Back to Journals » Cancer Management and Research » Volume 17
Iron-Dependent Cell Death: Exploring Ferroptosis as a Unique Target in Triple-Negative Breast Cancer Management
Authors Tan LK , Liu J, Ma CZ, Huang S, He FH, Long Y, Zheng ZS, Liang JL, Xu N, Wang G, Liu YF
Received 2 November 2024
Accepted for publication 25 January 2025
Published 19 March 2025 Volume 2025:17 Pages 625—637
DOI https://doi.org/10.2147/CMAR.S503932
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Li-kuan Tan,1 Jiaxing Liu,1 Cheng-zhi Ma,1 Shaolong Huang,1 Feng-hui He,1 Yang Long,1 Zhi-sheng Zheng,1 Jia-liang Liang,1 Nan Xu,1 Guanghui Wang,2 Yu-fei Liu1
1Breast Surgery, Tongren People’s Hospital, Tongren, People’s Republic of China; 2Breast Surgery, Guizhou Provincial People’s Hospital, Guiyang, People’s Republic of China
Correspondence: Guanghui Wang; Yu-fei Liu, Email [email protected]; [email protected]
Abstract: Triple-negative breast cancer (TNBC) is characterized by aggressive behavior, high metastatic potential, and frequent relapses, presenting significant treatment challenges. Ferroptosis, a unique form of programmed cell death marked by iron-dependent lipid peroxidation, has emerged as a crucial factor in cancer biology. Recent studies indicate that TNBC cells possess a distinct metabolic profile linked to iron and glutathione, which may render them more susceptible to ferroptosis than other breast cancer subtypes. Moreover, ferroptosis plays a role in the interactions between immune cells and tumor cells, suggesting its potential to modulate the tumor microenvironment and influence the immune response against TNBC.Evidence reveals that ferroptosis not only affects TNBC cell viability but also alters the tumor microenvironment by promoting the release of damage-associated molecular patterns (DAMPs), which can recruit immune cells to the tumor site. Specific ferroptosis-related genes and biomarkers, such as ACSL4 and GPX4, demonstrate altered expression patterns in TNBC tissues, offering promising avenues for diagnostic and prognostic applications. Furthermore, in preclinical models, the induction of ferroptosis has been shown to enhance the efficacy of existing therapies, indicating a synergistic effect that could be harnessed for therapeutic benefit. The compelling link between ferroptosis and TNBC underscores its potential as a novel therapeutic target. Future research should focus on developing strategies that exploit ferroptosis in conjunction with traditional therapies, including the identification of natural compounds and efficacious ferroptosis inducers for personalized treatment regimens. This review elucidates the multifaceted implications of ferroptosis in TNBC, providing valuable insights for improving both diagnosis and treatment of this formidable breast cancer subtype.
Keywords: triple-negative breast cancer, ferroptosis, programmed cell death, immune response, therapeutic strategies
Introduction
Triple-negative breast cancer (TNBC) is a complex subtype of breast cancer characterised by the absence of estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptor 2 (HER2).1 This classification sets TNBC apart from other breast cancer types, as it exhibits poor responses to hormonal and targeted therapies. Known for its aggressive nature, TNBC tumours often grow rapidly and tend to metastasise early, resulting in poor clinical outcomes and high recurrence rates. Notably, the survival rate of TNBC patients is significantly lower than that of patients with other breast cancer subtypes within the first five years following diagnosis.2 Among available treatments, chemotherapy remains the primary approach for TNBC; however, its side effects and varying efficacy underscore the urgent need for new strategies to improve patient prognosis.3
In recent years, ferroptosis has emerged as a newly recognised form of programmed cell death that has garnered considerable interest in the scientific community.4 Characterised by unique molecular mechanisms and pathological features, ferroptosis plays a significant role in tumour biology. Specifically, it is induced through iron-dependent lipid peroxidation, leading to cell death that differs from conventional forms like apoptosis and necrosis.5 The hallmark of ferroptosis includes plasma membrane rupture and excessive reactive oxygen species (ROS) production, underscoring its unique mechanism operating through cellular iron regulation, with profound implications for cancer research.6
Studies indicate that TNBC cells typically exhibit distinct iron metabolism traits, presenting relatively high iron levels and low antioxidant capacity.7 This characteristic makes TNBC cells particularly sensitive to ferroptosis, with research showing that inducing ferroptosis can significantly enhance the lethality of TNBC cells and inhibit tumour growth.8 Furthermore, ferroptosis may also regulate the tumour microenvironment, influencing immune cell function and tumour evasion mechanisms.9 Within the realm of TNBC, it is important to recognize that this subtype is not a singular entity but rather consists of distinct molecular subtypes, including basal-like, mesenchymal, and luminal androgen receptor (LAR) subtypes, each possessing unique features and susceptibilities.10 For instance, basal-like TNBC is generally characterized by the expression of epidermal growth factor receptor (EGFR) and a high proliferation index, making it particularly aggressive and more likely to benefit from therapies aimed at exploiting ferroptosis pathways. In contrast, the mesenchymal subtype exhibits enhanced epithelial-mesenchymal transition (EMT) markers and a greater capacity for metastasis; this metabolic flexibility may render them more sensitive to iron deprivation strategies that induce ferroptosis.11 The LAR subtype, which often presents with hormone receptor expression, indicates that different therapeutic approaches may be necessary to target these unique molecular profiles effectively.12 Therefore, delving deeper into the mechanisms of ferroptosis not only provides new treatment opportunities but may also open fresh avenues for managing TNBC.
This review aims to explore the potential and application of ferroptosis in TNBC management. We will synthesise current research advancements regarding ferroptosis and its biological roles in TNBC, analyse its potential as a diagnostic biomarker, and outline treatment strategies based on ferroptosis, ultimately offering new insights and directions for TNBC management. Through a comprehensive understanding of ferroptosis, we hope to advance research in TNBC, ultimately improving patient survival rates and quality of life.
The Mechanisms of Ferroptosis
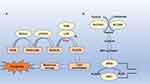
Ferroptosis is a form of regulated cell death distinct from apoptosis, necrosis, and other death forms.5 It is characterised by the accumulation of lipid peroxides to lethal levels in an iron-dependent manner. Unlike conventional cell death pathways, ferroptosis does not exhibit the morphological changes typical of apoptosis or necrosis; instead, it presents unique cellular and molecular features. The distinctive hallmark of ferroptosis is the excessive production of reactive oxygen species (ROS) due to lipid peroxidation, affecting polyunsaturated fatty acids in cellular membranes and ultimately leading to cell rupture and death13 (Figure 1).
At the molecular level, ferroptosis is closely linked to iron metabolism and oxidative stress. Intracellular iron levels are pivotal; an excess of ferrous iron enhances ROS generation through Fenton reactions, where hydrogen peroxide is converted to hydroxyl radicals.14 This process initiates a cascade of lipid peroxidation, wherein lipids rich in polyunsaturated fatty acids undergo oxidative degradation, forming toxic lipid peroxides.15 Elevated levels of these peroxidised lipids can disrupt cell membrane integrity, leading to ferroptotic cell death.
