Back to Journals » Journal of Pain Research » Volume 18
LASSO Logistic Regression for Predicting Postoperative Severe Pain After Hepatic Hemangioma Ablation
Authors Gao R, Xu F, Song Y, Ke S, Kong J, Wang S , Sun W, Gao J
Received 14 December 2024
Accepted for publication 1 April 2025
Published 9 April 2025 Volume 2025:18 Pages 1909—1921
DOI https://doi.org/10.2147/JPR.S510668
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Karina Gritsenko
Ruize Gao,1,* Fei Xu,2,* Yuntang Song,2 Shan Ke,2 Jian Kong,2 Shaohong Wang,2 Wenbing Sun,2 Jun Gao2
1Department of Interventional Radiology, Beijing Chaoyang Hospital Affiliated to Capital Medical University, Beijing, 100043, People’s Republic of China; 2Department of Hepatobiliary Surgery, Beijing Chaoyang Hospital Affiliated to Capital Medical University, Beijing, 100043, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wenbing Sun, Department of Hepatobiliary Surgery, Beijing Chaoyang Hospital Affiliated to Capital Medical University, 5 JingYuan Road, Shijingshan District, Beijing, 100043, People’s Republic of China, Email [email protected] Jun Gao, Department of Hepatobiliary Surgery, Beijing Chaoyang Hospital Affiliated to Capital Medical University, 5 JingYuan Road, Shijingshan District, Beijing, 100043, People’s Republic of China, Email [email protected]
Purpose: To develop a least absolute shrinkage and selection operator (LASSO) logistic regression to predict postoperative severe pain after thermal ablation of hepatic hemangioma (HH).
Patients and Methods: From January 2014 to March 2024, 285 patients with HH treated by thermal ablation were retrospectively recruited. Forty-seven patients with postoperative severe pain [visual analogue scale (VAS) score ≥ 5] were matched 1:2 with 94 patients with mild pain (VAS score < 5). The LASSO and multivariate logistic regression identified independent risk factors for severe pain after thermal ablation for HH. The model’s performance was evaluated using receiver operating characteristic (ROC) curves, calibration plots, and decision curve analysis (DCA). Internal validation was performed using the Bootstrap method.
Results: The ablation time (OR = 1.070, p = 0.046), postoperative levels of aspartate aminotransferase (AST) (OR = 1.012, p < 0.001), lactate dehydrogenase (LDH) (OR = 1.009, p = 0.001), neutrophil to lymphocyte ratio (NLR) (OR = 1.266, p = 0.034) were independent risk factors of severe pain. The model’s area under the curve (AUC) = 0.985 (95% CI, 0.971– 0.998). After internal verification by the Bootstrap method, the model still had a high discriminative ability (AUC = 0.979, 95% CI, 0.971– 0.985). The calibration curve illustrated good agreement between the predicted and observed probability of severe pain. DCA verified that the model possesses significant predictive value.
Conclusion: Our nomogram predicts postoperative severe pain for HH with good discrimination and calibration based on the easily available risk factors.
Keywords: thermal ablation, hepatic hemangioma, postoperative pain, LASSO logistic regression, nomogram
Introduction
Hepatic hemangioma (HH) is the most common benign tumor of the liver, with a reported incidence of 3%~20% in the general population. Although the overwhelming majority of HHs are asymptomatic and require no intervention, those with a large diameter (≥ 5 cm) along with continuous growth, severe symptoms, or a high risk of complications may necessitate active management.1–3
Traditionally, the gold-standard treatment for HH was surgical resection (SR). However, SR is invasive and has a high risk of complications. Recently, thermal ablation treatment using radiofrequency ablation (RFA) or microwave ablation (MWA) has been increasingly accepted for treating HH. Notably, this increasing acceptance is due to its unique advantages, including minimal invasiveness and definite efficacy.4–8
Despite the high efficacy of thermal ablation for treating HH, complications of varying degrees have been observed.8 Among the various complications, postoperative pain is frequently encountered yet often neglected.8–10 Postoperative pain impacts the physical and psychological well-being of patients and may also hinder postoperative recovery.11 Consequently, enhancing the management of postoperative pain holds significant importance. However, to our knowledge, no studies have yet reported on the predictors of postoperative pain following thermal ablation for the treatment of HH. Since variations exist in perceived pain and discomfort postoperatively, identifying factors associated with severe pain following thermal ablation could help predict its occurrence and improve pain management. Therefore, this study aimed to investigate postoperative pain in detail and evaluate the factors related to severe pain after thermal ablation for HH.
