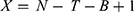Back to Journals » International Journal of Nanomedicine » Volume 20
Layer-by-Layer Engineering of Black Seed Oil Based SNEDDSs (BSO-SNEDDSs): Optimizing Chemical Stability and Bioavailability in Ramipril Formulations
Authors Shahba AAW , Sherif AY, Elzayat EM , Ali S, Kazi M
Received 19 December 2024
Accepted for publication 20 March 2025
Published 9 April 2025 Volume 2025:20 Pages 4415—4432
DOI https://doi.org/10.2147/IJN.S510918
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Ahmad Abdul-Wahhab Shahba,1,2 Abdelrahman Y Sherif,1,2 Ehab M Elzayat,1 Shaukat Ali,3 Mohsin Kazi1,2
1Department of Pharmaceutics, College of Pharmacy, King Saud University, Riyadh, 11451, Kingdom of Saudi Arabia; 2Kayyali Chair for Pharmaceutical Industries, College of Pharmacy, King Saud University, Riyadh, 11451, Kingdom of Saudi Arabia; 3Ascendia Pharma, Inc, North Brunswick, NJ, 08902, USA
Correspondence: Mohsin Kazi, Department of Pharmaceutics, College of Pharmacy, King Saud University, P.O. Box-2457, Riyadh, 11451, Kingdom of Saudi Arabia, Tel +966114677372, Email [email protected] Ahmad Abdul-Wahhab Shahba, Department of Pharmaceutics, College of Pharmacy, King Saud University, P.O. Box-2457, Riyadh, 11451, Kingdom of Saudi Arabia, Tel +966114677372, Email [email protected]
Purpose: The inherent chemical instability of ramipril (RMP) can lead to reduced therapeutic efficacy and safety, emphasizing the need for innovative formulation strategies for increased stability and bioavailability. This study aims to develop RMP-loaded liquid and solid self-nanoemulsifying formulations (SNEDDSs) that incorporate cardioprotective black seed oil (BSO) as a natural source of bioactive thymoquinone (THQ) for comprehensive chemical stability and pharmacokinetic evaluation.
Methods: A systematic approach was employed to transform liquid SNEDDSs into both single-layer (Single-SNEPs) and multilayer (Multi-SNEPs) self-nanoemulsifying pellets through fluid bed coating technology. Extensive characterization encompassing morphological analysis, dissolution studies, chemical stability assessments, and pharmacokinetic profiling, was conducted.
Results: In vitro dissolution studies demonstrated that the multilayered 5L-SNEPs formulation exhibited the highest dissolution efficiency compared with that of pure RMP (p > 0.05) and pure THQ (P < 0.05). Notably, the 5-layer pellets (5L-SNEPs) exhibited superior chemical stability of RMP (p < 0.05) compared with the liquid SNEDDS and other pellet variants. In-vivo pharmacokinetic analysis in rats revealed that liquid SNEDDS showed a numerically greater maximum plasma concentration (Cmax = 106 ± 34 ng/mL) and area under the curve (AUC = 454 ± 265 ng·h/mL) compared to pure RMP (Cmax = 90 ± 17 ng/mL; AUC = 308 ± 213 ng·h/mL), indicating a 1.5-fold higher AUC from the liquid SNEDDS. However, the difference was not statistically significant. Interestingly, 5L-SNEPs resulted in the lowest RMP exposure among the tested formulations, with a Cmax of 60 ± 18 ng/mL and an AUC of 155 ± 59 ng·h/mL, although the differences were not statistically significant compared to the other groups. The time to maximum concentration (Tmax) was 0.8 hours for liquid SNEDDS, 0.6 hours for the 5L-SNEPs, and 0.5 hours for pure RMP.
Conclusion: While liquid SNEDDSs exhibit promisingly greater oral bioavailability than crystalline drugs do, the performance of multilayer solid SNEDDSs necessitates further refinement. Nonetheless, this comprehensive investigation establishes a robust foundation for continued research on multifunctional bioactive oil-based SNEDDSs to enhance the bioavailability of drugs with limited water solubility.
Keywords: ramipril, solid self-nanoemulsifying formulations, SSNEDDSs, self-nanoemulsifying pellets, hypertension, oral bioavailability
Graphical Abstract:

Introduction
Ramipril (Figure 1), an angiotensin-converting enzyme (ACE) inhibitor, plays a pivotal role in the therapeutic landscape for hypertension and congestive heart failure. As an ethyl ester prodrug, ramipril undergoes enzymatic conversion to its active form, ramiprilat, which is crucial for its therapeutic effects.1 Maintaining chemical stability is crucial for ensuring effective systemic drug concentrations are achieved.2 Ramipril is unstable under acidic, neutral, and alkaline hydrolysis conditions, indicating significant degradation in solvents of varying pH, particularly under alkaline conditions.3 This instability highlights the need to develop effective formulation strategies that can provide appropriate protection.
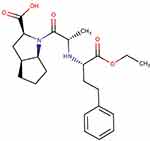 |
Figure 1 Chemical Structure of Ramipril, which represents an ethyl ester prodrug. Marvin JS was used for drawing and displaying the structure, Chemicalize, ChemAxon (https://chemicalize.com, accessed on 13 Feb 2025). |
Liquid SNEDDSs typically offer enhanced solubility and oral bioavailability. This is attributed to their capacity to form nanoemulsions upon dispersion, which can facilitate improved in vivo drug absorption.4–7 However, solid SNEDDSs may provide superior chemical stability8 and better patient compliance.9,10 In contrast, while liquid formulations provide immediate solubilization benefits, they may pose limitations such as lower stability and higher production costs, which can limit their practical application.4
Both liquid and solid SNEDDS formulations significantly enhanced RMP solubility and stability. Liquid SNEDDS maintained thermodynamic stability, with optimized formulations exhibiting nanoscale droplet sizes, preventing drug precipitation and phase separation.11,12 In addition, nanoemulsions have been shown to increase ramipril stability by reducing degradation rates, particularly at a pH of 5.0, which resulted in the lowest degradation over 180 days.13,14 Solid SNEDDS (S-SNEDDS) formulations further improved RMP stability by curtailing its degradation in liquid SNEDDS formulations during storage.8,12
Research indicates that BSO and its bioactive component thymoquinone (THQ) exhibit significant antihypertensive effects, primarily through its antioxidant and anti-inflammatory properties.15–17 Clinical trials have reported that supplementation with BSO can lead to significant reductions in both systolic and diastolic blood pressure, as well as improvements in lipid profiles, further supporting its role in cardiovascular health.15 These findings prompted the investigation of incorporating these compounds into the fabrication of ramipril-loaded self-nanoemulsifying drug delivery systems (RMP-SNEDDSs) to potentially synergize with the antihypertensive and cardioprotective effects of the formulations. A recent RMP BSO-loaded SNEDDS study showed good dissolution performance. However, the study also revealed significant RMP degradation in the SNEDDS formulation after 8 days of storage under accelerated conditions.18 According to recent studies, the degradation mechanism was suggested to involve amide hydrolysis, alkyl ester hydrolysis, and lactamization of the amino acid or derivative.19 These stability limitations represent a critical challenge for current RMP formulations that warrant further investigation. In this regard, the current study introduces a novel multi-layer SNEDDS system. This approach involved a RMP-free SNEDDS layer, which was separated from the RMP layer by a protective layer. The unique advantage of this approach is isolating the RMP from the lipid-based excipients of the SNEDDS, potentially enhancing the stability of the drug during storage.The noteworthy potential for ramipril to degrade into multiple impurities highlights the necessity of having accurate and specific analytical methods that can reliably quantify the active drug in presence of these degradation products.20,21 In the present study, researchers developed UPLC and LC-MS/MS methods to accurately quantify RMP and THQ in the presence of their degradant impurities within the formulation,19 along with the quantifying RMP and its active metabolite (ramiprilat) in plasma. This achievement marks a pivotal milestone toward precise drug quantification throughout in vitro and in vivo characterization.
