Back to Journals » Cancer Management and Research » Volume 17
Is Soft Tissue Density at the Margin of Abdominal Sarcomas Predictive of Recurrence After Tumor Resection
Authors Şentürk A, Harmantepe AT, Gonullu E , Canturk AO , Mutlu F, Taydas O
Received 11 November 2024
Accepted for publication 9 February 2025
Published 14 February 2025 Volume 2025:17 Pages 301—307
DOI https://doi.org/10.2147/CMAR.S502158
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Adem Şentürk,1 Ahmet Tarik Harmantepe,2 Emre Gonullu,2 Alp Omer Canturk,3 Fuldem Mutlu,4 Onur Taydas4
1Sakarya University Training and Research Hospital, Department of Surgical Oncology, Sakarya, Turkey; 2Sakarya University Training and Research Hospital, Department of Gastroenterology Surgery, Sakarya, Turkey; 3Sakarya University Training and Research Hospital, Department of General Surgery, Sakarya, Turkey; 4Sakarya University Training and Research Hospital, Department of Radiology, Sakarya, Turkey
Correspondence: Adem Şentürk, Sakarya University Training and Research Hospital, Sakarya, Turkey, Tel +905360290724, Email [email protected]
Background: The prognostic value of negative surgical margins in soft tissue sarcomas in terms of disease course is well known. However, there is a lack of consensus in the literature regarding the impact of preoperative radiological surgical margins on recurrence rates and overall survival The aim of the present study was to determine whether soft tissue density at the margin of abdominal sarcomas using Hounsfield Unit (HU) measurement on CT is associated with recurrence after tumor resection.
Material and Methods: Seventeen patients who underwent resectional surgery for abdominal sarcoma between May 2014 and May 2024 were retrospectively analyzed. Patients were compared with their preoperative CT scans for postoperative local recurrence according to soft tissue density at the margins of the sarcomas.
Results: Of the 17 patients, nine (52.9%) had recurrence. No significant difference was found for gender in terms of recurrence (p> 0.05). As the median age decreases, recurrence increases significantly. (60 years (23– 70) vs 73 years (44– 79); p= 0.044). Increased preoperative tissue density (width 3 to 5 cm) at sarcoma margin measured by CT was significantly associated with recurrence after tumor resection (with at margin: 3cm; p=0.047, 4cm; p=0.019, 5 cm; p=0.018). The cut-of value of density measured by preoperative CT for soft tissue at sarcoma margin with recurrence was − 98.8 hounsfield Unit (HU), whereas cut-of value of density was − 109.6 hU with a 91.5% sensitivity, 58.9% specificity, 23.2% positive predictive value (PPV), 76.8% negative predictive value (NPV), and 0.83 accuracy, respectively.
Conclusion: Study results suggest that the risk of recurrence after tumor resection can be predicted by measuring soft tissue density at the sarcoma margin on preoperative CT scans.There appears to be a linear relationship between increased preoperative soft tissue density at the sarcoma margin and recurrence after tumor resection. This measurement method offers a perspective that reveals a new approach to this subject. Multicenter studies consisted of larger patient populations are needed to reach a definitive conclusion.
Keywords: soft tissue sarcoma, recurrence, computerized tomography, Hounsfield Unit
Introduction
Sarcomas represent a rare and diverse category of malignant tumors originating from mesenchymal tissues, constituting less than 1% of adult malignancies.1 Approximately 80% of new sarcoma cases arise from soft tissues. According to the World Health Organization (WHO), soft tissue neoplasms encompass over 100 distinct histologic subtypes.2 Given this diversity, the concept of “tumor margins” plays a critical role, yet its evaluation and interpretation remain complex and inconsistently standardized. Multiple studies have highlighted the prognostic significance of achieving negative tumor margins at the time of diagnosis.3–7 Surgeons typically rely on initial imaging to assess the feasibility of tumor resection. However, there is a lack of consensus in the literature regarding the impact of radiological surgical margins on recurrence rates and overall survival. There may be many reasons for this, the main reason is the lack of enough prospective randomized studies in this rare and heterogeneous disease group. The American Joint Committee on Cancer (AJCC) has developed an extensive classification system for negative margins.8 However, the precise measurement of negative margins in millimeters is often absent from international databases and pathology reports. Even all these limited literature data indicate that the surgical margin, if any, the radiological margin, may be associated with disease-free survival and recurrence.9 The microscopic status of margins (positive or negative) is consistently identified as a significant histological factor influencing long-term outcomes. Negative margins should be the target whenever possible. However, different countries use different definitions for negative and positive margins.10–12 All these data show us that even if the definition of “negative margin” is variable, the prognostic value of this risk factor remains important in most studies and better outcomes are obtained for patients after “complete primary resection” than in cases of “incomplete surgery”.13 We will draw attention to a point that has never been touched upon before, namely the radiological margin. What is meant by this boundary is defined as the analysis of the transition zone between the HU measurement of the mass and healthy tissue. If there is a radiological margin, and it can be determined before surgery, this will be revolutionary data.
