Back to Journals » Therapeutics and Clinical Risk Management » Volume 21
Lessons Learned From Awake ECMO Approach in Covid-19-Related Acute Respiratory Distress Syndrome - a Scoping Review
Authors Sklienka P , Burša F, Frelich M, Máca J , Romanová T , Vodička V, Straková H, Bílená M, Jor O, Neiser J, Tomášková H
Received 18 November 2024
Accepted for publication 14 April 2025
Published 13 May 2025 Volume 2025:21 Pages 655—668
DOI https://doi.org/10.2147/TCRM.S507120
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Peter Sklienka,1– 3 Filip Burša,1– 3 Michal Frelich,1,2 Jan Máca,1– 3 Tereza Romanová,1– 3 Vojtěch Vodička,1 Hana Straková,1,2 Markéta Bílená,1,2 Ondřej Jor,1,2 Jan Neiser,1,2 Hana Tomášková4
1Department of Anesthesiology and Intensive Care Medicine, University Hospital Ostrava, Ostrava, Czech Republic; 2Department of Anesthesiology and Intensive Care Medicine, Faculty of Medicine, University of Ostrava, Ostrava, Czech Republic; 3Institute of Physiology and Pathophysiology, Faculty of Medicine, University of Ostrava, Ostrava, Czech Republic; 4Department of Epidemiology and Public Health, Faculty of Medicine, University of Ostrava, Ostrava, Czech Republic
Correspondence: Peter Sklienka, Department of Anesthesiology and Intensive Care Medicine, University Hospital of Ostrava, 17.listopadu 1790, Ostrava, 70800, Czech Republic, Tel +420 59 737 2702, Email [email protected]
Abstract: During the COVID-19 pandemic, specific COVID-19-related conditions renewed interest in the full-awake venovenous extracorporeal membrane oxygenation (faV–V ECMO) approach, in which ECMO is applied to awake, cooperative, and non-intubated patients. This scoping review aims to provide a descriptive overview of faV–V ECMO in patients with COVID-19-related acute respiratory distress syndrome (CARDS). We searched the PubMed, Web of Science, and Scopus databases using the keywords “awake ECMO” or “spontaneous breathing AND ECMO”, combined with “COVID-19”, “SARS-CoV-2” or “coronavirus”, utilizing the Boolean operator “AND”. The search included papers published from November 1, 2019, to December 31, 2024. Sixty-four papers were assessed for eligibility at the abstract level, and fourteen articles (seven small-sample cohort studies and seven case reports) comprising 95 patients were included in the final analysis. The most frequent reasons for preferring faV-V ECMO over mechanical ventilation were barotrauma and patient refusal of intubation and mechanical ventilation. The faV-V ECMO strategy was successful (ie, patients not intubated, disconnected from ECMO, and discharged from the hospital) in 36.4% of cases (cohort studies only). The incidence of defined severe adverse events (bleeding, thrombosis, cannula malposition, delirium, and progression of barotrauma) was considered low. The mortality rate for CARDS patients treated with faV-V ECMO (including only patients from cohort studies) reached 33.0%, notably lower than the 48% reported for CARDS patients treated with V-V ECMO in the ELSO registry. Patients who were intubated due to worsening respiratory failure during faV-V ECMO had significantly higher mortality. Infectious complications, sepsis, and multiorgan failure were the most frequent causes of death. However, significant heterogeneity in the definitions and reporting of management, ECMO-related complications, and outcomes was observed across the papers. Despite the heterogeneity of the data, faV-V ECMO in CARDS patients can be considered a safe approach associated with a lower mortality rate than that reported in the overall V-V ECMO CARDS population.
Keywords: awake venovenous extracorporeal membrane oxygenation, COVID-19-related acute respiratory distress syndrome, refusal of intubation, barotrauma, bleeding
Introduction
The COVID-19 pandemic brought about a remarkable increase in extracorporeal membrane oxygenation (ECMO) utilization. To date (March 15, 2025), 17,669 COVID-19 patients treated with veno-venous (V–V) ECMO have been reported in the Extracorporeal Life Support Organization (ELSO) Registry, with a mortality rate for these patients reaching 48%.1
Typically, the ECMO procedure for acute respiratory distress syndrome (ARDS) is initiated in patients who remain severely hypoxemic or hypercapnic despite being intubated and treated with invasive mechanical ventilation. During the COVID-19 pandemic, intensivists faced challenges associated with COVID-19-related conditions, which contributed to renewed interest in the full-awake ECMO (faV–V ECMO) approach, where ECMO is applied to awake, cooperative, non-intubated, and spontaneously breathing patients. First, a relatively high number of patients with severe COVID-19-related ARDS (CARDS) refused intubation and mechanical ventilation due to concerns stemming from misinformation spread by media, social networks, and even some healthcare professionals.2,3 Second, a significant number of patients presented with a condition termed “silent hypoxemia” (SH), characterized by severe hypoxemia without any subjective perception of dyspnea. Silent hypoxemia was observed in a range of 4.9% to 31.9% of all hypoxemic patients with COVID-19-related pneumonia who had severely abnormal initial chest X-ray or computed tomography scans, and the mortality rate in patients with SH was reported in the range of 17.6% to 25.9%.4,5 Although the hypoxemia-induced stimulation of the respiratory center in COVID-19-related silent hypoxemia is suppressed, inflammatory and mechanical signals from injured lungs may still provoke excessive respiratory drive, exposing the injured lung to the risk of further damage known as patient self-inflicted lung injury (P-SILI).6–8 The most severe cases of P-SILI are characterized by the disruption of lung parenchyma, initially leading to subtle collections of air contiguous to the bronchovascular sheath on chest CT scans (often referred to as Macklin lines), which precede the development of clinically apparent barotrauma (including pneumothorax, pneumomediastinum, pneumopericardium, and subcutaneous emphysema).9,10 Studies evaluating the incidence of spontaneous barotrauma in CARDS patients have reported that a significant portion of patients developed barotrauma before the initiation of any form of invasive ventilation, suggesting the link between excessive respiratory effort during silent hypoxemia and P-SILI-induced barotrauma.11,12 The application of intermittent positive pressure mechanical ventilation (IPPV), even in lung-protective invasive or noninvasive modes (NIV), further increases the risk of barotrauma.13,14 The faV–V ECMO approach allows to respect the patient´s preference not to be intubated. It combines multiple benefits from adequate gas exchange and preserved spontaneous ventilation while minimizing the risks associated with intermittent positive ventilation (Table 1).
 |
Table 1 Advantages and Disadvantages of Awake ECMO Approach |
We present a scoping review of the literature on the faV–V ECMO approach in CARDS patients. We aim to provide a descriptive overview of the current faV–V ECMO management, complications, and outcomes.
