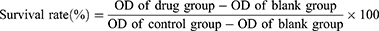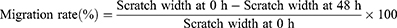Back to Journals » Cancer Management and Research » Volume 17
Jujuboside B Inhibits the Proliferation and Migration of Non-Small Cell Lung Cancer H1299 Cells Through Inhibiting PI3K/Akt and Wnt/β-Catenin Pathways
Authors Chen M, Li J, Hu S, Wang Y, Wang K, Wang T
Received 4 March 2025
Accepted for publication 5 June 2025
Published 16 June 2025 Volume 2025:17 Pages 1143—1153
DOI https://doi.org/10.2147/CMAR.S526130
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Kattesh Katti
Mengzhu Chen,1 Jian Li,1 Shaodan Hu,1 Yuhan Wang,2 Keju Wang,1 Tan Wang1
1Department of Respiratory, The Affiliated Hospital to Changchun University of Chinese Medicine, Changchun, 130021, People’s Republic of China; 2School of Traditional Chinese Medicine, Changchun University of Chinese Medicine, Changchun, 130117, People’s Republic of China
Correspondence: Tan Wang, Email [email protected]
Background: Lung cancer has high incidence and mortality rates. In consideration of the high toxicity and side effects of traditional chemical drugs and drug resistance, researchers have substituted some natural products for anti-lung cancer drugs. Ziziphi spinosae semen (ZSS) is a famous traditional Chinese herb, and ZSS-based formulas have been used against lung cancer in clinic, such as Xiaoyi Sanjie Formula. Jujuboside B (JUB) is one of the main bioactive components in ZSS; however, JUB’s roles in the treatment of lung cancer, and the mechanism have not been well reported.
Objective: This study intends to evaluate the effects of JUB on the proliferation and migration of non-small cell lung cancer H1299 cells (NC-1299 cells), as well as the potential related regulatory mechanism.
Methods: The viability, migration and invasive ability of NC-1299 cells were analyzed by CCK8, wound-healing, transwell invasion, and the expression of proteins was analyzed through Western blotting experiments.
Results: It showed that 160 and 320 μmol/L JUB significantly inhibited the proliferation of NC-1299 cells, reduced the cell migration in the wound healing test, and decreased the cell number of clonal assay (P < 0.05). JUB also significantly inhibited the expression of Vimentin, MMP2, and MMP9 proteins related to the migration and invasion of tumor cells, as well as inhibited the PI3K/AKT and Wnt/β-catenin pathways.
Conclusion: The results indicate JUB has a significant inhibitory effect on NC-1299 cells, and such inhibitory effect is associated with inhibiting the expression of migration and invasive proteins such as MMP2, as well as inhibiting the PI3K/AKT and Wnt/β-catenin pathways. The findings provide some references for the application of JUB in the substitution of anti-lung cancer drugs.
Keywords: Jujuboside B, non-small cell human lung cancer NC-1299 cells, proliferation, migration, PI3K/AKT, Wnt/β-catenin
Introduction
Lung cancer is a common type of cancer with a high incidence and mortality rate.1 In addition, lung cancer is often the direct cause of death when cancer from other organs spreads and metastasizes to the lungs.2 In clinical practice, non-small cell lung cancer is the main type of lung cancer, and the common treatment options are radiotherapy,3 chemotherapy,4 immunotherapy5 and surgical resection.6 However, the above treatment options are highly restrictive to the patient’s cancer stage and physical status, for example, surgery is not feasible for advanced or metastatic lung cancers, and radiotherapy and chemotherapy are too damaging to the body to be carried out if the patient is unable to withstand too much physical and mental pain. In addition, the drug resistance problem also restricts the efficacy of traditional anti-lung cancer drugs.7 Therefore, there is an urgent need and practical significance to find new treatment strategies for new lung cancers. At present, targeted inhibition of pathways that are closely related to tumor cell migration and invasion and other vitality has become a popular solution to fight tumors.8,9