Materials and Methods
Patient Cohort
We retrospectively reviewed the data of consecutive patients with HH treated by thermal ablation from January 2014 to March 2024. Data were collected from the clinical databases of Beijing Chaoyang Hospital affiliated to Capital Medical University. All patients provided informed consent to review and analyze their perioperative medical records. The inclusion and exclusion criteria and ablation procedures are described in a previous study.7
All 285 patients were categorized into two groups with severe pain and mild pain based on their postoperative visual analogue scale (VAS) scores, of whom 47 experienced severe pain and 238 experienced mild pain. Patients with severe pain were then matched 1:2 to patients with mild pain for gender, age, body mass index (BMI), Childe-Pugh class, comorbidities, and American Society of Anaesthesiologists (ASA) grade. Data were extracted by 2 independent reviewers using a standardized collection form.
The study was conducted in accordance with the Declaration of Helsinki. The study was approved by the institutional ethics committee of Beijing Chaoyang Hospital affiliated to Capital Medical University.
Data Collection
Biographical data and tumor characteristics were preoperatively collected, including the size, number, distribution, and location of tumor. The operation approach, ablation method, time, and site were collected intraoperatively. Postoperatively, the effectiveness of ablation, complications, hospital stay, VAS score, and analgesic treatment were collected. Additionally, laboratory results were collected for each patient on the day before ablation and the day after ablation, including aspartate transaminase (AST) level, alanine transaminase (ALT) level, white blood cell count (WBC), bilirubin, lactate dehydrogenase (LDH), neutrophil to lymphocyte ratio (NLR). The changes in those laboratory results refer to the variation from preoperative to postoperative levels.
Ablation Strategy
Two types of ablation systems were used in this study. Internally cooled cluster electrodes (Cool-tip ACTC2025 or ACTC1525; COVIDIEN, USA) were used for the RFA. A microwave therapeutic system (ECO-100A1, ECO Medical Instrument Co. Ltd., Nanjing, China) was used for the MWA. Overall, and whatever the ablation method, subcapsular hemangiomas were treated under laparoscopic guidance, while deep lesions were treated with computed tomography (CT) guidance.2,7
Measurements of Postoperative Pain
The use of the VAS score was explained to the patients before data were collected.12 Twenty-four hours after the thermal ablation procedure, patients were requested to recall the maximum pain level that they had felt within the last 24 hours as a number between 0 and 10 using the VAS score. Patients were then stratified into two groups based on their VAS scores: a severe pain group, which was defined as patient VAS scores of ≥ 5, and a mild pain group, which was defined as patient VAS scores of < 5.13
Statistical Analysis
Continuous data were expressed as mean ± SD and compared between groups using the Student’s t-test and analysis of variance. The Chi-square test or Fisher’s exact test analyzed differences in categorical data. P values < 0.05 were deemed significant.
Considering the multicollinearity among variables, the least absolute shrinkage and selection operator (LASSO) regression model was employed to screen for potential risk factors. The screened factors were then included in multivariate logistic regression to analyze the independent risk factors of severe pain. According to the results, the nomogram of the logistic regression model for predicting severe pain was drawn.
The model was internally validated using the Bootstrap method to replicate 1000 times. The model’s receiver operating characteristic (ROC) curve was developed to assess the discriminative power of the model. A calibration plot and Hosmer-Lemeshow test were used to evaluate the model’s accuracy. A decision curve analysis (DCA) plot was used to assess the clinic practicability of the model. Statistical analysis was performed using R 4.3.1 software.
Results
Baseline Characteristics of Patients
This retrospective cohort study included 285 patients in our institution from January 2014 to March 2024. We matched the total of 141 (1:2) patients from the severe pain group (n = 47) and mild pain group (n = 94). There were no significant differences in gender, age, BMI, comorbidities, ASA grade, Child-Pugh grade, number of tumor, whether the tumor was subcapsular or adjacent to the hepatic veins, approach of ablation, and ablation method between the two groups. However, there were significant differences in tumor size (6.77 ± 1.62 cm vs 9.70 ± 2.27 cm, p < 0.001) and whether the tumor was adjacent to the diaphragm (45.74% vs 65.96%, p = 0.037) or portal veins (25.53% vs 65.96%, p < 0.001) between two groups (Table 1).