Materials and Methods
The search adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement.16 We searched the PubMed, Web of Science, and Scopus databases using the keywords “awake extracorporeal membrane oxygenation” or “spontaneous breathing AND extracorporeal membrane oxygenation”, combined in a search string with the Boolean operator “AND” with “COVID-19”, “SARS-CoV-2” or “coronavirus”. The search included papers published from November 1, 2019 (COVID-19 outbreak) to December 31, 2024. Related articles from the retrieved citations and reference lists of the full texts were also assessed for further relevant studies without any language restrictions. We excluded reviews, editorials, letters to the editor, meeting abstracts, and studies that described awake ECMO in patients who were mechanically ventilated at the time of ECMO initiation and extubated later during the ECMO run. Studies on pediatric patients were also excluded. No language restrictions were imposed.
Three reviewers (O.J., T.R., and V.V.) independently searched and selected relevant studies. Full texts of the relevant articles were assessed for inclusion criteria by two authors (M.F. and F.B.). Eligible articles were further evaluated for potential biases and duplications by two co-authors (M.B. and H.S.). Any disagreements regarding the inclusion of articles were resolved by consensus, led by the main author (P.S.).
Evaluated Parameters and Definitions
Several aspects contributing to faV–V ECMO management and safety were evaluated:
- Specific parameters and indications for the faV-V ECMO approach instead of the „mechanical ventilation first” strategy
- cannulation strategy
- anticoagulation drug and anticoagulation goals used
- ventilatory support during faV-V ECMO
- sedation strategy
- physiotherapy during faV-V ECMO run
Life-threatening complications occurring during the faV-V ECMO procedure were also evaluated, including bleeding, thrombosis, cannula malposition, delirium, infection/sepsis, and barotrauma. Among the outcomes, we focused on the faV-V ECMO efficacy (successful awake treatment was defined as weaning the patient from ECMO without requiring intubation) and mortality.
Statistics
Statistical analysis was feasible only for comparing mortality differences between two groups of patients from cohort studies: those successfully disconnected from ECMO without requiring intubation and those who required intubation due to respiratory distress progression during the faV-V ECMO. A two-tailed chi-square test was used, with a p-value of less than 0.05 considered statistically significant. Statistical analysis was performed using Stata software, version 18.
Results
Studies
According to the given methodology, 64 papers were identified and assessed for eligibility at the abstract level. After evaluating for duplications and study characteristics, seven small-sample cohort studies and seven case reports met the inclusion criteria and were included in the final analysis.15,17–29 (Figure 1). The included papers report 95 patients, with 88 in cohort studies and 7 in case report papers. (Supplementary Table 1) Possible bias was identified in one patient reported in a case report, and the main author´s center participated in the multicentric retrospective study.19,28 Despite this uncertainty, both papers were included in the final analysis. Among the cohort studies, four reported solely on faV-V ECMO patients.15,17,19,22 One study provided a propensity score-matched comparison with a control group receiving conventional management with V-V ECMO and mechanical ventilation, and one study compared patients with pneumomediastinum treated with faV-V ECMO to patients treated by mechanical ventilation,18 and one study reported limited data of the faV-V ECMO patients from a large dataset CARDS ECMO patients.18,20,21
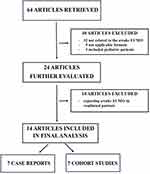 |
Figure 1 Flowchart for evaluated articles. Abbreviations: ECMO, Extracorporeal Membrane Oxygenation. |
All of the case report papers referred to successful faV-V ECMO treatment.23–29
Parameters Before ECMO Initiation
All papers noted that patients were conscious and cooperative at the time of decision-making, and informed consent for the faV-V ECMO procedure was obtained from all patients. The reported reasons for the faV-V ECMO instead of intubation and mechanical ventilation were: risk of barotrauma or presence of barotrauma in 38 patients (40.0%), refusal of intubation/mechanical ventilation in five patients (5.3%), and a combination of various clinical conditions (eg, neuromuscular disease, pulmonary disease, high BMI) leading to ECMO team consensus to prefer faV-V ECMO was mentioned in other cases. The Sequential Organ Failure Assessment (SOFA) score at the time of faV-V ECMO initiation was noted in three studies comprising nine patients (9.5%), with reported scores ranging from 4 to 5.7. (Supplementary Table 2) Before ECMO therapy, all patients for whom the type of respiratory support was specified received either a high-flow nasal oxygen cannula (HFNC) or non-invasive ventilation (NIV) with a high fraction of inspired oxygen. Patients were severely hypoxemic, with reported Horowitz index (PaO2/FiO2 ratio) values ranging from 39.0 to 95.7. The respiratory rate ranged from 28.3 to over 35.0 breaths per minute. Awake pronation before faV-V ECMO was reported in 11 patients (11.6%) (Table 2).
 |
Table 2 Respiratory Patterns Before Connection to Awake ECMO |
Awake ECMO Management
Femoral-internal jugular vein cannulation was used in 60 patients (63.2%), the femoral-femoral approach was used in 28 patients (29.5%), a dual-lumen cannula was inserted via the right internal jugular vein in six patients (6.3%), and a dual-lumen cannula allowing for right ventricular mechanical circulatory support was inserted through the right internal jugular vein in one case (1.1%).
