Back to Journals » Cancer Management and Research » Volume 17
Isolated Central Nervous System Infiltrated and Progressed to Acute Myeloid Leukemia from Chronic Myeloid Leukemia with e1a3 BCR-ABL1 Transcript: A Rare Case Report and Literature Review
Authors Qiang X, Wen Q, Li J, Chen S, Tao T, Zhang H, Wang P, Peng X, Feng Y , Zhang X
Received 14 October 2024
Accepted for publication 30 December 2024
Published 11 January 2025 Volume 2025:17 Pages 35—43
DOI https://doi.org/10.2147/CMAR.S499043
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Xing Qiang, Qin Wen, Jia Li, Siyu Chen, Tinglu Tao, Hongyang Zhang, Ping Wang, Xiangui Peng, Yimei Feng, Xi Zhang
Medical Center of Hematology, Xinqiao Hospital, Army Medical University, Chongqing, People’s Republic of China
Correspondence: Xiangui Peng; Yimei Feng, Email [email protected]; [email protected]
Abstract: The chronic myeloid leukemia (CML) is easily diagnosed by laboratory examination, however, rare BCR-ABL1 mRNA transcripts variants, such as e1a3 present diagnosis and therapeutic challenges. This case report details the diagnosis and management of a CML patient with the e1a3 transcript by FISH and RT-PCR. Following initial diagnosis, the patient was treated with the tyrosine kinase inhibitor (TKI) Flumatinib. During the treatment, although the FCM-MRD of the bone marrow kept negative, the e1a3 expression detected by PCR always remained positive. After eighteen months, the patient experienced headaches, vomiting, and blurred vision. Subsequent bone marrow analysis and flow cytometry detection of cerebrospinal fluid indicated that the patient had entered the blast phase, progressing to acute myeloid leukemia (AML). Treatment was switched to the third-generation TKI olverembatinib, combined with chemotherapy, followed by allogeneic hematopoietic stem cell transplantation. The patient remains disease-free following olverembatinib maintenance therapy. This case underscores the importance of comprehensive diagnostic apporsches and the potential efficacy of third-generation TKIs and allo-HSCT in the treatment of e1a3-type CML.
Keywords: CML-BC, e1a3 transcript, CNS, HSCT, olverembatinib
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative characterized by the presence of the Philadelphia chromosome, arising from a t(9;22)(q34;q11) chromosomal translocation. This translocation results in the formation of the BCR::ABL1 fusion gene, which encodes a constitutively active tyrosine kinase that drives the leukemic process.1 Variations in the breakpoints within the BCR or ABL1genes lead to diverse BCR::ABL1 mRNA transcripts, with the e1a2, e13a2, e14a2, and e19a2 being the most common. Less frequent variants, including e1a3, e13a3, and e6a2, have been identified.2–4 About 1% of patients with CML may have e1a2/a3 transcripts coding for the oncogenic protein P190, and these patients may have a worse prognosis.5,6 e1a3 is a rare but poor prognostic transcript, as shown in several cases.7
CML has been widely characterized as a triphasic disease, progressing from a chronic phase through an accelerated phase to a blastic phase (BP). BP-CML a poor-prognosis acute leukemia, which may be myeloid, lymphoid, or mixed phenotype.8 In addition to presenting with de novo BP-CML, patients may experience deterioration during therapy. CML can also progress towards the extramedullary blast phase due to the infiltration of blast cells, which can affect various parts of the body, including the bones, lymph nodes, gastrointestinal tract, skin, central nervous system (CNS), and soft tissues.9–11
Cases of CML invading the CNS are not rare,12–17 the CNS progression of e1a3 subtype CML, especially isolated CNS infiltration without any relapse in the hematological aspects has not been reported. The present case report describes a rare case of CML with the e1a3 BCR-ABL1 transcript, which is unique as it involves the emergence of an isolated CNS blast crisis during flumatinib maintenance therapy, despite achieving hematological and cytogenetic responses.