A critical regulatory factor in ferroptosis is glutathione (GSH), a potent antioxidant protecting cells from oxidative stress.16 GSH is synthesised from precursors, including cysteine, glycine, and glutamate. In ferroptosis, GSH depletion is significant as it disrupts the function of glutathione peroxidase 4 (GPX4), an enzyme detoxifying lipid peroxides.17 Without sufficient GPX4 activity, lipid peroxides accumulate, promoting ferroptosis. This dependency on GSH highlights the interplay between cellular redox balance and ferroptotic signalling. Furthermore, the amino acid cysteine, a limiting factor for GSH synthesis, emphasises the nutritional aspects influencing ferroptosis susceptibility.18
In addition to iron and GSH, other critical regulators associated with ferroptosis include acyl-CoA synthetase long-chain family member 4 (ACSL4) and ferritin. ACSL4 facilitates the incorporation of polyunsaturated fatty acids into membranes, increasing these lipids’ susceptibility to peroxidation.19 Higher expression levels of ACSL4 are often observed in various cancer cells undergoing ferroptosis, suggesting a potential role for its modulation in cancer therapy. Ferritin, which sequesters free iron within cells, serves as a protective mechanism against ferroptosis.20 When ferritin levels are low or dysfunctional, free iron accumulates, promoting oxidative stress and ferroptotic cell death.
The intricate balance between these factors dictates a cell’s fate in the context of ferroptosis. Cells that maintain robust antioxidant defences, high GSH levels, and functional ferritin are more resilient to ferroptotic stimuli.21 Conversely, cancer cells often exhibit altered iron metabolism and reduced antioxidant capacities, rendering them particularly susceptible to ferroptosis. This vulnerability opens new avenues for therapeutic interventions, especially in treating malignancies like TNBC, where conventional therapies may be ineffective.22
The Role of Ferroptosis in Triple-Negative Breast Cancer
Sensitivity of TNBC Cells to Ferroptosis
Recent studies have revealed a unique sensitivity of triple-negative breast cancer (TNBC) cells to ferroptosis, a regulated form of cell death driven by iron-dependent lipid peroxidation. This sensitivity is linked to the elevated intracellular iron levels and the altered lipid metabolism inherent in TNBC cells.23 Research indicates that TNBC cells respond distinctly to ferroptosis inducers, such as Erastin and RSL3, which inhibit the activity of glutathione peroxidase 4 (GPX4), resulting in the accumulation of lethal lipid peroxides. Unlike other breast cancer subtypes, the oxidative stress induced by these compounds leads to a pronounced decrease in cell viability specific to TNBC.24 This diminished ability of TNBC cells to tolerate oxidative stress renders them particularly vulnerable to ferroptosis. Understanding this vulnerability presents a promising opportunity for developing novel therapeutic strategies that target ferroptosis in TNBC.
Impact of Ferroptosis on the Tumor Microenvironment in TNBC
The tumour microenvironment (TME) of TNBC is complex, consisting of various cellular components, including cancer-associated fibroblasts (CAFs), the extracellular matrix, and immune cells. Recent evidence suggests that ferroptosis significantly influences the TME, particularly through its interactions with immune cells. The death of tumour cells via ferroptosis can lead to the release of damage-associated molecular patterns (DAMPs) that modulate immune responses.25
On one hand, ferroptosis may foster an immunosuppressive environment, as byproducts of lipid peroxidation can activate regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs). These immune cells can inhibit the function of effector T cells, thereby dampening the anti-tumour immune response.26 On the other hand, the release of certain DAMPs may activate dendritic cells and enhance the presentation of tumour antigens, potentially leading to a more robust anti-tumour immune response.27
This dual role of ferroptosis in shaping the TNBC TME highlights the need to carefully consider how ferroptosis-inducing therapies might influence not only cancer cell viability but also the dynamics of immune responses within the TME. Ultimately, harnessing the immune modulatory effects of ferroptosis could enhance the efficacy of existing immunotherapies and lead to novel treatment paradigms for TNBC.
Ferroptosis-Related Targets and Biomarkers
Ferroptosis is a complex form of regulated cell death that plays a crucial role in various pathological contexts, including cancer. Exploring ferroptosis has unveiled potential therapeutic targets and biomarkers, especially in aggressive cancer types like TNBC. This section delves into the expression patterns of genes and molecules associated with ferroptosis, identifying potential biomarkers for diagnosis and prognosis in TNBC.
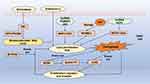
Zhang et al discovered that the overexpression of IDH2 in TNBC inhibits ferroptosis, promoting the proliferation and other malignant characteristics of TNBC cells.28 Studies have shown that the knockout of TFR2 enhances ferroptosis in TNBC cells.29 Thrombin, through the activation of cytosolic phospholipase A2α (cPLA2α), increases the release of arachidonic acid (AA), thereby inducing ferroptosis in TNBC cells.30 Research by Wang et al elucidated that low expression levels of PTGER3 protect TNBC cells from ferroptosis, consequently facilitating disease progression.31 Additionally, studies indicate that the knockout of TYMS provides new insights into inhibitory effects in TNBC.32 Evidence shows that WTAP upregulates LCN2 by modulating NUPR1 m(6)A methylation, thereby inhibiting ferroptosis and accelerating the progression of TNBC.33 Findings by Paris et al pave the way for further investigation of PROM2 as a potential biological target for metastatic TNBC.34 Mechanistically, PTPRG-AS1 targets miR-376c-3p to upregulate SLC7A11, suppressing ferroptosis and promoting TNBC progression, suggesting that PTPRG-AS1 could serve as a therapeutic target for TNBC.35 Additionally, research indicates that LncFASA plays a critical role in cancer development mediated by ferroptosis, providing new insights for treatment strategies in TNBC.36 Single-cell transcriptomic analyses reveal that mutant p53 can protect TNBC cells from ferroptosis.37 Furthermore, PGRMC1, by binding to intracellular iron, inhibits ferroptosis and increases the likelihood of TNBC occurrence, serving as a molecular basis for combination therapies in TNBC management38 (Table 1).
 |
Table 1 Mechanisms of Regulation of Ferroptosis by Various Gene/Biomarkers in TNBC |
MTHFD2 is a significant molecular biomarker for predicting the prognosis of patients with triple-negative breast cancer (TNBC) and serves as a promising therapeutic target for its treatment.39 Yuan et al found that STEAP3 is highly expressed in TNBC patients and is correlated with overall survival, establishing a foundation for targeted therapies in this patient population.40 Mechanistically, studies have shown that neutrophil extracellular traps (NETs) can interact with TLR9 to reduce Merlin phosphorylation, thereby helping TNBC cells resist ferroptosis.41 The overexpression of lncRNA HCP5, which encodes a protein, represents a crucial oncogenic event in TNBC as it regulates ferroptosis, suggesting new therapeutic avenues.42 Furthermore, TNBC cells can evade ferroptosis through the gankyrin/p53/SLC7A11/GPX4 signaling pathway, indicating that gankyrin may serve as a valuable biomarker for predicting TNBC prognosis or as a potential therapeutic target.43 Yang et al systematically described the heterogeneity of ferroptosis in TNBC and proposed innovative immunotherapeutic combination strategies for the luminal androgen receptor (LAR) subtype of TNBC.44 A prognostic model composed of 12 ferroptosis-related genes can predict the prognosis of TNBC patients, with seven of these genes—ASN, LAMP2, CAV1, DPP4, HELLS, TF, and ZFP69B—identified as potential new targets for therapy.45 Additionally, PGM5P3-AS1 promotes cellular ferroptosis by regulating MAP1LC3C, thereby inhibiting the malignant progression of TNBC.46 The signaling pathways involving ferroptosis-related metabolic and immune factors (IMRGs) may assist in the prognosis assessment of TNBC, providing insights into its molecular characteristics and guiding treatment strategies.7 Lastly, HLF enhances the resistance of TNBC cells to ferroptosis through GGT1, ultimately contributing to disease progression47 (Figure 2).