The aim of the present study was to determine whether soft tissue density at the margin of abdominal sarcomas is associated with local recurrence after tumor resection.
Materials and Methods
The data for this study were retrospectively analyzed over a 10-year period, spanning from September 2014 to May 2024. The research adhered to the ethical principles outlined in the Declaration of Helsinki. Approval for the study was granted by the Ethics Committee of Sakarya University (E-71522473-050.01.04-285316 - 299). The study encompassed patients who received curative surgical treatment for abdominal sarcoma. Written informed consent forms were obtained from the patients.
Demographic and clinical details, including age, gender, and comorbidities, were retrieved retrospectively from the hospital database and analyzed. Histopathological subtype of sarcoma, surgical data, pathological results, pathological and radiological negative surgical margins, tumor diameter, disease-free survival, local recurrence, morbidity and mortality data were analyzed. Patients were evaluated in two groups as those with and without intraabdominal recurrence at postoperative follow-up. Factors affecting recurrence and disease-free survival were statistically investigated and evaluated. The relationship between surgical resection margin, radiologic margin and postoperative recurrence was evaluated. Only intraabdominal sarcomas located intraperitoneal are included, and retroperitoneal located sarcomas are excluded.
Patients underwent an abdominopelvic CT scan using a 64-detector CT scanner (Aquilion 64, Toshiba Medical Systems, Japan). For each CT examination, 100 milliliters (mL) of intravenous (IV) contrast material (300 mg/mL Omnipaque, GE Healthcare, Ireland) was administered at a flow rate of 4 mL/sec, with no oral contrast used. Venous phase abdominal CT images were obtained 70 seconds following IV contrast administration, using the following parameters: tube voltage of 130 kV, effective mAs of 90, slice thickness of 5 mm, collimation of 4×2.5 mm, and pitch of 1.6. The scan area encompassed the body region from the upper diaphragm to the ischial tuberosities. HU measurements were performed on the workstation using a 1 cm diameter ROI. The first ROI was placed starting from the fat tissue where the lesion ended on the CT. Then, density measurements were performed towards the periphery, with a total of 5 adjacent ROIs. The mean density of each ROI was recorded and analysis was performed on this basis.
The CT images were retrospectively reviewed by an abdominal radiologist with 10 years of experience, who was blinded to patients’ physical examination findings, laboratory data, and pathology reports.
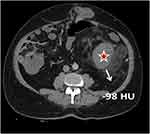
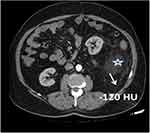
CT scans were directly sent to the workstation (Centricity Universal Viewer, GE Healthcare, USA) to measurements. Density measurement was made with region of interest (ROI) at every cm from the edges of the tumor (Figures 1 and 2).
Statistical Analysis
Data analysis was performed using SPSS version 26 (IBM Corporation). Descriptive statistics for the distribution of responses to independent variables were expressed as frequencies and percentages for categorical data, while numerical data were represented using means, standard deviations, and medians. The normality of continuous variables was assessed using the Kolmogorov–Smirnov test. For comparisons involving binary and multiple groups, the Chi-square test or Fisher’s exact test was utilized for categorical variables, while the One-Way ANOVA or Kruskal–Wallis test was applied for quantitative data. Results were considered statistically significant at a p-value of less than 0.05, with a 95% confidence interval. ROC (Recipient Operating Characteristic) analysis was used to determine the cutoff values. The area under the ROC curves (AUC) values obtained as a result of the ROC analysis were evaluated as 0.9–1: excellent, 0.8–0.9: good, 0.7–0.8: moderate, 0.6–0.7: poor and 0.5–0.6: unsuccessful. The best cutoff point (maximum sensitivity and specificity) was determined in the ROC analysis. Sensitivity, specificity, positive-negative predictive values (PPV, NPV) and likelihood ratio (+) values were calculated to evaluate the success of the cutoff points determined after the ROC analysis.