Anticoagulation management was mentioned in five papers covering 39 patients. Unfractionated heparin (targeted to an anti-Xa level ranging from 0.2 to 0.4 IU/mL and 0.3 to 0.5 U/mL, respectively) was used in two cohort studies comprising sixteen patients. Argatroban, titrated to an anti-IIa level of 0.4 to 0.6 µg/mL, was used in one cohort study (ten patients), and bivalirudin was used in one cohort study (targeted to partial thromboplastin time in the range 50–80 s) and one case report (goals not reported).
One study (n = 10 patients) reported routine use of sedation targeting a Richmond Agitation-Sedation Scale (RASS) score of 0 to −2, enabling daytime activities and induction of sleep during the night and controlling the respiratory rate <20 breaths per minute (all patients received a combination of an opiate and dexmedetomidine from the start faV-V ECMO run). Another cohort study reported “minimized sedation” (n = 7 patients), while one case report noted the patient was “sedated”. On the contrary, two cohort studies (n = 43 patients) reported avoiding sedation during faV-V ECMO.
The high-flow nasal cannula was used for respiratory support in 66 patients (69.5%), non-invasive mechanical ventilation was applied in 11 patients (11.6%), and exact data were missing for the remaining 18 (18.9%) patients.
In most cases, ECMO settings were adjusted according to blood gas analysis or peripheral oxygen saturation (SpO2). Two papers (n = 28 patients) described respiratory effort (respiratory rate and/or respiratory mechanics) as a relevant parameter for adjusting ECMO settings; however, the exact goal (respiratory rate <20 breaths per minute) was mentioned in one study only (n = 10 patients).
Early physiotherapy in bed was reported in six papers (three cohort studies and three case reports) comprising 38 patients. Of these, eight patients could also stand and walk in the ICU during the ECMO run.
Overall, the management of patients during ECMO support was reported with high heterogeneity. The available data are summarized for clarity in Supplementary Table 3.
Complications
Complications during the ECMO run were defined and reported highly inconsistently across the publications.
Combined hemostatic complications (both bleeding and thrombosis) were reported in 13 patients (13.7%), bleeding events alone were reported in 18 patients (18.9%), and isolated thrombotic events occurred in five patients (5.3%). Accidental decannulation occurred in one patient (1.1%), and cannula malposition requiring intubation was described also in one case (1.1%).
Delirium or encephalopathy was strictly reported in 18 patients; in 11 of these, subsequent intubation was documented (in the remaining seven, the link between delirium and subsequent intubation was not mentioned). The secondary infection events were reported highly inhomogeneously. Fifty infection events were reported in sixty-seven patients from cohort studies (two studies did not report secondary infection occurrence); case reports mainly did not focus on infections). In cases of secondary infections, it is not feasible to determine if the individual events overlap. The publications do not indicate whether the infectious episodes occurred during awake ECMO or even after intubation.
Barotrauma progression occurred in five patients (5.3%), but it is unclear whether barotrauma progressed during the faV-V ECMO procedure or after intubation. A detailed summary of the reported adverse events is provided in Table 3.
 |
Table 3 Reported Complications |
Outcomes
In the case report papers, all patients were reported to have been successfully treated (ie, not intubated, disconnected from ECMO, and discharged from the hospital). In the cohort studies, 32 out of 88 patients (36.4%) completed ECMO treatment without requiring intubation due to respiratory failure, 53 patients required intubation due to respiratory failure, and three patients died while not intubated. The reported duration of faV-V ECMO varied from 8 [IQR 5–12] days to 23.3 ± 7.2 days in cohort studies and from six days to over 60 days (with ECMO still ongoing at publication later) in the case reports (Table 4).
 |
Table 4 Awake ECMO outcomes |
Among the 53 patients who required intubation due to respiratory failure, the reported main reasons were as follows:
- Hypoxemia and excessive respiratory effort persisting despite maximal ECMO support in 12 patients (21.8% of the intubated)
- Sepsis or septic shock in 7 patients (12.7% of the intubated)
- Airway protection in 5 patients (9.1% of the intubated)
- A combination of hypoxemia and delirium in 4 patients (7.3% of the intubated)
- Delirium alone in 4 patients + encephalopathy in 4 patients (14.5% of the intubated)
- Patient request for intubation in 3 patients (5.5% of the intubated)
- Subclavian cannulation in 3 patients (5.5% of the intubated)
- Heparin-induced thrombocytopenia resulting in oxygenator clotting in 2 patients (3.6% of the intubated)
- ECMO cannula malposition in one case (1.8% of the intubated)
- Stabilize prior to CT scan in one case (1.8% of the intubated)
- A combination of multiple factors in the remaining patients
The efficacy of faV-V ECMO in preventing the progression of barotrauma was addressed explicitly in two evaluated studies involving sixteen patients who had spontaneous pneumomediastinum or Macklin lines on CT scans performed before the initiation of ECMO; no progression of barotrauma during the faV-V ECMO therapy was reported.15,18
Two studies, which included six patients intubated due to respiratory failure, reported post-intubation respiratory system compliance ranging from 9–12 mL/cm H2O and 8 (6.75–11) mL/cm H2O, respectively.
For the mortality evaluation, only data from cohort studies were used. Overall, 29 out of 88 patients (33.0%) from the cohort studies died. Of the patients intubated due to respiratory failure, 26 out of 53 (mortality rate of 49.1%) died. In comparison, only three out of 35 patients (mortality rate of 8.6%) who completed faV-V ECMO without intubation died finally. The difference between the groups of patients who were intubated from respiratory causes and those who did not require intubation was statistically significant (p = 0.001).