Targeting Ferroptosis: Therapeutic Strategies
Inducing Ferroptosis: Molecular Drugs and Mechanisms
The ability of UA to induce ferroptosis by inhibiting the NRF2 pathway presents a promising strategy for treating breast cancer stem cells (BCSCs), potentially addressing metastasis and drug resistance in TNBC.8 Research indicates that Na2SeO₄-induced ferroptosis is mediated by the ATM protein in MDA-MB-231 cells.48 Uridine-modified Ru(II) complexes have been developed as potential LIMP-2-targeting agents for TNBC treatment by promoting the generation of reactive oxygen species (ROS) and inducing ferroptosis.49 Tang et al discovered that pitavastatin can trigger autophagy-dependent ferroptosis in TNBC cells via the mevalonate pathway, suggesting a potential adjunctive treatment option for these patients.50 The senescence-associated secretory phenotype (SASP) effectively induces autophagy-related ferroptosis; understanding the mechanisms by which SASP regulates cell death may offer new strategies for TNBC therapy and drug repositioning.51 G-4 inhibits the malignant phenotype of TNBC by inducing apoptosis through the suppression of the EGFR pathway, subsequently promoting LCN2-dependent ferroptosis.52 Additionally, senescent cells catalyze specific redox reactions that drive membrane peroxidation and disrupt the cellular detoxification of lipid hydroperoxides, including those found in treatment-resistant cancer cells.53 A chloride nitrilated compound has been shown to decrease intracellular glutathione levels in TNBC cells, inhibiting cell proliferation, downregulating GPX4 expression, and increasing lipid peroxidation, ultimately inducing ferroptosis.54 Compound 4d induces both apoptosis and ferroptosis by reducing mitochondrial membrane potential and downregulating GPX4 expression.55 Studies suggest that hTERT G4-targeting ligands can disrupt mitochondrial function in cancer cells, impairing iron metabolism and activating ferroptosis.56 HTB50-2 exerts its antitumor effects through the FOSL2/FOXC1 signaling pathway, highlighting its significant therapeutic potential in TNBC treatment.57 Furthermore, research indicates that compound 9o is an effective and selective CA IX inhibitor and a ferroptosis inducer for TNBC.58 SBFI26 leads to the accumulation of intracellular fatty acids, resulting in an excess of ferrous ions and subsequent lipid peroxidation, thereby triggering ferroptosis59 (Table 2).
 |
Table 2 The Mechanisms of Emerging Ferroptosis Inducers in TNBC Treatment |
Research indicates that ononin may represent a promising strategy for treating TNBC by inducing ferroptosis, which disrupts the Nrf2/SLC7A11 axis.60 BA-Fe(III) activates ferroptosis in tumor cells by downregulating the enzymatic activity of ferritin heavy chain 1 and glutathione peroxidase.61 HCL-23 promotes TNBC cell death by mediating apoptosis through caspase activation and HO-1-dependent ferroptosis.62 Hinokitiol-chelated Fe(hino)(3), exhibiting redox activity, enhances the generation of free radicals via Fenton reactions, thus acting as a ferroptosis inducer with demonstrated anti-TNBC efficacy.63 So-2 induces ferroptosis in TNBC by downregulating E2F7 expression, demonstrating inhibitory effects on TNBC both in vitro and in vivo. Isolated from traditional Chinese medicine, the natural compound So-2 shows promise as a candidate drug for TNBC treatment.64 Hu et al discovered that the natural product Til exerts antitumor activity against TNBC by promoting ferroptosis, with the HO-1/SLC7A11 pathway playing a critical role in Til-induced cell death.65 Moreover, researchers have proposed a therapeutic ferroptosis inducer, IR780-SPhF, which enables simultaneous imaging and treatment of TNBC through mitochondrial targeting.66 DSF/Cu enhances lipid peroxidation in TNBC cells, significantly increasing HMOX1 activity and thereby inducing ferroptosis to promote TNBC cell death.67 Additionally, AC induces autophagy-dependent ferroptosis by ubiquitinating GPX4, thereby inhibiting the progression and metastasis of TNBC and presenting AC as a novel candidate for therapy.68 The natural compound Eup shows potential as a TNBC treatment by inducing apoptosis and ferroptosis through the ubiquitination of mutant p53.69 Furthermore, a reported small molecule photosensitizer induces ferroptosis through a self-amplifying process, enhancing the immunotherapeutic profile of tumors.70 SIM suppresses HMGCR expression, downregulates the mevalonate (MVA) pathway and GPX4, facilitating ferroptosis in cancer cells.71 GA promotes the generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) by activating NADPH oxidase and iNOS, leading to reduced GSH and GPX activity, exacerbating lipid peroxidation, and ultimately triggering ferroptosis in TNBC cells.72
Combining Ferroptosis with Existing Treatment Modalities
Research conducted by Wang et al has demonstrated that Pt-3 is a promising candidate drug, serving as an exemplary model for the development of cancer therapeutics.73 Additionally, Yu et al discovered that exosome-based erastin formulations offer both targeted delivery and biocompatibility, providing an innovative and robust platform for anticancer therapy.74
Recent studies have confirmed the occurrence of ferroptosis in TNBC and revealed a novel combination immunotherapy strategy for treating refractory LAR tumors.75 The activation of PRMT5 plays a critical role in regulating iron metabolism, promoting resistance to ferroptosis inducers and immunotherapy, thereby positioning PRMT5 as a potential target for overcoming immune resistance in TNBC.76
Zhu et al designed a multifunctional nanoplatform (MP-FA@R-FNPs) that represents a potential synergistic strategy for iron/chemical/immunotherapy, enhanced by MRI-guided tumor treatment.77 Anti-cancer results demonstrated that ATO/SRF@BSA exhibited tumor-specific cytotoxic efficacy, significantly improving the tumor hypoxic microenvironment while alleviating the side effects of SRF.78 High-intensity focused ultrasound (HIFU)-driven nanomotors have shown great potential for inducing ferroptosis in immunotherapy for TNBC.79 Studies have highlighted the substantial potential of iron-based ternary sulfides as a new therapeutic platform for a combination of photothermal therapy, iron therapy, and immunotherapy aimed at suppressing TNBC.80 Near-infrared (NIR)-enhanced ferroptosis synergistic treatment strategies bring hope for TNBC therapy.22 Wang et al discovered a novel strategy utilizing CT/CDT nanoparticles to induce ferroptosis for treating TNBC.81 Cancer nanomedicines targeting ferroptosis typically rely on the direct delivery of Fenton catalysts to drive lipid peroxidation in cancer cells.82