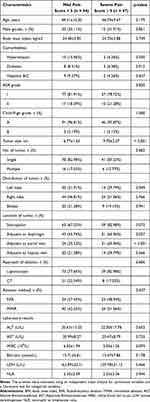 |
Table 1 Baseline Characteristics of the Groups Stratified by Pain Score |
The Outcome of Thermal Ablation
The perioperative data of thermal ablation are shown in Table 2. There were no technical failures in either group. The ablation time in the mild pain group was significantly shorter than that in the severe pain group (28.10 ± 10.89 min vs 47.94 ± 13.28 min, p < 0.001). Additionally, the ablation sites in the mild pain group were significantly less than those in the severe pain group (5.29 ± 1.23 vs 7.91 ± 1.98, p < 0.001). Postoperatively, hemolysis-related complications, including hemoglobinuria, systemic inflammatory response syndrome (SIRS), and anemia occurred. Fifty-one patients in the mild pain group and 34 patients in the severe pain group developed hemoglobinuria without statistical difference and were treated with adequate hydration. In our observation, all cases of hemoglobinuria spontaneously subsided within 72 h. Fifteen patients in the mild pain group and 20 patients in the severe pain group (15.96% vs 42.55%, p < 0.001) experienced SIRS and were treated with infection control, rehydration, oxygen inhalation, and corticosteroids. After treatment, all cases of SIRS spontaneously subsided within 72 h. Seven patients in the mild pain group and 4 patients in the severe pain group had anemia (p > 0.05). All cases resolved spontaneously and required no treatment. One patient in the mild pain group and 1 patient in the severe pain group had asymptomatic pleural effusion and needed no treatment (p > 0.05). Patients in the mild pain group also had shorter hospital stays (4.03 ± 1.49 days vs 6.36 ± 2.66 days, p < 0.001) and received less opioid treatment (5.32% vs 91.49%, p < 0.001).
 |
Table 2 Intraoperative and Postoperative Results of Groups Stratified by Pain Score |
We further investigated the postoperative laboratory results between the two groups. Patients in the severe pain group suffered from more severe liver injuries. Significant differences were observed in the levels of ALT, AST, and bilirubin, with the mild pain group showing markedly lower levels compared to the severe pain group (ALT: 88.04 ± 52.29 U/L vs 219.47 ± 169.44 U/L, p < 0.001; AST: 127.82 ± 106.89 U/L vs 408.73 ± 285.44 U/L, p < 0.001; bilirubin: 26.33 ± 12.39 mmol/L vs 35.35 ± 17.71 mmol/L, p = 0.002). WBC, LDH and NLR were also significantly higher in the severe pain group, indicating a pronounced inflammatory response (WBC: 8.50 ± 2.22,109/L vs 10.93 ± 2.48,109/L, p < 0.001; LDH: 285.39 ± 107.17 U/L vs 616.06 ± 236.69 U/L, p < 0.001; NLR: 6.17 ± 3.11 vs 13.28 ± 9.01, p < 0.001). Furthermore, the changes in the levels of these parameters between the day before and the day after ablation were also significantly greater in the severe pain group (all p < 0.05).
Screening for Independent Predictors of Intense Pain
Using 10-fold cross-validation through LASSO logistic regression, the lambda value of 0.107 corresponding to the one standard error was taken as the optimal value of the model (Figure 1).
From all the preoperative, intraoperative, and postoperative variables, LASSO logistic regression selected ablation time and postoperative levels of AST, LDH, and NLR as potential risk factors for postoperative severe pain after thermal ablation. Multivariate logistic regression analysis showed that ablation time (OR = 1.070, p = 0.046), postoperative levels of AST (OR = 1.012, p < 0.001), LDH (OR = 1.009, p = 0.001), NLR (OR = 1.266, p = 0.034) were independent risk factors of severe pain, as shown in Table 3. We conducted a correlation analysis on variables that showed statistical differences in the t-test or Chi-square test. The results indicated that tumor size (R = 0.93, p < 0.001) and ablation site (R = 0.82, p < 0.001) are correlated with ablation time (Figure 2).