Two papers addressed the mortality difference between faV-V ECMO and conventional management. A significantly lower mortality rate was found in patients with spontaneous pneumomediastinum treated with faV-V ECMO than those treated with mechanical ventilation.18 Another study found no difference in the mortality of the faV-V ECMO patients compared to a propensity score-matched control group receiving conventional management combining V-V ECMO and invasive mechanical ventilation.21
Among the death causes, 15 patients (50.0%) died from infectious complications/sepsis and subsequent multiorgan failure (in two cases in combination with bleeding), and one from intracerebral hemorrhage (3.0%). The exact cause of death was not mentioned for the remaining 13 patients (Table 4).
Discussion
We present a comprehensive review of papers reporting the use of the full-awake V-V ECMO approach in COVID-19-related ARDS patients. Based on predefined criteria, seven small cohort studies and seven case reports involving 74 patients met the inclusion criteria for analysis. The main reasons for using the faV-V ECMO approach were the presence or risk of barotrauma and patient refusal of intubation or mechanical ventilation. The faV-V ECMO strategy was successful (ie, patient not intubated from the respiratory cause, disconnected from ECMO, and discharged from the hospital) in 37.5% of cases (cohort studies included only). The incidence of defined serious adverse events (bleeding, thrombosis, cannula malposition, delirium, and barotrauma progression) was relatively low. The mortality rate for CARDS patients treated by the faV-V ECMO approach (patients from cohort studies included only) reached 34.1%, notably lower than the overall mortality rate of CARDS patients on ECMO reported in the ELSO registry (48%). Infectious complications, sepsis, and multiorgan failure were the most frequent causes of death. Patients who were intubated due to worsening respiratory failure during the faV-V ECMO run had significantly higher mortality outcomes compared to those who did not require intubation during the faV-V ECMO support. However, a significant heterogeneity in the definitions and reporting of both management, ECMO-related complications and outcomes was observed across the papers.
During the COVID-19 pandemic, a significant number of COVID-19 patients refused intubation and mechanical ventilation, even in the face of severe respiratory distress.30 The full-awake V-V ECMO represents an option for specific circumstances, particularly for patients who are severely hypoxemic but still conscious, cooperative, and breathing spontaneously at the point when mechanical respiratory support becomes urgent. In the evaluated studies, refusal of intubation and mechanical ventilation was mentioned as a reason for the faV-V ECMO strategy in five patients. Due to the increasing influence of social media and misinformation, it can be assumed that intensivists will continue to see patients with severe hypoxemia but refuse intubation and mechanical ventilation.
The faV-V ECMO mitigates the risks associated with invasive positive pressure ventilation, notably the risks of barotrauma. In CARDS patients, barotrauma developed frequently during the period of spontaneous ventilation, and barotrauma was associated with higher in-hospital mortality.31–33 These concerns were the most frequent reasons for opting for faV-V ECMO instead of IPPV. During the ECMO run, pneumothorax requiring a chest tube was reported in five cases; however, it was documented that this occurred before intubation (ie, during the awake ECMO run) in only one case. The efficacy of faV-V ECMO in preventing the progression of barotrauma was explicitly addressed in two evaluated studies involving sixteen patients who had spontaneous pneumomediastinum or Macklin lines on CT scans performed before the initiation of ECMO; notably, no progression of barotrauma during the faV-V ECMO therapy was reported.15,18 Additionally, mortality rates among patients with spontaneous pneumomediastinum were reported to be lower in the faV-V ECMO group compared to those undergoing invasive mechanical ventilation.18 Although there are currently no randomized studies directly comparing the efficacy and safety of faV-V ECMO against IPPV+ECMO strategy in patients at risk for or presenting with barotrauma, the presented data suggest that maintaining spontaneous breathing during ECMO treatment could represent a viable strategy to help prevent the progression of life-threatening barotrauma in this patient population.
In the papers that reported the SOFA score, respiratory failure was the only organ dysfunction when the faV-V ECMO was initiated. The retrospective studies reporting data on CARDS ECMO patients revealed that the pre-ECMO SOFA scores ranged from 8 to 12, with a high proportion of patients having a renal and hemodynamic component of the SOFA score of 3 or greater.34,35 Although the mortality rate in the evaluated studies is notably lower than that reported in the ELSO database, further studies are warranted to explore the possible effects of early faV-V ECMO (ie, when organ dysfunction has not yet developed) on outcomes.
Patients in the evaluated studies that reported the mode of respiratory support were supported by high-flow nasal oxygen cannula or non-invasive positive pressure ventilation during the faV-V ECMO run. Compared to NIV, HFNC reduces the risk of barotrauma in patients with COVID-19-related acute respiratory failure.14 Moreover, HFNC provides a flow-dependent improvement in lung mechanics and homogeneity, thereby reducing the work of breathing and the risk of patient self-inflicted lung injury (P-SILI).36–38 Among the evaluated papers, two papers (28 patients) described various parameters of the respiratory effort (respiratory rate and/or respiratory mechanics) as relevant parameters for adjusting ECMO settings. Despite the gas exchange provided by ECMO and the use of HFNC/NIV support, forty patients still progressed to intubation and mechanical ventilation. In these intubated patients, the mechanical properties of the respiratory system immediately after intubation were reported in five patients, and catastrophic values of respiratory system compliance were observed after intubation. Due to the small sample size and the absence of a control group, it is difficult to determine whether these patients had more severe initial lung injury or progressed to catastrophic “solid lung” due to unrecognized excessive respiratory effort generating P-SILI. These facts highlight the need to identify early clinical indicators that define the point at which spontaneous breathing becomes harmful and leads to further self-inflicted lung injury, even in patients receiving ECMO treatment. Worsening of respiratory mechanics (an increase of respiratory rate; signs of vigorous effort—nasal flaring, tracheal tug, sternocleidomastoid muscle phasic activity, and abdominal muscle use), tidal swings of central venous pressure, and nasal pressure swings findings might represent simple non-invasive or minimally invasive methods for detecting vigorous respiratory effort and unfavorable progress requiring reassessment of therapeutic strategy.39–41 Moreover, the lung ultrasound findings correlate with CT scan findings, and the frequent lung ultrasound score (LUS) evaluation may also help identify patients with worsening lung tissue pathology and unfavorable disease progression.42,43
The faV-V ECMO approach supports behavioral management but increases the risk of anxiety and delirium, with intense, exaggerated movements further elevating the risk of fatal complications, such as cannula malposition. Approximately 30% of all COVID-19 patients experience neurological manifestations, even in the absence of respiratory symptoms, with delirium being the most common neuropsychiatric diagnosis in hospitalized COVID-19 patients. The prevalence of delirium among hospitalized adults with COVID-19 ranges from 10.2 to 80.2, and delirium development was identified as an independent risk factor for unfavorable outcomes.44–46 On the contrary, the pooled prevalence rate of delirium among critically ill patients who received various modes of ECMO support reaches 40.8%.47 In the evaluated studies, the various forms of delirium or encephalopathy were documented in 18 out of 74 patients. Although the papers do not provide precise data on the mortality rates of patients presenting with delirium, the observed links between delirium, the need for subsequent intubation, and higher mortality in intubated suggest that delirium should be considered a warning sign of a potentially unfavorable course.