Understanding the pharmacokinetics of Ramipril, including its absorption, distribution, metabolism, and excretion, is vital for evaluating formulation effectiveness.1 A thorough evaluation of both chemical stability and pharmacokinetics can guide the fabrication of optimal Ramipril formulations. The current study embarks on the development of RMP-SNEDDSs, incorporating BSO as the oil phase and utilizing fluid bed coating techniques, with a focus on comprehensive chemical stability and pharmacokinetic evaluation in rat models. Liquid SNEDDSs, single-layer pellets, and multilayer pellets incorporating both drug-free and protective layers were manufactured via fluid bed coating. Extensive characterization, spanning morphological analysis, dissolution studies, chemical stability assessments, and pharmacokinetic profiling were subsequently performed, ensuring a comprehensive understanding of formulation behavior.
Materials and Methods
Materials
Chemicals and Reagents
Ramipril (RMP) was sourced from Jai Radhe Sales (Ahmedabad, India), the active metabolite Ramiprilat of RMP and Thymoquinone (THQ) from Sigma‒Aldrich (St. Louis, MO, USA), and other chemicals were obtained from the following suppliers: Imwitor 988 (I988), Imwitor 308 (I308), Kolliphor EL (KrEL), Kolliphor ELP high purity (KrEL (HP), Kolliphor RH40 (Kr-RH40) and Kollicoat® Smartseal 30 D from BASF, (Ludwigshafen, Germany), Hydrogenated castor oil (HCO30) from Nicole chemical co., (Tokyo, Japan), Tween 20 (T20) from BDH, England, Tween 85 (T85) from Merck-Schuchardt OHG, Germany, Transcutol® P (TCP) from Gattefossé (Lyon, France), Super refined Tween 80 (SR-T80) from CRODA (Dusseldorf, Germany), Vcaps Plus® HPMC and fish gelatin capsules from Capsugel (South Carolina, USA).
Black Seed Oil (BSO) Extraction
The seeds of Nigella sativa (also known as black seeds), weighing approximately 500 grams, were procured from Kaligonj Bazar (Kaligonj, Gazipur, Bangladesh). The black seed oil (BSO) was then extracted from the seeds using a cold pressing method at Masum Oil Mill. The extracted oil was later stored in a sealed amber glass bottle for future use.22 A voucher specimen (#15966) of the Nigella sativa seeds was previously deposited in the Herbarium of the College of Pharmacy, King Saud University, Saudi Arabia, and its identity was confirmed by the taxonomist, Dr. Mohammed Yusuf, College of Pharmacy, King Saud University.
Black Seed Oil (BSO) Standardization
Thymoquinone (2-isopropyl-5-methylbenzo-1, 4-quinone, THQ) is one of the principal bioactive constituents of N. sativa and was, therefore, used to standardize the BSO. Standard Thymoquinone (THQ) was purchased from Sigma‒Aldrich (St. Louis, MO, USA). A THQ stock solution (500.0 μg/mL) was used as a reference solution for the standardization of BSO. Serial THQ concentrations (0.5–50.0 μg/mL) were prepared, and the actual THQ amount in BSO was calculated based on the THQ calibration curve. The amount of thymoquinone (THQ) present in the currently used black seed oil was approximately 11.7 ± 1.2 mg/g, which is consistent with the previously reported THQ amounts in BSO.23,24
Preparation of Liquid Self-Nanoemulsifying Drug Delivery Systems (L-SNEDDSs) and RMP Loading
RMP-free liquid SNEDDSs were initially prepared using bioactive oil (BSO) with a nonionic surfactant, cosolvent, and/or cosurfactant at the BSO/TCP/HCO-30 ratio (32.25/27.75/40), as described previously.25 The resulting mixture was vigorously homogenized (vortexed for approximately 1 min) to achieve maximum miscibility. For drug-loaded SNEDDSs, RMP was loaded into the formulation at 10 mg per gram w/w ratio. The components were then thoroughly mixed and sonicated for 1 h to ensure complete drug solubilization and homogenization. The prepared mixtures were finally transferred and stored in 1.5 mL Eppendorf tubes for further characterization.26
Fluid Bed Coating: Transforming Liquid SNEDDSs into Solid Single-SNEPs
Single-layer self-nanoemulsifying pellets (Single-SNEPs) were prepared via a bottom-spray fluid-bed coater (GPCC 1.1 fluid bed processor lab model, ACG Pharma Technologies Pvt. Ltd., Maharashtra, India), as illustrated in Figure 2A and Table 1.27–29 The coating solution was precisely prepared in three sequential steps. First, a 20% aqueous solution of the coating polymer was prepared. Second, freshly prepared RMP-loaded SNEDDSs and/or an anti-tacking agent were incorporated into the polymer solution while under mechanical stirring. Third, distilled water was added to accomplish the desired coating solution concentration. Preheated nonpareil sugar spheres were introduced into the fluid bed chamber 10 minutes prior to coating. Subsequently, the coating solution was bottom-sprayed onto the fluidizing spheres via a peristaltic pump. Finally, the coated pellets were dried for a minimum of 10 minutes.26,28
 |
Table 1 Layer Composition of the Single-SNEPs and Multi-SNEPs Formulations |
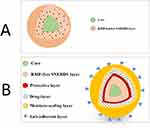 |
Figure 2 Schematic diagram of (A) Single-SNEPs and (B) 5L-SNEPs. |
Optimizing Solid Multi-Layer SNEPs (Multi-SNEPs) Preparation
Multi-SNEPs were prepared via a bottom-spray fluid-bed coater (GPCC 1.1 fluid bed processor lab model; ACG Pharma Technologies Pvt. Ltd., Maharashtra, India), as illustrated in Figure 2B and Table 1.26,30 Each layer’s coating solution was freshly prepared, blending appropriate components via mechanical stirring. The final volume was adjusted with the necessary neutral/alkaline solution under continuous stirring. Ten minutes prior to coating, sugar spheres (800–1000 µm) were introduced into the preheated fluid bed chamber. Subsequently, the coating solution was sprayed onto the fluidizing spheres via a peristaltic pump. Generally, the optimum operating conditions include batch size (450--500), airflow (45--55 cfm), atomizing air pressure (1.2--2.5 bar), column height (25--40), and product temperature (23--38 °C). Following coating, the pellets were dried for a minimum of 10 minutes.26,28
The fluid bed coating process aimed to fabricate multilayered pellets in which a drug-free SNEDDS layer was shielded from RMP by a protective layer (Figure 2B, Table 1).29,31 Optionally, moisture sealing and anti-adherent layers were applied to safeguard against drug hydrolysis and pellet adherence during storage (Figure 2B, Table 1).32 Consequently, various multilayered pellets were prepared were developed by altering the number and composition of the coating layers.