Case Presentation
A 56-year-old Chinese female patient visited our center with the primary complaint of a high white blood cell(WBC) count discovered during a physical examination in June 2021. The initial laboratory investigations revealed a WBC count (WBC) of 226.0×109/L, hemoglobin level of 109 g/L, and a platelet count of 164×109/L. Myeloid blasts were identified in 2% of the peripheral blood and 3% of the bone marrow aspirate. The karyotype was 46 XX t (9;22) (q34; q11) in all 20 metaphases analyzed. [20/20] Fluorescence in situ hybridization (FISH) analysis detected BCR::ABL1 translocations in approximately 93% of nucleated cells. Notably, the quantitative real-time polymerase chain reaction (RT-PCR) yielded negative results for the BCR::ABL1 transcript (P190, P210, and P230). Therefore, we conducted a new PCR detection test for some rare subtypes of expression, and finally find that of positive results for rare e1a3 BCR::ABL1 transcript. An amplified band of 136 base pairs (bp) was observed using specific primers: the forward primer 5`CTGGCCCAACGATGGCGA` and reverse primer 5`GATGTAGTTGCTTGGGACCCA3`, which were parts of BCR e1 and ABL a3 regions, respectively. The fluorescent probe primer sequence used for quantitative analysis is: CCATTTTTGGTTTGGGCTTCACACCAT.
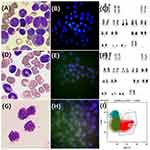
Eventually, the patient was diagnosed with chronic phase – CML (CP-CML) with e1a3 BCR::ABL1 transcript, as confirmed by morphology, FISH and cytogenetic analyses (Figure 1A-C). She was promptly treated with regimens consisting of hydroxyurea and first-line TKI (tyrosine kinase inhibitor) Flumatinib at a daily dosage of 600mg. Notably, at the time of diagnosis in June 2021, the diagnostic workup was limited to bone marrow examinations, including morphology, flow cytometry, FISH and RT-PCR. Given the absence of symptom like headaches indicating central nervous system involvement, no imaging or lumbar puncture was initially performed. Consequently, the patient received standard treatment for CML with oral Flumatinib 600mg/day.
After three, six, nine, and twelve months of TKI treatment, the patient exhibited normal bone marrow morphology, flow cytometry-minimal residual disease (FCM-MRD), and FISH analysis, indicating the absence of accelerated phases or blast phase. Nevertheless, PCR assays persistently demonstrated a positive result of e1a3 expression. Regrettably, the level of e1a3 was only qualitatively analyzed and not quantitatively analyzed, and the patient has been maintained on oral treatment with flumatinib.
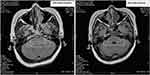
The patient exhibited symptoms of headaches, vomiting, and blurred vision up to 17 months after oral flumatinib (December 2022). Given the possibility of CNS invasion, the diagnostic tests were conducted. A brain magnetic resonance imaging (MRI) scan revealed abnormal signals in the medulla oblongata and bilateral pontine arms (Figure 2). The diagnostic lumbar puncture indicated that the patient has progressed to the chronic myeloid leukemia blast phase (BP-CML), revealing that the total number of nucleated cells in cerebrospinal fluid (CSF) was 4091×106/L, and closely resembled myeloid blasts morphologically. The immunophenotyping analysis of CSF revealed the presence of 86.8% myeloid blasts expressed CD34 and CD117. FISH analysis of CSF also demonstrated BCR::ABL1 translocations in approximately 70% of nucleated cells (Figure 1G-I). Concurrently, a PCR quantitative analysis of e1a3 was performed and found that 3.18% of expression in the bone marrow (Table 1). Meanwhile, the bone marrow puncture was submitted, including the evaluation of bone marrow morphology, flow cytometry for minimal residual disease (MRD), and FISH tests. Notably, all these assessments from bone marrow returned negative results (Figure 1D-F). At present, the patient has been diagnosed with isolated CNS infiltrated and progressed to AML with e1a3 BCR::ABL1 transcript.