Results
In the present study, among the 17 patients with intra-abdominal sarcoma, 47.1% (Group 1, n=8) did not experience recurrence after tumor resection, while 52.9% (Group 2, n=9) had a recurrence. A comparison of the recurrence status of these patients with their demographic characteristics is detailed in Table 1.
 |
Table 1 Patients Characteristics and Demographic Data |
In the present study, the mean age of patients in the non-recurrence developed group was 67.13±14.14 years, whereas the mean age of those in the recurrence group was 55.44±15.27 years. This difference between the two groups was found to be statistically significant (p<0.05). The mean tumor size for patients in the non-recurrence group was 16.50±9.18 cm, compared to 15.67±6.91 cm for patients in the recurrence group. The difference between the two groups was not statistically significant (p>0.05). The mean postoperative recurrence time was observed to be 22.45±20.68 months (min-max=1-60 months, median=13). Disease-free survival was 26.75±28.78 (6–84) months in non-recurrence group, whereas 22.44±20.68 (1–60) months in recurrence developed group, and the difference was significant (p=0.047), (Table 2).
 |
Table 2 Association of Recurrence with Demographic Variables |
In the present study, as the preoperative density of soft tissue at the sarcoma margin –measured by CT at a distance of 3cm to 5 cm from the edge of the majin increases, the local recurrence rate increases after tumor resection significantly (Figure 1). While no significant difference was found in the density (HU) in terms of recurrence in those with a preoperative sarcoma margin with a width of 1 cm (p=0.033) and 2 cm (p=0.58), postoperative recurrence increased significantly in those with a width of 3 cm (p=0.047), 4 cm (p=0.019) and 5 cm (p=0.018), respectively. (Table 3).
 |
Table 3 Comparison of Soft Tissue Density at the Sarcoma Margin in Patients with and without Recurrence Using HU Measurement on Preoperative CT |
The sensitivity, selectivity, PPV and NPV values and likelihood ratio (+) values calculated for the classification success in response prediction for the mean values of density and width at margin of sarcomas according to 1 cm, 2 cm, 3 cm, 4 cm and 5 cm on CT, (Table 3). The cut-of value of density measured by preoperative CT for soft tissue at sarcoma margin in patients with without recurrence was −109.6 hU with a 91.5% sensitivity, 58.9% specificity, 23.2% positive predictive value (PPV), 76.8% negative predictive value (NPV), and 0.83 accuracy On the other hand, The cut-of value of density measured by preoperative CT for soft tissue at sarcoma margin in patients with recurrence was −98.8 hU with a 98.8% sensitivity, 51.7% specificity, 31% positive predictive value (PPV), 85% negative predictive value (NPV), and 0.87 accuracy, (Table 4).
 |
Table 4 Comparison of Soft Tissue Density and Width at the Sarcoma Margin in Patients with and without Recurrence Using HU Measurement on Preoperative CT Scans |
Discussion
The present study evaluated the predictivity of soft tissue density at the margin of abdominal sarcomas, utilizing Hounsfield unit measurements by preoperative CT to assess their impact on local recurrence after tumor resection. Although the use and sensitivity of CT in differentiating soft tissue tumors from benign to malignant lesions is limited, and is not as sensitive as MRI, an increasing number of studies have been reported in recent years with the introduction of high-resolution CTs.14–16
Because soft tissue sarcomas are relatively less common than other tumors and are divided into many subgroups, CT diagnosis based on the tumor histopathological structure becomes difficult. In the present study, we observed a relationship between the tissue density and at the margin of abdominal sarcomas with local recurrence after tumor resection. As the preoperative density of soft tissue at the sarcoma margin –measured by CT at a distance of 3 to 5 cm from the edge of the margin- increases, the local recurrence rate significantly increases after tumor resection. The fact that tissue density does not create a significant difference at the edge of the tumor margin (1–2 cm) in recurrence cases, but creates a significant difference at 3–5 cm, may have developed due to the desmoplastic reaction caused by the tumor. This draws our attention as a valuable finding that the progression of the disease may be poor. Although it is certainly not possible to reach a definitive conclusion with such a small sample group, the results are promising and should be supported by other studies consisting of larger series.
According to our literature review, we did not find any other study examining the effect of tissue density and at margins soft tissue sarcomas on local recurrence. Therefore, we think that the current study is the first reported research topic in this field and offers a new perspective on this subject. If the study results are supported by other studies consisting of larger series, an idea about the course of the disease can be obtained in the preoperative period and the current status of the disease and the possibility of local recurrence after tumor resection can be taken into account. Therefore, a more accurate prediction about the course of the disease can be shared with patients.
One of the striking results of this study is that the local recurrence rate decreases with aging. It may be due to the weakening of the immune system with advanced age. However, since the number of samples is small, our theory regarding this claim remains weak. This theory can be made meaningful as a result of multi-center studies consisting of much larger populations.
This study has several limitations. Firstly, it is retrospective in nature. Secondly, the sample size is relatively small. Lastly, the sarcoma subgroups are heterogeneous, which may influence the findings.
Conclusion
The results of the present study suggest that the detection of denser tissue at the margin of sarcomas on preoperative CT may increase the risk of local recurrence after tumor resection. This measurement method offers a perspective that reveals a new approach to this subject. Prospective randomized studies consisting of larger series are needed to reach a definitive conclusion.
