Back to Journals » Journal of Pain Research » Volume 18
Ketamine Infusion as an Adjunct to Opioid Analgesia in Pediatric Patients with High-Risk Neuroblastoma Undergoing Treatment with Dinutuximab: Adverse Effects and Safety in a Non-ICU Setting
Authors Streby KA, Tobias JD , McPhaden E, Downie S, Stanek J, Roth C , Patel PO
Received 7 September 2024
Accepted for publication 13 January 2025
Published 20 January 2025 Volume 2025:18 Pages 283—292
DOI https://doi.org/10.2147/JPR.S487724
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jinlei Li
Keri A Streby,1,* Joseph D Tobias,2,3,* Evan McPhaden,4 Shannon Downie,4 Joseph Stanek,1 Catherine Roth,2 Priyal O Patel1,2
1Department of Pediatrics- Division of Pediatric Oncology, Nationwide Children’s Hospital and The Ohio State University College of Medicine, Columbus, OH, USA; 2Department of Anesthesiology & Pain Medicine, Nationwide Children’s Hospital, Columbus, OH, USA; 3Department of Anesthesiology & Pain Medicine, The Ohio State University College of Medicine, Columbus, OH, USA; 4Ohio University Heritage College of Osteopathic Medicine, Dublin Campus and Ohio University, Athens, OH, USA
*These authors contributed equally to this work
Correspondence: Priyal O Patel, DO Department of Anesthesiology & Pain Medicine Nationwide Children’s Hospital, 700 Children’s Drive, Columbus, Ohio, 43205, USA, Tel +1-(614) 722-3728, Fax +1-(614) 722-4203, Email [email protected]
Introduction: Anti-GD2 immunotherapy has improved outcomes for children with high-risk neuroblastoma (HRNBL). Dinutuximab promotes complement-mediated reaction against disialoganglioside GD2, which is expressed in peripheral nerves and over-expressed in neuroblastoma. Dinutuximab is associated with ≥grade 3 neuropathic pain. Targeting GD2 stimulates the NMDA receptor, which makes ketamine useful in treatment of associated pain. The objective of this retrospective study is to describe the use of ketamine for pain uncontrolled by opioids, and ketamine’s impact on total opioid usage for patients receiving dinutuximab. In addition, the secondary objective is to describe the toxicities of pain management with opioids versus opioid plus ketamine.
Methods: A retrospective chart review of 40 hRNBL patients receiving dinutuximab at Nationwide Children’s Hospital, from 2010 to 2022, was conducted. Demographics, pain scores, medication records, and total daily IV morphine milligram equivalents (IVMME) with and without a ketamine adjunct were collected. Linear mixed effect regression was used to model IVMME use for pain management across dinutuximab cycles and explore the effect of ketamine.
Results: The study cohort included 187 dinutuximab hospitalizations from 40 patients. Age at diagnosis ranged from 1.2 to 11.4 years. 66/187 hospitalizations included ketamine. The average daily IVMME during post-consolidation dinutuximab infusions was greater in admissions with ketamine (median 11.67 mg/day vs 6.09 mg/day; p = 0.0005). Ketamine was not significantly associated (p = 0.77) with daily IVMME when examining opioid use longitudinally over dinutuximab cycles and controlling for patient age. Fever/chills was more frequent in admissions that utilized ketamine (79% vs 63%; p = 0.0297). No other significant statistical differences in adverse effects were observed in patients’ receiving opioids versus opioids plus ketamine.
Conclusion: Findings suggest ketamine is safe in a non-ICU setting for treatment of complex pain during anti-GD2 immunotherapy. Additional prospective studies are needed to quantify the effect of reducing opioid side effects by including ketamine in pain management plans.
