Back to Journals » Journal of Multidisciplinary Healthcare » Volume 18
J-Shaped Association Between Non-HDL Cholesterol to HDL Cholesterol Ratios and Gout in US Adults With Gout
Authors Tian RN, Zhang SX, Zhang N, Shi Y , Guo HQ, Wang C, Duan ZG
Received 26 November 2024
Accepted for publication 5 February 2025
Published 18 February 2025 Volume 2025:18 Pages 933—946
DOI https://doi.org/10.2147/JMDH.S508765
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ruo-Nan Tian,1,2 Sheng-Xiao Zhang,2– 5 Nan Zhang,6 Yan Shi,1 Hua-Qing Guo,1 Chen Wang,1 Zhi-Guang Duan6
1College of Humanities and Social Sciences, Shanxi Medical University, Taiyuan, Shanxi, 030001, People’s Republic of China; 2Key Laboratory of Cellular Physiology at Shanxi Medical University, Ministry of Education, Taiyuan, Shanxi Province, 030001, People’s Republic of China; 3Department of Rheumatology and Immunology, The Second Hospital of Shanxi Medical University, Taiyuan, 030001, People’s Republic of China; 4Shanxi Provincial Key Laboratory of Rheumatism Immune Microecology, Taiyuan, Shanxi Province, 030001, People’s Republic of China; 5SXMU-Tsinghua Collaborative Innovation Center for Frontier Medicine, Shanxi Medical University, Taiyuan, Shanxi Province, 030001, People’s Republic of China; 6School of Management, Shanxi Medical University, Taiyuan, Shanxi Province, 030001, People’s Republic of China
Correspondence: Zhi-Guang Duan, School of Management, Shanxi Medical University, No. 56, Xinjiannan Road, Taiyuan, Shanxi Province, People’s Republic of China, Email [email protected]
Background and Aim: This study aims to assess the potential association between NHHR and gout risk among the US adult population.
Methods and Results: Utilizing data from the NHANES spanning from 2007 to 2018, we performed a cross-sectional analysis. A weighted multivariable logistic regression model, generalized additive model (GAM) and a restricted cubic spline model were applied to elucidate the association between NHHR and gout risk. In addition, subgroup and sensitivity analyses were conducted to ensure the stability of our findings. This study cohort included 27,731 participants. Multivariate logistic regression analysis indicated a significant correlation between NHHR and the likelihood of gout. This association was sustained after accounting for a range of potential confounding confounders. The risk of gout was observed to escalate with increasing quartiles of NHHR quartiles, with a 67% increased risk in the fourth quartile. Both RCS and curve fitting results indicated a J-shaped relationship between NHHR and gout. The association remained significant in several subgroup analyses. The interaction test did not yield statistically significant effects on this association.
Conclusion: The NHHR is nonlinearly correlated with the risk of gout in US adults. Further investigation research into the role of NHHR in gout could offer new perspectives on the prevention and treatment of gout. However, additional large-scale prospective studies are necessary to validate and reinforce these results.
Keywords: non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol, gout, National health and nutrition examination survey, cross-sectional study
Introduction
Gout, a form of inflammatory arthritis precipitated by hyperuricemia (HUA), is characterized by the deposition of monosodium urate crystals in the joints and soft tissues.1 The etiology of gout is multifactorial, with dysregulated uric acid metabolism and impaired renal excretion identified as pivotal factors2,3. The United States bears a high burden of gout, with the highest age-standardized prevalence rates globally.4 The Global Burden of Disease Study 2019 has reported a significant increase in the annual incidence rate from 38.71 to 45.94 cases per 100,000 people between 1990 and 2019, predominantly affecting men.5 Gout is not only marked by severe joint pain but also frequently comorbid with conditions such as obesity, metabolic syndrome, osteoporosis, hyperlipidemia, chronic kidney disease, type 2 diabetes mellitus, and obstructive sleep apnea.6,7 These comorbidities amplify the disease’s impact, increasing the risk of morbidity and mortality.8 Moreover, gout has been established as an independent risk factor for cardiovascular diseases, with higher cardiovascular mortality rates observed in affected individuals.9 Given the substantial impact of gout on quality of life, delineating its risk factors is imperative for enhancing treatment strategies and guiding the development of novel therapeutics.