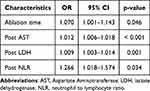 |
Table 3 Risk Factor for Multivarious Analysis |
Development and Validation of the Nomogram Prediction Model
According to the multivariate logistic regression analysis results, a regression equation of the logistic regression model was obtained as logistic (p) = −11.867 + 0.067 × ablation time + 0.012 × postoperative AST + 0.009 × postoperative LDH + 0.236 × postoperative NLR. The model is presented in a nomogram (Figure 3). From this, one can obtain the individual score corresponding to four independent risk factors through the scale positioned. The predicted probability associated with the score represents the likelihood of experiencing severe pain following the thermal ablation for HH. When a patient’s score reaches 60 points, the probability of severe pain exceeds 90%.
 |
Figure 3 Nomogram for predicting postoperative severe pain after thermal ablation. |
The area under the ROC curve (AUC) of this prediction model was 0.985 (95% CI, 0.971–0.998), indicating satisfactory predictive performance (Figure 4A). The Bootstrap method (resampling: 1000) was used for internal validation to evaluate the predictive performance of the developed model. The AUC of internal validation using the Bootstrap method was 0.979 (95% CI, 0.971–0.985) (Figure 4B). The calibration plot showed that the predicted curve was in good agreement with the actual observed curve. Notably, the calibration curve presented good concordance between the model prediction results and actual clinical observation (Figure 5). Additionally, the Hosmer-Lemeshow test showed no statistical difference between the actual and the predicted probability of postoperative severe pain after thermal ablation (p = 0.295). Furthermore, DCA was performed to evaluate the models further (Figure 6). DCA visually showed that the nomogram had a superior overall net benefit within the wide and practical ranges of threshold probabilities and impacted patient outcomes. Thus, this indicates that the prediction model possesses significant predictive value.
Discussion
In our study, 47 patients with HH who developed severe pain postoperatively were included and matched 1:2 to 94 patients with mild pain. Using LASSO and multivariate logistic regression analysis, 4 independent risk factors (ablation time, postoperative levels of AST, LDH, and NLR) associated with severe pain after thermal ablation for HH were finally identified. We further established a clinical prediction model, which is presented as a nomogram with good discrimination, calibration, and clinical predictive value.
Thermal ablation has been increasingly accepted for treating HH due to its safety and efficiency. However, postoperative pain is not rare. Kong et al5 utilized RFA or MWA to treat HH (5–10 cm) and observed that 8.33% of patients in the RFA group and 4.17% of patients in the MWA group experienced postoperative pain. Shi et al10 also treated HH (5–10 cm) with MWA, in which 21.9% of patients experienced postoperative pain. When encountering HH larger than 10 cm, the incidence of postoperative pain may be higher. Wu et al8 reported the experience of treating giant HH (≥ 10 cm) with RFA, 18.42% developed postoperative pain. Similarly, another study on MWA for the treatment of giant HH reported that 30.8% of patients developed pain following the procedure.14 Notably, the location of the tumor may also be related to postoperative pain. In the study of Gao et al9 where RFA was applied to HH abutting the diaphragm, 25% of patients experienced postoperative pain. However, previous literature has not addressed the severity of pain and analgesic treatment. In our cohort, 16.5% of patients (47 / 285) experienced postoperative severe pain, with a VAS score ≥ 5. Furthermore, 91.49% of aforementioned patients received opioid treatment.
Effective postoperative pain management not only alleviates the patient’s pain and facilitates recovery from the disease but also has significant social benefits. A study in China reported that most patients lack a proper understanding of postoperative pain and analgesics, about 50% received no treatment for their postoperative pain, and about 20% were unsatisfied with their pain management.15 Another study from the USA reported that of the patients included, approximately 86% experienced pain after surgery. Additionally, 74% of these patients still had moderate to severe pain after discharge. Among those who received pain control treatment, about 40% still had moderate or severe pain after their first treatment.16 Similarly, pain control treatment following interventional procedures was often inadequate.17 Nowadays, pain-free is an inevitable trend in the development of medicine; a comfortable experience is a fundamental right that patients are entitled to enjoy. This is especially true for benign diseases like HH, where strengthening postoperative pain management is particularly important. Herein, enhanced early recognition and active pain management following thermal ablation treatment in patients with HH are essential for facilitating disease recovery and improving postoperative quality of life.