Furthermore, T-LMD-induced ferroptosis was shown to be accompanied by the release of the immunogenic cell death marker HMGB1, suggesting its potential to enhance antitumor immune activation in TNBC.83 Nanoparticles that synergize ferroptosis and cuproptosis have been found to enhance tumor immunotherapy.84 Intracellular GSH reduction of Fe(3+)/Cu(2+) can further amplify the production of hydroxyl radicals (·OH). The depletion of GSH may inhibit GPX-4-mediated antioxidant responses, thereby inducing ferroptosis. This modulation within the tumor microenvironment enhances the efficacy of ferroptosis/cuproptosis/CDT as an effective therapeutic approach.85 The intelligent nanodrug GOx-IA@HMON@IO exhibits good biocompatibility and safety, making it suitable for MRI-guided tumor therapies.86 Deng et al identified a new strategy combining a closed-loop enhanced treatment pathway (CCLT@FT) to induce ferroptosis, regulate lactate metabolism, and facilitate immune checkpoint blockade (ICB) therapy, providing a vital alternative for effective immunotherapy in TNBC.87 Calcium-manganese dual-ion hybrid nanosensors (CMS) enhance antitumor immunity by inducing ferroptosis and stimulating intrinsic immune awakening, serving simultaneously as ferroptosis inducers and immune adjuvants for TNBC.88 Further analysis indicated that nFeAPG improved the suppressed immune microenvironment by enhancing the responses of dendritic cells (DCs) and T cells.89 The FTM@AM nanoplatform demonstrated efficient cytotoxic effects and invasion suppression against ultrasound-mediated aggressive TNBC, showcasing significant potential for clinical translation.90 Nanocrystals, through integrated image-guided interventions, aim to streamline the tumor treatment process, addressing challenges posed by the lack of therapeutic targets and the tendency for multidirectional metastasis in TNBC.91
The novel magnetic-targeting nanotherapy platform developed by Zhang et al shows promise as a new paradigm for treating TNBC.92 The shRNA@Fe(3)O(4) MNPs exhibit both magnetic hyperthermia and gene therapy functionality, demonstrating satisfactory therapeutic effects against TNBC with no significant toxic side effects.93 Moreover, the activation of tumor immune responses through self-sustaining nanoreactors in conjunction with ferroptosis presents a potential strategy for clinical application.94 N-S Alb nanoparticles (NPs) possess the ability to promote a “mixed” type of cell death and show promising prospects in enhancing delivery capacity and targeting efficacy for TNBC treatment.95 Y(2)O(3)-NPs have been shown to be safe for normal REP1 and HDF cells while inducing DNA damage and apoptosis in TNBC MDA-MB-231 cells by increasing the production of reactive oxygen species (ROS), exhibiting potent selective cytotoxicity.96 Chen et al developed a small-molecule self-assembling nano precursor drug for the combined delivery of the chemotherapeutic agent camptothecin (CPT), ferrocene (Fc), and ferroptosis inhibitors (RSL3). This approach leverages the synergistic effects of ferroptosis and apoptosis to overcome drug resistance in chemotherapy and enhance therapeutic efficacy.97 The CPT/Fe@PDA-FA10plus formulation can significantly kill resistant tumor cells while inhibiting the growth of in situ resistant TNBC through apoptosis, ferroptosis, and photothermal therapy, with no noticeable toxic side effects on major organs.98 Additionally, this research provides the first report on RSL-3 as a mitochondrial-targeted sonodynamic therapy (SDT) activator for ferroptosis induction.99 AGuIX nanoparticles may modulate the anti-ferroptotic system by inhibiting the NRF2-GSH-GPX4 signaling pathway.100 In a 4T1 tumor-bearing mouse model, HMTBF exhibited good in vivo antitumor effects, indicating that the nanodevice could serve as an effective inducer of ferroptosis and apoptosis for the combined treatment of TNBC.101
Research conducted by Song et al elucidated the molecular mechanisms by which serine/arginine-rich splicing factor 1 (SRSF1) influences cisplatin sensitivity in TNBC cells.102 Treatment with THP alone exhibits significant anti-TNBC effects, and its combination with doxorubicin (DOX) effectively enhances the sensitivity of TNBC cells to DOX.103 Furthermore, the combination of DOX and DBT may promote ferroptosis through the Nrf2/HO-1/GPX4 signaling axis.104 The composition of the tumor microenvironment significantly impacts patients’ responses to immunotherapy, suggesting that DUSP1 could be a potential target for overcoming drug resistance.105
Conclusions and Perspectives
Ferroptosis has emerged as a promising strategy for managing TNBC, a subtype known for its resistance to conventional therapies. The unique mechanistic pathways of ferroptosis, defined by iron-dependent lipid peroxidation leading to cell death, provide a novel therapeutic angle to exploit the vulnerabilities of TNBC cells. This form of regulated cell death not only provides insights into the metabolic adaptations of cancer cells but also presents opportunities for novel therapeutic interventions, particularly through the development of ferroptosis inducers and the exploration of natural compounds with ferroptotic properties.106 As our understanding of ferroptosis expands, future research should focus on elucidating the specific molecular mechanisms regulating this process in TNBC. This will facilitate the identification of potential biomarkers for patient stratification and response prediction. Moreover, combining ferroptosis inducers with established treatment modalities, such as chemotherapy and immunotherapy, may enhance therapeutic efficacy and patient outcomes.107
The data presented in the tables highlight various molecules and their mechanisms related to ferroptosis in TNBC. Notably, several compounds—including UA, Na2SeO₄, and pitavastatin—demonstrate potential for inducing ferroptosis, with mechanisms ranging from oxidative stress to autophagy activation. The prospect of utilizing these molecules in conjunction with conventional therapies could pave the way for more effective treatment regimens. These findings underscore the importance of continued exploration of ferroptosis in the context of TNBC, as they may lead to the development of novel therapeutic strategies that significantly impact patient care and survival outcomes.Thus, the integration of ferroptosis into the therapeutic landscape for TNBC holds significant promise, with the potential to revolutionize our approach to treating this aggressive form of breast cancer and ultimately improve survival rates.108 As we delve into this promising area of research, interdisciplinary efforts will be essential to translate these findings into clinical applications, paving the way for innovative treatment strategies that could substantially impact patient care in TNBC.
Data Sharing Statement
Data availability is not applicable to this article as no new data were created or analyzed in this study.
Ethical Approval
This study did not involve human or animal subjects, and thus, no ethical approval was required.