Gout is usually associated with elevated blood uric acid levels alongside metabolic disturbances, such as insulin resistance and hyperlipidemia, which are risk factors for atherosclerosis.10 These metabolic derangements are reflected in lipid abnormalities, including elevated triglycerides (TG), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) levels, and reduced high-density lipoprotein cholesterol (HDL-C).10 High cholesterol levels are known to exacerbate inflammatory responses, potentially influencing the initiation and progression of chronic metabolic diseases like gout.11 Studies have shown a strong association between blood uric acid and lipid profiles, with non-HDL-C recognized as a critical predictor of cardiovascular events12. The nexus between uric acid and dyslipidemia is further underscored by findings that longer durations of gout and lower HDL-C levels are associated with an increased risk of gouty attacks, with low serum HDL-C levels remaining a significant predictor even after adjusting for confounding factors.13
The non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) is an emerging metric that provides a comprehensive assessment of atherogenic and anti-atherogenic lipid particles.14 NHHR has demonstrated superior predictive capabilities for various metabolic diseases over traditional lipid parameters, including cerebrovascular disease,15 coronary atherosclerosis,16 chronic kidney disease17, insulin resistance and metabolic syndrome.18 Given the metabolic breadth of gout’s impact, investigating the relationship between NHHR and gout is of significant interest. The current understanding of this association remains limited. To address this knowledge gap, we conducted a cross-sectional study using data from the National Health and Nutrition Examination Survey (NHANES) to explore the correlation between NHHR and the risk of gout.
Materials and Methods
Study Design and Participants
The National Health and Nutrition Examination Survey (NHANES), conducted by the National Center for Health Statistics (NCHS), is a cross-sectional survey designed to assess the health and nutritional status of the civilian noninstitutionalized population in the United States. It employs a multistage sampling strategy to collect interview, examination, and laboratory data from participants. The NHANES protocol is approved by the NCHS Research Ethics Review Board, and informed consent is obtained from each participant. Data are publicly accessible via the NHANES website (https://www.cdc.gov/nchs/nhanes/).19
For this study, we utilized six cycles of NHANES data from 2007 to 2018, encompassing 59,744 participants. The inclusion criteria were adults aged 20 years or older. Exclusions were applied for participants with missing data on TC, HDL-C, gout diagnosis, covariates including BMI, race, marital status, poverty income ratio (PIR), education, physical activity (PA), diabetes, hypertension, smoking status, alcohol use, steroid use, and statin use, as well as for pregnant women. The participant selection process is detailed in Figure 1.
 |
Figure 1 Flowchart of the participant selection from NHANES 2007–2018. |
Assessment of NHHR
The NHHR served as the primary independent variable in this study. NHHR was calculated using the formula: NHHR = Non-HDL-C/HDL-C. Non-HDL-C = total cholesterol (TC) − HDL-C, where Non-HDL-C was determined by subtracting HDL-C from TC. Lipid profiles were analyzed using enzymatic tests on an automated biochemical analyzer, with TC and HDL-C levels measured according to standardized procedures.
Definition of Gout
Gout diagnosis was ascertained through NHANES home interviews, where participants were asked whether a doctor or health professional had ever diagnosed them with gout. Affirmative responses were considered indicative of gout.
Assessment of Covariates
A comprehensive set of covariates known to influence gout risk was selected, including demographic and health-related factors. These covariates comprised gender (female, male), age, race (Mexican American, non-Hispanic White, non-Hispanic Black, Other race), education (Less than 9th grade, 9–11th grade, High school graduate, Some college, College graduate or above), body mass index (BMI, kg/m2) categorized as underweight (<18.50), normal (18.50–25), overweight (25–30), or obese (≥30), marital (Married, Never married, Widowed, Separated, Living with partner, Divorced), income-to-poverty ratio (PIR, 0–1.29, 1.30–3.49, ≥3.50), physical activity (PA, MET-min/week), total cholesterol (TC, mmol/L), high-density lipoprotein cholesterol (HDL-C, mmol/L), Non-HDL-C (mmol/L), smoke (current, former, never), alcohol (yes, no),20 hypertension (yes, no), diabetes (yes, no), steroid use (yes, no), statin use (yes, no), estimated glomerular filtration rate (eGFR, mL/min/1.73m2).21
PA was data were derived from the Physical Activity questionnaire (PAQ) in NHANES. Based on these values, participants are categorized into three groups: no PA (<60), Low intensity PA (60–2880), and High intensity PA (>2880).22,23 Medication use in the past 30 days was ascertained through questionnaire responses, with the use of statin lipid-lowering drugs and steroidal lipid-lowering drugs categorized accordingly. For detailed descriptions of these variables (Table S1), the NHANES website serves as the reference source.