Keywords: anti-GD2 immunotherapy, dinutuximab, neuropathic pain, pediatric analgesia, ketamine, high-risk neuroblastoma, opioid
Introduction
Ketamine was synthesized in the 1960’s and introduced for clinical use in 1970 as a derivative and alternative intravenous anesthetic agent to phencyclidine, as the latter had significant psychomimetic effects thereby limiting its clinical utility. Given its ability to provide amnesia and analgesia with generally limited effects on cardiac and respiratory function, it became and has remained a popular anesthetic agent in various clinical scenarios, including infants with congenital heart disease.1–4 These same properties have led to its popularity as a primary agent or adjunct to propofol, midazolam, or dexmedetomidine for procedural sedation.5–10
In addition to its role in the induction/maintenance of anesthesia and procedural sedation, the varied end-organ effects of ketamine have led to its use as an adjunct to primary pain management in various acute and chronic clinical scenarios.11–17 Ketamine has been proposed to have analgesic effects at both central and peripheral sites, including antagonism at the
Various acute and chronic conditions can result in escalating pain in pediatric patients. This is commonly seen in pediatric patients with complex hematology and oncology diagnoses, and progression of pain burden may be related to the primary disease process or aggressive therapeutic regimens. For example, studies have shown that the use of low-dose ketamine as an adjunct for sickle cell pain crises has contributed to better pain control and reduction in the need for opioid escalation in the treatment of complex pain symptoms.23 However, studies focusing on the use of ketamine in pediatric patients with oncologic diagnoses are lacking. In efforts to treat aggressive oncologic diseases, novel therapies have been developed as primary, adjunctive, or salvage therapies to conventional chemotherapeutic agents. One such therapy, chimeric 14.18 human/murine anti-GD2 monoclonal antibody (dinutuximab), has significantly improved outcomes in patients with high-risk neuroblastoma (HRNBL).24 The treatment protocol for dinutuximab consists of at least five to six cycles – each four days in length, with infusions running for 10–20 hours per day. This therapy promotes a complement-mediated reaction against the sphingolipid disialoganglioside GD2, which is highly expressed on neuroblastoma cells.25 However, as disialoganglioside GD2 is also expressed in the in the central nervous system and on peripheral nerves, the therapy can result in significant neuropathic pain, which may not be amenable to treatment with standard regimens including opioids, sometimes necessitating cessation of therapy.26–28 In fact, neuropathy is reported in up to 80% patients undergoing treatment with this medication.25,29 The pain is thought to be caused by the drug’s impact on C and Aδ fibers in the peripheral nervous system. Typically, the pain is limited to the time of infusion, starting within the first hour of infusion and dissipating shortly after the infusion is complete.30 In addition, the severity of pain tends to decrease with subsequent infusions.
Due to the risk of rapid onset of pain with dinutuximab infusions, it is essential to have a pain regimen that takes effect quickly. Therefore, short-acting opioids, and in particular patient- or nurse-controlled analgesia pumps, are routinely used as first-line treatment for dinutuximab-associated pain.30 In addition, due to risk of severe, complicated pain with use of anti-GD2 therapy, the addition of adjunctive analgesic therapies to systemic opioids is frequently necessary to control pain.31,32
Recent retrospective studies have suggested that using gabapentin and ketamine as adjuncts in the pain regimen has been effective in treating pain in patients undergoing treatment with dinutuximab. In vivo neuroblastoma studies have suggested that gabapentin helps decrease substance P-induced activation in neuroblastoma cells, thus assisting with overall pain control.33 A challenge to gabapentin use for dinutuximab-related, quick onset pain is that gabapentin typically needs to be started at a lower dose and titrated up to therapeutic doses to decrease risk of sedation. In addition, it may take several hours to days for gabapentin to take full effect. Therefore, if it is used, it needs to be started 3–5 days in advance and should be continued at least 2 days after completion of dinutuximab.34 Often, parental worry about time to reach full effect, risk factors such as increased sedation in the home setting, and oral medication burden tend to be barriers against consistent compliance with patient use of gabapentin in this setting.
Ketamine is a fast acting N-methyl-D-aspartate (NMDA) receptor antagonist.35 Ketamine is thought to reduce the mean channel opening time and decrease the frequency of channel opening and leads to desensitization of central pain pathways.22 Furthermore, blockade of NMDA receptors with ketamine may prevent the development of pain sensitivity and opioid tolerance.14,22 IV Ketamine has been noted to be successful in treating complex pain during dinutuximab infusions.34 At low doses, IV ketamine is an excellent analgesic with minimal sedative or cardio-respiratory effects. However, a major challenge for the use of IV ketamine infusions at several institutions is that it typically requires admission to the intensive care unit. In addition, ketamine infusion management requires trained provider and frequent monitoring, both of which add to the burdens of use for the management of dinutuximab-related pain.13,34 At our institution, in addition to ICU and anesthesiology providers, the palliative care team has been trained to use ketamine infusions to manage complex pain that is under- or not responsive to opioids or other analgesics. These drips may be managed outside of the ICU setting by trained palliative care providers, and this has allowed better access to ketamine for management of complex pain in patients with HRNB undergoing anti-GD2 treatment. In this retrospective study, we present our preliminary experience with the addition of a ketamine infusion to intravenous opioids to treat pain in patients with neuroblastoma undergoing therapy with dinutuximab. The objective of this study is to describe the utility of ketamine use for complex pain uncontrolled by opioids and its impact on total opioid usage for patients on dinutuximab. In addition, the secondary objective is to describe the side effect profile of pain management with opioids vs opioid plus ketamine.