The results of the present study suggested that ablation time, and postoperative levels of AST, LDH, and NLR are associated with severe pain for HH after thermal ablation. Previous studies have indicated that the incidence and severity of postoperative pain are associated with larger tumors, larger ablation volume, multiple ablations, and longer duration of ablation, where thermal ablation was applied to liver cancer.18–21 In our study, significant differences were observed in tumor size, ablation time, and ablation site between the two groups, however, only ablation time retained significance in LASSO regression and multivariate logistic regression analysis. Thus, we hypothesize that there is multicollinearity among those three variables, and conduct a correlation analysis. The results showed that ablation time is significantly correlated with both tumor size (R = 0.93, p < 0.001) and the ablation site (R = 0.82, p < 0.001) (Figure 2). Theoretically, the larger tumor usually needs to be treated with multiple ablations and requires a longer duration of ablation. In clinical practice, the pursuit of reduced ablation durations cannot come at the expense of therapeutic efficacy. Therefore, when extended ablation times are inevitable, it is imperative to prioritize the early recognition and aggressive management of pain. Interestingly, in our observation, tumors with a high extent of intratumoral enhancement on contrast-enhanced CT tend to collapse significantly during ablation and have short ablation times. However, this phenomenon still requires further verification.
In the present study, liver injury, represented by elevated AST level, is another independent risk factor for postoperative severe pain. AST is found in the cytosol and mitochondria of hepatocytes and is released into the bloodstream in the event of hepatocellular damage and cell death.22 Moreover, thermal ablation procedures are associated with tissue destruction, and pain is primarily due to ischemia of the treated organ.23 A similar result has also been reached in studies about postoperative pain after the thermal ablation of malignant liver tumors.13,18
Furthermore, inflammatory pain occurs in response to tissue injury and may develop 1–3 days after the procedure.17,24 LDH is a key biomarker of inflammation and tissue injury.25–28 Yang et al29 found that after RFA for HH, the level of LDH increased significantly, and was associated with SIRS. NLR, a novel inflammatory marker, has also been proven to be involved in the occurrence and development of various inflammatory responses.28,30 Rathee et al31 demonstrated a significant correlation between NLR and postoperative pain. However, a rare study focuses on the influence of inflammatory markers on post-ablation pain of HH. In the present study, LDH and NLR were identified as independent risk factors for postoperative severe pain. Previous studies have found that RFA for HH induces inflammatory responses, represented by heme-induced endothelial cell pyroptosis.32,33 Hence, we hypothesize that as the duration of ablation increases, the pyroptosis of endothelial cells is gradually triggered, increasing the release of inflammatory factors and ultimately causing pain. Hence, anti-inflammatory treatment may assist analgesics in relieving pain after ablation for HH, which needs further investigation.
Previous studies on thermal ablation treatment for liver cancer suggest that the location of the tumor is correlated with the occurrence of postoperative pain, especially when the tumor is adjacent to large blood vessels or the peritoneum.34 However, in our study, proximity to large blood vessels was not defined as a risk factor for severe pain after thermal ablation treatment for HH. This may be related to the nature of the hemangioma. Compared with liver cancer, the main component of HH is blood sinusoids, which collapse and atrophy rapidly during the ablation process, causing the tumor to move farther away from large blood vessels and reducing thermal damage. Additionally, in our study, thermal ablation was performed under laparoscopic guidance for subcapsular HH. The laparoscopic technique offers a direct vision of the entire procedure, allowing the isolation of the tumor from the peritoneum.
The nomogram based on clinical data described in this study has not been previously reported. Importantly, it provides a novel predictive tool for severe pain after thermal ablation for HH, which is anticipated to assist in pain management, enhance patient comfort, and accelerate recovery.
We must acknowledge some limitations of our study: First, the study adopted a retrospective design. Second, variations between individuals might cause inconsistencies when measuring subjective pain levels using a VAS score. Third, the single-center study and small sample sizes may affect the accuracy of interpretation. Further multi-center, prospective, randomized controlled studies are needed.
Conclusion
In conclusion, this study found that postoperative pain after thermal ablation for HH is associated with prolonged ablation time and increased levels of AST, LDH, and NLR after ablation. The clinical prediction model based on these four variables performed well in terms of both good discrimination and calibration. The results of this study should help in developing strategies for pain management after thermal ablation for HH and enable physicians to provide better counseling to patients regarding reasonable pain expectations.
Data Sharing Statement
Data are available upon reasonable request and contacting the correspondent authors.
Acknowledgments
The authors thank AiMi Academic Services (www.aimieditor.com) for English language editing and review services.
Disclosure
The authors did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors and declare no conflicts of interest.
References
1. Toro A, Mahfouz AE, Ardiri A, et al. What is changing in indications and treatment of hepatic hemangiomas. A review. Ann Hepatol. 2014;13(4):327–339.