Statistical Analyses
Analyses were conducted using R Software (V.4.3.2), with statistical significance defined as P < 0.05. We use multiple imputation to fill in missing data. Given the complex survey design of NHANES, all analyses were weighted to account for the multistage sampling strategy. The sampling weight was calculated by the following formula: WM12YR = 1/6*MEC2YR. (MEC2YR is the 2-year sample weight in each survey period; WM12YR is the sample weight calculated after combining the six periods).
Weighted means ± standard deviation was used to report continuous variables, while categorical variables were expressed as frequencies and percentages. Student’s t-test and chi-square test were used to compare baseline characteristics between participants with and without gout. Multivariate logistic regression was employed to evaluate the association between NHHR and gout, with models adjusted for potential confounders. Three models were specified: model 1 (unadjusted), model 2 (adjusted for age, gender, and race), and model 3 (fully adjusted for all covariates). The relationship between NHHR and gout was further explored using generalized additive model (GAM) and restricted cubic spline (RCS) regression to assess for nonlinearity. The piecewise regression model and logarithmic likelihood ratio test were was applied to define intervals and identify threshold effects. Receiver operating characteristic (ROC) curve analysis were applied to measure the efficacy of using non-HDLc, HDL-c, TC and the NHHR to determine the risk of developing gout.
Subgroup analyses were conducted to ensure the consistency of findings across different demographic and clinical subgroups. Sensitivity analyses were also performed, including assessments in participants without chronic kidney disease (CKD) and an unweighted logistic analysis for all participants to test the robustness of the results.
Results
Participants’ Baseline Characteristics
A total of 27,731 participants were included from the NHANES, a group that is representative of approximately 194.6 million noninstitutionalized US residents. The baseline characteristics of the study population, stratified by gout status, are presented in Table 1. Among the participants 1327 had gout (4.70%), and 26,404 did not. Gout was more prevalent among males (68.26%) than females (31.74%). Gout patients were significantly older, with a mean age of 60.98 ± 0.48 years, compared to non-gout participants with a mean age of 46.99 ± 0.25 years (p < 0.001). A higher proportion of gout patients were current or former smokers and had a lower prevalence of alcohol consumption compared to those without gout. Additionally, individuals with gout more frequently had hypertension, were less physically active, more likely to have diabetes, and more likely to be obese. The mean values for key metabolic parameters were as follows: estimated glomerular filtration rate (eGFR) was 94.20 ± 0.32 mL/min/1.73m², total cholesterol (TC) was 5.00 ± 0.01 mmol/L, high-density lipoprotein cholesterol (HDL-C) was 1.38 ± 0.01 mmol/L, non-HDL-C was 3.62 ± 0.01 mmol/L, and the non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) was 2.93 ± 0.02. There were no significant differences in education level and poverty income ratio (PIR) between participants with and without gout (p > 0.05).
 |
Table 1 Weighted Baseline Characteristics of Included Participants |
Association of NHHR With Gout
Weighted multivariate logistic regression analysis, was conducted to evaluate the association between NHHR and the presence of gout. As detailed in Table 2, in the continuous model, both the unadjusted (OR = 1.14; 95% CI: 1.09–1.19; P < 0.001) and the minimally adjusted model (OR = 1.15; 95% CI: 1.09–1.21; P < 0.001) demonstrated a significant positive association between NHHR and gout. This association persisted and remained robust in the fully adjusted model (OR = 1.21; 95% CI: 1.08–1.35; P < 0.001), indicating that for each unit increase in NHHR, there is a 21% increase in the odds of having gout.
