly observed in psoriasis.
Supporting these findings, our immunohistochemical analyses indicated that all of the test substances effectively reduced inflammation and inhibited the proliferation of epidermal cells,10,11 as evidenced by the downregulations of IL-1β and WNT, respectively. Furthermore, a marked reduction in the antimicrobial peptide β-defensin, a key pro-inflammatory amplifier and immune activator in psoriasis,38 was particularly noted in the rice extract and ReS treatment groups compared to the sericin and nanosericin groups. This reduction likely indicates that rice extract modulates the immune response and suppresses inflammation-driven pathways associated with psoriasis. Notably, ReS treatment was also effective in reducing β-defensin levels, similar to rice extract, suggesting retention of rice’s anti-inflammatory properties.
In contrast, the treatment groups receiving sericin and nanosericin exhibited a more pronounced reduction in the expression of caspase-3, a protein involved in regulating cell death that significantly increases in severe grades of psoriasis,39 compared to those treated with rice extract and ReS. This suggests that sericin plays a critical role in regulating cell death processes, which is essential for maintaining skin barrier function, thereby rendering it particularly beneficial for chronic skin conditions such as psoriasis. Moreover, the antioxidant properties of the formulations were highlighted by increased expression of Nrf-2, while the anti-inflammatory effects were demonstrated by elevated TGF-β expression,6,10,11 both of which were enhanced in the nanoformulation of nanosericin and ReS, respectively. The nanoparticle formulation likely improves cellular uptake and bioavailability, resulting in more potent therapeutic effects.12 Thus, the abilities of nanosericin and ReS to enhance Nrf-2 and TGF-β expression, respectively, suggest their crucial role in reducing oxidative stress, a known contributor to psoriasis pathogenesis.40,41
Proteomic analysis was employed to investigate the specific mechanisms by which each test substance alleviates psoriasis. We focused on proteins whose abundance changed across different treatment groups, including ReS at 40 µg/mL, sericin at 50 µg/mL, and nanosericin at 150 µg/mL. Notably, the majority of these proteins function as extracellular exosomes and play a key role in regulating innate immunity-driven inflammatory mechanisms. Exosomes, known for their role in intercellular communication, could facilitate the transfer of these regulatory proteins between keratinocytes and immune cells, thereby promoting skin recovery and mitigating the chronic inflammatory response in psoriasis.42–47
In terms of protein-protein interactions, sericin’s primary mechanism for reducing psoriasis severity involves regulating cell death by upregulating proteasome proteins. These proteins are responsible for degrading damaged or misfolded proteins, specifically enhancing the activity of the ‘regulation of activated p21-activated kinase (PAK)-2p34 pathway. By controlling apoptosis via PAK-2p34,48 sericin is postulated to restore the balance between cell survival and death, inhibit caspase-3 activation in keratinocytes, and mitigate oxidant-induced cell death through keratinocyte growth factor. These effects ultimately reduce the excessive proliferation of skin cells that characterizes psoriatic lesions. Sericin’s therapeutic effect on psoriasis also involves enhancing antioxidant defenses through the selenocysteine synthesis pathway, the predominant form of the antioxidant trace element selenium. This is achieved via the upregulation of cytoplasmic ribosomal proteins and extracellular exosomes, indicating that exosomes may act as critical mediators in the regulation of apoptosis and antioxidant activity during sericin treatment.
For nanosericin, its efficacy in psoriasis treatment is primarily driven by enhancing cellular metabolism, particularly through the upregulation of glycolysis and gluconeogenesis in skin cells, mediated by increased extracellular exosome proteins. Psoriasis involves metabolic reprogramming, notably an increased reliance on glycolysis and disruptions in the TCA cycle, amino acid, and fatty acid metabolism.49 Nanosericin’s ability may regulate these metabolic shifts presents a promising therapeutic strategy for managing psoriasis, potentially reducing hyperproliferation and inflammation while improving skin homeostasis with minimal side effects. Its nanoparticle form also likely offers enhanced potency and bioavailability compared to traditional sericin.35–37
Furthermore, when rice extract was encapsulated in nanosericin, its antioxidant effects remained consistent, primarily through the selenoamino acid metabolism pathway mediated by exosome upregulation. Exosomes likely help deliver antioxidant enzymes and protective molecules, enhancing anti-inflammatory and skin-protective effects.50 Interestingly, proteomic analysis also showed that ReS effectively reduced keratinocyte differentiation by decreasing proteins linked to the cornified envelope, more so than nanosericin or untreated groups. This modulation of keratinocyte differentiation, crucial for restoring skin barrier function, aligns with the observed reduction in antimicrobial peptides and WNT levels in the ReS-treated groups.