Studies have shown that lung cancer is closely related to the Wnt/β-catenin signaling pathway.10 Key components of the pathway, Wnt1, β-catenin, and cell cycle protein D1 show obvious disordered expression, and abnormal hyperactivation of the Wnt/β-catenin pathway is associated with tumor metastasis, migration, invasion, and chemotherapy resistance, and it promotes the occurrence and development of lung cancer.11 Meanwhile, PI3K/AKT pathway also plays a key role in the development of lung tumors, and this pathway was often hyperactivated in lung cancer due to some components of it disordered.12 For the above targeted pathways, the traditional strategy is to inhibit or block the pathway with chemical drugs such as betalain,13 but chemical drugs have side effects such as high biotoxicity and drug resistance for prolonged use, which limits their use. Recent studies have shown that the use of natural products that can target the aforementioned pathways as an alternative to chemical drugs offers a new possibility against lung cancer, such as curcumin,14 nobiletin,15 and lycorine.16
Ziziphi spinosae semen is a traditional Chinese medicine, and it is one of the main ingredients of the Xiaoyi Sanjie formula that used for the treatment of lung cancer. Jujuboside B (JUB), a natural product saponin, is one of the main bioactive components in Ziziphi spinosae semen.17 Studies have shown that JUB can exert anti-tumor effects through the PI3K/AKT and Wnt/β-catenin pathways,18,19 but its resistance to lung cancer has not been reported. Human non-small cell lung cancer H1299 cells (NC-1299 cells) are often used as a cell model for in vitro investigation of non-small cell lung cancer. In this study, we proposed to treat NC-1299 cells with JUB firstly, and then comprehensively assess the inhibitory effect of JUB on NC-1299 cancer cells and related mechanisms by determining the tumor cell viability, migration and invasion ability, as well as the expression of Wnt/β-catenin and PI3K/AKT pathways, which may provide some reference bases for JUB application in the treatment of lung cancers.
Materials and Methods
Cell Culture and Reagents
NC-1299 cells were got from Peking Union Medical College Cell Bank (Beijing, China), and maintained at 37 °C, 5% CO2 condition. JUB was got from Aladdin Biochemical Technology Co., Ltd (Shanghai, China), No. J114069. Cell Counting Kit-8 (CCK-8 Kit) was got from Beyotime Biotechnology (Shanghai, China), No. C0038. Antibodies for Cyclin D1 (No. ab16663) were got from Abcam company (Cambridge, UK); antibodies for E-cadherin (No. A20798), Vimentin (No. A19607) were got from ABclonal Technology (Wuhan, China); antibodies for MMP2 (No. bs0412R), MMP9 (No. bs4593R), p-PI3K (No. bs5570R), PI3K (No. bs10657R) were got from Bioss company (Beijing, China); antibodies for p-AKT (No. mAb 4060), AKT (No. mAb2118), β-catenin (No. mAb9562), c-Myc (No. mAb5605), GAPDH (No. mAb2118) were got from Cell Signaling Technology (Danvers, MA, USA).
Cytotoxicity Analysis Through CCK8 Assay
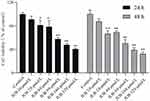
The cytotoxicity of JUB on NC-1299 cells was assessed using the CCK8 kit. Briefly, a density of 1×10 3 cells were cultured in 96-well plates, per well, and treated with different concentrations of JUB (0, 10, 20, 40, 80, 160 and 320 μmol/L) for 24 or 48 h. Then, CCK8 was added in the wells and NC-1299 cells were incubated for another 4 h. Subsequently, the optical density (OD) value of each well was analyzed using a Varioskan™ LUX microplate reader (Thermo Fisher Scientific, Massachusetts, USA) at 450 nm. The cell survival rate and the cell inhibition rate were calculated based on the OD values. The half inhibitory concentration (IC50) of JUB on NC-1299 cells was analyzed using GraphPad Prism 8.0 software, based on the drug concentration and the corresponding calculated inhibition rate of each group. The IC50 value was 65.03 μmol/L and 55.27 μmol/L, for 24 h and 48 h, respectively.
Wound Healing Assay
About 1×105 cells per well were cultured in 6-well plates and treated with different concentrations of JUB (0, 80, 160 and 320 μmol/L). A 200 µL pipette tip was applied to create wound through scratching cells in a straight line, and an inverted microscope imaging system (IX-51, Olympus, Japan) was used to taken images of the wound scratch immediately. The cells were cultured for another 48 h, and the wound scratch images at 48 h were taken with the aforementioned microscope imaging system again. The migration rate of cells was calculated with the following formula:
Colony Formation Assay
About 5×102 cells per well were cultured in 6-well plates and treated with different concentrations of JUB (0, 80, 160 and 320 μmol/L). After 2 weeks culture, the cells were then fixed with 4% paraformaldehyde for 30 min, and they were further stained with crystal violet for 10 min. And the images of cells were taken with an inverted microscope imaging system (IX-51, Olympus, Japan), and further analyzed with ImageJ software (National Institutes of Health, Bethesda, USA).