2. Gao J, Fan RF, Yang JY, et al. Radiofrequency ablation for hepatic hemangiomas: a consensus from a Chinese panel of experts. World J Gastroenterol. 2017;23(39):7077–7086. doi:10.3748/wjg.v23.i39.7077
3. Hasan HY, Hinshaw JL, Borman EJ, Gegios A, Leverson G, Winslow ER. Assessing normal growth of hepatic hemangiomas during long-term follow-up. JAMA Surg. 2014;149(12):1266–1271. doi:10.1001/jamasurg.2014.477
4. Qu C, Liu H, Li XQ, Feng K, Ma K. Percutaneous ultrasound-guided ‘three-step’ radiofrequency ablation for giant hepatic hemangioma (5-15 cm): a safe and effective new technique. Int J Hyperthermia. 2020;37(1):212–219. doi:10.1080/02656736.2020.1732484
5. Kong J, Gao R, Wu S, et al. Safety and efficacy of microwave versus radiofrequency ablation for large hepatic hemangioma: a multicenter retrospective study with propensity score matching. Eur Radiol. 2022;32(5):3309–3318. doi:10.1007/s00330-021-08425-4
6. Wen SQ, Wan M, Len KM, et al. Safety and efficacy of laparoscopic radiofrequency ablation for hepatic hemangiomas: a multicenter retrospective study. Ann Hepatol. 2018;17(2):268–273. doi:10.5604/01.3001.0010.8653
7. Xu L, Wu SL, Kong J, et al. Thermal ablation of hepatic hemangioma: a multi-center experience with long-term outcomes. Eur J Radiol. 2023;164.
8. Wu S, Gao R, Yin T, et al. Complications of radiofrequency ablation for hepatic hemangioma: a multicenter retrospective analysis on 291 cases. Front Oncol. 2021;11:706619. doi:10.3389/fonc.2021.706619
9. Gao J, Kong J, Ding XM, et al. Laparoscopic vs computerized tomography-guided radiofrequency ablation for large hepatic hemangiomas abutting the diaphragm. World J Gastroenterol. 2015;21(19):5941–5949. doi:10.3748/wjg.v21.i19.5941
10. Shi Y, Song J, Ding M, et al. Microwave ablation versus transcatheter arterial embolization for large hepatic hemangiomas: clinical outcomes. Int J Hyperthermia. 2020;37(1):938–943. doi:10.1080/02656736.2020.1766122
11. Aydede M, Shriver A. Recently introduced definition of “nociplastic pain” by the international association for the study of pain needs better formulation. Pain. 2018;159(6):1176–1177. doi:10.1097/j.pain.0000000000001184
12. Miller MD, Ferris DG. Measurement of subjective phenomena in primary care research: the visual analogue scale. Fam Pract Res J. 1993;13(1):15–24.
13. Hsieh YC, Yap YS, Hung CH, Chen CH, Lu SN, Wang JH. Factors related to postoperative pain among patients who underwent radiofrequency ablation of hepatocellular carcinoma. Clin Radiol. 2013;68(6):600–607. doi:10.1016/j.crad.2012.12.006
14. Wang Z, Tang X, Qi X, et al. Feasibility, safety, and efficacy of ultrasound-guided percutaneous microwave ablation for giant hepatic hemangioma. Int J Hyperthermia. 2018;35(1):246–252. doi:10.1080/02656736.2018.1493541
15. Weiran L, Lei Z, Woo SM, et al. A study of patient experience and perception regarding postoperative pain management in Chinese hospitals. Patient Prefer Adherence. 2013;7:1157–1162. doi:10.2147/PPA.S53235
16. Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30(1):149–160. doi:10.1185/03007995.2013.860019
17. Faramarzalian A, Armitage KB, Kapoor B, Kalva SP. Medical management of tumor lysis syndrome, postprocedural pain, and venous thromboembolism following interventional radiology procedures. Semin Intervent Radiol. 2015;32(2):209–216. doi:10.1055/s-0035-1549379
18. Andreano A, Galimberti S, Franza E, et al. Percutaneous microwave ablation of hepatic tumors: prospective evaluation of postablation syndrome and postprocedural pain. J Vasc Interv Radiol. 2014;25(1):97–105.e101–102. doi:10.1016/j.jvir.2013.09.005
19. Hori M, Tanaka M, Suzuki Y, et al. Periprocedural pain of radiofrequency ablation for hepatocellular carcinoma. Gastroenterol Nurs. 2011;34(2):129–134. doi:10.1097/SGA.0b013e318210c084
20. Lee S, Rhim H, Kim YS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinomas: factors related to intraprocedural and postprocedural pain. AJR Am J Roentgenol. 2009;192(4):1064–1070. doi:10.2214/AJR.08.1350
21. Ongiem A, Siriussawakul A, Aungsumat wangdee BW, Homsud S, Jaiyen T. Assessment of pain severity after radiofrequency ablation in patients with hepatocellular carcinoma. J Med Assoc Thai. 2016;99(5):572–577.