Author Contributions
Li-kuan Tan and Jia-xing Liu contributed equally to this work. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (nos. 81802479 and 82360501) and Guizhou Provincial Department of Health Project (gzwjkj2019-1-124), and Guizhou Provincial Department of Science and Technology Project (Qianke He Foundation [2020] 1Y343).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Derakhshan F, Reis-Filho JS. Pathogenesis of Triple-Negative Breast Cancer. Ann Rev Pathol. 2022;17(1):181–204. doi:10.1146/annurev-pathol-042420-093238
2. Li X, Yang J, Peng L, et al. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res Treat. 2017;161(2):279–287. doi:10.1007/s10549-016-4059-6
3. Marra A, Curigliano G. Adjuvant and Neoadjuvant Treatment of Triple-Negative Breast Cancer With Chemotherapy. Cancer J. 2021;27(1):41–49. doi:10.1097/PPO.0000000000000498
4. Kajarabille N, Latunde-Dada GO. Programmed Cell-Death by Ferroptosis: antioxidants as Mitigators. Int J mol Sci. 2019;20(19):4968. doi:10.3390/ijms20194968
5. Liu J, Hong M, Li Y, Chen D, Wu Y, Hu Y. Programmed Cell Death Tunes Tumor Immunity. Front Immunol. 2022;13:847345. doi:10.3389/fimmu.2022.847345
6. Wang B, Wang Y, Zhang J, et al. ROS-induced lipid peroxidation modulates cell death outcome: mechanisms behind apoptosis, autophagy, and ferroptosis. Arch Toxicol. 2023;97(6):1439–1451. doi:10.1007/s00204-023-03476-6
7. Li XF, Fu WF, Zhang J, Song CG. An iron metabolism and immune related gene signature for the prediction of clinical outcome and molecular characteristics of triple-negative breast cancer. BMC Cancer. 2022;22(1):619. doi:10.1186/s12885-022-09679-x
8. Yang X, Liang B, Zhang L, et al. Ursolic acid inhibits the proliferation of triple‑negative breast cancer stem‑like cells through NRF2‑mediated ferroptosis. Oncol Rep. 2024;52(1). doi:10.3892/or.2024.8753.
9. Niu X, Chen L, Li Y, Hu Z, He F. Ferroptosis, necroptosis, and pyroptosis in the tumor microenvironment: perspectives for immunotherapy of SCLC. Semi Cancer Biol. 2022;86(Pt 3):273–285. doi:10.1016/j.semcancer.2022.03.009
10. Vtorushin S, Dulesova A, Krakhmal N. Luminal androgen receptor (LAR) subtype of triple-negative breast cancer: molecular, morphological, and clinical features. J Zhejiang Univ Sci B. 2022;23(8):617–624. doi:10.1631/jzus.B2200113
11. Haerinck J, Berx G. Partial EMT takes the lead in cancer metastasis. Dev. Cell. 2021;56(23):3174–3176. doi:10.1016/j.devcel.2021.11.012
12. Martorana F, Di Grazia G, Rosano GN, et al. More Than Meets the Eye: a Case of Breast Cancer Switching from Being Luminal-Androgen-Receptor-Positive to Being Hormone-Receptor-Positive. Medicina. 2023;59(10). doi:10.3390/medicina59101875.
13. Niu B, Liao K, Zhou Y, et al. Application of glutathione depletion in cancer therapy: enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials. 2021;277:121110. doi:10.1016/j.biomaterials.2021.121110
14. Liu J, Kang R, Tang D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2022;289(22):7038–7050. doi:10.1111/febs.16059
15. Lei G, Zhuang L, Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer. 2022;22(7):381–396. doi:10.1038/s41568-022-00459-0
16. Ursini F, Maiorino M. Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic Biol Med. 2020;152:175–185. doi:10.1016/j.freeradbiomed.2020.02.027
17. Liang D, Feng Y, Zandkarimi F, et al. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell. 2023;186(13):2748–2764.e2722. doi:10.1016/j.cell.2023.05.003
18. Koppula P, Zhuang L, Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021;12(8):599–620. doi:10.1007/s13238-020-00789-5
19. Liao P, Wang W, Wang W, et al. CD8(+) T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell. 2022;40(4):365–378.e366. doi:10.1016/j.ccell.2022.02.003
20. Fuhrmann DC, Mondorf A, Beifuß J, Jung M, Brüne B. Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol. 2020;36:101670. doi:10.1016/j.redox.2020.101670
21. Xu Y, Li Y, Li J, Chen W. Ethyl carbamate triggers ferroptosis in liver through inhibiting GSH synthesis and suppressing Nrf2 activation. Redox Biol. 2022;53:102349. doi:10.1016/j.redox.2022.102349
22. Wei R, Fu G, Li Z, et al. Au-Fe(3)O(4) Janus nanoparticles for imaging-guided near infrared-enhanced ferroptosis therapy in triple negative breast cancer. J Colloid Interface Sci. 2024;663:644–655. doi:10.1016/j.jcis.2024.02.201
23. Gao M, Yi J, Zhu J, et al. Role of Mitochondria in Ferroptosis. Molecular Cell. 2019;73(2):354–363.e353. doi:10.1016/j.molcel.2018.10.042
24. Ye S, Hu X, Sun S, Su B, Cai J, Jiang J. Oridonin promotes RSL3-induced ferroptosis in breast cancer cells by regulating the oxidative stress signaling pathway JNK/Nrf2/HO-1. Eur J Pharmacol. 2024;974:176620. doi:10.1016/j.ejphar.2024.176620
25. Cheng Z, Xue C, Liu M, et al. Injectable microenvironment-responsive hydrogels with redox-activatable supramolecular prodrugs mediate ferroptosis-immunotherapy for postoperative tumor treatment. Acta Biomater. 2023;169:289–305. doi:10.1016/j.actbio.2023.08.002
26. Morello S, Pinto A, Blandizzi C, Antonioli L. Myeloid cells in the tumor microenvironment: role of adenosine. Oncoimmunology. 2016;5(3):e1108515. doi:10.1080/2162402X.2015.1108515
27. Gong D, Chen M, Wang Y, Shi J, Hou Y. Role of ferroptosis on tumor progression and immunotherapy. Cell Death Discovery. 2022;8(1):427. doi:10.1038/s41420-022-01218-8
28. Zhang C, Zhou Y, Chen T, et al. Isocitrate dehydrogenase 2 regulates the proliferation of triple-negative breast cancer through the ferroptosis pathway. Sci Rep. 2024;14(1):4732. doi:10.1038/s41598-024-55561-0
29. Yang Y, Du J, Huang YF, et al. Identification of TFR2 as a novel ferroptosis‑related gene that serves an important role in prognosis and progression of triple‑negative breast cancer. Oncol Lett. 2024;27(2):43. doi:10.3892/ol.2023.14176
30. Xu S, Tuo QZ, Meng J, Wu XL, Li CL, Lei P. Thrombin induces ferroptosis in triple-negative breast cancer through the cPLA2α/ACSL4 signaling pathway. Transl Oncol. 2024;39:101817. doi:10.1016/j.tranon.2023.101817
31. Wang S, Zhang Y, Zhang D, et al. PTGER3 knockdown inhibits the vulnerability of triple-negative breast cancer to ferroptosis. Cancer Sci. 2024;115(6):2067–2081. doi:10.1111/cas.16169
32. Wang L, Wu Z, Wang Y, et al. TYMS Knockdown Suppresses Cells Proliferation, Promotes Ferroptosis via Inhibits PI3K/Akt/mTOR Signaling Pathway Activation in Triple Negative Breast Cancer. Cell Biochem Biophys. 2024;82(3):2717–2726. doi:10.1007/s12013-024-01388-5