 |
Table 2 The Association Between NHHR and Gout |
For sensitivity analysis, NHHR was categorized into quartiles. Participants in the highest quartile clearly faced a greater risk, with a 0.67-fold increase compared to those in the lowest quartile (OR = 1.67, 95% CI: 1.67–2.15, P < 0.001). In both the unadjusted and minimally adjusted models, participants in the second and third quartiles of NHHR also showed an increased risk of gout, but only participants in the fourth quartile were statistically significant. However, in the fully adjusted model, while the risk estimates were elevated, they did not reach statistical significance (Table 2). Based on the ROC curve results, the areas under the curve (AUCs) for the NHHR, non-HDL-c, and HDL-c were 61.16%, 49.70%, and 38.80%, respectively (Figure 2).
 |
Figure 2 ROC results. |
Smooth Curve Fitting and Threshold Effect Analysis
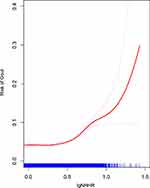
A four-knot restricted cubic spline analysis revealed a non-linear dose-response relationship between NHHR and the risk of gout (P for non-linearity <0.001) (Figure 3). Figure 4 shows the results of smooth curve fitting based on the generalized additive models. Interestingly, the results showed a J-shaped relationship between NHHR after logarithmic transformation and the risk of gout across the entire population, with a turning point before the risk of gout changes less with the increase of NHHR levels. After reaching the turning point, the increase in NHHR levels led to an increased risk of gout prevalence. The maximum likelihood method was used to find the turning point of 0.31 for the smoothing curve, adjusted for sex, age, race and the results are shown in Table 3.
 |
Table 3 Threshold Effect of NHHR Levels on Gout |
 |
Figure 3 The RCS curve of the association between lgNHHR and gout among all participants. RCS regression was adjusted for age, gender, race. |
Subgroup Analysis and Interaction Testing
Subgroup analyses revealed varying associations between NHHR and gout across different demographic and clinical subgroups. In gender-stratified analyses, a significant positive correlation was observed among females across all models. However, in males, no significant association was found in the unadjusted and partially adjusted models (OR = 1.03; 95% CI: 0.97–1.09, p=0.301). When stratified by age, a significant positive correlation was noted for participants aged 20–59 years, whereas for those aged 80 years and above, the association was not statistically significant in any model (Table 4).
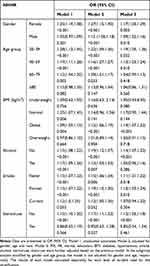 |
Table 4 Subgroup Analysis for the Association Between NHHR and Gout |
Analyses by smoking and drinking status showed robust positive correlations between NHHR and gout in non-smokers (both never and former smokers) and non-drinkers across all models. Additionally, a significant relationship was observed in participants using steroid drugs (p<0.001).
To assess the stability of the observed associations, interaction testing was conducted, as depicted in Figure 5. The results indicated that subgroups defined by age group (20–39, 40–59, 60–79, ≥80 years), race, body mass index, marital status, poverty income ratio (PIR), education, physical activity (PA), diabetes, hypertension, smoking, alcohol consumption, steroid use, and statin use did not significantly influence the relationship between NHHR and gout (P interaction > 0.05). However, a significant interaction was identified between hypertension, gender, and NHHR (P interaction < 0.05), suggesting that the effect of NHHR on gout risk may vary by these factors.
 |
Figure 5 Subgroup analysis of the association between NHHR and gout. |
Sensitivity Analysis
Sensitivity analyses were conducted to assess the robustness of the relationship between NHHR and gout risk after excluding participants with chronic kidney disease (CKD).24 In the weighted fully adjusted categorical model, the association between NHHR and gout was not statistically significant for the second (Q2: OR = 0.87; 95% CI, 0.64–1.15, P=0.291), third (Q3: OR = 0.90; 95% CI, 0.63–1.29, P=0.567), and fourth (Q4: OR = 1.32; 95% CI, 0.94–1.85, P=0.104) quartiles (Table 5).
 |
Table 5 Sensitivity Analyses |
Additionally, unweighted logistic regression for all participants revealed a positive association between NHHR and gout (OR = 1.09; 95% CI, 1.05–1.13, P<0.001), which is consistent with primary analysis, suggesting the observed relationship between NHHR and gout risk is stable and reliable, even after accounting for potential confounders and excluding individuals with CKD.