Transwell Invasion Assay
The invasion assay was performed according to an earlier research.20 Briefly, transwell chambers coated with Matrigel were used for invasion assays. NC-1299 cells were pretreated with JUB (0, 80, 160 and 320 μmol/L) for 48 h. Then, 5×104 NC-1299 cells that were resuspended with 100 μL serum-free medium were seeded in the upper chamber, and 600 μL of medium containing 10% FBS was added to the lower chamber as a chemoattractant. The cells then were incubated at 37°C for 24 h and invaded into the lower chamber, and they were fixed with 4% paraformaldehyde for 20 min, and stained with 0.1% crystal violet for 30 min. And 5 randomly selected fields were used to count the number of cells.
Western Blotting Assay
NC-1299 cells were cultured in 6-well plates and treated with different concentrations of JUB (0, 80, 160 and 320 μmol/L) for 48 h. Cells from all groups were collected and the total proteins of cells were obtained, and the concentration of protein samples was calculated with bicinchoninic acid method (BCA). The SDS-PAGE gel was then used to separate the proteins which were further transferred to PVDF membrane. After incubating with 5% nonfat milk for 1 h, the membranes were further incubated with primary antibody at 4°C overnight, secondary antibody for 1 h, respectively. The protein brands were visualized with ECL luminescent solution, and the brand density was evaluated with Image Lab software (Bio-Rad, Richmond, CA, USA).
Statistical Analysis
Data were statistically analyzed with SPSS 25.0 software (SPSS Inc., Chicago, IL, USA). A one-way analysis of variance was used for comparison of difference, and P < 0.05 was considered as statistically significant difference.
Results
JUB Reduces the Cell Viability, Migration, Invasion, Proliferation of Human Lung Cancer NC-1299 Cells
The CCK-8, wound healing, and colony formation assays were used to evaluating the effects of JUB on the cell viability and proliferation of NC-1299 cells. Figure 1 illustrated that 20, 40, 80, 160 and 320 μmol/L JUB could remarkably inhibit NC-1299 cells both at 24 and 48 h, especially when the dose of JUB were 80, 160 and 320 μmol/L (Figure 1, P < 0.05). Hence, the aforementioned three doses were chosen for subsequent scratch wound healing, and colony formation assays. Figure 2 illustrated that the migration and invasion ability of NC-1299 cells was remarkably inhibited by JUB, as the scratch width (Figure 2A) was wider and the migration rate was remarkably reduced in the JUB groups (Figure 2B, P < 0.05), and the invasion rate was remarkably reduced in the JUB groups (Figure 2C and D, P < 0.05). Figure 3 illustrated that JUB could reduce the clonal formation of NC-1299 cells as the cell number was remarkably decreased in the JUB groups (Figure 3A and B, P < 0.01). Taken together, these findings indicated JUB could reduce the cell viability, migration, invasion, proliferation of NC-1299 cells.
JUB Inhibits the Protein Expression Associated with Tumor Cell Migration and Invasion in NC-1299 Cells
Tumor migration and invasion ability is an important indicator for assessing the viability and damage of tumor cells. Researches have shown that E-cadherin, Vimentin, MMP2, and MMP9 proteins are closely related to tumor migration and invasion.21–23 Figure 4 illustrated that the expression of these proteins changed significantly when the JUB concentration was 160 or 320 μmol/L, especially at 320 μmol/L, as the level of Vimentin, MMP2, and MMP9 was remarkably down-regulated in the JUB 160 and 320 groups (Figure 4A–E, P < 0.05). Taken together, these findings indicated JUB could inhibit the protein expression associated with tumor cell migration and invasion in NC-1299 cells.
JUB Inhibits the PI3K/AKT Signaling Pathway in NC-1299 Cells
The abnormal activation of PI3K/AKT signaling pathway was a common phenomenon in tumors,24 and high phosphorylation ratios of key proteins are important signs of activation of this pathway. Figure 5 illustrated that the phosphorylation ratio of PI3K and AKT proteins was remarkably down-regulated in the JUB 160 and 320 groups (Figure 5A–C, P < 0.05). Taken together, these findings indicated JUB could inhibit the PI3K/AKT signaling pathway in NC-1299 cells.