22. Li Y, Li M, Mao J, et al. The processing mechanism of vinegar-processed curcumae rhizome enhances anti hepatic fibrotic effects through regulation of PI3K/Akt/mTOR signaling pathway. Phytomedicine. 2024;135:156098. doi:10.1016/j.phymed.2024.156098
23. Cashman JN, Ng L. The management of peri- and postprocedural pain in interventional radiology: a narrative review. Pain Manag. 2017;7(6):523–535.
24. Lv N, Kong Y, Mu L, Pan T, Xie Q, Zhao M. Effect of perioperative parecoxib sodium on postoperative pain control for transcatheter arterial chemoembolization for inoperable hepatocellular carcinoma: a prospective randomized trial. Eur Radiol. 2016;26(10):3492–3499. doi:10.1007/s00330-016-4207-8
25. Li S, Zhang Y, Li M, Xie C, Serum Albumin WH. a good indicator of persistent organ failure in acute pancreatitis. BMC Gastroenterol. 2017;17(1):59. doi:10.1186/s12876-017-0615-8
26. He Q, Wang M, Zhu H, et al. Mediation effect of stroke recurrence in the association between post-stroke lactate dehydrogenase and functional disability. Front Aging Neurosci. 2024;16:1450863. doi:10.3389/fnagi.2024.1450863
27. Cuddihy J, Wu G, Ho L, et al. Lactate dehydrogenase activity staining demonstrates time-dependent immune cell infiltration in human ex-vivo burn-injured skin. Sci Rep. 2021;11(1):21249. doi:10.1038/s41598-021-00644-5
28. Wang S, Liu J, Hu S, Mao Y. LDH and NLR, as inflammatory markers, the independent risk factors for COVID-19 complicated with respiratory failure in elderly patients. Pak J Med Sci. 2024;40(9):2112–2117. doi:10.12669/pjms.40.9.8728
29. Yang M, Yang X, Wang S, et al. HMGB1-induced endothelial cell pyroptosis is involved in systemic inflammatory response syndrome following radiofrequency ablation of hepatic hemangiomas. Am J Transl Res. 2019;11(12):7555–7567.
30. Lin L, Yang J, Fu W, Liu X, Liu Y, Zou L. Association between neutrophil-to-lymphocyte ratio and short-term all-cause mortality in patients with cerebrovascular disease admitted to the intensive care unit-a study based on the MIMIC-IV database. Front Med. 2024;11:1457364. doi:10.3389/fmed.2024.1457364
31. Rathee A, Chaurasia MK, Singh MK, Singh V, Kaushal D. Relationship between pre- and post-operative C-Reactive Protein (CRP), Neutrophil-to-Lymphocyte Ratio (NLR), and Platelet-to-Lymphocyte Ratio (PLR) with post-operative pain after total hip and knee arthroplasty: an observational study. Cureus. 2023;15(8):e43782. doi:10.7759/cureus.43782
32. Yao C, Kong J, Xu F, et al. Heme-inducing endothelial pyroptosis plays a key role in radiofrequency ablation of hepatic hemangioma leading to systemic inflammatory response syndrome. J Inflamm Res. 2024;17:371–385. doi:10.2147/JIR.S435486
33. Yang X, Liu J, Yang MM, et al. Heme is involved in the systemic inflammatory response following radiofrequency ablation of hepatic hemangiomas. Eur J Gastroenterol Hepatol. 2020;32(9):1200–1206. doi:10.1097/MEG.0000000000001636
34. Jung CFM, Liverani E, Binda C, et al. Non-Operating Room Anesthesia (NORA) for ultrasound-guided liver radiofrequency ablation. Diagnostics. 2024;14(16). doi:10.3390/diagnostics14161783
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