Materials and Methods
This retrospective study was approved by the Institutional Review Board of Nationwide Children’s Hospital (STUDY00002219) in accordance with the principles stated in the Declaration of Helsinki. As a retrospective study using de-identified data, the need for individual written informed consent was waived. The study cohort included patients ≤18 years of age who received a dinutuximab infusion, who required a continuous opioid infusion ± a ketamine infusion for pain management from January 1st, 2010, to May 31st, 2022. The start date for the study followed the development of an institutional policy and procedure for the administration of a continuous infusion as an adjunct to opioid analgesia for acute pain related to medical or surgical conditions. Adverse events were collected from patients’ charts as documented by the pain or palliative care team, or graded for other clinical trials by oncology. Patients in this study received ketamine infusions for pain management while receiving dinutuximab on the inpatient ward (outside of the Pediatric ICU setting). Information regarding the development of this protocol and its requirements are outlined below.
Potential patients were identified from records from the Acute Pain Service, pharmacy database, the Oncology patient lists, and an electronic medical record (EMR) data query. For each patient, demographic data were collected including age, gender, weight, height, race/ethnicity, and comorbid conditions. Data regarding ketamine infusion included dose, whether a bolus of ketamine was administered, and the initial infusion rate (mg/kg/hour). Adjustments to the ketamine infusion rate during its administration on the inpatient ward were also recorded – in particular, decreases in the infusion rate related to adverse effects during therapy were noted. Information regarding pain and analgesia included pain scores and analgesic agents administered (opioids and adjunctive agents), including the dose, opioid continuous infusion rates, and frequency of the medication. Additional data regarding clinical efficacy were obtained from reviewing the daily progress notes. Adverse events, including the need to stop the ketamine infusion or decrease the infusion rate, were also recorded each day for patients while on the ketamine.
Ketamine Protocol
Prior to the start of this initiative, a standardized ketamine policy and protocol was created for the hospital, and outlined the use of a ketamine infusion on the inpatient hematology/oncology ward as an adjunct to pain management for acute pain unrelieved by an opioid infusion. Mandatory education was provided to inpatient nursing staff prior to caring for these patients through the hospital online learning system. This education provided information about the use of ketamine for analgesia, dosing, adverse effects, patient monitoring, and documentation requirements in the EMR. After completion of the module, the nurses completed a post-test to receive credit for completion. Information about the use of ketamine for analgesia was also added to the hospital’s pain management intranet website. The use of ketamine infusions started on the inpatient hematology/ oncology ward in March 2020 and was then expanded to other inpatient floors in September 2020. Ketamine infusions for analgesia were managed with a consultation to either the Acute Pain Service or the Palliative Care Service.
Per institutional policy, ketamine infusions were indicated as the use of a multimodal regimen for severe pain un- or under-responsive to common standard practice for analgesia, such as opioid use. Ketamine infusion dosing orders and monitoring recommendations were entered as an order set in the EMR. The order set was created to reflect the institutional ketamine policy. Recommended starting dose for a continuous IV ketamine infusion was 0.025–0.1mg/kg/hr. The maximum suggested ketamine infusion rate for use on inpatient wards was 0.4mg/kg/hr or 40mg/hr. Patients on ketamine were placed on continuous oxygen saturation monitoring during the infusion. Blood pressure, heart rate, respiratory rate, sedation, and level of consciousness were monitored closely. Per protocol, these data were collected by nursing at the following intervals: every 30 minutes for the first hour, then hourly for hours two to three of infusion, followed by every 4 hours for the remainder of the infusion. The presence of any symptoms of central nervous system toxicity (ex. sedation level, altered mental status) was documented routinely (every 30 mins for the first hour, and then hourly for the duration of the infusion). Pain was monitored with use of standardized pain scales in patients who are able to self-report scores and/or through clinical assessment.