 |
Table 1 E1A3 Expression and FCM-MRD Monitoring of This Patient |
Based on the above findings, we amended the treatment protocol. Initially, we transitioned the patient from flumatinib to third-generation TKIs olverembatinib (a novel small molecular oral third-generation BCR::ABL1 inhibitor) at a daily dosage of 40mg.18 Subsequently, the patient was promptly initiated on a triple intrathecal regimen comprising methotrexate (10mg), dexamethasone (5mg), and cytarabine (50mg). After 13 cycles of intrathecal chemotherapy, the FCM-MRD of CSF cytology continued to detect myeloid blasts, therefore, to the decision to administer intravenous chemotherapy to the patient initially, followed by allogeneic hematopoietic stem cell transplantation treatment (Allo-HSCT) was prompted. She received a supplementary IA chemotherapy regimen, comprising Idarubicin 10 mg on days 1–3 and Cytarabine 150mg every 12 hours on days 1–3. Radiotherapy was strongly recommended, but the patient expressed a disagreement with the recommended course of treatment. After another 3 cycles of intrathecal chemotherapy, the CSF cytology turned to negative with no detectable myeloid blasts. Subsequently, the patient underwent an unrelated HLA-matched Allo-HSCT. On day 12 post-transplant, hematopoietic reconstruction was required, and the patient was discharged on day 45. To date, the patient has undergone eight lumbar punctures for detection post-transplantation, with negative FCM-MRD results in the cerebrospinal fluid and negative expression of e1a3 in the bone marrow sample simultaneously, remaining disease-free and alive. Commencing 60 days post-transplant, the patient began taking olverembatinib at an initial dose of 20mg every other day (qod), which was escalated to 30mg qod. By day 100 post-transplant, the dosage was increased to 40mg qod, a dose that has been maintained to date. The patient has expressed satisfaction with the treatment approach and the current outcomes.
Discussion
One of the key features of the e1a3 translocation is the absence of exon a2, and the deletion of ABL1 exon a2 from the fusion transcript is predicted to lead to a protein lacking approximately two-thirds of the N terminus in the Src homology (SH3) domain.19 The latter is crucial for full leukemogenesis in vivo. Because BCR::ABL1 mutants deficient in the SH3 domain induce growth factor independence, leading to reduced proliferation of tumor cells in vivo due to diminished tissue invasiveness.20 Therefore, the clinical course of CML patients with e1a3 BCR::ABL1 fusion transcripts is characterized by a lower clinical progression, lower white blood cells (WBC)counts, and an extended duration in the chronic phase even in the absence of treatment.21 However, the e1a3 and e1a2 (p190) transcripts have been reported to possess similar molecular weight and probably a similar clinical profile, thus it has been hypothesized that e1a3 fusion may predispose extramedullary infiltration and disease acceleration.22 We present a case of CML with the e1a3 BCR-ABL1 transcript, which is unique as it involves the emergence of an isolated CNS blast crisis, despite achieving hematological and cytogenetic responses.
Accurate diagnosis e1a3 CML is imperative. One concern is that it might be disregarded during routine testing procedures. The FISH assay employs probes that specifically target the breakpoints at both ends of the genes, thereby enabling the detection of various breakpoints within the BCR and ABL1 genes. Moreover, the next-generation sequencing (NGS) can identify atypical translocations, rectifying the previously overlooked detection of this significant genetic events. If a BCR::ABL1 negative result is obtained by conventional testing, then RT–PCR and NGS sequencing of RNA can facilitate the detection of this breakpoint to avoid misdiagnosis.23–25 The case we reported was also diagnosed clearly through RT-PCR. Honestly, FCM plays an important role in the initial diagnosis of CML, in the blast phase, and in the differential diagnosis of MPN, mixed cell leukemia, and other conditions. However, PCR is more recommended for MRD monitoring, especially during the stable period.