Moreover, the proteomic analysis also identified several proteins pivotal to the therapeutic effects observed in psoriasis treatment, including members of the S100A family, filaggrin, caspase-14, and involucrin. These proteins are central to the structural integrity, inflammation, and barrier function of the skin, all of which are dysregulated in psoriasis.6,10,11 The consistency between proteomic findings and psoriasis-related gene expression profiles obtained via qRT-PCR further underscores their relevance.
The S100A protein family plays a key role in psoriasis pathogenesis, particularly in inflammation and keratinocyte proliferation. Notably, converting sericin into its nanoparticle form significantly reduced the expression of S100A-6 and S100A-7 in psoriatic skin. S100A-7 (psoriasin) is a biomarker associated with keratinocyte differentiation and psoriasis-involved skin severity,31,51 while S100A-6 (calcyclin) is a calcium-binding protein involved in cell stress, proliferation, and differentiation.52 This reduction suggests that nanosericin may dampen inflammation, as elevated S100A-7 is linked to increased inflammation and keratinocyte activation in psoriasis. Rice extract alone similarly reduced S100A-6 and S100A-7 expression, but when combined with nanosericin, it unexpectedly increased S100A-7, indicating a potential antagonistic interaction.
Conversely, the combination of rice extract and nanosericin proved beneficial for other members of the S100A family, particularly S100A-8 and S100A-11, whose expression was significantly reduced in the ReS treatment group compared to nanosericin alone. S100A-8 (MRP8) is a calcium- and zinc-binding protein that plays an important role in the molecular damage pattern, especially in psoriatic keratinocytes.51,53 S100A-11, another calcium-binding protein, contributes to enhanced cell infiltration and the IL-17 signaling pathway,54 which is a main mechanism for keratinocyte proliferation in psoriasis.10 This suggests that rice extract may provide additional anti-inflammatory benefits by targeting these proteins involved in leukocyte recruitment and keratinocyte activation.
Additionally, the expression of filaggrin, caspase-14, and involucrin, crucial for skin barrier function and keratinocyte differentiation, was elevated in both sericin and nanosericin treatment groups. This elevation supports skin recovery in psoriasis by maintaining the skin barrier and preventing excessive keratinocyte turnover.6,10,11 While encapsulating rice extract within nanosericin slightly reduced their expression, levels remained higher than in the untreated group, indicating that nanosericin’s beneficial effects on barrier function are largely preserved in this combination therapy.
As discussed above, the overall findings of this study can be summarized in the graphical abstract, which clearly illustrates that the key therapeutic properties of each treatment for psoriasis include anti-inflammatory effects, anti-proliferative activity, antioxidant properties, restoration of cell survival and death balance, enhanced skin recovery, and regulation of metabolic shifts. These findings, consistent across both the current study and previous research by the team, demonstrate that therapeutic formulations showing efficacy in psoriasis treatment share a common mechanism: the reduction of critical pathophysiological changes, such as epidermal thickening resulting from hyperproliferation of keratinocytes, acanthosis, and parakeratosis, albeit to varying extents. The differences among the formulations lie in the specific mechanisms that confer their distinct properties. For example, sericin’s mechanism in reducing disease severity involves the modulation of cell survival and death through the PAK-2p34 pathway, mediated by caspase-3. When formulated as nanoparticles, sericin also enhances disease improvement through the regulation of metabolic shifts, alongside a synergistic mechanism of antioxidant activity via selenoamino acid synthesis in both sericin and ReS. Conversely, rice extract in a nanovehicle formulation exerts its therapeutic effects primarily by modulating keratinocyte proliferation through the reduction of cornified envelope proteins and S100A6 & S100A7 genes, in addition to lowering WNT levels, which is observed in conventional rice extract. An important consideration is the variation in host responses, as evidenced by the distinct mechanisms of action observed across different models (in vitro vs in vivo). This suggests that the clinical outcomes of these therapeutic forms in clinical trials may vary based on detailed mechanistic actions. Therefore, the development of new therapeutic options for psoriasis remains valuable in addressing current treatment limitations, and ongoing research is essential for advancing these formulations.