Opioid Pain Pump Guidelines
Prior to the start of this study, the comprehensive pain and palliative care services had established guidelines around patient-, nurse-, and/or caregiver-controlled analgesia (PCA/NCA/CCA) pain pumps. Mandatory education was provided to inpatient nursing staff prior to caring for these patients through the hospital online learning system. This education provided information about the use of opioid pain pumps, dosing, adverse effects, patient monitoring, and documentation requirements in the EMR. After completion of the module, the nurses completed a post-test to receive credit for completion. Per institutional policy, pain pumps had to be managed by either the comprehensive pain team or palliative care. Like the policy around ketamine use, dosing orders and monitoring recommendations for PCA/NCA/CCAs were entered as an order set in the EMR. The order set was created to reflect the institutional practice guidelines. Vitals were monitored similarly to what was described above for the ketamine infusions.
Under most clinical circumstances, PCA/NCA/CCAs were considered for complex pain not responsive to intermittent dosing of IV or PO opioids. Caretakers, patients, and nurses were all provided education on pain pumps. Patients who had the capacity to understand how to use a pain pump properly were placed on PCA. If patients were unable to reliably be in control of their own pain pump, they were either started on NCAs or CCAs. NCAs were primarily used over CCAs when parents were unwilling or unavailable to manage the pain pumps. However, in patients undergoing treatment with dinutuximab, use of PCA/NCA/CCAs was the standard for first-line use during the cycles. Patients on dinutuximab were primarily started on both a basal and demand dose due to the nature and risk of severe basal and acutely worsening pain during the dinutuximab infusion. PCA/NCA/CCA settings were started at starting doses and titrated up as needed and as tolerated for adequate pain control. If pain was at a level of adequate control based on patient and/or caretaker report and providers’ clinical assessment, attempts were made to wean down on opioid demand dose and basal settings as clinically tolerated.
Morphine, hydromorphone, or fentanyl pain pumps were available on formulary. For opioid naïve patients, the standard practice at our institution was to start on a morphine pain pump. If patients had a history of using other opioids, such as hydromorphone or fentanyl, then they were started on the opioid they tolerated best. Also of note, after being placed on a particular opioid pain pump, if the patient had an undesirable side effect, such as uncontrolled opioid-induced pruritus, rotation to a different opioid was considered. If rotated to a different type of opioid, dose conversions were used to find an equivalent MME dose.
For opioid naïve patients with weights less than 50kg, the standard starting dosing recommendations for morphine, hydromorphone, and fentanyl pain pumps basal rates were 0.01 −0.03mg/kg/hr, 0.003 to 0.005 mg/kg/hr, 0.25 to 1 mcg/kg/hr, respectively. For opioid naïve patients with weights greater than 50kg, the standard starting dosing recommendations for morphine, hydromorphone, and fentanyl pain pumps basal rates were 0.5–1mg/hr, 0.1–0.2mg/hr, and 10–50 mcg/hr, respectively.
For patients below the weight of 50kg, the demand starting dose recommendations for morphine, hydromorphone, and fentanyl pain pumps were 0.01–0.03mg/kg, 0.003 to 0.005 mg/kg, 0.25 to 1 mcg/kg, respectively. For patients above the weight of 50kg, the demand starting dose recommendations for morphine, hydromorphone, and fentanyl pain pumps were 0.5–1mg, 0.1–0.2mg, and 10–50 mcg, respectively. Recommendations around lockout intervals for demand doses were every 12–20 mins.
In patients where pain was uncontrolled despite appropriate changes to the pain pump settings and/or further increase in opioids was hindered by undesirable side effects such as increased sedation, providers considered initiation of ketamine as an adjunct.
Opioid Conversion
All data on opioid use was converted to IV morphine milligram equivalents (MME) using pediatric-appropriate opioid conversion rates.36
Statistical Analysis
All data were summarized using standard descriptive statistics. Frequency and percentage for qualitative variables and median and interquartile range (IQR) or range. Nonparametric methods were used to compare opioid usage among those with and without ketamine. Linear mixed effects models were used to longitudinally assess opioid needs across dinutuximab cycles while including ketamine, age, cycle, and ketamine by cycle interaction as fixed effects. A random intercept was included for each individual patient. P-values were two-sided and those less than 0.05 were considered statistically significant. Data analyses were performed using the SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
We studied 40 patients with high-risk neuroblastoma who underwent treatment with dinutuximab consisting of 187 total hospital admissions. Included patients were mostly male (65%) with a median age at neuroblastoma diagnosis being 3.3 years (range: 1.2–11.4 years) (Table 1). Dinutuximab cycles were predominately used in the setting of primary neuroblastoma post-consolidation treatment (n = 137, 73%) or extended induction (n = 13, 7%); however, there were 37 (20%) dinutuximab cycles of relapse therapy among 11 patients. Dinutuximab was used along with IL-2 (47%), or in combination with chemotherapy (23%). When not given with IL-2, dinutuximab was given with GM-CSF (simultaneously during post-consolidation and for 1 week after completion of dinutuximab during chemoimmunotherapy).