Consent
Written informed consent forms were obtained from the patients.
Funding
This research received no funding.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. PMID: 36633525. doi:10.3322/caac.21763
2. World Health Organization Classification of Tumours Editorial Board. Soft Tissue and Bone Tumours.
3. Brecht IB, Ferrari A, Int-Veen C, et al. Grossly-resected synovial sarcoma treated by the German and Italian pediatric soft tissue sarcoma cooperative groups: discussion on the role of adjuvant therapies. Pediatr Blood Cancer. 2006;46:11–17. doi:10.1002/pbc.20502
4. Orbach D, Mc Dowell H, Rey A, Bouvet N, Kelsey A, Stevens MC. Sparing strategy does not compromise prognosis in pediatric localized synovial sarcoma: experience of the International Society of Pediatric Oncology, malignant mesenchymal tumors (SIOP-MMT) working group. Pediatr Blood Cancer. 2011;57:1130–1136. doi:10.1002/pbc.23138
5. Ferrari A, Casanova M, Collini P, et al. Adult-type soft tissue sarcomas in pediatric-age patients: experience at the Istituto Nazionale Tumori in Milan. J Clin Oncol. 2005;23:4021–4030. doi:10.1200/JCO.2005.02.053
6. Koscielniak E, Harms D, Henze G, et al. Results of treatment for soft tissue sarcoma in childhood and adolescence: a final report of the German cooperative soft tissue sarcoma study CWS-86. J Clin Oncol. 1999;17:3706–3719. doi:10.1200/JCO.1999.17.12.3706
7. Spunt SL, Hill DA, Motosue AM, et al. Clinical features and outcome of initially unresected nonmetastatic pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Clin Oncol. 2002;20:3225–3235. doi:10.1200/JCO.2002.06.066
8. Gundle KR, Kafchinski L, Gupta S, et al. Analysis of margin classification systems for assessing the risk of local recurrence after soft tissue Sarcoma resection. J Clin Oncol. 2018;36(7):704–709. PMID: 29346043. doi:10.1200/JCO.2017.74.6941
9. Spunt SL, Poquette CA, Hurt YS, et al. Prognostic factors for children and adolescents with surgically resected nonrhabdomyosarcoma soft tissue sarcoma: an analysis of 121 patients treated at St Jude Children’s Research Hospital. J Clin Oncol. 1999;17(12):3697–3705. PMID: 10577841. doi:10.1200/JCO.1999.17.12.3697
10. Sadoski C, Suit HD, Rosenberg A, Mankin H, Efird J. Preoperative radiation, surgical margins, and local control of extremity sarcomas of soft tissues. J Surg Oncol. 1993;52(4):223–230. PMID: 8468983. doi:10.1002/jso.2930520405
11. Cecchetto G, Guglielmi M, Inserra A, et al.; Italian Cooperative Group on Soft-tissue Sarcomas. Primary re-excision: the Italian experience in patients with localized soft-tissue sarcomas. Pediatr Surg Int. 2001;17(7):532–534. PMID: 11666052. doi:10.1007/s003830100580
12. Chui CH, Spunt SL, Liu T, et al. Is reexcision in pediatric nonrhabdomyosarcoma soft tissue sarcoma necessary after an initial unplanned resection? J Pediatr Surg. 2002;37(10):1424–1429. PMID: 12378447. doi:10.1053/jpsu.2002.35405
13. Sparber-Sauer M, Ferrari A, Spunt SL, et al. The significance of margins in pediatric Non-Rhabdomyosarcoma soft tissue sarcomas: Consensus on surgical margin definition harmonization from the INternational Soft Tissue SaRcoma ConsorTium (INSTRuCT). Kanser Med. 2023;12(10):11719–11730. PMID: 36744538; PMCID: PMC10242312. doi:10.1002/cam4.5671
14. Liu DN, Li ZW, Wang HY, Zhao M, Zhao W, Hao CY. Use of 18F-FDG-PET/CT for Retroperitoneal/Intra-abdominal soft tissue Sarcomas. Contrast Media mol Imaging. 2018;2018:2601281. doi:10.1155/2018/2601281
15. Wu G, Xie R, Li Y, Hou B, Morelli JN, Li X. Histogram analysis with computed tomography angiography for discriminating soft tissue sarcoma from benign soft tissue tumor. Medicine. 2020;99(2):e18742. doi:10.1097/MD.0000000000018742
16. Tsubakimoto M, Yamashiro T, Tamashiro Y, et al. Quantitative CT density histogram values and standardized uptake values of FDG-PET/CT with respiratory gating can distinguish solid adenocarcinomas from squamous cell carcinomas of the lung. Eur J Radiol. 2018;100:108–115. doi:10.1016/j.ejrad.2018.01.021
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.