Conclusion
In summary, our study underscores the therapeutic potential of sericin-based nanoformulations, particularly nanosericin and ReS, in treating psoriasis. These formulations significantly reduce inflammation, modulate keratinocyte activity, mitigate oxidative stress, and enhance metabolic function, all of which contribute to improved disease management. Our findings highlight the ability of these targeted nanotechnologies to address key pathological mechanisms in psoriasis, offering a promising alternative to current treatments. This work positions nanosericin and ReS as valuable candidates for advancing psoriasis therapy, with the potential to offer clinicians and patients more effective, tailored treatment options. Future research should focus on optimizing the clinical scalability of these formulations, assessing their long-term safety and efficacy in large animal models, and exploring potential synergistic effects with existing therapies. Further investigation into the molecular mechanisms driving their therapeutic action will be essential to fully harness the promise of these nanoformulations and expand their clinical application in psoriasis.
Data Sharing Statement
The data sets used in the current study may be shared upon a reasonable request to Sumate Ampawong, Ph.D.
Funding
This research project has been funded by Mahidol University (Fundamental Fund: fiscal year 2024 by National Science Research and Innovation Fund (NSRF)): SA and partially supported by Health Systems Research Institute (HSRI 67-027): PS.
Disclosure
The authors declare no competing interests.
References
1. Monteleone G, Pallone F, MacDonald TT, Chimenti S, Costanzo A. Psoriasis: from pathogenesis to novel therapeutic approaches. Clin Sci. 2011;120(1):1–11. doi:10.1042/CS20100163
2. Damiani G, Bragazzi NL, Karimkhani Aksut C, et al. The global, regional, and national burden of psoriasis: results and insights from the global burden of disease 2019 study. Front Med Lausanne. 2021;8:743180. doi:10.3389/fmed.2021.743180
3. Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385. doi:10.1038/jid.2012.339
4. Sewerin P, Brinks R, Schneider M, Haase I, Vordenbaumen S. Prevalence and incidence of psoriasis and psoriatic arthritis. Ann Rheum Dis. 2019;78(2):286–287. doi:10.1136/annrheumdis-2018-214065
5. Egeberg A, Andersen YMF, Thyssen JP. Prevalence and characteristics of psoriasis in Denmark: findings from the Danish skin cohort. BMJ Open. 2019;9(3):e028116. doi:10.1136/bmjopen-2018-028116
6. Ampawong S, Kengkoom K, Sukphopetch P, et al. Evaluating the effect of rice (Oryza sativa L.: SRNC05053-6-2) crude extract on psoriasis using in vitro and in vivo models. Sci Rep. 2020;10(1):17618. doi:10.1038/s41598-020-74634-4
7. Prasunnakarn P. Prevalence and incidence of psoriasis in Udonthani Hospital. Udonthani Hospital Med J. 2017;25(2):143–150.
8. Rajatanavin N, Wongpraparut C, Rattanakaemakorn P, et al. Expert opinion on psoriasis management, 2020 and beyond. Thai J Dermatol. 2022;38(1):1–16.