 |
Table 1 Study Cohort Demographics |
A total of 122 dinutuximab infusions of the 187 (65%) encounters utilized only opioids for pain management whereas the other 65 (35%) admissions incorporated ketamine along with an opioid for pain management. The median starting dose of ketamine was 0.1mg/kg/hr. The median cumulative dose of ketamine per hospitalization was 205 mg (IQR: 150–332mg), and the median dose of ketamine per hospital day was 34.18 mg/day (IQR: 27.93–50.33mg). Table 2 highlights the adverse effect profile in the ketamine and no ketamine groups. The most common side effects in both the opioid-only and opioids plus ketamine groups were rash/itching, fevers/chills, vomiting/diarrhea, and hypotension. Symptoms of delirium and altered mental status were rare for both groups. Fever/chills was more frequent in admissions that utilized ketamine along with opioids (79% vs 63%; p = 0.0297). No other significant statistical differences in rates of adverse effects were observed in those patients receiving opioids alone versus opioids plus ketamine.
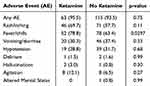 |
Table 2 Adverse Events with Ketamine Administration |
Overall, hospitalizations utilized a median of 50.7 IV morphine milligram equivalents during admission (MME) (IQR: 4.59–13.09). Cumulative MME in encounters that utilized only opioids for pain management was significantly lower than those which also incorporated ketamine for pain control (median 46.49 vs 57.61; p = 0.0002). When looking specifically at standard post-consolidation dinutuximab infusions, the median daily IV morphine milligram equivalent (IVMME) was significantly lower for hospitalizations with opioids only compared to those with opioids along with ketamine (6.09 vs 11.67; p = 0.0005) (Figure 1). This difference holds true across primary post-consolidation dinutuximab cycles (Figure 2). However, when looking in the context of relapse therapy – no significant difference in daily opioid usage was observed between admissions with and without ketamine for pain management (medians: 7.09 vs 7.48; p = 0.92).
 |
Figure 1 Median (IQR) morphine equivalent opioid usage (mg/day) between admissions with and without ketamine utilization. |
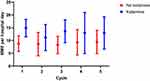 |
Figure 2 Median (IQR) morphine equivalent opioid usage (mg/day) across dinutuximab cycles with and without ketamine utilization. |
Discussion
Treatment with dinutuximab for HRNBL has notably improved the five-year event-free survival and overall survival for patients in addition to increasing responses in patients with relapsed or refractory HRNBL.37–39 Additionally, anti-GD2 antibody immunotherapies are currently being investigated for use as initial treatment of HRNBL in combination with chemotherapy.36 However, the introduction of this immunotherapy has also had an adverse impact on symptom burden for patients with HRNBL. Previous studies have noted a side effect profile of pain, fever, nausea/vomiting, hypotension, and capillary leak syndrome in patients undergoing treatment with this immunotherapy.37,40 Specifically, hyperalgesia and neuropathic pain have been noted in patients undergoing treatment with dinutuximab, especially during their first infusion cycle.40 For example, Blom et al demonstrated in their study that 88% of patients on dinutuximab infusions experienced grade ≥3 pain in their first infusion cycle. While historically, continuous opioid infusions with or without oral adjuncts such as gabapentin have been used as the primary method of pain treatment, our retrospective study demonstrates that the addition of ketamine infusions to the pain plan can be safe in treating complex pain symptoms in patients with HRNBL.