JUB Inhibits the Wnt/β-Catenin Signaling Pathway in NC-1299 Cells
The Wnt/β-catenin signaling pathway is closely associated with tumors, and suppression of this pathway was a common strategy against cancers.25,26 And c-Myc, Cyclin D1, β-catenin are three key proteins in the Wnt/β-catenin pathway, Figure 6 illustrated that the level of the aforementioned key proteins was remarkably down-regulated in the JUB 160 and 320 groups (Figure 6A–D, P < 0.05). Taken together, these findings indicated JUB could inhibit the Wnt/β-catenin signaling pathway in NC-1299 cells.
Discussion
Consistent with the conjecture of this study, the results illustrated that JUB exhibited significant inhibitory effects on NC-1299 lung cancer cells, as 160 and 320 μmol/L JUB significantly inhibited the proliferation, invasive and migratory abilities of NC-1299 cells. In the present study, JUB, the main active ingredient in Ziziphi spinosae semen, was investigated, and its results indicated that JUB is one of the key components in the anticancer process of Ziziphi spinosae semen. These findings provide some reference bases and research directions for the subsequent in-depth investigation of the anticancer mechanism of Ziziphi spinosae semen.
The PI3K/AKT and Wnt/β-catenin pathways are two critical pathways that are frequently involved in cancer,27,28 and they are often transitionally activated in cancer cells, resulting in enhanced proliferation, migration, and invasion of tumor cells.29 Therefore, the development of novel anticancer drugs is often pre-screened on the basis of their ability to specifically target and inhibit these two pathways.30,31 Based on this, many natural products with anticancer potential have been discovered, and evidences showed that flavonoids berberine,32 marmesin,33 and dynactin34 all can target the above two key pathways to inhibit cancer. The results of the present study showed that the anticancer effects of JUB were also associated with targeting and inhibiting the PI3K/AKT and Wnt/β-catenin pathways, which also laterally corroborates the value of PI3K/AKT and Wnt/β-catenin in screening for new anticancer drugs.
Notably, although the present study initially demonstrated the anti-lung cancer properties and potential mechanisms of JUB at the cellular level, there are still many limitations. First, the current works did not further explore which proteins in the pathways play the most critical regulatory functions. Subsequent studies will focus on evaluating the key factors in the PI3K/AKT and Wnt/β-catenin pathways with the help of pathway blockers,35 protein inhibitors,36 and protein overexpression systems,37 in order to better utilize the anti-tumor properties of JUB. Secondly, this study did not conduct in vivo animal experiments, but only in vitro validation, which means that there is still a lot of work to be supplemented for the clinical application of JUB, such as clarifying the dosage, suitable dosage form, and route of administration in vivo, etc., and we will also focus on the related content in the subsequent work. In addition, there are other active ingredients in Ziziphi spinosae semen, eg, Sanjoinine,38 etc., and their effects and mechanisms in the process of anti-tumor are also worth studying, which will provide more comprehensive references for the clinical application of Ziziphi spinosae semen in the treatment of lung cancer.
Conclusion
In conclusion, this study revealed the inhibitory effect of JUB on the proliferation and migration of NC-1299 lung cancer cells and found that it was associated with the inhibition of PI3K/AKT and Wnt/β-catenin pathways. These findings offer a partial reference for the application of JUB and Ziziphi spinosae semen as alternative drugs against lung cancer in the clinic, as well as provide some evidence for the use of Ziziphi spinosae semen as a homology of medicine and food in the prevention and treatment of lung cancer.
Acknowledgments
The authors thank the Affiliated Hospital to Changchun University of Chinese Medicine for the supporting.
Funding
The present study was supported by the Science and Technology Development Plan item from the Science and Technology Department of Jilin Province (YDZJ202301ZYTS459).
Disclosure
There is no conflict of interest to declare.