Formulation of Coating Layers
The formulation for drug-free SNEDDS layer followed the refined protocol for RMP solid lipid self-nanoemulsifying pellets (Single-SNEPs) but without RMP (Table 1).26,29 The optimum coating solution comprised liquid SNEDDS:HPMC E3:Plasacryl T20 at ratios of 40/54.5/5.5, achieving a total concentration of 10%. The protective layer was constructed using HPMC E3 at a concentration of 5%, aiming to balance structural integrity and permeability. Through systematic experimentation, the concentration was fine-tuned to ensure effective protection while facilitating the desired release kinetics.3 For the drug layer, the coating solution was prepared following Sun et al’s (2008) methodology, using HPMC E3 at a (4/1) polymer/drug ratio and a total concentration of 3.5% at pH 8.33
Additional moisture-sealing and anti-adherent layers were also formed to shield RMP from moisture exposure and pellet agglomeration. Kollicoat® Smartseal 30D was chosen to shield RMP from moisture exposure, with a meticulously set total concentration of 15%. This approach aims to increase stability and shelf-life, safeguarding therapeutic efficacy and quality. On the basis of the insights of Kablitz et al (2006),34 the coated pellets were dry mixed with silicon dioxide at 1% w/w to mitigate pellet agglomeration during storage.
In vitro Dissolution Studies of L-SNEDDSs, Single-SNEDDSs and ML-SNEDDSs
These studies were conducted to assess the dissolution performance of optimized L-SNEDDS, Single-SNEPs, and Multi-SNEPs formulations.18,30 The dissolution tests were performed using a USP dissolution apparatus II (Model: UDT-804, LOGAN Inst. Corp., USA) equipped with a paddle stirrer operating at a speed of 50 rpm. The dissolution medium comprised using simulated gastric fluid (500 mL), 0.1M HCL with no enzymes, maintained at 37 ± 0.5 °C. Samples were collected at intervals of 5, 10, 15, 30, 45, and 60 minutes and subsequently filtered using a filter syringe. The filtered samples were then appropriately diluted in solvent and analyzed using a validated UPLC method.19 Dissolution profiles were evaluated in terms of dissolution efficiency (DE%).35
Accelerated Stability Studies
The chemical and physical stabilities of the L-SNEDDS, Single-SNEPs, and Multi-SNEPs were evaluated under accelerated storage conditions. Both the liquid SNEDDS and solid SNEPs (packaged in airtight amber glass vials) were subjected to storage in climatic stability chambers Walk-in Chamber, Thermolab, India) maintained at 40 °C ± 2 °C and a relative humidity (RH) of 75% ± 5%.2,29 Samples were collected after a minimum of 2 and 6 months and allowed to equilibrate to room temperature prior to conducting the required investigations.
The chemical RMP and THQ stabilities were evaluated on the basis of the percentage of intact drug remaining in the formulation. Liquid SNEDDS samples were directly dissolved in acetonitrile and assayed via UPLC.2,19,29 While solid SNEPs were initially crushed via a mill, an aliquot was subsequently dissolved in acetonitrile, sonicated for at least 15 minutes, and assayed via UPLC.19,36 All stability assessments were conducted in triplicate. Additionally, the formulation physical appearance was visually inspected to record any changes in turbidity, color, or pellet agglomeration.
In vivo Study in Rats
Analysis of Ramipril and Ramiprilat in Plasma
Sample Preparation
The plasma samples were processed via the protein precipitation method. Briefly, 100 µL of the plasma sample was combined with 50 µL of the internal standard prednisolone (50 µg/mL) and 3.5 mL of methanol and then vortexed for approximately 20 sec. After centrifugation at 10,000 rpm for 10 min, 350 µL of the supernatant was transferred to an Eppendorf tube and placed under hued for evaporation. The residue was dissolved in 100 µL of methanol and sonicated for 5 minutes to ensure complete drug solubilization. Then, the samples were centrifuged for 10 min, the filtrate was removed and placed into UPLC vial inserts, and 5 µL was injected into the LC‒MS/MS for quantitative analysis.
Chromatographic Conditions
In this study, a UPLC‒MS/MS method (UPLC: Waters Acquity, Milford, MA, USA) was employed to calculate the concentrations of ramipril and ramiprilat in rat plasma. The method involved using a BEH HILIC column (50 mm × 2.1 mm, 1.7 µm) with a mobile phase of acetonitrile and 0.1% formic acid (50:50 v/v) in an isocratic elution running at a flow rate of 0.25 mL/min over a total run time of 2 min. Prednisolone was employed as the internal standard. The eluted analytes were detected and quantified using a tandem mass spectrometer (TQ detector, Waters Corp., Milford, MA) equipped with an electrospray ionization (ESI) source, which was operated in the positive ionization mode. The quantification was performed in the multiple reaction monitoring (MRM) mode.
Pharmacokinetic Study in Rats
Study Design and Animal Handling
The oral bioavailability of the optimized RMP solid SNEPs was compared with that of its liquid counterpart (liquid SNEDDS), the raw ramipril powder and the marketed product Ramipril® Sandoz (2.5 mg). Male SD rats (Sprague–Dawley rats, 180–220 g) were obtained from the Laboratory Animal Center of Pharmacy College, KSU. The rats were randomly allocated to four groups: the liquid formulation (liquid SNEDDS) (group A), the leading RMP solid SNEPs formula (group B), the Ramipril® Sandoz tablet (2.5 mg) (group C) and the pure ramipril powder (Group D). The animals were fasted for 12 h prior to the oral administration of RMP formulations. The study was conducted without any pretreatment of the animals. The experimental procedures were conducted under the approval of the KSU Animal Ethical Committee and in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Reference no#: KSU- SE- 23-04).
In the current study, the “resource equation method” could be an alternative approach to sample size determination. As recommended in the published guidelines, the error degrees of freedom (X) should be maintained within the recommended range of 10 to 20 (Equation 1).37
Where N = total observations count, T = treatments number, B = number of blocks and X = the error degrees of freedom (should be between 10–20).