The most common adverse effects noted in our study were rash/itching, fevers/chills, vomiting/diarrhea, and hypotension. Largely, there was no statistical difference in adverse effects between the opioid-only group as compared to the ketamine plus opioid group. It is important to note that given the retrospective nature of this study, and polypharmacy needed in treatment of patients with HRNBL, it was difficult to discern with complete accuracy if the adverse effects were from ketamine, opioids, dinutuximab, or other agents. In particular, dinutuximab is known to be associated with the risks of hypotension, fevers, vomiting, diarrhea, skin rashes/changes, and capillary leak syndrome.37,40 Therefore, it is likely that the common adverse effects noted in our study were related to dinutuximab, rather than solely to ketamine and/or opioids.
Another limitation of this retrospective study includes missing data due to reliance on pre-existing charting. In particular, much inconsistency existed with documentation of pain scores. The majority of patients did not have consistently recorded pain scores. Therefore, we were unable to report how use of opioids versus opioids plus ketamine impacted pain scores. Based on subjective data from notes, a trend towards pain improvement was noted with the addition of ketamine in patients with severe, complex pain who were previously treated with opioids only.
Further, additional limitations include potential provider bias with determining the timing of ketamine initiation. While only providers with ketamine training were allowed to manage ketamine infusions for these patients, there was likely variance in practice styles and comfort with using ketamine as an adjunct. In addition, due to the retrospective nature of the study, it was difficult to control for confounding factors. For example, there was no control over the consistency of use of other adjunctive medical treatments for pain management.
In addition, while previous studies have suggested that ketamine is opioid sparing, our data showed that the patients who were on ketamine + opioids had higher MME requirement as compared to the opioid-only group. The likely reason for this is that the patients who required initiation of ketamine had more complex pain burden. Even prior to ketamine initiation, these patients were requiring frequent escalation of opioids due to hyperalgesia and uncontrolled discomfort. Ketamine was started when opioid escalation alone was ineffective in controlling pain. In fact, our data did show that the addition of ketamine was associated with lower need to further increase opioid dose in patients with severe, complex pain.
Our team is planning to conduct a randomized-controlled, prospective trial investigating the efficacy of ketamine monotherapy vs opioids monotherapy vs ketamine plus opioids for management of pain in patients undergoing treatment with dinutuximab. This prospective study utilizing patient-reported outcomes, including consistent use of documenting pain scores using validated pain scales, would better help determine the efficacy of ketamine use in management of pain and to delineate how different medications contribute to the specific side effect profile seen in patients undergoing treatment with this immunotherapy. In addition, a prospective randomize-controlled study would better allow us to investigate to what extent, if any, ketamine is opioid-sparing.
Conclusions
In conclusion, our study showed that ketamine can be safely administered and shows signs of improved pain control in patients with HRNBL undergoing dinutuximab immunotherapy. Collaboration with nursing, palliative care/pain providers, and pediatric oncologists is imperative to the successful administration of ketamine outside of the intensive care unit setting.
Acknowledgments
Keri A Streby and Joseph D Tobias are co-first authors of this study. The abstract of this paper was presented at the American Society of Pediatric Hematology/Oncology (ASPHO) 2023 Conference as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Pediatric Blood & Cancer. https://doi.org/10.1002/pbc.30390
Disclosure
The authors report no conflicts of interest in this work.
References
1. Roelofse JA. The evolution of ketamine applications in children. Paediatr Anaesth. 2010;20(3):240–245. doi:10.1111/j.1460-9592.2009.03145.x
2. Lin C, Durieux ME. Ketamine and kids: an update. Paediatr Anaesth. 2005;15(2):91–97. doi:10.1111/j.1460-9592.2005.01475.x