9. Rujimongkon K, Ampawong S, Reamtong O, Buaban T, Aramwit P. The therapeutic effects of Bombyx mori sericin on rat skin psoriasis through modulated epidermal immunity and attenuated cell proliferation. J Tradit Complement Med. 2021;11(6):587–597. doi:10.1016/j.jtcme.2021.06.007
10. Aramwit P, Fongsodsri K, Tuentam K, et al. Sericin coated thin polymeric films reduce keratinocyte proliferation via the mTOR pathway and epidermal inflammation through IL17 signaling in psoriasis rat model. Sci Rep. 2023;13(1):12133. doi:10.1038/s41598-023-39218-y
11. Tuentam K, Aramwit P, Reamtong O, et al. Sericin-based poly(Vinyl) alcohol relieves plaque and epidermal lesions in psoriasis; a chance for dressing development in a specific area. Int J Mol Sci. 2022;24(1). doi:10.3390/ijms24010145.
12. Das G, Shin HS, Campos EVR, et al. Sericin based nanoformulations: a comprehensive review on molecular mechanisms of interaction with organisms to biological applications. J Nanobiotechnol. 2021;19(1):30. doi:10.1186/s12951-021-00774-y
13. Hu D, Xu Z, Hu Z, Hu B, Yang M, Zhu L. pH-triggered charge-reversal silk sericin-based nanoparticles for enhanced cellular uptake and doxorubicin delivery. ACS Sust Chem Engineer. 2017;5:1638–1647. doi:10.1021/acssuschemeng.6b02392
14. Huang L, Tao K, Liu J, et al. Design and fabrication of multifunctional sericin nanoparticles for tumor targeting and pH-responsive subcellular delivery of cancer chemotherapy drugs. ACS Appl Mater Interfaces. 2016;8(10):6577–6585. doi:10.1021/acsami.5b11617
15. Parisi OI, Fiorillo M, Scrivano L, et al. Sericin/poly(ethylcyanoacrylate) nanospheres by interfacial polymerization for enhanced bioefficacy of fenofibrate: in vitro and in vivo studies. Biomacromolecules. 2015;16(10):3126–3133. doi:10.1021/acs.biomac.5b00746
16. Hu D, Li T, Xu Z, Liu D, Yang M, Zhu L. Self-stabilized silk sericin-based nanoparticles: in vivo biocompatibility and reduced doxorubicin-induced toxicity. Acta Biomater. 2018;74:385–396. doi:10.1016/j.actbio.2018.05.024
17. Bhuyan D, Greene GW, Das RK. Dataset on the synthesis and physicochemical characterization of blank and curcumin encapsulated sericin nanoparticles obtained from Philosamia ricini silkworm cocoons. Data Brief. 2019;26:104359. doi:10.1016/j.dib.2019.104359
18. Suktham K, Koobkokkruad T, Wutikhun T, Surassmo S. Efficiency of resveratrol-loaded sericin nanoparticles: promising bionanocarriers for drug delivery. Int J Pharm. 2018;537(1–2):48–56. doi:10.1016/j.ijpharm.2017.12.015
19. Rioux G, Simard M, Morin S, Lorthois I, Guerin SL, Pouliot R. Development of a 3D psoriatic skin model optimized for infiltration of IL-17A producing T cells: focus on the crosstalk between T cells and psoriatic keratinocytes. Acta Biomater. 2021;136:210–222. doi:10.1016/j.actbio.2021.09.018
20. Scheurer J, Sauer B, Focken J, et al. Histological and functional characterization of 3D human skin models mimicking the inflammatory skin diseases psoriasis and atopic dermatitis. Dis Model Mech. 2024;17(1). doi:10.1242/dmm.050541.
21. Soboleva AG, Mezentsev A, Zolotorenko A, Bruskin S, Pirusian E. Three-dimensional skin models of psoriasis. Cells Tissues Organs. 2014;199(5–6):301–310. doi:10.1159/000369925
22. Aramwit P, Siritientong T, Srichana T. Potential applications of silk sericin, a natural protein from textile industry by-products. Waste Manag Res. 2012;30(3):217–224. doi:10.1177/0734242X11404733
23. Ampawong S, Tirawanchai N, Kanjanapruthipong T, et al. Sericin enhances ammonia detoxification by promotes urea cycle enzyme genes and activates hepatic autophagy in relation to CARD-9/MAPK pathway. Heliyon. 2023;9(11):e21563. doi:10.1016/j.heliyon.2023.e21563
24. Fongsodsri K, Tiyasatkulkovit W, Chaisri U, et al. Sericin promotes chondrogenic proliferation and differentiation via glycolysis and Smad2/3 TGF-beta signaling inductions and alleviates inflammation in three-dimensional models. Sci Rep. 2024;14(1):11553. doi:10.1038/s41598-024-62516-y
25. Tena N, Martin J, Asuero AG. State of the art of anthocyanins: antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants. 2020;9(5):451.