Discussion
This cross-sectional study for the first time revealed a positive correlation between the NHHR and the prevalence of gout, even after adjusting for a multitude of confounding variables. Interestingly, we found a J-shaped correlation between NHHR and gout risk. Notably, the prevalence of gout was substantially higher in males (68.26%) compared to females (31.74%), a disparity potentially attributed to the uric acid-lowering effect of estrogen25 Age was also identified as a significant factor, with the prevalence of gout increasing with advancing years. The association between NHHR and gout was consistent across various subgroups, including age, race, BMI, education, marital status, physical activity, diabetes, smoking status, and use of statins and steroids. Subgroup analyses confirmed the stability of these findings across different demographic and clinical settings. The sensitivity analyses further bolstered the robustness of our findings. While the association remained significant in the unweighted analysis including all participants, it became non-significant when individuals with chronic kidney disease (CKD) were excluded, indicating the potential influence of CKD on the observed relationship.
NHHR, a novel lipid ratio, has emerged as a valuable tool for assessing atherogenic lipids and has been increasingly recognized as a biomarker for various diseases through extensive studies utilizing the NHANES database.14,26,27 Although our study is among the first to investigate the relationship between NHHR and gout, previous research has established links between gout and lipid-related factors.28,29 Dyslipidemia has been associated with a higher prevalence and incidence of gout, and patients with gout often exhibit a history of dyslipidemia.30 Notably, Mak et al have demonstrated that low serum HDL-C levels are a strong predictor of gouty attacks,13 a finding supported by the notion that the acute phase response can influence lipid profiles.31 Our study’s design aimed to mitigate this confounding effect, leading to the conclusion that serum HDL-C levels are not significantly affected by the acute phase response associated with gouty attacks.
Our findings also highlight the influence of other factors strongly associated with gout, such as male gender, non-Hispanic black race, obesity, low poverty income ratio (PIR), diabetes, hypertension, and physical inactivity. The lower prevalence of gout in women may be due to the protective effect of estrogen on uric acid excretion.32 PIR, a reflection of socioeconomic status, has been implicated as a risk factor for gout, potentially due to its impact on healthcare access and treatment adherence.33 Furthermore, bariatric surgery has been shown to reduce the incidence of gout in obese subjects by 40%,34 underscoring the role of obesity in gout development. Mendelian randomization studies have reinforced the causal link between increased BMI and elevated serum uric acid levels, thereby increasing gout risk.35–37 Alcohol consumption, known to increase blood uric acid levels, also emerged as a significant risk factor for gout.38
The relationship between physical activity and gout is complex, with varying intensities and frequencies of exercise exerting differential effects on gout risk. Mild to moderate exercise has been shown to reduce serum uric acid levels through anti-inflammatory mechanisms, while vigorous exercise can lead to a transient increase in blood uric acid levels39,40. Similarly, observational studies have indicated that hypertensive patients are at an increased risk of developing gout.41 This shows that a healthy lifestyle plays an important role in the prevention of gout.
Dyslipidemia is posited to influence the inflammatory response, a key component in the pathogenesis of gout, by modulating the activity of the NLRP3 inflammasome and the subsequent release of interleukin 1β.42–45 This underscores the importance of lipid metabolism in the development of gout and suggests that NHHR, as a novel lipid ratio, may serve as a more effective tool for assessing the impact of lipid metabolism on gout risk.
Strengths and Limitations
The present study offers several notable strengths. Its foundation on the NHANES database provides a robust platform for generalizing findings to the broader US noninstitutionalized population, bolstered by strict adherence to the NHANES sampling and weighting protocols. This enhances the external validity of our results. Additionally, by focusing on the NHHR as a potential marker for gout prevalence, our study sheds light on the significance of lipid metabolism in gout pathogenesis, a relatively underexplored area. The meticulous control for a wide array of confounding covariates, selected based on prior research, further strengthens the reliability and validity of our findings. Moreover, the large sample size and comprehensive data collection contribute to the robustness of our results.