3. WHO definition of Palliative Care. 2020. Available from: https://www.who.int/cancer/palliative/definition/en/.
4. Maldini B. Ketamine anesthesia in children with acute burns and scalds. Acta Anaesthesiol Scand. 1996;40(9):1108–1111. doi:10.1111/j.1399-6576.1996.tb05572.x
5. Hodges SC, Walker IA, Bosenberg AT. Paediatric anaesthesia in developing countries. Anaesthesia. 2007;62 Suppl 1:26–31. doi:10.1111/j.1365-2044.2007.05294.x
6. McCarty EC, Mencio GA, Walker LA, Green NE. Ketamine sedation for the reduction of children’s fractures in the emergency department. J Bone Joint Surg Am. 2000;82-A(7):912–918. doi:10.2106/00004623-200007000-00002
7. Pena BM, Krauss B. Adverse events of procedural sedation and analgesia in a pediatric emergency department. Ann Emerg Med. 1999;34(4 Pt 1):483–491. doi:10.1016/s0196-0644(99)80050-x
8. Krauss B, Green SM. Sedation and analgesia for procedures in children. N Engl J Med. 2000;342(13):938–945. doi:10.1056/NEJM200003303421306
9. Tosun Z, Aksu R, Guler G, et al. Propofol-ketamine vs propofol-fentanyl for sedation during pediatric upper gastrointestinal endoscopy. Paediatr Anaesth. 2007;17(10):983–988. doi:10.1111/j.1460-9592.2007.02206.x
10. Tobias JD. Dexmedetomidine and ketamine: an effective alternative for procedural sedation? Pediatr Crit Care Med. 2012;13(4):423–427. doi:10.1097/PCC.0b013e318238b81c
11. James PJ, Howard RF, Williams DG. The addition of ketamine to a morphine nurse- or patient-controlled analgesia infusion (PCA/NCA) increases analgesic efficacy in children with mucositis pain. Paediatr Anaesth. 2010;20(9):805–811. doi:10.1111/j.1460-9592.2010.03358.x
12. Vadivelu N, Schermer E, Kodumudi V, Belani K, Urman RD, Kaye AD. Role of ketamine for analgesia in adults and children. J Anaesthesiol Clin Pharmacol. 2016;32(3):298–306. doi:10.4103/0970-9185.168149
13. Finkel JC, Pestieau SR, Quezado ZM. Ketamine as an adjuvant for treatment of cancer pain in children and adolescents. J Pain. 2007;8(6):515–521. doi:10.1016/j.jpain.2007.02.429
14. Sheehy KA, Lippold C, Rice AL, Nobrega R, Finkel JC, Quezado ZM. Subanesthetic ketamine for pain management in hospitalized children, adolescents, and young adults: a single-center cohort study. J Pain Res. 2017;10:787–795. doi:10.2147/JPR.S131156
15. Zempsky WT, Loiselle KA, Corsi JM, Hagstrom JN. Use of low-dose ketamine infusion for pediatric patients with sickle cell disease-related pain: a case series. Clin J Pain. 2010;26(2):163–167. doi:10.1097/AJP.0b013e3181b511ab
16. Taylor M, Jakacki R, May C, Howrie D, Maurer S. Ketamine PCA for treatment of end-of-life neuropathic pain in pediatrics. Am J Hosp Palliat Care. 2015;32(8):841–848. doi:10.1177/1049909114543640
17. Dahmani S, Michelet D, Abback PS, et al. Ketamine for perioperative pain management in children: a meta-analysis of published studies. Paediatr Anaesth. 2011;21(6):636–652. doi:10.1111/j.1460-9592.2011.03566.x
18. Mion G, Villevieille T. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci Ther. 2013;19(6):370–380. doi:10.1111/cns.12099
19. Sarton E, Teppema LJ, Olievier C, et al. The involvement of the mu-opioid receptor in ketamine-induced respiratory depression and antinociception. Anesth Analg. 2001;93(6):1495–1500. doi:10.1097/00000539-200112000-00031
20. Hirota K, Okawa H, Appadu BL, Grandy DK, Devi LA, Lambert DG. Stereoselective interaction of ketamine with recombinant mu, kappa, and delta opioid receptors expressed in Chinese hamster ovary cells. Anesthesiology. 1999;90(1):174–182. doi:10.1097/00000542-199901000-00023
21. Minami K, Sudo Y, Shiraishi S, Seo M, Uezono Y. Analysis of the effects of anesthetics and ethanol on mu-opioid receptor. J Pharmacol Sci. 2010;112(4):424–431. doi:10.1254/jphs.10003fp
22. Bell RF, Kalso EA. Ketamine for pain management. Pain Rep Sep-Oct. 2018;3(5):e674. doi:10.1097/PR9.0000000000000674
23. Hagedorn JM, Monico EC. Ketamine infusion for pain control in acute pediatric sickle cell painful crises. Pediatr Emerg Care. 2019;35(1):78–79. doi:10.1097/PEC.0000000000000978