26. Wu LT, Tsai SC, Ho TJ, et al. Advanced whole transcriptome sequencing and artificial intelligence/machine learning (AI/ML) in imiquimod-induced psoriasis-like inflammation of human keratinocytes. Biomedicine. 2024;14(4):36–50. doi:10.37796/2211-8039.1468
27. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi:10.1016/0022-1759(83)90303-4
28. Lohitthai S, Rungruengkitkun A, Jitprasutwit N, et al. Type VI secretion system accessory protein TagAB-5 promotes Burkholderia pseudomallei pathogenicity in human microglia. Biomedicines. 2023;11(11). doi:10.3390/biomedicines11112927.
29. Kitisin T, Muangkaew W, Ampawong S, Sukphopetch P. Utilization of an in vitro biofabricated 3D skin as a pathological model of cutaneous candidiasis. New Microbiol. 2020;43(4):171–179.
30. Muangkaew W, Thanomsridetchai N, Tangwattanachuleeporn M, Ampawong S, Sukphopetch P. Unveiling lodderomyces elongisporus as an emerging yeast pathogen: a holistic approach to microbiological diagnostic strategies. Mycopathologia. 2024;189(6):94. doi:10.1007/s11046-024-00901-x
31. Madsen P, Rasmussen HH, Leffers H, et al. Molecular cloning, occurrence, and expression of a novel partially secreted protein “psoriasin” that is highly up-regulated in psoriatic skin. J Invest Dermatol. 1991;97(4):701–712. doi:10.1111/1523-1747.ep12484041
32. Kengkoom K, Angkhasirisap W, Kanjanapruthipong T, et al. Streptozotocin induces alpha-2u globulin nephropathy in male rats during diabetic kidney disease. BMC Vet Res. 2021;17(1):105. doi:10.1186/s12917-021-02814-z
33. Mohammed S, Hegab M, Ou R. Nanofiltration performance of glutaraldehyde crosslinked graphene oxide-cellulose nanofiber membrane. Chem Eng Res Des. 2022;183:1–12. doi:10.1016/j.cherd.2022.04.039
34. Cho KY, Moon JY, Lee YW, et al. Preparation of self-assembled silk sericin nanoparticles. Int J Biol Macromol. 2003;32(1–2):36–42. doi:10.1016/S0141-8130(03)00023-0
35. Chen Y, Lu Y, Lee RJ, Xiang G. Nano encapsulated curcumin: and its potential for biomedical applications. Int J Nanomed. 2020;15:3099–3120. doi:10.2147/IJN.S210320
36. Li C, Luo T, Zheng Z, Murphy AR, Wang X, Kaplan DL. Curcumin-functionalized silk materials for enhancing adipogenic differentiation of bone marrow-derived human mesenchymal stem cells. Acta Biomater. 2015;11:222–232. doi:10.1016/j.actbio.2014.08.009
37. Nadeem Butt E, Ali S, Summer M, Siddiqua Khan A, Noor S. Exploring the mechanistic role of silk sericin biological and chemical conjugates for effective acute and chronic wound repair and related complications. Drug Dev Ind Pharm. 2024;50(7):577–592. doi:10.1080/03639045.2024.2387814
38. Shelley JR, Davidson DJ, Dorin JR. The dichotomous responses driven by beta-defensins. Front Immunol. 2020;11:1176. doi:10.3389/fimmu.2020.01176
39. Bebars SMM, Al-Sharaky DR, Gaber MA, Afify DR. Immunohistochemical Expression of Caspase-3 in Psoriasis. J Clin Diagn Res. 2017;11(7):EC01–EC05. doi:10.7860/JCDR/2017/25609.10145
40. Cannavo SP, Riso G, Casciaro M, Di Salvo E, Gangemi S. Oxidative stress involvement in psoriasis: a systematic review. Free Radic Res. 2019;53(8):829–840. doi:10.1080/10715762.2019.1648800
41. Dobrica EC, Cozma MA, Gaman MA, Voiculescu VM, Gaman AM. The involvement of oxidative stress in psoriasis: a systematic review. Antioxidants. 2022;11(2). doi:10.3390/antiox11020282
42. Chen XM, Zhao Y, Wu XD, et al. Novel findings from determination of common expressed plasma exosomal microRNAs in patients with psoriatic arthritis, psoriasis vulgaris, rheumatoid arthritis, and gouty arthritis. Discov Med. 2019;28(151):47–68.