33. Tan M, He Y, Yi J, et al. WTAP Mediates NUPR1 Regulation of LCN2 Through m(6)A Modification to Influence Ferroptosis, Thereby Promoting Breast Cancer Proliferation, Migration and Invasion. Biochem Genet. 2024;62(2):876–891. doi:10.1007/s10528-023-10423-8
34. Paris J, Wilhelm C, Lebbé C, et al. PROM2 overexpression induces metastatic potential through epithelial-to-mesenchymal transition and ferroptosis resistance in human cancers. Clin Translat Med. 2024;14(3):e1632. doi:10.1002/ctm2.1632
35. Li J, Li PT, Wu W, et al. POU2F2-mediated upregulation of lncRNA PTPRG-AS1 inhibits ferroptosis in breast cancer via miR-376c-3p/SLC7A11 axis. Epigenomics. 2024;16(4):215–231. doi:10.2217/epi-2023-0100
36. Fan X, Liu F, Wang X, et al. LncFASA promotes cancer ferroptosis via modulating PRDX1 phase separation. Sci China Life Sci. 2024;67(3):488–503. doi:10.1007/s11427-023-2425-2
37. Dibra D, Xiong S, Moyer SM, et al. Mutant p53 protects triple-negative breast adenocarcinomas from ferroptosis in vivo. Sci Adv. 2024;10(7):eadk1835. doi:10.1126/sciadv.adk1835
38. Zhao Y, Ruan X, Cheng J, Xu X, Gu M, Mueck AO. PGRMC1 promotes triple-negative breast cancer cell growth via suppressing ferroptosis. Climacteric. 2023;26(2):135–142. doi:10.1080/13697137.2023.2170225
39. Zhang H, Zhu S, Zhou H, Li R, Xia X, Xiong H. Identification of MTHFD2 as a prognostic biomarker and ferroptosis regulator in triple-negative breast cancer. Front Oncol. 2023;13:1098357. doi:10.3389/fonc.2023.1098357
40. Yuan L, Liu J, Bao L, Qu H, Xiang J, Sun P. Upregulation of the ferroptosis-related STEAP3 gene is a specific predictor of poor triple-negative breast cancer patient outcomes. Front Oncol. 2023;13:1032364. doi:10.3389/fonc.2023.1032364
41. Yao L, Sheng X, Dong X, et al. Neutrophil extracellular traps mediate TLR9/Merlin axis to resist ferroptosis and promote triple negative breast cancer progression. Apoptosis. 2023;28(9–10):1484–1495. doi:10.1007/s10495-023-01866-w
42. Tong X, Yu Z, Xing J, et al. LncRNA HCP5-Encoded Protein Regulates Ferroptosis to Promote the Progression of Triple-Negative Breast Cancer. Cancers. 2023;15(6):1880. doi:10.3390/cancers15061880
43. Lei M, Zhang YL, Huang FY, et al. Gankyrin inhibits ferroptosis through the p53/SLC7A11/GPX4 axis in triple-negative breast cancer cells. Sci Rep. 2023;13(1):21916. doi:10.1038/s41598-023-49136-8
44. Jiang L, Gao XM, Cao J. The Achilles heel of TNBCs: ferroptosis heterogeneity. Cell Metab. 2023;35(1):1–2. doi:10.1016/j.cmet.2022.11.014
45. Xu N, Li B, Liu Y, et al. Ferroptosis and triple-negative breast cancer: potential therapeutic targets. Front Oncol. 2022;12:1017041. doi:10.3389/fonc.2022.1017041
46. Qi L, Sun B, Yang B, Lu S. PGM5P3-AS1 regulates MAP1LC3C to promote cell ferroptosis and thus inhibiting the malignant progression of triple-negative breast cancer. Breast Cancer Res Treat. 2022;193(2):305–318. doi:10.1007/s10549-021-06501-3
47. Li H, Yang P, Wang J, et al. HLF regulates ferroptosis, development and chemoresistance of triple-negative breast cancer by activating tumor cell-macrophage crosstalk. J Hematol Oncol. 2022;15(1):2. doi:10.1186/s13045-021-01223-x
48. Xu M, Gao X, Yue L, et al. Sensitivity of Triple Negative Breast Cancer cells to ATM-dependent Ferroptosis Induced by Sodium Selenite. Exp Cell Res. 2024;442(2):114222. doi:10.1016/j.yexcr.2024.114222
49. Wu Q, Yuan C, Wang J, et al. Uridine-Modified Ruthenium(II) Complex as Lysosomal LIMP-2 Targeting Photodynamic Therapy Photosensitizer for the Treatment of Triple-Negative Breast Cancer. JACS Au. 2024;4(3):1081–1096. doi:10.1021/jacsau.3c00808
50. Tang WJ, Xu D, Liang MX, et al. Pitavastatin induces autophagy-dependent ferroptosis in MDA-MB-231 cells via the mevalonate pathway. Heliyon. 2024;10(5):e27084. doi:10.1016/j.heliyon.2024.e27084
51. Takatani-Nakase T, Ikushima C, Sakitani M, Nakase I. Regulatory network of ferroptosis and autophagy by targeting oxidative stress defense using sulfasalazine in triple-negative breast cancer. Life Sci. 2024;339:122411. doi:10.1016/j.lfs.2023.122411
52. Sun G, Wang J, Liu F, et al. G-4 inhibits triple negative breast cancer by inducing cell apoptosis and promoting LCN2-dependent ferroptosis. Biochem Pharmacol. 2024;222:116077. doi:10.1016/j.bcp.2024.116077
53. Su F, Descher H, Bui-Hoang M, et al. Iron(III)-salophene catalyzes redox cycles that induce phospholipid peroxidation and deplete cancer cells of ferroptosis-protecting cofactors. Redox Biol. 2024;75:103257. doi:10.1016/j.redox.2024.103257
54. Mathew M, Sivaprakasam S, Dharmalingam-Nandagopal G, et al. Induction of Oxidative Stress and Ferroptosis in Triple-Negative Breast Cancer Cells by Niclosamide via Blockade of the Function and Expression of SLC38A5 and SLC7A11. Antioxidants. 2024;13(3):291. doi:10.3390/antiox13030291
55. Ma LF, Xu LL, Yuan LJ, et al. Discovery of NO Donor-Aurovertin Hybrids as Dual Ferroptosis and Apoptosis Inducers for Treating Triple Negative Breast Cancer. J Med Chem. 2024;67(15):13089–13105. doi:10.1021/acs.jmedchem.4c01070
56. Long W, Zeng YX, Zheng BX, et al. Targeting hTERT Promoter G-Quadruplex DNA Structures with Small-Molecule Ligand to Downregulate hTERT Expression for Triple-Negative Breast Cancer Therapy. J Med Chem. 2024;67(15):13363–13382. doi:10.1021/acs.jmedchem.4c01255
57. Liu N, Yin WY, Duan WQ, et al. HTB50-2 Inhibits Growth and Migration of Triple-negative Breast Cancer via FOSL2/FOXC1 Signaling Axis and Subsequent Ferroptosis. Curr Med Chem. 2024;31. doi:10.2174/0109298673306760240802062909
58. Liang Q, Zhang S, Liu J, et al. Discovery of novel 1,8-naphthalimide piperazinamide based benzenesulfonamides derivatives as potent carbonic anhydrase IX inhibitors and ferroptosis inducers for the treatment of triple-negative breast cancer. Bioorg Chem. 2024;150:107596. doi:10.1016/j.bioorg.2024.107596
59. He G, Zhang Y, Feng Y, et al. SBFI26 induces triple-negative breast cancer cells ferroptosis via lipid peroxidation. J Cell Mol Med. 2024;28(7):e18212. doi:10.1111/jcmm.18212
60. Gong G, Wan Y, Liu Y, Zhang Z, Zheng Y. Ononin triggers ferroptosis-mediated disruption in the triple negative breast cancer both in vitro and in vivo. Int Immunopharmacol. 2024;132:111959. doi:10.1016/j.intimp.2024.111959
61. Chai J, Hu J, Wang T, Bao X, Luan J, Wang Y. A Multifunctional Liposome for Synergistic Chemotherapy with Ferroptosis Activation of Triple-Negative Breast Cancer. Mol Pharmaceut. 2024;21(2):781–790. doi:10.1021/acs.molpharmaceut.3c00903
62. Zhao P, Song H, Gao F, et al. A Novel Derivative of Curcumol, HCL-23, Inhibits the Malignant Phenotype of Triple-Negative Breast Cancer and Induces Apoptosis and HO-1-Dependent Ferroptosis. Molecules. 2023;28(8):1.