Considering the cannulation strategy in patients treated with faV-V ECMO, clinicians should be aware of the risk of artificial cannula malpositioning or even decannulation due to spontaneous movement, which can cause cessation of blood flow through the circuit or even immediate death due to exsanguination. According to data from large databases, accidental decannulation represents the most frequent life-threatening mechanical complication of ECMO.48,49 In the evaluated papers, accidental decannulation occurred in only one patient, and malposition requiring intubation due to respiratory distress in another patient, even though the majority of patients underwent routine early physiotherapy, including standing or ambulation in seven patients. These data align with studies reporting the feasibility and safety of early mobilization and ambulation in ECMO patients undertaken by an experienced multi-professional team.50–52
Thrombotic and bleeding events (TBE) are common and potentially fatal complications associated with ECMO therapy. In this study, the incidence rate of TBE reached 32.4%, comparable to that reported for patients treated with ECMO in large databases and meta-analyses.53–55 Although the rates of intracranial hemorrhage (ICH) in COVID-19 patients receiving ECMO are higher compared to similar controls, only one episode of ICH was reported among the evaluated studies.56 These results are particularly significant given that full-awake ECMO patients are conscious, move spontaneously, and often undergo intensive physiotherapy. The risk of TBE is further increased by the routine anticoagulation required for ECMO patients. Due to a lack of robust evidence, controversies about drug choices, therapeutic targets, or monitoring still exist, leading to significant variability in practice among ECMO centers.57,58 Currently, unfractionated heparin (UFH) is the most commonly used anticoagulant for ECMO.59 However, direct thrombin inhibitors (DTIs), such as bivalirudin and argatroban, may provide advantages due to their more predictable pharmacokinetics and the absence of risk for heparin-induced thrombocytopenia.60,61 This variability in practice is reflected in our findings—only two of the cohort studies reported on anticoagulation management, including targeted ranges for anti-Xa levels in UFH administration and anti-IIa activity for argatroban treatment. Among the case reports, only one mentioned the drug used for anticoagulation (bivalirudin). The low incidence of thrombotic and hemorrhagic events observed in the evaluated studies suggests that practical local guidelines can reduce the risk of TBE, regardless of the anticoagulant used.
In the cohort studies analyzed, 29 out of 88 (33.0%) patients ultimately died. However, this number is notably lower than the overall mortality rate of CARDS patients on ECMO reported in the ELSO registry (48%).1 Moreover, patients who required intubation had significantly higher mortality compared to those who did not require intubation. These results are in accordance with data from a meta-analysis of awake ECMO patients with ARDS, where the mortality rate for those who failed the awake ECMO strategy (patients extubated during ECMO run also included) was 57.2%. On the contrary, overall mortality in the ARDS group reached only 20.2%.62 Fifteen of the reported deaths were related to infectious complications, sepsis, and multiorgan failure, while the cause of death was not specified for six patients. These findings are consistent with current knowledge that patients with ARDS typically die from sepsis and multiorgan failure rather than from hypoxemia or hypercapnia.63,64
The presented paper has several limitations:
- The number of studies and patients is low for robust statistical analysis.
- The design of the evaluated studies is primarily retrospective, except for one prospective study that involved seven patients.
- All seven of the case reports described successful faV-V ECMO treatments.
- A significant heterogeneity in the definitions and reporting of both management, ECMO-related complications, and outcomes were observed across the papers.
Given the aforementioned limitations, this article provides a descriptive overview of current full-awake V-V ECMO practices and outlines hypotheses for further research rather than presenting exact evidence. Based on the presented results, we suggest that further research should focus on identifying patients who are likely to benefit the most from the faV-V ECMO approach, establishing the clinical criteria linked to detrimental respiratory effort, and objectively evaluating the effectiveness of faV-V ECMO in relation to traditional ECMO strategies (eg, in intubated patients) for ARDS patients on mechanical ventilation. Nonetheless, despite the identified limitations, the data presented reflect real-world clinical situations and could assist in decision-making when immediate invasive mechanical support for respiratory function is necessary.
Conclusion
The main reasons for using the faV-V ECMO approach in COVID-19-related ARDS were the presence or risk of barotrauma and patient refusal of intubation or mechanical ventilation. The faV-V ECMO strategy was successful (ie, patients were not intubated from the respiratory cause, disconnected from ECMO, and discharged from the hospital) in 40.3% of cases (cohort studies included only). The incidence of defined serious adverse events (bleeding, thrombosis, cannula malposition, delirium, and barotrauma progression) was considered low. The mortality rate for CARDS patients treated by the faV-V ECMO approach (patients from cohort studies included only) was notably lower than that of CARDS patients on ECMO reported in the ELSO registry. Infectious complications, sepsis, and multiorgan failure were the most frequent causes of death. Patients who were intubated due to worsening respiratory failure during the awake ECMO run had worse outcomes compared to those who did not require intubation during the ECMO support.