43. Jacquin-Porretaz C, Cordonnier M, Nardin C, et al. Increased levels of interleukin-17A exosomes in psoriasis. Acta Derm Venereol. 2019;99(12):1143–1147. doi:10.2340/00015555-3300
44. Jiang M, Fang H, Shao S, et al. Keratinocyte exosomes activate neutrophils and enhance skin inflammation in psoriasis. FASEB J. 2019;33(12):13241–13253. doi:10.1096/fj.201900642R
45. Jonoush ZA, Mahdavi R, Farahani M, Zeinali F, Shayan E, Amari A. The implications of exosomes in psoriasis: disease: emerging as new diagnostic markers and therapeutic targets. Mol Biol Rep. 2024;51(1):465. doi:10.1007/s11033-024-09449-x
46. Sawamura S, Tselmeg Mijiddorj M, Kajihara I, et al. Clinical significance of circulating exosomal interleukin-23 and tumour necrosis factor-alpha messenger RNA in psoriasis. J Eur Acad Dermatol Venereol. 2023;37(6):e815–e817. doi:10.1111/jdv.18945
47. Shao S, Fang H, Zhang J, et al. Neutrophil exosomes enhance the skin autoinflammation in generalized pustular psoriasis via activating keratinocytes. FASEB J. 2019;33(6):6813–6828. doi:10.1096/fj.201802090RR
48. Lu Y, Pan ZZ, Devaux Y, Ray P. p21-activated protein kinase 4 (PAK4) interacts with the keratinocyte growth factor receptor and participates in keratinocyte growth factor-mediated inhibition of oxidant-induced cell death. J Biol Chem. 2003;278(12):10374–10380. doi:10.1074/jbc.M205875200
49. Sarandi E, Krueger-Krasagakis S, Tsoukalas D, et al. Psoriasis immunometabolism: progress on metabolic biomarkers and targeted therapy. Front Mol Biosci. 2023;10:1201912. doi:10.3389/fmolb.2023.1201912
50. Zhang W, Liu R, Chen Y, Wang M, Du J. Crosstalk between Oxidative Stress and Exosomes. Oxid Med Cell Longev. 2022;2022:3553617. doi:10.1155/2022/3553617
51. Liang H, Li J, Zhang K. Pathogenic role of S100 proteins in psoriasis. Front Immunol. 2023;14:1191645. doi:10.3389/fimmu.2023.1191645
52. Wang Y, Kang X, Kang X, Yang F. S100A6: molecular function and biomarker role. Biomark Res. 2023;11(1):78. doi:10.1186/s40364-023-00515-3
53. Grantham HJ, Hussain AB, Reynolds NJ. Serum S100A8/A9 may act as biomarker of atherosclerosis severity in psoriasis. J Invest Dermatol. 2022;142(11):2848–2850. doi:10.1016/j.jid.2022.06.018
54. He J, Lei Y, Li X, Wu B, Tang Y. Exploring the prognostic value of S100A11 and its association with immune infiltration in breast cancer. Sci Rep. 2023;13(1):22922. doi:10.1038/s41598-023-50160-x
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.