To maximize statistical power, and in alignment with the above “resource equation method” along with the guideline’s recommendation of a minimum of 5 independent observations per group for datasets undergoing statistical analysis, 5 animals were considered per each treatment group.37
Sampling Procedure
The freshly prepared formulations were dispersed in normal saline (0.9% NaCl) and administered to the animals via oral gavage. Blood samples were collected at the following time points: 0 (prior to dose administration), 15 minutes, 30 minutes, 1, 2, 4, 6, 8, 12, and 24 hours. Approximately 0.3 mL of blood was collected into heparinized tubes at each time point. The blood samples were then centrifuged at 3000 × g for 15 minutes to separate the plasma, which was subsequently stored at −80 °C until analysis. The key pharmacokinetic parameters, including Cmax, Tmax, and AUC, were determined from the plasma concentration data.
Pharmacokinetic Data Analysis
Microsoft Excel was used to calculate the pharmacokinetic parameters from the experiments. The area under the plasma concentration-time curve (AUC) was calculated via the trapezoidal method. The relative bioavailability of the representative SNEDDS to the control was calculated as follows: relative bioavailability % =100 * AUCSNEDDS/AUC control. The maximum plasma concentration (Cmax) and time to maximum concentration (Tmax) after oral administration were determined directly from the concentration versus time curve.
Computational and Statistical Analyses
The dataset was analyzed via Python 3.9.13 in a Jupyter Notebook environment. Normality was assessed via the Shapiro‒Wilk test and homogeneity of variance was evaluated with Levene’s test.38,39
The statistical analyses were conducted via Python libraries, including Scipy.stats, pingouin, and statsmodels. The significance level was set at a p-value less than 0.05. For data with a normal distribution and homogeneous variances, one-way ANOVA with Tukey’s post hoc test was used to compare multiple group means. When the assumption of homogeneity was violated, Welch’s ANOVA was performed, followed by the Games–Howell post hoc test. For nonnormally distributed data, the Kruskal‒Wallis test, followed by Dunn’s post hoc test, was employed to assess differences in medians across groups.
Certain parts of the manuscript text were composed and/or refined with the assistance of a Claude AIbot (Anthropic/Poe). Nonetheless, the authors maintained full accountability for the overall conceptualization and findings.
Results
Fluid Bed Coating of Liquid SNEDDSs into Single-Layer (Single-SNEPs) and Multilayer Self-Nanoemulsifying Pellets (Multi-SNEPs)
Microscopic analysis of single-layer (Single-SNEPs) and multilayer (Multi-SNEPs) self-nanoemulsifying pellets revealed their structural integrity and morphological characteristics. Both Single-SNEPs and Multi-SNEPs exhibited spherical shapes with smooth surfaces, indicative of uniform coating and encapsulation. The pellets displayed well-defined boundaries, suggesting thorough and consistent coating of the liquid SNEDDS within the polymeric matrix. Notably, the pellets exhibited a solidified appearance, indicating successful fluid bed coating and subsequent solidification of the encapsulated SNEDDS.
In vitro Dissolution Studies
RMP Release Findings
The dissolution profiles of all SNEDDS formulations, including L-SNEDDS, Single-SNEPs, and 5L-SNEPs, were not significantly different from pure RMP (p > 0.05). Notably, among these formulations, 5L-SNEPs showed the highest dissolution efficiency percentage (DE%) for RMP. This finding suggests that the multilayered structure of 5L-SNEPs could enhance RMP release, resulting in improved drug dissolution characteristics. A graphical representation of these results can be found in Figure 3A and its corresponding detail, Figure 3A.
THQ Release Findings
The dissolution efficiency (DE) of all SNEDDS formulations, namely, L-SNEDDS, Single-SNEPs, and 5L-SNEPs, was significantly greater (p < 0.05) than that of pure THQ (Figure 4A and its detailed view, Figure 4A). Remarkably, among these formulations, 5L-SNEPs demonstrated the most notable enhancement in THQ DE%, surpassing pure THQ by a four-fold increment in DE%. This substantial increase underscores the efficacy of the multilayered structure of 5L-SNEPs in facilitating the release of THQ, highlighting its potential as a promising formulation for enhanced drug delivery.
Accelerated Stability Study
RMP Chemical Stability Findings
RMP exhibited substantial degradation across all formulations, which was evident in both the 2-month and the 6-month samples (Figure 5). By the 6-month mark, complete degradation of RMP occurred in the L-SNEDDS, Single-SNEPs, and even 3L-SNEPs. In stark contrast, 4L-SNEPs and 5L-SNEPs significantly enhanced RMP stability, with improvements of 30% and 37%, respectively, compared with the other formulations. Notably, silicon dioxide incorporation into the Multi-SNEPs demonstrated a beneficial stabilization effect on RMP within the formulation, further enhancing its stability. These findings underscore the potential of the moisture sealing layer and anti-adherent layer in improving the stability of RMP-containing formulations upon storage.
While data was not collected at the 4-month time point, the degradation pattern observed between the 2-month and 6-month time points for the 5L-SNEP formulation suggests a consistent reduction of around 20% of intact RMP every two months under the accelerated storage conditions for this particular formulation. The observed trend in the stability data lends support to the hypothesis of a potential linear reduction in RMP stability, within 5L-SNEPs, over time.
THQ Chemical Stability Findings
Compared with RMP, THQ had a better stability profile in all formulations (Figure 6). In contrast to RMP, liquid SNEDDS maintained the highest intact THQ, with a significant (p < 0.05) difference from most of the other formulations. By the end of the study, the L-SNEDDS maintained 70% intact THQ within the formulation.
Physical Stability Findings
By the study end, the physical appearance of liquid SNEDDS had not changed (Table S1, Figure 7A). Single-SNEPs showed significant pellet agglomeration and adherence to the container wall, as shown in Figure 7B. Neither RMP-free Single-SNEPs nor 3L-SNEPs significantly changed the pellet appearance (Figure 7C and D). Interestingly, 4L-SNEPs showed significant pellet agglomeration and adherence to the container wall, which was reversibly deagglomerated by the spatula (Figure 7E). In contrast, 5L-SNEPs maintained good pellet flowability until the end of the study (Figure 7F). These findings reveal the beneficial effect of the addition of silicone dioxide as an anti-adherent layer on top of the pellets.
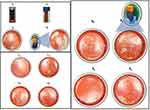 |
Figure 7 Physical appearance of (A0, A6) L-SNEDDS, (B0, B6) RMP-loaded Single-SNEPs, (C, C0) RMP-free Single-SNEPs, (D0, D6) 3L-SNEPs, (E0, E6) 4L-SNEPs, and (F0, F6) 5L-SNEPs at 0 and 6 months. |
In vivo Study in Rats
Analysis of Ramipril and Ramiprilat in Plasma
Method Performance and Assay Validation
The optimum ionization parameters were as follows: Ramipril: ionization pairs (m/z) 417.09→234.4 (cone voltage 40 V, collision energy 20 V) (Figure 8A); Ramiprilat: 389.25→206.06 (cone voltage 34 V, collision energy 52 V) (Figure 8B); and prednisolone: 403.09→220.35 (cone voltage 42 V, collision energy 13 V) (Figure 8C). Several combinations of acetonitrile and 0.1% formic acid were evaluated as possible mobile phases. The combination of acetonitrile and 0.1% formic acid in an isocratic elution program (50:50 v/v) was found to be the most suitable for resolution of the peaks of ramipril, ramiprilat and the internal standard prednisolone. Under the described chromatographic conditions, the retention times were approximately 0.61, 0.57 and 0.56 min for ramipril, ramiprilat and prednisolone, respectively (Figure S1).
 |
Figure 8 Ionization pairs (m/z) of (A) ramipril, (B) ramiprilat, and (C) prednisolone. |
Good linearity (r2>0.999) was observed for ramipril over the range of 10–500 ng/mL and for ramiprilat over the range of 20–1000 ng/mL in an aliquot amount as low as 50 µL of rat plasma, which could be described by the following regression equations: Y = 0.0571x + 0.4815 (Ramipril, Figure S2), and Y = 0.0052x + 0.9755 (ramiprilat, Figure S2), in which Y was the peak area ratio of the drug to the internal standard, and X was the analyte concentration in ng/mL in the plasma.