24. Park JR, Eggert A, Caron H. Neuroblastoma: biology, prognosis, and treatment. Hematol Oncol Clin North Am. 2010;24(1):65–86. doi:10.1016/j.hoc.2009.11.011
25. Ozkaynak MF, Sondel PM, Krailo MD, et al. Phase I study of chimeric human/murine anti-ganglioside G(D2) monoclonal antibody (ch14.18) with granulocyte-macrophage colony-stimulating factor in children with neuroblastoma immediately after hematopoietic stem-cell transplantation: a children’s cancer group study. J Clin Oncol. 2000;18(24):4077–4085. doi:10.1200/JCO.2000.18.24.4077
26. Sorkin LS, Yu AL, Junger H, Doom CM. Antibody directed against GD(2) produces mechanical allodynia, but not thermal hyperalgesia when administered systemically or intrathecally despite its dependence on capsaicin sensitive afferents. Brain Res. 2002;930(1–2):67–74. doi:10.1016/s0006-8993(01)03408-4
27. Ari P, Kars M, Meany H, Pestieau S. Treatment of transient peripheral neuropathy during chimeric 14.18 antibody therapy in children with neuroblastoma: a case series. J Pediatr Hematol Oncol. 2018;40(2):e113–e116. doi:10.1097/MPH.0000000000000889
28. Anghelescu DL, Goldberg JL, Faughnan LG, et al. Comparison of pain outcomes between two anti-GD2 antibodies in patients with neuroblastoma. Pediatr Blood Cancer. 2015;62(2):224–228. doi:10.1002/pbc.25280
29. Keyel ME, Reynolds CP. Spotlight on dinutuximab in the treatment of high-risk neuroblastoma: development and place in therapy. Biologics. 2019;13:1–12. doi:10.2147/BTT.S114530
30. Bertolizio G, Otis A, Tam K, Aswar S, Garbin M, Ingelmo P. Multimodal analgesic plan for children undergoing chimeric 14.18 immunotherapy. J Pediatr Hematol Oncol. 2021;43(2):e169–e172. doi:10.1097/MPH.0000000000001722
31. Gorges M, West N, Deyell R, Winton P, Cheung W, Lauder G. Dexmedetomidine and hydromorphone: a novel pain management strategy for the oncology ward setting during anti-GD2 immunotherapy for high-risk neuroblastoma in children. Pediatr Blood Cancer. 2015;62(1):29–34. doi:10.1002/pbc.25197
32. Wallace MS, Lee J, Sorkin L, Dunn JS, Yaksh T, Yu A. Intravenous lidocaine: effects on controlling pain after anti-GD2 antibody therapy in children with neuroblastoma--a report of a series. Anesth Analg. 1997;85(4):794–796. doi:10.1097/00000539-199710000-00014
33. Park S, Ahn ES, Han DW, et al. Pregabalin and gabapentin inhibit substance P-induced NF-kappaB activation in neuroblastoma and glioma cells. J Cell Biochem. 2008;105(2):414–423. doi:10.1002/jcb.21837
34. Nysom K, Morad AG, Rafael MS, et al. Pain mitigation and management strategies for anti-GD2 infusions: an expert consensus. Pediatr Blood Cancer. 2023;70(5):e30217. doi:10.1002/pbc.30217
35. Prakash S, Gupta AK, Meena JP, Seth R. A review of the clinical applications of ketamine in pediatric oncology. Pediatr Blood Cancer. 2021;68(1):e28785. doi:10.1002/pbc.28785
36. Friedrichsdorf SJ. Pediatric Pain Master Class. UCSF;2023.
37. Blom T, Lurvink R, Aleven L, et al. Treatment-related toxicities during anti-gd2 immunotherapy in high-risk neuroblastoma patients. Front Oncol. 2020;10:601076. doi:10.3389/fonc.2020.601076
38. Yu AL, Gilman AL, Ozkaynak MF, et al. Long-term follow-up of a phase III study of ch14.18 (Dinutuximab) + Cytokine immunotherapy in children with high-risk neuroblastoma: COG study ANBL0032. Clin Cancer Res. 2021;27(8):2179–2189. doi:10.1158/1078-0432.CCR-20-3909
39. Mody R, Yu AL, Naranjo A, et al. Irinotecan, temozolomide, and dinutuximab with GM-CSF in children with refractory or relapsed neuroblastoma: a report from the children’s oncology group. J Clin Oncol. 2020;38(19):2160–2169. doi:10.1200/JCO.20.00203
40. Mastrangelo S, Rivetti S, Triarico S, et al. Mechanisms, characteristics, and treatment of neuropathic pain and peripheral neuropathy associated with dinutuximab in neuroblastoma patients. Int J mol Sci. 2021;22(23):12648. doi:10.3390/ijms222312648
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