Acknowledgments
Supported by MH CZ – DRO (FNOs/2025).
Contributions of authors to the paper
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
University Hospital Ostrava.
Disclosure
The authors report no conflicts of interest in this work.
References
1. The Extracorporeal Life Support Organization. Extracorporeal Membrane Oxygenation (ECMO) in COVID-19. Available from: https://www.elso.org/covid-19.aspx.
2. Gisondi MA, Chambers D, La TM, et al.. A stanford conference on social media, ethics, and COVID-19 misinformation (INFODEMIC): qualitative thematic analysis. J Med Internet Res. 2022;24(2):e35707. doi:10.2196/35707
3. Chou WS, Gaysynsky A, Vanderpool RC. The COVID-19 misinfodemic: moving beyond fact-checking. Health Educ Behav. 2021;48(1):9–13. doi:10.1177/1090198120980675
4. Busana M, Gasperetti A, Giosa L, et al.. Prevalence and outcome of silent hypoxemia in COVID-19. Minerva Anestesiol. 2021;87(3):325–333. doi:10.23736/S0375-9393.21.15245-9
5. Brouqui P, Amrane S, Million M, et al.. Asymptomatic hypoxia in COVID-19 is associated with poor outcome. Int J Infect Dis. 2021;102:233–238. doi:10.1016/j.ijid.2020.10.067
6. Porzionato A, Emmi A, Contran M, et al. Case report: the carotid body in COVID-19: histopathological and virological analyses of an autopsy case series. Front Immunol. 2021;12:736529. doi:10.3389/fimmu.2021.736529
7. Kallet RH, Branson RD, Lipnick MS. Respiratory drive, dyspnea, and silent hypoxemia: a physiological review in the context of COVID-19. Respir Care. 2022;67(10):1343–1360. doi:10.4187/respcare.10075
8. Spinelli E, Mauri T, Beitler JR, et al.. Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med. 2020;46(4):606–618. doi:10.1007/s00134-020-05942-6
9. Paternoster G, Belmonte G, Scarano E, et al.. COVID-macklin study group. macklin effect on baseline chest CT scan accurately predicts barotrauma in COVID-19 patients. Respir Med. 2022;197(106853):106853. doi:10.1016/j.rmed.2022.106853
10. Casadiego Monachello FJ, de la Torre Terron MC, Mendez Barraza JA, et al.. Macklin effect as an early radiological predictor of barotrauma in ARDS COVID-19 patients in invasive mechanical ventilation. Med Intensiva. 2023;47(4):235–236. doi:10.1016/j.medin.2022.07.003
11. Melhorn J, Achaiah A, Conway FM, et al.. Pneumomediastinum in COVID-19: a phenotype of severe COVID-19 pneumonitis? The results of the United Kingdom (POETIC) survey. Eur Respir J. 2022;60(3):2102522. doi:10.1183/13993003.02522-2021
12. Woo W, Kipkorir V, Marza AM, et al.. International covid-pneumothorax working group Icp-Wg. prognosis of spontaneous pneumothorax/pneumomediastinum in coronavirus disease 2019: the CoBiF score. J Clin Med. 2022;11(23):7132. doi:10.3390/jcm11237132
13. Serck N, Piagnerelli M, Augy JL, et al.. Barotrauma in COVID-19 acute respiratory distress syndrome: retrospective analysis of the COVADIS prospective multicenter observational database. BMC Anesthesiol. 2023;23(1):138. doi:10.1186/s12871-023-02093-1
14. Vetrugno L, Castaldo N, Fantin A, et al.. Ventilatory associated barotrauma in COVID-19 patients: a multicenter observational case-control study (COVI-MIX-study). Pulmonology. 2023;29(6):457–468. doi:10.1016/j.pulmoe.2022.11.002
15. Paternoster G, Bertini P, Belletti A, et al. Venovenous extracorporeal membrane oxygenation in awake non-intubated patients with COVID-19 ARDS at high risk for barotrauma. J Cardiothorac Vasc Anesth. 2022;36(8):2975–2982. doi:10.1053/j.jvca.2022.03.011