63. Zhao H, Zhang M, Zhang J, et al. Hinokitiol-iron complex is a ferroptosis inducer to inhibit triple-negative breast tumor growth. Cell Biosci. 2023;13(1):87. doi:10.1186/s13578-023-01044-0
64. Liu N, Jing Z, Wen-Qi D, et al. Natural compound So-2 suppresses triple-negative breast cancer through inducing ferroptosis via downregulating transcription factor E2F7. Arch Biochem Biophys. 2023;744:109694. doi:10.1016/j.abb.2023.109694
65. Hu C, Zhao JF, Wang YM, Wu XL, Ye L. Tiliroside induces ferroptosis to repress the development of triple-negative breast cancer cells. Tissue Cell. 2023;83:102116. doi:10.1016/j.tice.2023.102116
66. Gan H, Huang X, Luo X, et al. A Mitochondria-Targeted Ferroptosis Inducer Activated by Glutathione-Responsive Imaging and Depletion for Triple Negative Breast Cancer Theranostics. Adv Healthc Mater. 2023;12(22):e2300220. doi:10.1002/adhm.202300220
67. Chu M, An X, Fu C, et al. Disulfiram/Copper Induce Ferroptosis in Triple-Negative Breast Cancer Cell Line MDA-MB-231. Front Biosci. 2023;28(8):186. doi:10.31083/j.fbl2808186
68. Chen YM, Xu W, Liu Y, et al. Anomanolide C suppresses tumor progression and metastasis by ubiquitinating GPX4-driven autophagy-dependent ferroptosis in triple negative breast cancer. Int J Bio Sci. 2023;19(8):2531–2550. doi:10.7150/ijbs.82120
69. Wei Y, Zhu Z, Hu H, Guan J, Yang B, Zhao H. Eupaformosanin induces apoptosis and ferroptosis through ubiquitination of mutant p53 in triple-negative breast cancer. Eur J Pharmacol. 2022;924:174970. doi:10.1016/j.ejphar.2022.174970
70. Ling YY, Wang WJ, Hao L, et al. Self-Amplifying Iridium(III) Photosensitizer for Ferroptosis-Mediated Immunotherapy Against Transferrin Receptor-Overexpressing Cancer. Small. 2022;18(49):e2203659. doi:10.1002/smll.202203659
71. Yao X, Xie R, Cao Y, et al. Simvastatin induced ferroptosis for triple-negative breast cancer therapy. J Nanobiotechnol. 2021;19(1):311. doi:10.1186/s12951-021-01058-1
72. Wen Y, Chen H, Zhang L, et al. Glycyrrhetinic acid induces oxidative/nitrative stress and drives ferroptosis through activating NADPH oxidases and iNOS, and depriving glutathione in triple-negative breast cancer cells. Free Radic Biol Med. 2021;173:41–51. doi:10.1016/j.freeradbiomed.2021.07.019
73. Wang FY, Yang LM, Wang SS, et al. Cycloplatinated (II) Complex Based on Isoquinoline Alkaloid Elicits Ferritinophagy-Dependent Ferroptosis in Triple-Negative Breast Cancer Cells. J Med Chem. 2024;67(8):6738–6748. doi:10.1021/acs.jmedchem.4c00285
74. Yu M, Gai C, Li Z, et al. Targeted exosome-encapsulated erastin induced ferroptosis in triple negative breast cancer cells. Cancer Sci. 2019;110(10):3173–3182. doi:10.1111/cas.14181
75. Wuguo T, Jianjie Z, Donglin L. Correlation between ferroptosis-related genes and PDL-1 immunotherapy in triple negative breast cancer. Asian J Surg. 2023;46(10):4595–4597. doi:10.1016/j.asjsur.2023.05.036
76. Wang Z, Li R, Hou N, et al. PRMT5 reduces immunotherapy efficacy in triple-negative breast cancer by methylating KEAP1 and inhibiting ferroptosis. J Immun Ther Cancer. 2023;11(6):e006890. doi:10.1136/jitc-2023-006890
77. Zhu X, Xie L, Tian J, Jiang Y, Song E, Song Y. A multi-mode Rhein-based nano-platform synergizing ferrotherapy/chemotherapy-induced immunotherapy for enhanced tumor therapy. Acta Biomater. 2024;180:383–393. doi:10.1016/j.actbio.2024.03.030
78. Zhou TJ, Zhang MM, Liu DM, et al. Glutathione depletion and dihydroorotate dehydrogenase inhibition actuated ferroptosis-augment to surmount triple-negative breast cancer. Biomaterials. 2024;305:122447. doi:10.1016/j.biomaterials.2023.122447
79. Yu X, Li X, Chen Q, et al. High Intensity Focused Ultrasound-Driven Nanomotor for Effective Ferroptosis-Immunotherapy of TNBC. Adv Sci. 2024;11(15):e2305546. doi:10.1002/advs.202305546
80. Wu Q, Li Z, Zhou X, et al. Photothermal Ferrotherapy - Induced Immunogenic Cell Death via Iron-Based Ternary Chalcogenide Nanoparticles Against Triple-Negative Breast Cancer. Small. 2024;20(20):e2306766. doi:10.1002/smll.202306766
81. Wang N, Zhang Q, Wang Z, et al. A chemo/chemodynamic nanoparticle based on hyaluronic acid induces ferroptosis and apoptosis for triple-negative breast cancer therapy. Carbohydr Polym. 2024;329:121795. doi:10.1016/j.carbpol.2024.121795
82. Wang H, Liu X, Yan X, et al. An ATPase-Mimicking MXene nanozyme pharmacologically breaks the ironclad defense system for ferroptosis cancer therapy. Biomaterials. 2024;307:122523. doi:10.1016/j.biomaterials.2024.122523
83. Shetake NG, Das SK, Kumar A, Pandey BN. Nano-inducer of ferroptosis for targeted chemotherapy of human triple negative breast carcinoma. Biomaterials Advances. 2024;161:213868. doi:10.1016/j.bioadv.2024.213868
84. Li Y, Liu J, Chen Y, Weichselbaum RR, Lin W. Nanoparticles Synergize Ferroptosis and Cuproptosis to Potentiate Cancer Immunotherapy. Adv Sci. 2024;11(23):e2310309. doi:10.1002/advs.202310309
85. Huang H, Guo H, Liu J, et al. Dendrimer/metal-phenolic nanocomplexes encapsulating CuO(2) for targeted magnetic resonance imaging and enhanced ferroptosis/cuproptosis/chemodynamic therapy by regulating the tumor microenvironment. Acta Biomater. 2024;183:252–263. doi:10.1016/j.actbio.2024.05.035