Cases harboring the e1a3 BCR-ABL1 transcripts have been reported in Acute Lymphoblastic Leukemia (ALL), AML, and CML. We conducted a literature review and found no reports of central nervous system invasion in several cases of the e1a3 subtype CML. This is the first reported case, and the treatment methods for these cases are not consistent (Table 2). The standard treatment for this condition has not been established. The e1a3 BCR-ABL gene encodes the ATP binding site of ABL kinase, which can be utilized for TKI-targeted therapy. ALL patients with e1a3 BCR-ABL gene-positive who receive TKI-targeted therapy have a higher survival rate compared to those who do not receive TKI treatment, and the combination with allogeneic hematopoietic stem cell transplantation is more effective.22,23 A case report has illustrated that the administration of dasatinib led to the regression of extramedullary blast crisis (cervical mass) in CML with e1a3 BCR-ABL1 fusion transcript.26 If the T315i mutation was developed after treatment with dasatinib monotherapy, ponatinib therapy can be replaced.27
 |
Table 2 Cases of e1a3- CML in Literature |
This is exactly the issue of TKI treatment resistance in CML, not limited to the e1a3 subtype. Imatinib has demonstrated efficacy in the e1a3 subtype of CML or ALL,27 and our patient was initially treated with flumatinib. Flumatinib is a chemically derived form of imatinib with two modifications, enhances the specificity of its BCR::ABL1 inhibition.28 It is recommended as the first line drug according to CML related consensus in China.29 However, in this case, the treatment fails to produce satisfactory effects and progresses to an isolated CNS blast crisis in this case. The extramedullary proliferation of blast is an additional diagnostic indicator of BP-CML, regardless of the disease stage present in the bone marrow. The dissemination of leukemic cells in the CNS is linked to high WBC counts during the initial diagnosis.30,31 One possible reason for the CNS infiltration observed in this case was the marked elevation in WBC count (226.0×109/L). Patients present with an isolated relapse in the CNS, underscoring the significant role of the CNS as a refuge for leukemic stem cells. In addition, flumatinib rarely pass through the blood-brain barrier, which is also the main reason. In cases with high white blood cell counts and persistent positivity in RT-PCR testing for e1a3, early MRI of the head and intrathecal chemotherapy should be performed to prevent central nervous system leukemia. This case provides a valuable lesson in the management of CML patients with e1a3 BCR::ABL1 transcripts.
The blood-brain barrier (BBB) permeability of Flumatinib was about 0–6%,32 and the BBB permeability of olverembatinib was 2–12%, higher than the former.33 Accordingly, when our patient presented with symptoms of CNS, we promptly transitioned from flumatinib to olverembatinib with the aim of increasing the drug concentration in the CSF. Simultaneously, systemic intravenous medication was administered to enhance the therapeutic effect. Finally, allo-HSCT was performed and olverembatinib was administered for continued maintenance treatment post-transplantation. On day 150 after transplantation, FCM-MRD of CSF was negative, and e1a3 expression of bone marrow was also negative (Table 1).
The advent of TKIs has transformed the treatment landscape for patients with BP-CML, providing new options and enabling remission for older and more fragile individuals, although it may be of shorter duration, through the use of single agents. However, there is a significant risk of relapse within a few months without allo-HSCT consolidation. The combination of allo-HSCT with TKI maintenance therapy has been shown to be an effective approach for treating BP-CML, especially for extramedullary disease.34–37 Although studies on extramedullary BP-CML lack extensive data and mainly rely on case reports, it is crucial to treat patients with the same level of care as other BP-CML patients. Irrespective of the specific TKI selected, it is essential to incorporate systemic and /or intrathecal therapy within the overall approach to preventing and treating CNS leukemia. This is also the reason why our patient was successfully treated. Till today, the e1a3 expression of our patient is still negative and quantity analysis of PB and BM are 0% after transplantation. Unfortunately, the e1a3 detection was inconsistency in this report, due to the absence of quantitative testing in the initial stage, the precise expression level remained unclear, and treatment measure was not promptly adjusted until the patient exhibited symptoms of the central nervous system. Most of the time, peripheral blood and bone marrow were tested, while cerebrospinal fluid testing was more important, which is the limitation of this case.