Pharmacokinetic Study in Rats
The pharmacokinetic study involved four formulations: liquid SNEDDS, multilayer SNEPs (Multi-SNEPs), marketed RMP tablets, and pure RMP. The AUC (ng/h/mL) reflects the total exposure, Cmax (ng/mL) is the peak plasma concentration, and Tmax (h) is the time to reach Cmax. The investigation into the pharmacokinetics of liquid SNEDDSs [BSO/TCP/HCO-30] has yielded substantial enhancement of drug bioavailability, as illustrated in Table 2 and Figure 9.
 |
Table 2 Comparative Pharmacokinetics of Ramiprilat After the Oral Administration of Solid SNEDDS Pellets, Liquid SNEDDSs, and Marketed Capsules in Rats |
 |
Figure 9 Pharmacokinetic study of ramiprilat in rat plasma. The data are shown as the means ± SDs; n = 4–5. |
In the context of raw RMP powder administered orally, the maximum concentration (Cmax) was measured at 90 ng/mL, with a corresponding time to reach the maximum concentration (Tmax) of 0.5 hours. In contrast, when representative Liquid SNEDDSs of RMP were orally administered, the Cmax substantially improved to 105.5 ng/mL (p > 0.05), and the Tmax was 0.8 hours (p > 0.05). On the other hand, Multi-SNEPs showed a substantial decrease in the C max to 59.5 ng/mL, and the t max was 0.6 hours.
Moreover, the area under the concentration-time curve from time zero to the last measurable concentration (AUC0–t) of RMP was notably greater in the liquid SNEDDS group than in the RMP powder-treated group (147%, P > 0.05). Specifically, the AUC0–t increased from 307.6 ± 212.7 ng h/mL to 454.2 ± 264.9 ng h/mL. This increase in relative bioavailability for RMP was also consistent with that of the RMP-marketed tablet Sandoz. In contrast, compared with pure RMP, Multi-SNEPs substantially decreased the AUC to 155 ng/mL, which was an approximately 50% reduction in the AUC (p > 0.05).
The observed overlapping ± values reported for pharmacokinetic parameters could be attributed to inherent biological variability between individual subjects, small sample sizes, and/or potential heterogeneity within the study population. This variability may have impacted the clarity of the statistical conclusions and the ability to reliably detect differences between the formulations.
Discussion
The development of stable and bioavailable RMP formulations, a widely prescribed ACE inhibitor, is of significant clinical importance in hypertension and congestive heart failure management. The inherent chemical instability of ramipril, which can lead to diminished therapeutic efficacy and safety, emphasizes the need for innovative formulation strategies to protect the drug until it is absorbed and converted into the body.40
The current study embarked on a comprehensive evaluation of liquid and solid self-nanoemulsifying drug delivery systems (SNEDDSs) for ramipril, incorporating cardioprotective black seed oil (BSO) as the oil phase. Researchers have utilized fluid bed coating techniques to transform liquid SNEDDSs into both single-layer (Single-SNEPs) and multilayer (Multi-SNEPs) self-nanoemulsifying pellets, with a focus on assessing formulation chemical stability and pharmacokinetic profiles.
The fluid bed coating process successfully transformed the liquid SNEDDSs into both Single-SNEPs and Multi-SNEPs, as evidenced by the spherical shape and acceptable morphological characteristics of the pellets. The solidified appearance of the pellets suggested that the liquid SNEDDS was effectively transformed into a solidified state through the fluid bed coating technique.
The in vitro dissolution studies provided a valued understanding of the drug release profiles of the different SNEDDS formulations. Compared with those of pure RMP, the dissolution profiles of the liquid SNEDDS, Single-SNEPs, and multilayered 5L-SNEPs were not significantly different. Notably, 5L-SNEPs exhibited the highest dissolution efficiency percentage (DE%) for RMP, suggesting that the multilayered structure could increase RMP release and improve its dissolution characteristics.
In the case of THQ, all SNEDDS formulations (L-SNEDDS, Single-SNEPs, and 5L-SNEPs) demonstrated statistically significant improvements in dissolution efficiency (DE%) compared with that of pure THQ. Remarkably, 5L-SNEPs showed the most substantial increase in THQ DE%, surpassing pure THQ by a four-fold increase. This significant increase in THQ release underscores the effectiveness of the multilayered structure of 5L-SNEPs in enhancing the release of this bioactive compound.
The stability study provided crucial insights into the formulation chemical stability. RMP exhibited substantial degradation across all formulations, with complete degradation observed in L-SNEDDS, Single-SNEP, and even 3L-SNEPs groups by the 6-month mark. The depletion of RMP in these formulations is very crucial, as it imparts a 100% loss of medication efficacy and raises significant safety concerns with respect to the formed degradation products.
For the L-SNEDDS, the RMP degradation was likely driven by a combination of amide hydrolysis, alkyl ester hydrolysis, and lactamization of the amino acid or derivative, as suggested by the literature.19 In the case of the Single-SNEP formulation, a combination of the above-mentioned mechanisms, along with hydrolysis facilitated by atmospheric humidity, may have contributed to the complete degradation of RMP observed by the 6-month time point. For the 3L-SNEP formulation, the isolation between the RMP layer and the SNEDDS layer may have diminished the likelihood of SNEDDS-related degradation mechanisms. However, the lack of sufficient protection against hydrolysis by atmospheric moisture appears to be the primary driver of the rapid depletion of RMP in this formulation.
In contrast, compared with the other formulations, 4L-SNEPs and 5L-SNEPs significantly increased RMP stability, with improvements of 30% and 37%, respectively. The incorporation of moisture-sealing and silicon dioxide layers into the Multi-SNEPs formulations was found to have a beneficial stabilization effect on RMP, further enhancing its stability under accelerated storage conditions.
In the case of THQ, the compound exhibited better stability than RMP across all formulations. Notably, the liquid SNEDDS maintained the highest intact THQ, with a significant difference from most other formulations. By the end of the study, the L-SNEDDS retained 70% of the intact THQ within the formulation.