However, our study is not without limitations. The reliance on self-reported data for gout diagnosis and medication use could introduce recall bias, potentially affecting the accuracy of our estimates. The cross-sectional design limits our ability to infer causality and establish temporality between NHHR and gout. Additionally, we were unable to account for certain variables such as detailed medication histories, including anti-gout drugs like allopurinol or diuretics, which could influence uric acid levels. Despite our efforts to adjust for known confounders, residual or unmeasured confounding factors may persist. Lastly, the predominance of American participants in our study suggests that our findings may not be directly generalizable to other racial or ethnic groups, underscoring the need for future research in diverse populations.
Conclusion
In summary, this study adds to the burgeoning evidence linking NHHR to an increased risk of gout among US adults. It provides a fresh perspective on disease prevention, gout symptom reduction, and scientific health management for American adults. It underscores the clinical relevance of NHHR as a predictor of gout and highlights the importance of examining the interplay between lipid metabolism and gout within the context of population health. The cross-sectional nature of our study, however, calls for further research to confirm the causality of this association and explore the underlying mechanisms. Longitudinal studies and investigations in diverse populations will be crucial to validate our findings and expand our understanding of the relationship between NHHR and gout.
Abbreviations
NHHR, Non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio; NHANES, National Health and Nutrition Examination Survey; non-HDL-C, Non-high-density lipoprotein cholesterol; HDL-C, High-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HUA, Hyperuricemia; TG, Triglycerides; TC, Total cholesterol; CKD, Chronic kidney disease; eGFR, Estimated glomerular filtration rate; PIR, Poverty-to-income ratio; BMI, Body mass index; PIR, Poverty income ratio ; PA, Physical activity; RCS, Restricted cubic spline.
Data Sharing Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
This study was conducted at Shanxi medical university, China, and received ethical approval from the Ethics Committee of Shanxi medical university (Approval No. 2005001). All procedures followed the ethical standards set forth in the Declaration of Helsinki and relevant institutional guidelines. Written informed consent was obtained from all participants prior to their involvement in the study.
Acknowledgments
We thank the NHANES participants and staff for their contributions. We also thank Zhang Jing Second Department of Infectious Disease, Shanghai Fifth People’s Hospital, Fudan University) for his work on the NHANES database. His outstanding work, NHANES R package, and web page, makes it easier for us to explore NHANES database.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This project was supported by grants from the Natural Science Foundation of Shanxi Province (No. 202203021221269) and the National Natural Science Foundation of China (No. 82001740).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016;388(10055):2039–2052. doi:10.1016/S0140-6736(16)00346-9
2. Terkeltaub RA. Clinical practice. Gout N Engl J Med. 2003;349(17):1647–1655. doi:10.1056/NEJMcp030733
3. Liu R, Han C, Wu D, et al. Prevalence of hyperuricemia and gout in mainland China from 2000 to 2014: a systematic review and meta-analysis. Biomed Res Int. 2015;2015:762820. doi:10.1155/2015/762820
4. Safiri S, Kolahi AA, Cross M, et al. Prevalence, incidence, and years lived with disability due to gout and its attributable risk factors for 195 countries and territories 1990–2017: a systematic analysis of the global burden of disease study 2017. Arthritis Rheumatol. 2020;72(11):1916–1927. doi:10.1002/art.41404