References
1. Thandra KC, Barsouk A, Saginala K, Aluru JS, Barsouk A. Epidemiology of lung cancer. Contemp Oncol. 2021;25(1):45–52. doi:10.5114/wo.2021.103829
2. Leiter A, Veluswamy RR, Wisnivesky JP. The global burden of lung cancer: current status and future trends. Nat Rev Clin Oncol. 2023;20(9):624–639. doi:10.1038/s41571-023-00798-3
3. Altorki NK, McGraw TE, Borczuk AC, et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. Lancet Oncol. 2021;22(6):824–835. doi:10.1016/S1470-2045(21)00149-2
4. Provencio M, Nadal E, González-Larriba JL, et al. Perioperative nivolumab and chemotherapy in stage III non–small-cell lung cancer. N Engl J Med. 2023;389(6):504–513. doi:10.1056/NEJMoa2215530
5. Wakelee H, Liberman M, Kato T, et al. Perioperative pembrolizumab for early-stage non–small-cell lung cancer. N Engl J Med. 2023;389(6):491–503. doi:10.1056/NEJMoa2302983
6. Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet. 2022;399(10335):1607–1617. doi:10.1016/S0140-6736(21)02333-3
7. Wu J, Lin Z. Non-small cell lung cancer targeted therapy: drugs and mechanisms of drug resistance. Int J Mol Sci. 2022;23(23):15056. doi:10.3390/ijms232315056
8. Iksen, Pothongsrisit S, Pongrakhananon V. Targeting the PI3K/AKT/mTOR signaling pathway in lung cancer: an update regarding potential drugs and natural products. Molecules. 2021;26(13):4100. doi:10.3390/molecules26134100
9. Ko E-B, Jang Y-G, Kim C-W, Go R-E, Lee HK, Choi K-C. Gallic acid hindered lung cancer progression by inducing cell cycle arrest and apoptosis in a549 lung cancer cells via PI3K/Akt pathway. Biomol Ther. 2022;30(2):151–161. doi:10.4062/biomolther.2021.074
10. Teng Y, Wang X, Wang Y, Ma D. Wnt/beta-catenin signaling regulates cancer stem cells in lung cancer A549 cells. Biochem Biophys Res Commun. 2010;392(3):373–379. doi:10.1016/j.bbrc.2010.01.028
11. Stewart DJ. Wnt signaling pathway in non–small cell lung cancer. J Natl Cancer Inst Monogr. 2014;106(1):djt356. doi:10.1093/jnci/djt356
12. Sarris EG, Saif MW, Syrigos KN. The biological role of PI3K pathway in lung cancer. Pharmaceuticals. 2012;5(11):1236–1264. doi:10.3390/ph5111236
13. Yin Z, Yang Y, Guo T, Veeraraghavan VP, Wang X. Potential chemotherapeutic effect of betalain against human non‐small cell lung cancer through PI3K/Akt/mTOR signaling pathway. Environ Toxicol. 2021;36(6):1011–1020. doi:10.1002/tox.23100
14. Wang J-Y, Wang X, Wang X-J, Zheng B-Z, Wang Y, Liang B. Curcumin inhibits the growth via Wnt/β-catenin pathway in non-small-cell lung cancer cells. Eur Revi Med Pharmaco. 2018;22(21):7492–7499. doi:10.26355/eurrev_201811_16290
15. Han SH, Han JH, Chun WJ, Lee SS, Kim HS, Lee JW. Nobiletin inhibits non‐small‐cell lung cancer by inactivating WNT/β‐catenin signaling through downregulating miR‐15‐5p. Evid Based Complement Alternat Med. 2021;2021(1):7782963. doi:10.1155/2021/7782963
16. Sun Y, Wu P, Sun Y, et al. Lycorine possesses notable anticancer potentials in on-small cell lung carcinoma cells via blocking Wnt/β-catenin signaling and epithelial-mesenchymal transition (EMT). Biochem Biophys Res Commun. 2018;495(1):911–921. doi:10.1016/j.bbrc.2017.11.032
17. Liu M-H, Jin H-X, Song Z, Wang J-Y, Gao D-J. Phytochemical, pharmacological, pharmacokinetic and toxicological characteristics of Ziziphi Spinosae Semen: a review. Front Pharmacol. 2024;15:1504009. doi:10.3389/fphar.2024.1504009
18. Yang Z, Cai W, Chen Y, et al. Jujuboside B reverse CUMS‐promoted tumor progression via blocking PI3K/Akt and MAPK/ERK and dephosphorylating CREB signaling. J Immunol Res. 2022;2022(1):5211368. doi:10.1155/2022/5211368
19. Wang C, Chen J-C, Xiao -H-H, et al. Jujuboside a promotes proliferation and neuronal differentiation of APPswe-overexpressing neural stem cells by activating Wnt/β-catenin signaling pathway. Neurosci Lett. 2022;772:136473. doi:10.1016/j.neulet.2022.136473