Conclusion
Conventional diagnostic methods may overlook the e1a3-type CML, requiring a combination of RT-PCR, FISH, and other methods to ensure a definitive diagnosis about e1a3-type CML. Previous reports have shown that e1a3-type CML has a protracted disease course and is sensitive to TKIs. However, due to the high white blood cell count at diagnosis, the extramedullary invasion characteristic of the e1a3 subtype, and the limited ability of flumatinib to cross the blood-brain barrier, this case experienced a CNS blast crisis. Currently, no standardized treatment has been established. Moreover, allo-HSCT together with third-generation TKI maintenance therapy is suggested as an effective treatment approach.
Abbreviations
CML, Chronic myeloid leukemia; BP, blastic phase; CP, chronic phase; WBC, white blood cell count; CNS, central nervous system; FISH, Fluorescence in situ hybridization; RT-PCR, real-time polymerase chain reaction; FCM-MRD, flow cytometry-minimal residual disease; CSF, cerebrospinal fluid; Allo-HSCT, allogeneic hematopoietic stem cell transplantation treatment.
Ethics and Informed Consent
Consent for publication was obtained from the patient for her data included in the study. This retrospective study was approved by the Ethics Committee of the Second Affiliated Hospital of Army Medical University.
Acknowledgments
The authors express gratitude to the team from the hematological laboratory of Xinqiao hospital for their diagnostic contributions. We would like to express our gratitude to Professor Cai Yuanqing from the Radiology Department of Xinqiao Hospital for his assistance in publication.
Funding
This work was supported by Chongqing Technology Innovation and Application Development Project (CSTB2023TIAD-STX0008), National Natural Science Foundation of China (No. 82020108004), Youth Project of National Health Commission (WKZX2023CX080001), General Project of Chongqing Natural Science (cstc2019jcyjmsxmX0273; cstc2020jcyj-msxmX1086), and Chongqing Science and Health Joint Medical Research Program (2021MSXM140).
Disclosure
The authors have no conflicts of interest to disclose in this work.
References
1. Cortes J, Pavlovsky C, Saussele S. Chronic myeloid leukaemia. Lancet. 2021;398(10314):1914–1926. doi:10.1016/S0140-6736(21)01204-6
2. Burmeister T, Schwartz S, Taubald A, et al. Atypical BCR-ABL mRNA transcripts in adult acute lymphoblastic leukemia. Haematologica. 2007;92(12):1699–1702. doi:10.3324/haematol.11737
3. Demehri S, Paschka P, Schultheis B, et al. e8a2 BCR-ABL: more frequent than other atypical BCR-ABL variants? Leukemia. 2005;19(4):681–684. doi:10.1038/sj.leu.2403604
4. Gong Z, Medeiros LJ, Cortes JE, et al. Clinical and prognostic significance of e1a2 BCR-ABL1 transcript subtype in chronic myeloid leukemia. Blood Cancer J. 2017;7(7):e583. doi:10.1038/bcj.2017.62
5. Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2025 update on diagnosis, therapy, and monitoring. Am J Hematol. 2024;99(11):2191–2212. doi:10.1002/ajh.27443
6. Qin YZ, Jiang Q, Jiang H, et al. Prevalence and outcomes of uncommon BCR - ABL 1 fusion transcripts in patients with chronic myeloid leukaemia: data from a single centre. Br J Haematol. 2018;182(5):693–700. doi:10.1111/bjh.15453