The physical stability assessment revealed that 5L-SNEPs maintained good pellet flowability until the end of the study, indicating the beneficial effect of the silicon dioxide anti-adherent layer added on top of the pellets.
These data demonstrate that by carefully selecting such protective polymers, the degradation of sensitive drugs such as RMP can be further minimized. Most specifically, the use of multifunctional taste masking and moisture-protective polymers, such as Kollicoat Smartseal, further enhanced the performance of the coated pellets. By fine-tuning the processing parameters outlined above, one can achieve the desired coating levels with maximum drug loading and improved stability of highly moisture-sensitive drugs such as RMP. Taken together, the results of this study open avenues for several other drugs that are insoluble, sensitive to moisture, and highly bitter. Using innovative polymers such as Kollicoat Smartseal can help increase API stability by acting as a moisture barrier to prevent hydrolysis or degradation.
The pharmacokinetic study in rats provided important insights into the in vivo performance of the different ramipril (RMP) formulations. While the liquid SNEDDS group presented a numerically greater Cmax (105.5 ng/mL) and AUC (454.2 ng·h/mL) with a 1.5-fold increase in the AUC compared with the raw RMP powder group (AUC: 307.6 ng·h/mL), these differences were not statistically significant. Interestingly, the multilayered 5L-SNEPs formulation, which demonstrated the highest in vitro dissolution efficiency for RMP, did not show greater in vivo bioavailability than the raw RMP powder. In fact, 5L-SNEPs presented a lower Cmax (59.5 ng/mL) and AUC (155.1 ng·h/mL) than RMP powder did. However, statistical analysis revealed no significant differences between any of the formulations for the parameters AUC, Cmax and Tmax. The high variability in the data, as evident from the large standard deviation values, could have contributed to the lack of significant differences between the formulations. Therefore, while numerically, the liquid SNEDDS showed greater exposure, the high intersubject variability masked the statistical significance of this difference compared with the other groups.
Some factors can be suggested to anticipate the discrepancy between the superior in vitro dissolution but lower in vivo exposure of Multi-SNEPs than that of the pure drug:
- Digestion and Absorption: In vitro tests lack the complex digestive dynamics of the gastrointestinal tract. Factors such as emulsification, the digestion of excipients, the precipitation of drugs from nanoemulsions, and interactions with intestinal transporters influence in vivo absorption but are not reflected in simple dissolution tests.
- Excipient Effects: Certain excipients in Multi-SNEPs may interact negatively with intestinal transporters and reduce permeation, an effect not observed in vitro.
Accordingly, further optimization is needed to bridge the gap between the enhanced in vitro characteristics and the in vivo performance of the multi-SNEPs. This includes:
- Investigation of the impact of changing the polymers used for SNEDDS solidification, drug layering, moisture-sealing, and/or anti-adherent layers on the in vivo performance of Multi-SNEPs. This can help identify the most suitable excipients to improve the in vivo absorption and pharmacokinetic behavior of RMP.
- Evaluation of the effect of changing the order and number of coating layers in Multi-SNEPs on the in vivo pharmacokinetic profile. This can provide more insights into the optimal multilayer structure that can enhance RMP bioavailability.
- Inclusion of drug-loaded single SNEPs in the in vivo study to compare its in-vivo performance against 5L-SNEPs. This can help elucidate whether the isolation of between RMP and SNEDDS layer in Multi-SNEP poses any challenges in vivo that may inhibit the drug from migrating into the formed nanoemulsion droplets.
The observed enhancement in the bioavailability of RMP from the liquid SNEDDS could be attributed to the improved solubility and dissolution profiles of the drug. The liquid SNEDDS could have facilitated better uptake of the nanoemulsion by enterocytes at the absorption site, as proposed by Elgart, Cherniakov et al (2013).41 This finding aligns with previous reports supporting the notion of improved bioavailability through enhanced solubility and absorption, as documented by previous publications.42–44
In this study, the AUC ratio of the liquid SNEDDS to pure RMP was 1.47, suggesting that the bioavailability of the liquid SNEDDS was approximately 47% greater than that of the pure drug, although the difference was not statistically significant. Overall, the lack of significant improvement in exposure from SNEDDS formulations could be attributed to the high variability, complex in vivo performance of lipid-based systems and/or dependence on the digestion process. The pharmacokinetic results highlight the need to evaluate the in vivo performance of solidified SNEDDS formulations carefully, as the additional processing steps and coating layers may not always translate to improved bioavailability, despite the enhanced in vitro characteristics.
Limitations of the Study
While this investigation provided valuable insights into the development and performance of liquid and solidified SNEDDS formulations for ramipril, several limitations should be acknowledged:
- The high variability observed in the pharmacokinetic data may have masked potentially significant differences between the formulations. Increasing the sample size or exploring alternative animal models could help elucidate the in vivo performance more robustly.
- The study focused on the oral bioavailability of ramipril but did not evaluate the pharmacodynamic effects or clinical outcomes associated with the different SNEDDS formulations. Incorporating such assessments, particularly related to the potential blood pressure reduction effects of the incorporated bioactive black seed oil, could provide a more comprehensive understanding of the therapeutic implications of the developed delivery systems.
The results of this comprehensive investigation highlight the need for further optimization and evaluation of solidified SNEDDS formulations to fully harness their potential for improving the oral bioavailability of poorly water-soluble drugs. Future studies could involve the following:
- Conducting in vitro lipolysis studies to better understand the behavior of the SNEDDS formulations under simulated gastrointestinal conditions, including the effects of digestive enzymes and surfactants on drug release and absorption mechanisms.
- Performing pharmacokinetic evaluations in higher-order animal species to validate the findings and potentially uncover any species-specific differences in the in vivo performance of the SNEDDS formulations.
- The interaction of the formulation components with intestinal transporters and their impact on drug permeation should be investigated to elucidate the reasons for the discrepancy between the in vitro and in vivo results.
- The use of alternative moisture-protective polymers, such as Kollicoat Protect, to further enhance the chemical stability of sensitive drugs such as ramipril within solidified SNEDDSs.
Overall, this study marks a significant milestone in the pursuit of enhanced drug delivery strategies for poorly water-soluble drugs. While liquid SNEDDSs have demonstrated remarkable promise in enhancing oral bioavailability, the transition to multilayer solid SNEDDSs poses challenges that demand careful refinement. Nonetheless, this comprehensive investigation lays a robust foundation for continued research and development of multifunctional bioactive oil-based SNEDDSs, promising to revolutionize poorly water-soluble drug delivery and improve patient outcomes.
Conclusion
This comprehensive investigation represents a significant stride in the development of innovative delivery strategies for poorly water-soluble drugs such as ramipril. The fluid bed coating technique successfully transformed liquid SNEDDS formulations into both single-layer (Single-SNEPs) and multilayer (Multi-SNEPs) self-nanoemulsifying pellets, as evidenced by the acceptable morphological characteristics of the resulting pellets.
The in vitro dissolution studies demonstrated that the multilayered 5L-SNEPs exhibited the highest dissolution efficiency percentage (DE%) for both ramipril and the incorporated bioactive compound, thymoquinone. This finding suggests that the multilayered structure can effectively improve the release and dissolution of these active ingredients.