5. Zhang J, Jin C, Ma B, et al. Global, regional and national burdens of gout in the young population from 1990 to 2019: a population-based study. RMD Open. 2023;9(2):e003025. doi:10.1136/rmdopen-2023-003025
6. Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol. 2020;16(380):90. doi:10.1038/s41584-020-0441
7. Choi HK, McCormick N. Beyond joint pain, could each gout flare lead to heart attack? Nat Rev Rheumatol. 2022;18(11):619–620. doi:10.1038/s41584-022-00844-x
8. Singh JA, Gaffo A. Gout epidemiology and comorbidities. Semin Arthritis Rheum. 2020;50(3S):S11–S16. doi:10.1016/j.semarthrit.2020.04.008
9. Lottmann K, Chen X, Schädlich PK. Association between gout and all-cause as well as cardiovascular mortality: a systematic review. Curr Rheumatol Rep. 2012;14:195–203. doi:10.1007/s11926-011-0234-2
10. Son M, Seo J, Yang S, Magni P. Association between dyslipidemia and serum uric acid levels in Korean adults: Korea National Health and Nutrition Examination Survey 2016–2017. PLoS One. 2020;15(2):e0228684. doi:10.1371/journal.pone.0228684
11. Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15(2):104–116. doi:10.1038/nri3793
12. Ke D, Chen Q, Wu Q, et al. Analysis of the correlation between nonhigh-density lipoprotein cholesterol and coronary heart disease in elderly Chinese. Intern Med. 2011;50(12):1279–1285. doi:10.2169/internalmedicine.50.4988
13. Mak A, Ho RC, Tan JY, et al. Atherogenic serum lipid profile is an independent predictor for gouty flares in patients with gouty arthropathy. Rheumatology. 2009;48(3):262–265. doi:10.1093/rheumatology/ken471
14. Sheng G, Liu D, Kuang M, Zhong Y, Zhang S, Zou Y. Utility of non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio in evaluating incident diabetes risk. Diabetes Metab Syndr Obes. 2022;15:1677–1686. doi:10.2147/DMSO.S355980
15. Liu Z, Lin X, Zeng L, et al. Elevated non-HDL-C/HDL-C ratio increases the 1-year risk of recurrent stroke in older patients with non-disabling ischemic cerebrovascular events: results from the Xi’an Stroke Registry Study of China. BMC Geriatr. 2023;23(1):410. doi:10.1186/s12877-023-04102-x
16. Mardi P, Abdi F, Ehsani A, et al. Is non-high-density lipoprotein associated with metabolic syndrome? A systematic review and meta-analysis. Front Endocrinol. 2022;13:957136. doi:10.3389/fendo.2022.957136
17. Zuo PY, Chen XL, Liu YW, et al. Non-HDL-cholesterol to HDL-cholesterol ratio as an independent risk factor for the development of chronic kidney disease. Nutr Metab Cardiovasc Dis. 2015;25(6):582–587. doi:10.1016/j.numecd.2015.03.003
18. Kim SW, Jee JH, Kim HJ, et al. Non-HDL-cholesterol/HDL-cholesterol is a better predictor of metabolic syndrome and insulin resistance than apolipoprotein B/apolipoprotein A1. Int J Cardiol. 2013;168(3):2678–2683. doi:10.1016/j.ijcard.2013.03.027
19. Centers for disease control and prevention. about the National health and nutrition examination survey. NHANES. Available from: https://www.cdc.gov/nchs/nhanes/index.htm.
20. Hicks CW, Wang D, Matsushita K, et al. Peripheral neuropathy and all-cause and cardiovascular mortality in u.s. adults: a prospective cohort study. Ann Internal Med. 2021;174(2):167–174. doi:10.7326/M20-1340
21. Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi:10.7326/0003-4819-150-9-200905050-00006
22. Chen L, Cai M, Li H, et al. Risk/benefit tradeoff of habitual physical activity and air pollution on chronic pulmonary obstructive disease: findings from a large prospective cohort study. BMC Med. 2022;20(1):70. doi:10.1186/s12916-022-02274-8
23. Ran J, Zhang Y, Han L, et al. The joint association of physical activity and fine particulate matter exposure with incident dementia in elderly Hong Kong residents. Environ Int. 2021;156:106645. doi:10.1016/j.envint.2021.106645
24. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4S):S1–S276. doi:10.1016/j.kint.2021.05.021
25. Dalbeth N, Choi HK, Joosten LAB, et al. Gout. Nat Rev Dis Primers. 2019;5(1):69. doi:10.1038/s41572-019-0115-y
26. Qi X, Wang S, Huang Q, et al. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and risk of depression among US adults: a cross-sectional NHANES study. J Affect Disord. 2024;344:451–457. doi:10.1016/j.jad.2023.10.064