7. Hu K, Wang Y, Teng X, et al. Cell subsets and cytokine dynamics in cerebrospinal fluid after CAR-T cell therapy for B-cell acute lymphoblastic leukemia with central nervous system involvement. Bone Marrow Transplant. 2021;56(12):3088–3090. doi:10.1038/s41409-021-01471-y
8. DeFilipp Z, Khoury HJ. Management of advanced-phase chronic myeloid leukemia. Curr Hematol Malig Rep. 2015;10(2):173–181. doi:10.1007/s11899-015-0249-2
9. Chiba A, Toya T, Mizuno H, et al. Chronic myelogenous leukemia presenting with central nervous system infiltration, successfully treated with central nervous system-directed chemotherapy followed by allogeneic stem cell transplantation. Int J Hematol. 2018;108(6):640–646. doi:10.1007/s12185-018-2511-6
10. Sahu KK, Malhotra P, Uthamalingam P, et al. Chronic myeloid leukemia with extramedullary blast crisis: two unusual sites with review of literature. Indian J Hematol Blood Transfus. 2016;32(S1):89–95. doi:10.1007/s12288-014-0471-4
11. Mbekeani JN, Abdel Fattah M, Al Nounou RM, et al. Chronic myelogenous leukemia relapse presenting with central nervous system blast crisis and bilateral optic nerve infiltration. J Neuroophthalmol. 2016;36(1):73–77. doi:10.1097/WNO.0000000000000326
12. Zhou HS, Dai M, Wei Y, et al. Isolated central nervous system relapse in patient with blast-crisis chronic myeloid leukemia in durable complete cytogenetic remission on dasatinib treatment: pharmacokinetics and ABL mutation analysis in cerebrospinal fluid. Leuk Lymphoma. 2013;54(7):1557–1559. doi:10.3109/10428194.2012.745933
13. Bujassoum S, Rifkind J, Lipton JH. Isolated central nervous system relapse in lymphoid blast crisis chronic myeloid leukemia and acute lymphoblastic leukemia in patients on imatinib therapy. Leuk Lymphoma. 2004;45(2):401–403. doi:10.1080/10428190310001593184
14. Lai SW, Huang TC, Chen JH, et al. Dasatinib as the salvage therapy for chronic myeloid leukemia with blast crisis and central nervous system involvement: a case report. Oncol Lett. 2015;9(4):1957–1961. doi:10.3892/ol.2015.2928
15. Musleh M, Alhussien Q. A chronic myeloid leukemia present with an unusual relapse in central nervous system: a case report. Clin Case Rep. 2024;12(4):e8671. doi:10.1002/ccr3.8671
16. Boudiaf H, Ezziane K, Rouis NO, et al. Isolated blast crisis relapse in the central nervous system of a patient treating for a chronic myelogenous leukemia. Pan Afr Med J. 2020;36:142. doi:10.11604/pamj.2020.36.142.24155
17. Chatterjee G, Rastogi N, Thakkar D, et al. Successful haploidentical stem cell transplant with posttransplant cyclophosphamide for isolated central nervous system blast crisis in a child with chronic myeloid leukemia. J Pediatr Hematol Oncol. 2021;43(1):e146–e147. doi:10.1097/MPH.0000000000001675
18. Jiang Q, Li ZR, Qin YZ, et al. Olverembatinib (HQP1351), a well-tolerated and effective tyrosine kinase inhibitor for patients with T315I-mutated chronic myeloid leukemia: results of an open-label, multicenter Phase 1/2 trial (vol 15, 113, 2022). J hematol oncol. 2022;15:13.