86. Guo S, Li Z, Zhou R, et al. MRI-Guided Tumor Therapy Based on Synergy of Ferroptosis, Immunosuppression Reversal and Disulfidptosis. Small. 2024;20(29):e2309842. doi:10.1002/smll.202309842
87. Deng X, Zhu Y, Dai Z, et al. A Bimetallic Nanomodulator to Reverse Immunosuppression via Sonodynamic-Ferroptosis and Lactate Metabolism Modulation. Small. 2024;20(47):e2404580. doi:10.1002/smll.202404580
88. Deng X, Liu T, Zhu Y, et al. Ca & Mn dual-ion hybrid nanostimulator boosting anti-tumor immunity via ferroptosis and innate immunity awakening. Bioact Mater. 2024;33:483–496. doi:10.1016/j.bioactmat.2023.11.017
89. Chen R, Jiang Z, Cheng Y, et al. Multifunctional iron-apigenin nanocomplex conducting photothermal therapy and triggering augmented immune response for triple negative breast cancer. Int J Pharm. 2024;655:124016. doi:10.1016/j.ijpharm.2024.124016
90. Cao C, Lu Y, Pan X, et al. Time and Space Dual-Blockade Strategy for Highly Invasive Nature of Triple-Negative Breast Cancer in Enhanced Sonodynamic Therapy Based on Fe-MOF Nanoplatforms. Adv Healthc Mater. 2024;13(15):e2304249. doi:10.1002/adhm.202304249
91. Zhao C, Liu Z, Chang CC, et al. Near-Infrared Phototheranostic Iron Pyrite Nanocrystals Simultaneously Induce Dual Cell Death Pathways via Enhanced Fenton Reactions in Triple-Negative Breast Cancer. ACS nano. 2023;17(5):4261–4278. doi:10.1021/acsnano.2c06629
92. Liu R, Shi D, Guo L, et al. Ultrasound-Targeted Microbubble Disruption with Key Nanodroplets for Effective Ferroptosis in Triple-Negative Breast Cancer Using Animal Model. Int J Nanomed. 2023;18:2037–2052. doi:10.2147/IJN.S400495
93. Li Z, Guo T, Zhao S, Lin M. The Therapeutic Effects of MUC1-C shRNA@Fe(3)O(4) Magnetic Nanoparticles in Alternating Magnetic Fields on Triple-Negative Breast Cancer. Int J Nanomed. 2023;18:5651–5670. doi:10.2147/IJN.S426849
94. Li K, Xu K, He Y, et al. Oxygen Self-Generating Nanoreactor Mediated Ferroptosis Activation and Immunotherapy in Triple-Negative Breast Cancer. ACS nano. 2023;17(5):4667–4687. doi:10.1021/acsnano.2c10893
95. Ghadi R, Pandey PK, Gabhale A, et al. Genipin-crosslinked albumin nanoparticles containing neratinib and silibinin: a dual-death therapy for triple negative breast cancer. Int J Pharm. 2023;648:123570. doi:10.1016/j.ijpharm.2023.123570
96. Emad B, WalyEldeen AA, Hassan H, et al. Yttrium Oxide nanoparticles induce cytotoxicity, genotoxicity, apoptosis, and ferroptosis in the human triple-negative breast cancer MDA-MB-231 cells. BMC cancer. BMC Cancer. 2023;23(1):1151. doi:10.1186/s12885-023-11649-w
97. Chen Y, Yao Z, Liu P, et al. A self-assembly nano-prodrug for triple-negative breast cancer combined treatment by ferroptosis therapy and chemotherapy. Acta Biomater. 2023;159:275–288. doi:10.1016/j.actbio.2023.01.050
98. Cai Z, Huan ML, Zhang YW, et al. Tumor targeted combination therapeutic system for the effective treatment of drug resistant triple negative breast cancer. Int J Pharm. 2023;636:122821. doi:10.1016/j.ijpharm.2023.122821
99. Wang J, Zhao Z, Liu Y, et al. ‘Mito-Bomb’: a novel mitochondria-targeting nanosystem for ferroptosis-boosted sonodynamic antitumor therapy. Drug Delivery. 2022;29(1):3111–3122. doi:10.1080/10717544.2022.2126027
100. Sun H, Cai H, Xu C, et al. AGuIX nanoparticles enhance ionizing radiation-induced ferroptosis on tumor cells by targeting the NRF2-GPX4 signaling pathway. J Nanobiotechnol. 2022;20(1):449. doi:10.1186/s12951-022-01654-9
101. Zhou Y, Yang J, Chen C, et al. Polyphyllin III-Induced Ferroptosis in MDA-MB-231 Triple-Negative Breast Cancer Cells can Be Protected Against by KLF4-Mediated Upregulation of xCT. Front Pharmacol. 2021;12:670224. doi:10.3389/fphar.2021.670224
102. Song X, Wang X, Chen X, Yu Z, Zhou Y. SRSF1 inhibits ferroptosis and reduces cisplatin chemosensitivity of triple-negative breast cancer cells through the circSEPT9/GCH1 axis. J Proteom. 2024;292:105055. doi:10.1016/j.jprot.2023.105055
103. Shang Y, Zhao M, Chen S, et al. Tetrastigma hemsleyanum polysaccharide combined with doxorubicin promote ferroptosis and immune function in triple-negative breast cancer. Int J Biol Macromol. 2024;275(Pt 1):133424. doi:10.1016/j.ijbiomac.2024.133424
104. Gong G, Ganesan K, Liu Y, et al. Danggui Buxue Tang improves therapeutic efficacy of doxorubicin in triple negative breast cancer via ferroptosis. J Ethnopharmacol. 2024;323:117655. doi:10.1016/j.jep.2023.117655
105. Liu Z, He S, Huang Z, et al. Regulation of ferroptosis-related genes in CD8+ NKT cells and classical monocytes may affect the immunotherapy response after combined treatment in triple negative breast cancer. Thoracic Cancer. 2023;14(34):3369–3380. doi:10.1111/1759-7714.15128
106. Chen X, Kang R, Kroemer G, Tang D. Ferroptosis in infection, inflammation, and immunity. J Exp Med. 2021;218(6). doi:10.1084/jem.20210518
107. Yang F, Xiao Y, Ding JH, et al. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metab. 2023;35(1):84–100.e108. doi:10.1016/j.cmet.2022.09.021
108. Li J, He D, Li S, Xiao J, Zhu Z. Ferroptosis: the emerging player in remodeling triple-negative breast cancer. Front Immunol. 2023;14:1284057. doi:10.3389/fimmu.2023.1284057
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.