20. Wang W, Yang X, Dai J, Lu Y, Zhang J, Keller ET. Prostate cancer promotes a vicious cycle of bone metastasis progression through inducing osteocytes to secrete GDF15 that stimulates prostate cancer growth and invasion. Oncogene. 2019;38(23):4540–4559. doi:10.1038/s41388-019-0736-3
21. Guan H, Tan J, Zhang F, et al. Myofibroblasts from salivary gland adenoid cystic carcinomas promote cancer invasion by expressing MMP 2 and CXCL 12. Histopathology. 2015;66(6):781–790. doi:10.1111/his.12519
22. Ashkar F, Wu J. E-Cadherin and its signaling pathways: a novel target of dietary components in modulating cell migration and proliferation. Trends Food Sci Tech. 2024;146:104398. doi:10.1016/j.tifs.2024.104398
23. Richardson AM, Havel LS, Koyen AE, et al. Vimentin is required for lung adenocarcinoma metastasis via heterotypic tumor cell–cancer-associated fibroblast interactions during collective invasion. Clin Cancer Res. 2018;24(2):420–432. doi:10.1158/1078-0432.CCR-17-1776
24. Noorolyai S, Shajari N, Baghbani E, Sadreddini S, Baradaran B. The relation between PI3K/AKT signalling pathway and cancer. Gene. 2019;698:120–128. doi:10.1016/j.gene.2019.02.076
25. Lu W, Tinsley HN, Keeton A, Qu Z, Piazza GA, Li Y. Suppression of Wnt/β-catenin signaling inhibits prostate cancer cell proliferation. Eur J Pharmacol. 2009;602(1):8–14. doi:10.1016/j.ejphar.2008.10.053
26. Chen Y, Chen M, Deng K. Blocking the Wnt/β-catenin signaling pathway to treat colorectal cancer: strategies to improve current therapies. Int J Oncol. 2022;62(2):24. doi:10.3892/ijo.2022.5472
27. Mayer IA, Arteaga CL. The PI3K/AKT pathway as a target for cancer treatment. Ann Rev Med. 2016;67(1):11–28. doi:10.1146/annurev-med-062913-051343
28. Yu F, Yu C, Li F, et al. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct Target Ther. 2021;6(1):307. doi:10.1038/s41392-021-00701-5
29. Fleming-de-moraes CD, Rocha MR, Tessmann JW, de Araujo WM, Morgado-Diaz JA. Crosstalk between PI3K/Akt and Wnt/β-catenin pathways promote colorectal cancer progression regardless of mutational status. Cancer Biol Ther. 2022;23(1):1–13. doi:10.1080/15384047.2022.2108690
30. Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4(12):988–1004. doi:10.1038/nrd1902
31. Takahashi-Yanaga F, Sasaguri T. The Wnt/β-catenin signaling pathway as a target in drug discovery. J Pharmacol Sci. 2007;104(4):293–302. doi:10.1254/jphs.cr0070024
32. Huang J, Feng W, Li S, et al. Berberine exerts anti-cancer activity by modulating adenosine monophosphate-activated protein kinase (AMPK) and the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) signaling pathways. Curr Pharm Des. 2021;27(4):565–574. doi:10.2174/1381612826666200928155728
33. Wang Q, Zhong S, Wu H, Wu Q. In vitro anti-cancer effect of marmesin by suppression of PI3K/Akt pathway in esophagus cancer cells. Esophagus. 2022;19(1):163–174. doi:10.1007/s10388-021-00872-8
34. Hussain A, Dar MS, Bano N, et al. Identification of dinactin, a macrolide antibiotic, as a natural product-based small molecule targeting Wnt/β-catenin signaling pathway in cancer cells. Cancer Chemother Pharmacol. 2019;84:551–559. doi:10.1007/s00280-019-03870-x
35. Wu P, Hu Y-Z. PI3K/Akt/mTOR pathway inhibitors in cancer: a perspective on clinical progress. Curr Med Chem. 2010;17(35):4326–4341. doi:10.2174/092986710793361234
36. Dienstmann R, Rodon J, Serra V, Tabernero J. Picking the point of inhibition: a comparative review of PI3K/AKT/mTOR pathway inhibitors. Mol Cancer Ther. 2014;13(5):1021–1031. doi:10.1158/1535-7163.MCT-13-0639
37. Wu Y, Ginther C, Kim J, et al. Expression of Wnt3 activates Wnt/β-catenin pathway and promotes EMT-like phenotype in trastuzumab-resistant HER2-overexpressing breast cancer cells. Mol Cancer Ther. 2012;10(12):1597–1606. doi:10.1158/1541-7786.MCR-12-0155-T
38. Mesmar J, Abdallah R, Badran A, Maresca M, Shaito A, Baydoun E. Ziziphus nummularia: a comprehensive review of its phytochemical constituents and pharmacological properties. Molecules. 2022;27(13):4240. doi:10.3390/molecules27134240
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.