19. Al-Ali HK, Leiblein S, Kovacs I, et al. CML with an e1a3 BCR-ABL fusion: rare, benign, and a potential diagnostic pitfall. Blood. 2002;100(3):1092–1093. doi:10.1182/blood-2002-03-0930
20. Skorski T, Nieborowska-Skorska M, Wlodarski P, et al. The SH3 domain contributes to BCR/ABL-dependent leukemogenesis in vivo: role in adhesion, invasion, and homing. Blood. 1998;91(2):406–418. doi:10.1182/blood.V91.2.406
21. Roman J, Jimenez A, Barrios M, et al. E1A3 as a unique, naturally occurring BCR-ABL transcript in an indolent case of chronic myeloid leukaemia. Br J Haematol. 2001;114(3):635–637. doi:10.1046/j.1365-2141.2001.02971.x
22. Lopez-Andrade B, Sartori F, Gutierrez A, et al. Acute lymphoblastic leukemia with e1a3 BCR/ABL fusion protein. A report of two cases. Exp Hematol Oncol. 2015;5(1):21. doi:10.1186/s40164-016-0049-y
23. Chen Z. The e1a3 BCR-ABL1 fusion transcript in Philadelphia chromosome-positive acute lymphoblastic leukaemia: a case report. Hematology. 2023;28(1):2186040. doi:10.1080/16078454.2023.2186040
24. Martinelli G, Amabile M, Terragna C, et al. Concomitant expression of the rare E1/A3 and B2/A3 types of BCR/ABL transcript in a chronic myeloid leukemia (CML) patient. Leukemia. 1999;13(9):1463–1464. doi:10.1038/sj.leu.2401509
25. Martinez-Serra J, Del Campo R, Gutierrez A, et al. Chronic myeloid leukemia with an e1a3 BCR-ABL fusion protein: transformation to lymphoid blast crisis. Biomark Res. 2014;2(1):14. doi:10.1186/2050-7771-2-14
26. Miyashita N, Onozawa M, Suto K, et al. Aleukemic extramedullary blast crisis as an initial presentation of chronic myeloid leukemia with E1A3 BCR-ABL1 fusion transcript. Intern Med. 2022;61(7):1049–1054. doi:10.2169/internalmedicine.8319-21
27. Sheets JW, Eulitt P, He R, et al. Philadelphia chromosome-positive acute myeloid leukemia with e1a3 BCR-ABL1 fusion transcript. Hemasphere. 2020;4(6):e484. doi:10.1097/HS9.0000000000000484
28. Zhang L, Meng L, Liu B, et al. Flumatinib versus imatinib for newly diagnosed chronic phase chronic myeloid leukemia: a phase III, randomized, open-label, multi-center FESTnd Study. Clin Cancer Res. 2021;27(1):70–77. doi:10.1158/1078-0432.CCR-20-1600
29. Chinese Society of Hematology CMA. The guidelines for diagnosis and treatment of chronic myelogenous leukemia in China (2020 edition). Zhonghua Xue Ye Xue Za Zhi. 2020;41(5):353–364. in Chinese. doi:10.3760/cma.j.issn.0253-2727.2020.05.001
30. Altintas A, Cil T, Kilinc I, et al. Central nervous system blastic crisis in chronic myeloid leukemia on imatinib mesylate therapy: a case report. J Neurooncol. 2007;84(1):103–105. doi:10.1007/s11060-007-9352-0
31. Garcia-Gutierrez V, Breccia M, Jabbour E, et al. A clinician perspective on the treatment of chronic myeloid leukemia in the chronic phase. J Hematol Oncol. 2022;15(1):90. doi:10.1186/s13045-022-01309-0
32. Wang J, Wu JF, Wang YJ, et al. Basic and clinical study of efficacy and adverse effects of flumatinib in Ph+ ALL. Front Pharmacol. 2023;14:1178393. doi:10.3389/fphar.2023.1178393
33. Xiang D, Zhao T, Wang J, et al. Determination of olverembatinib in human plasma and cerebrospinal fluid by an LC-MS/MS method: validation and clinical application. J Pharm Biomed Anal. 2023;230:115382. doi:10.1016/j.jpba.2023.115382
34. Copland M. Treatment of blast phase chronic myeloid leukaemia: a rare and challenging entity. Br J Haematol. 2022;199(5):665–678. doi:10.1111/bjh.18370
35. Zhang XH, Chen J, Han MZ, et al. The consensus from The Chinese society of hematology on indications, conditioning regimens and donor selection for allogeneic hematopoietic stem cell transplantation: 2021 update. J hematol oncol. 2021;14(1):145. doi:10.1186/s13045-021-01159-2
36. Zhang C, Zhong JF, Zhang X. Revealing the molecular mechanism of central nervous system leukemia with single-cell technology. Crit Rev Oncol Hematol. 2020;153:103046. doi:10.1016/j.critrevonc.2020.103046
37. Wang XQ, Huang RH, Zhang XH, et al. Current status and prospects of hematopoietic stem cell transplantation in China. Chin Med J (Engl). 2022;135(12):1394–1403. doi:10.1097/CM9.0000000000002235
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.