The accelerated stability study revealed a critical difference in the chemical stability of ramipril across the various formulations. While the L-SNEDDS, Single-SNEP, and 3L-SNEP groups exhibited complete degradation of ramipril by the 6-month timepoint, the 4L-SNEPs and 5L-SNEPs formulations significantly improved ramipril stability, with 30% and 37% intact ramipril remaining, respectively. This enhanced stability achieved with the multilayered SNEP formulations can be attributed to the incorporation of moisture-sealing and silicon dioxide layers, which effectively shielded the drug from degradation mechanisms such as hydrolysis.
In contrast to the improved in vitro performance, the pharmacokinetic study in rats did not demonstrate a significant enhancement in the oral bioavailability of ramipril from the solidified SNEDDS formulations compared to the raw drug powder. This discrepancy between the in vitro and in vivo results highlights the need for further optimization to bridge the gap between the enhanced physicochemical characteristics and the in vivo performance of the solidified SNEDDS.
Potential strategies to address the bioavailability gap in solidified SNEDDSs may include investigating the impact of alternative polymers, coating layers, and their order on in vivo performance. Additionally, a direct comparison of drug-loaded single SNEPs and multi-SNEPs in vivo could provide insights into the potential limitations of physical separation between the drug and the SNEDDS layer. These targeted investigations can help identify the optimal multilayer structure and formulation composition to maximize the in vivo performance of solidified SNEDDS.
Institutional Review Board Statement
The animal study protocol was approved by the KSU Animal Ethical Committee and in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Reference no#: KSU- SE- 23-04).
Acknowledgments
This work was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (2-17-03-001-0048).
Funding
This research is funded by the National Plan for Science, Technology, and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (2-17-03-001-0048). The APC is funded by the National Plan for Science, Technology, and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (2-17-03-001-0048).
Disclosure
Dr Ahmad Shahba has a patent “Multi-layer self-nanoemulsifying pellets for dual enhancement of drug solubility and stability, Saudi Patent Office, Patent No# 6357” issued. The authors declare that they have no other conflicts of interest in this work.
References
1. Desmoulins PO, Burgaud S, Horspool LJ. Pharmacokinetics and pharmacodynamics of ramipril and ramiprilat in healthy cats. J Vet Pharmacol Ther. 2008;31(4):349–358. doi:10.1111/j.1365-2885.2008.00959.x
2. Shahba AA, Alanazi FK, Mohsin K, Abdel-Hamid M. Stability assessment of cinnarizine in self-emulsifying drug delivery systems. Latin Am J Pharm. 2012;31.
3. De Diego M, Godoy G, Mennickent S, Olivares M, Godoy R. Stress degradation studies of ramipril by a validated stability-indicating liquid chromatographic method. J Chilean Chem Soc. 2010;55(4):450–453. doi:10.4067/S0717-97072010000400008
4. Krstić M, Medarević Đ, Đuriš J, Ibrić S. Self-nanoemulsifying drug delivery systems (SNEDDS) and self-microemulsifying drug delivery systems (SMEDDS) as lipid nanocarriers for improving dissolution rate and bioavailability of poorly soluble drugs. Lipid Nanocarriers for Drug Targeting, Elsevier; 2018.
5. Betageri GV. Self-emulsifying drug delivery systems and their marketed products: a review. Asian J Pharma. 2019;13.
6. Morakul B. Self-nanoemulsifying drug delivery systems (SNEDDS): an advancement technology for oral drug delivery. Pharm Sci Asia. 2020;47(3):205–220. doi:10.29090/psa.2020.03.019.0121
7. Gupta R, Jain V, Nagar JC, et al. Bioavailability enhancement techniques for poorly soluble drugs: a review. Asian J Pharm Res Dev. 2020;8(2):75–78. doi:10.22270/ajprd.v8i2.664
8. Alhasani KF, Kazi M, Ibrahim MA, Shahba AA, Alanazi FK. Self-nanoemulsifying ramipril tablets: a novel delivery system for the enhancement of drug dissolution and stability. Int J Nanomed. 2019;14:5435–5448. doi:10.2147/IJN.S203311
9. Buya AB, Beloqui A, Memvanga PB, Préat V. Self-nano-emulsifying drug-delivery systems: from the development to the current applications and challenges in oral drug delivery. Pharmaceutics. 2020;12(12):1194. doi:10.3390/pharmaceutics12121194
10. Ibrahim T, El-Megraba N, Abdallaha M. Self-emulsifying drug delivery formulations. Zagazig J Pharm Sci. 2018;27(1):1–21. doi:10.21608/zjps.2018.38102
11. Madhavi K, Shikha A, Yadav JK. Self-Nano emulsifying drug delivery system of ramipril: formulation and in vitro evaluation. Int J Pharm Pharm Sci. 2016;8:291–296.
12. Kasturi M, Agrawal S, Janga KY. Development and characterization of ramipril loaded solid self nanoemulsifying drug delivery system (SNEDDS) for improved oral delivery of lipophilic drugs. Int J Pharm Biol Sci Arch. 2015;6:10–17.
13. Shafiq S, Shakeel F. Stability and self-nanoemulsification efficiency of ramipril nanoemulsion containing labrasol and plurol oleique. Clin Res Regul Affairs. 2010;27:12–17.
14. Shafiq S, Shakeel F, Talegaonkar S, Khar RK, Ali M. Nanoemulsion as carrier for stability enhancement of ramipril. J Dispers Sci Technol. 2010;31:975–979.
15. Derosa G, D’Angelo A, Maffioli P, Cucinella L, Nappi RE. The use of nigella sativa in cardiometabolic diseases. Biomedicines. 2024;12(2):405. doi:10.3390/biomedicines12020405
16. Hannan MA, Rahman MA, Sohag AAM, et al. Black cumin (nigella sativa L.): a comprehensive review on phytochemistry, health benefits, molecular pharmacology, and safety. Nutrients. 2021;13. doi:10.3390/nu13061784
17. Yimer EM, Tuem KB, Karim A, Ur-Rehman N, Anwar FNSL. (Black cumin): a promising natural remedy for wide range of illnesses. Evid Based Comple Alternat Med. 2019;2019:1528635. doi:10.1155/2019/1528635
18. Shahba AA, Sherif AY, Elzayat EM, Kazi M. Combined ramipril and black seed oil dosage forms using bioactive self-nanoemulsifying drug delivery systems (Bio-SNEDDSs). Pharmaceuticals. 2022;15(9):1120. doi:10.3390/ph15091120
19. Elzayat EM, Sherif AY, Shahba AA-W, Kazi M, Alyahya M, Darwish HW. Development and validation of a stability indicating UPLC-DAD method coupled with MS-TQD for ramipril and thymoquinone in bioactive SNEDDS with in silico toxicity analysis of ramipril degradation products. Open Chem. 2024;22:20240070.