16. Page MJ, McKenzie JE, Bossuyt PM, et al.. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:372.
17. Assanangkornchai N, Slobod D, Qutob R, et al.. Awake venovenous extracorporeal membrane oxygenation for coronavirus disease 2019 acute respiratory failure. Crit Care Explor. 2021;3(7):e0489. doi:10.1097/CCE.0000000000000489
18. Attou R, Redant S, Velissaris D, et al.. Extracorporeal membrane oxygenation versus invasive ventilation in patients with COVID-19 acute respiratory distress syndrome and pneumomediastinum: a cohort trial. Artif Organs. 2024;48(9):1038–1048. doi:10.1111/aor.14760
19. Galante O, Hasidim A, Almog Y, et al.. for Awake ECMO research team. extracorporal membrane oxygenation in nonintubated patients (Awake ECMO) With COVID-19 adult respiratory distress syndrome: the israeli experience. ASAIO J. 2023;69(8):e363–e367. doi:10.1097/MAT.0000000000001996
20. Kunavarapu C, Yeramaneni S, Melo J, et al.. Clinical outcomes of severe COVID-19 patients receiving early VV-ECMO and the impact of pre-ECMO ventilator use. Int J Artif Organs. 2021;44(11):861–867. doi:10.1177/03913988211047604
21. Mang S, Reyher C, Mutlak H, et al.. AWECO-study group. awake extracorporeal membrane oxygenation for COVID-19-induced acute respiratory distress syndrome. Am J Respir Crit Care Med. 2022;205(7):847–851. doi:10.1164/rccm.202105-1189LE
22. Sklienka P, Burša F, Frelich M, et al.. Optimizing the safety and efficacy of awake venovenous extracorporeal membrane oxygenation in patients with COVID-19-related ARDS. Ther Adv Respir Dis. 2024;18:17534666241282590. doi:10.1177/17534666241282590
23. Aziz JE, Dellavolpe J, Aziz S, et al. An extracorporeal membrane oxygenation first strategy in COVID-19 acute respiratory distress syndrome. ASAIO J. 2021;67(10):1097–1099. doi:10.1097/MAT.0000000000001554
24. Azzam MH, Mufti HN, Bahaudden H, et al.. Awake extracorporeal membrane oxygenation in coronavirus disease 2019 patients without invasive mechanical ventilation. Crit Care Explor. 2021;3(6):e0454. doi:10.1097/CCE.0000000000000454
25. Ghizlane EA, Manal M, Sara B, et al.. Early initiation of awake venovenous extracorporeal membrane oxygenation (ECMO) in critical COVID-19 pneumonia: a case reports. Ann Med Surg Lond. 2021;68:102641. doi:10.1016/j.amsu.2021.102641
26. Loyalka P, Cheema FH, Rao H, et al.. Early usage of extracorporeal membrane oxygenation in the absence of invasive mechanical ventilation to Treat COVID-19-related hypoxemic respiratory failure. ASAIO J. 2021;67(4):392–394. doi:10.1097/MAT.0000000000001393
27. Schmidt M, de Chambrun MP, Lebreton G, et al.. Extracorporeal membrane oxygenation instead of invasive mechanical ventilation in a patient with severe COVID-19-associated acute respiratory distress syndrome. Am J Respir Crit Care Med. 2021;203(12):1571–1573. doi:10.1164/rccm.202102-0259LE
28. Soroksky A, Tocut M, Rosman Z, et al.. Awake extracorporeal membrane oxygenation in a patient with COVID-19 pneumonia and severe hypoxemic respiratory failure. Eur Rev Med Pharmacol Sci. 2022;26(5):1761–1764. doi:10.26355/eurrev_202203_28246
29. Umlauf J, Eilenberger S, Spring O. successful treatment of a patient with COVID-19-induced severe ARDS, Pneumothorax, and pneumomediastinum with awake vv-ECMO implantation. Case Rep Crit Care. 2022;2022:6559385. doi:10.1155/2022/6559385
30. Fletcher JJ, Aughenbaugh A, Svabek C, et al.. Ventilator avoidance among critically ill COVID-19 patients with acute respiratory distress syndrome. J Int Med Res. 2022;50(11):3000605221135446. doi:10.1177/03000605221135446
31. Cai Z, Guo X, Lv X, et al.. Patient self-inflicted lung injury associated pneumothorax/pneumomediastinum is a risk factor for worse outcomes of severe COVID-19: a case-control study. Sci Rep. 2024;14(1):15437. doi:10.1038/s41598-024-66229-0
32. Shrestha DB, Sedhai YR, Budhathoki P, et al.. Pulmonary barotrauma in COVID-19: a systematic review and meta-analysis. Ann Med Surg Lond. 2022;73:103221.
33. Umbrello M, Venco R, Antonucci E, et al. Incidence, clinical characteristics and outcome of barotrauma in critically ill patients with COVID-19: a systematic review and meta-analysis. Minerva Anestesiol. 2022;88(9):706–718. doi:10.23736/S0375-9393.22.16258-9
34. Schmidt M, Hajage D, Lebreton G, et al. Groupe de recherche clinique en reanimation et soins intensifs du patient en insuffisance respiratoire aiguE (GRC-RESPIRE) sorbonne université; paris-sorbonne ECMO-COVID investigators. extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med. 2020;8(11):1121–1131. doi:10.1016/S2213-2600(20)30328-3
35. Hajage D, Combes A, Guervilly C, et al.. for COVID-ICU investigators. extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: an emulated target trial analysis. Am J Respir Crit Care Med. 2022;206(3):281–294. PMID: 35533052; PMCID: PMC9890253. doi:10.1164/rccm.202111-2495OC
36. Öner Ö, Ergan B, Kizil AS, et al.. Investigation of high flow nasal cannule efficiency with electric impedance tomography based parameters in COVID-19 adults patients: a retrospective study. PeerJ. 2023;14:11.