27. Qing G, Deng W, Zhou Y, Zheng L, Wang Y, Wei B. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and suicidal ideation in adults: a population-based study in the United States. Lipids Health Dis. 2024;23(1):17. doi:10.1186/s12944-024-02012-4
28. Kvasnička A, Friedecký D, Brumarová R, et al. Alterations in lipidome profiles distinguish early-onset hyperuricemia, gout, and the effect of urate-lowering treatment. Arthritis Res Ther. 2023;25(1):234. doi:10.1186/s13075-023-03204-6
29. Rho YH, Choi SJ, Lee YH, et al. The prevalence of metabolic syndrome in patients with gout: a multicenter study. J Korean Med Sci. 2005;20(6):1029–1033. doi:10.3346/jkms.2005.20.6.1029
30. Choi HG, Kwon BC, Kwon MJ, et al. Association between gout and dyslipidemia: a nested case-control study using a national health screening cohort. J Pers Med. 2022;12(4):605. doi:10.3390/jpm12040605
31. Van Lenten BJ, Reddy ST, Navab M, Fogelman AM. Understanding changes in high density lipoproteins during the acute phase response. Arterioscler Thromb Vasc Biol. 2006;26(8):1687–1688. doi:10.1161/01.ATV.0000232522.47018.a6
32. Rodríguez-Sosa E, De Miguel E, Borrás F, Andrés M. Filling gaps in female gout: a cross-sectional study of comorbidities in 192,037 hospitalised patients. RMD Open. 2023;9(2):e003191. doi:10.1136/rmdopen-2023-003191
33. Bowen-Davies Z, Muller S, Mallen CD, Hayward RA, Roddy E. Gout severity, socioeconomic status, and work absence: a cross-sectional study in primary care. Arthritis Care Res. 2018;70(12):1822–1828. doi:10.1002/acr.23562
34. Maglio C, Peltonen M, Neovius M, et al. Effects of bariatric surgery on gout incidence in the Swedish obese subjects study: a non-randomised, prospective, controlled intervention trial. Ann Rheum Dis. 2017;76(4):688–693. doi:10.1136/annrheumdis-2016-209958
35. Larsson SC, Burgess S, Michaëlsson K. Genetic association between adiposity and gout: a Mendelian randomization study. Rheumatology. 2018;57(12):2145–2148. doi:10.1093/rheumatology/key229
36. Lyngdoh T, Vuistiner P, Marques-Vidal P, et al. Serum uric acid and adiposity: deciphering causality using a bidirectional Mendelian randomization approach. PLoS One. 2012;7(6):e39321. doi:10.1371/journal.pone.0039321
37. Palmer TM, Nordestgaard BG, Benn M, et al. Association of plasma uric acid with ischaemic heart disease and blood pressure: mendelian randomisation analysis of two large cohorts. BMJ. 2013;347:f4262. doi:10.1136/bmj.f4262
38. Nieradko-Iwanicka B. The role of alcohol consumption in pathogenesis of gout. Crit Rev Food Sci Nutr. 2021;19:1–9. doi:10.1080/10408398.2021.1911928
39. Jablonski K, Young NA, Henry C, et al. Physical activity prevents acute inflammation in a gout model by downregulation of TLR2 on circulating neutrophils as well as inhibition of serum CXCL1 and is associated with decreased pain and inflammation in gout patients. PLoS One. 2020;15(10):e0237520. doi:10.1371/journal.pone.0237520
40. Saladini F, Mos L, Fania C, Garavelli G, Casiglia E, Palatini P. Regular physical activity prevents development of hypertension in young people with hyperuricemia. J Hypertens. 2017;35(5):994–1001. doi:10.1097/HJH.0000000000001271
41. Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Comorbidities in patients with gout prior to and following diagnosis: case-control study. Ann Rheum Dis. 2016;75(1):210–217. doi:10.1136/annrheumdis-2014-206410
42. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241. doi:10.1038/nature04516
43. Dalbeth N, Gosling AL, Gaffo A, Abhishek A. Gout. Lancet. 2021;397(10287):1843–1855. doi:10.1016/S0140-6736(21)00569-9
44. Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117(5):561–574. doi:10.1016/j.cell.2004.05.004
45. Cipolletta E, Tata LJ, Nakafero G, Avery AJ, Mamas MA, Abhishek A. Association between gout flare and subsequent cardiovascular events among patients with gout. JAMA. 2022;328(5):440–450. doi:10.1001/jama.2022.11390
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.