20. Shetty SK, Surendranath KV, Radhakrishnanand P, et al. stress degradation behavior of a polypill and development of stability indicating UHPLC method for the simultaneous estimation of aspirin, atorvastatin, ramipril and metoprolol succinate. Am J Anal Chem. 2011;2:401–410.
21. Mukherjee R, Patil S, Shetgiri N, Bhure S, Anwwari A, Rakhe A. Synthesis and structural elucidation of impurities in ramipril tablets. 2008.
22. Kazi M, Shahba AA, Alrashoud S, Alwadei M, Sherif AY, Alanazi FK. Bioactive self-nanoemulsifying drug delivery systems (Bio-SNEDDS) for combined oral delivery of curcumin and piperine. Molecules. 2020;25. doi:10.3390/molecules25071703.
23. Alkhatib H, Mawazi SM, Al-Mahmood SMA, Zaiter A, Doolaanea A. Thymoquinone content in marketed black seed oil in Malaysia. J Pharm Bioallied Sci. 2020;12(3):284–288. doi:10.4103/jpbs.JPBS_208_20
24. Ahmad N, Ahmad R, Al-Layly A, et al. Ultra-high-performance liquid chromatography-based identification and quantification of thymoquinone in Nigella sativa extract from different geographical regions. Pharmacogn Mag. 2018;14(57):471. doi:10.4103/pm.pm_119_18
25. Alshadidi A, Shahba AA, Sales I, Rashid MA, Kazi M. Combined curcumin and lansoprazole-loaded bioactive solid self-nanoemulsifying drug delivery systems (Bio-SSNEDDS). Pharmaceutics. 2021;14(1):2. doi:10.3390/pharmaceutics14010002
26. Shahba AA, Ahmed AR, Mohsin K, Abdel-Rahman SI, Alanazi FK. Solidification of cinnarizine self-nanoemulsifying drug delivery systems by fluid bed coating: optimization of the process and formulation variables. Pharmazie. 2017;72(3):143–151. doi:10.1691/ph.2017.6089
27. Lei Y, Lu Y, Qi J, et al. Solid self-nanoemulsifying cyclosporin A pellets prepared by fluid-bed coating: preparation, characterization and in vitro redispersibility. Int J Nanomed. 2011;6:795–805. doi:10.2147/ijn.S17711
28. Mukherjee T, Plakogiannis FM. Effects of process parameters on solid self-microemulsifying particles in a laboratory scale fluid bed. Pharm DevelopTechnol. 2012;17(4):511–520. doi:10.3109/10837450.2010.550625
29. Shahba AA-W, Alanazi FK, Abdel-Rahman SI. Stabilization benefits of single and multi-layer self-nanoemulsifying pellets: a poorly-water soluble model drug with hydrolytic susceptibility. PLoS One. 2018;13(7):e0198469. doi:10.1371/journal.pone.0198469
30. Shahba AA-W, Ahmed AR, Alanazi FK, Mohsin K, Abdel-Rahman SI. Multi-layer self-nanoemulsifying pellets: an innovative drug delivery system for the poorly water-soluble drug cinnarizine. AAPS Pharm Sci Tech. 2018;19(5):2087–2102. doi:10.1208/s12249-018-0990-7
31. Ahmed AR, Mota JP, Shahba AA-W, Irfan M. Chapter 3 - Aqueous polymeric coatings: new opportunities in drug delivery systems. In: Shegokar R, editor. Drug Delivery Aspects. Elsevier; 2020:33–56.
32. Shi S, Chen H, Cui Y, Tang X. Formulation, stability and degradation kinetics of intravenous cinnarizine lipid emulsion. Int J Pharm. 2009;373(1–2):147–155. doi:10.1016/j.ijpharm.2009.02.006
33. Sun N, Wei X, Wu B, Chen J, Lu Y, Wu W. Enhanced dissolution of silymarin/polyvinylpyrrolidone solid dispersion pellets prepared by a one-step fluid-bed coating technique. Powder Technol. 2008;182:72–80. doi:10.1016/j.powtec.2007.05.029
34. Kablitz CD, Harder K, Urbanetz NA, et al. Dry coating in a rotary fluid bed. Eur J Pharm Sci. 2006;27(2–3):212–219. doi:10.1016/j.ejps.2005.10.001
35. El Maghraby GM, Elzayat EM, Alanazi FK. Development of modified in situ gelling oral liquid sustained release formulation of dextromethorphan. Drug Dev Ind Pharm. 2012;38(8):971–978. doi:10.3109/03639045.2011.634811
36. Kayaert P, Anne M, Van den Mooter G, et al. Bead layering as a process to stabilize nanosuspensions: influence of drug hydrophobicity on nanocrystal reagglomeration following in-vitro release from sugar beads. J Pharm Pharmacol. 2011;63(11):1446–1453. doi:10.1111/j.2042-7158.2011.01351.x
37. Panos GD, Boeckler FMJDD, et al. Development therapy statistical analysis in clinical and experimental medical research: simplified guidance for authors and reviewers. Drug Design Develop Therapy. 2023;1959–1961.
38. Mishra P, Pandey CM, Singh U, Gupta A, Sahu C, Keshri A. Descriptive statistics and normality tests for statistical data. Ann Card Anaesth. 2019;22(1):67–72. doi:10.4103/aca.ACA_157_18
39. Sherif AY, Shahba AA-W. Development of a multifunctional oral dosage form via integration of solid dispersion technology with a black seed oil-based self-nanoemulsifying drug delivery system. Biomedicines. 2023;11(10):2733. doi:10.3390/biomedicines11102733
40. Regulska K, Musiał J, Stanisz BJ. Solid-state stability profiling of ramipril to optimize its quality efficiency and safety. Pharmaceutics. 2021;13(10):1600. doi:10.3390/pharmaceutics13101600
41. Elgart A, Cherniakov I, Aldouby Y, Domb AJ, Hoffman A. Improved oral bioavailability of BCS class 2 compounds by self nano-emulsifying drug delivery systems (SNEDDS): the underlying mechanisms for amiodarone and talinolol. Pharm Res. 2013;30(12):3029–3044. doi:10.1007/s11095-013-1063-y
42. Zhang J, Li J, Ju Y, Fu Y, Gong T, Zhang Z. Mechanism of enhanced oral absorption of morin by phospholipid complex based self-nanoemulsifying drug delivery system. Mol Pharmaceut. 2015;12(2):504–513. doi:10.1021/mp5005806
43. Mohsin K, Alamri R, Ahmad A, Raish M, Alanazi FK, Hussain MD. Development of self-nanoemulsifying drug delivery systems for the enhancement of solubility and oral bioavailability of fenofibrate, a poorly water-soluble drug. Int J Nanomed. 2016;11:2829–2838. doi:10.2147/IJN.S104187
44. Zhang XW, Chen GJ, Zhang TP, Ma ZG, Wu BJ. Effects of PEGylated lipid nanoparticles on the oral absorption of one BCS II drug: a mechanistic investigation. Int J Nanomed. 2014;9:5503–5514. doi:10.2147/IJN.S73340
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.