37. Mauri T, Alban L, Turrini C, et al. Optimum support by high-flow nasal cannula in acute hypoxemic respiratory failure: effects of increasing flow rates. Intensive Care Med. 2017;43(10):1453–1463. doi:10.1007/s00134-017-4890-1
38. Basile MC, Mauri T, Spinelli E, et al.. Nasal high flow higher than 60 L/min in patients with acute hypoxemic respiratory failure: a physiological study. Crit Care. 2020;24(1):654. doi:10.1186/s13054-020-03344-0
39. Tobin MJ. Why physiology is critical to the practice of Medicine: a 40-year personal perspective. Clin Chest Med. 2019;40(2):243–257. doi:10.1016/j.ccm.2019.02.012
40. Colombo J, Spinelli E, Grasselli G, et al. Detection of strong inspiratory efforts from the analysis of central venous pressure swings: a preliminary clinical study. Minerva Anestesiologica. 2020;86(12):1296–1304. doi:10.23736/S0375-9393.20.14323-2
41. Tonelli R, Cortegiani A, Marchioni A, et al.. Nasal pressure swings as the measure of inspiratory effort in spontaneously breathing patients with de novo acute respiratory failure. Crit Care. 2022;26(1):70. doi:10.1186/s13054-022-03938-w
42. Gil-Rodríguez J, Pérez de Rojas J, Aranda-Laserna P, et al.. Ultrasound findings of lung ultrasonography in COVID-19: a systematic review. Eur J Radiol. 2022;148:110156. doi:10.1016/j.ejrad.2022.110156
43. Dargent A, Chatelain E, Si-Mohamed S, et al.. for COVIDLUS study group. Lung ultrasound score as a tool to monitor disease progression and detect ventilator-associated pneumonia during COVID-19-associated ARDS. Heart Lung. 2021;50(5):700–705. doi:10.1016/j.hrtlng.2021.05.003
44. Guo Y, Lin J, Wu T, et al.. Risk factors for delirium among hospitalized adults with COVID-19: a systematic review and meta-analysis of cohort studies. Int J Nurs Stud. 2023;148:104602. doi:10.1016/j.ijnurstu.2023.104602
45. Xu Z, Wang H, Jiang S, et al.. Brain pathology in COVID-19: clinical manifestations and potential mechanisms. Neurosci Bull. 2024;40(3):383–400. doi:10.1007/s12264-023-01110-0
46. Rego LLD, Salluh JIF, Souza-Dantas VC, et al.. Delirium severity and outcomes of critically ill COVID-19 patients. Crit Care Sci. 2023;35(4):394–401. doi:10.5935/2965-2774.20230170-en
47. Ho MH, Lee JJ, Lai PCK, et al.. Prevalence of delirium among critically ill patients who received extracorporeal membrane oxygenation therapy: a systematic review and proportional meta-analysis. Intensive Crit Care Nurs. 2023;79:103498. doi:10.1016/j.iccn.2023.103498
48. Hadano H, Kamio T, Fukaguchi K, et al.. Analysis of adverse events related to extracorporeal membrane oxygenation from a nationwide database of patient-safety accidents in Japan. J Artif Organs. 2023;16:1–8.
49. Kim DH, Cho WH, Son J, et al.. Catastrophic mechanical complications of extracorporeal membrane oxygenation. ASAIO J. 2021;67(9):1000–1005. doi:10.1097/MAT.0000000000001354
50. Braune S, Bojes P, Mecklenburg A, et al.. Feasibility, safety, and resource utilisation of active mobilisation of patients on extracorporeal life support: a prospective observational study. Ann Intensive Care. 2020;10(1):161. doi:10.1186/s13613-020-00776-3
51. Cucchi M, Mariani S, De Piero ME, et al.. Awake extracorporeal life support and physiotherapy in adult patients: a systematic review of the literature. Perfusion. 2023;38(5):939–958. doi:10.1177/02676591221096078
52. Bonizzoli M, Lazzeri C, Drago A, et al.. Effects of a physiotherapic program in patients on veno-venous extracorporeal membrane oxygenation: an 8-year single-center experience. Minerva Anestesiol. 2019;85(9):989–994. doi:10.23736/S0375-9393.19.13287-7
53. Nunez JI, Gosling AF, O’Gara B, et al.. Bleeding and thrombotic events in adults supported with venovenous extracorporeal membrane oxygenation: an ELSO registry analysis. Intensive Care Med. 2022;48(2):213–224. doi:10.1007/s00134-021-06593-x
54. Jin Y, Zhang Y, Liu J, et al.. Thrombosis and bleeding in patients with COVID-19 requiring extracorporeal membrane oxygenation: a systematic review and meta-analysis. Res Pract Thromb Haemost. 2023;7(2):100103. doi:10.1016/j.rpth.2023.100103
55. Mansour A, Flecher E, Schmidt M, et al.. ECMOSARS investigators. bleeding and thrombotic events in patients with severe COVID-19 supported with extracorporeal membrane oxygenation: a nationwide cohort study. Intensive Care Med. 2022;48(8):1039–1052. doi:10.1007/s00134-022-06794-y
56. Lannon M, Duda T, Greer A, et al.. Intracranial hemorrhage in patients treated for SARS-CoV-2 with extracorporeal membrane oxygenation: a systematic review and meta-analysis. J Crit Care. 2023;77:154319. doi:10.1016/j.jcrc.2023.154319
57. Tonna JE, Abrams D, Brodie D, et al. Management of adult patients supported with venovenous extracorporeal membrane oxygenation (VV ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO J. 2021;67(6):601–610. doi:10.1097/MAT.0000000000001432
58. Chlebowski MM, Baltagi S, Carlson M, et al.. Clinical controversies in anticoagulation monitoring and antithrombin supplementation for ECMO. Crit Care. 2020;24(1):19. doi:10.1186/s13054-020-2726-9
59. Shekar K, Badulak J, Peek G, et al.. ELSO guideline working group. extracorporeal life support organization coronavirus disease 2019 interim guidelines: a consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. ASAIO J. 2020;66(7):707–721. doi:10.1097/MAT.0000000000001193
60. Diaz D, Martinez J, Bushman G, et al.. Anticoagulation strategies in COVID-19 infected patients receiving ECMO support. J Extra Corpor Technol. 2023;55(3):121–129. doi:10.1051/ject/2023027
61. Burša F, Máca J, Sagan J, et al.. A safety comparison of heparin and argatroban anticoagulation in veno-venous extracorporeal membrane oxygenation with a focus on bleeding. Transfus Med. 2024:75–81. PMID: 39375884. doi:10.1111/tme.13102
62. Belletti A, Sofia R, Cicero P, et al.. Extracorporeal membrane oxygenation without invasive ventilation for respiratory failure in adults: a systematic review. Crit Care Med. 2023;51(12):1790–1801. doi:10.1097/CCM.0000000000006027
63. Vincent JL, Zambon M. Why do patients who have acute lung injury/acute respiratory distress syndrome die from multiple organ dysfunction syndrome?Implications for management. Clin Chest Med. 2006;27(4):725–731. doi:10.1016/j.ccm.2006.06.010
64. Del Sorbo L, Slutsky AS. Acute respiratory distress syndrome and multiple organ failure. Curr Opin Crit Care. 2011;17(1):1–6. doi:10.1097/MCC.0b013e3283427295
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

