Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Lipoprotein(a) is Associated with Sarcopenia in Patients with Type 2 Diabetes: A cross-Sectional Study
Authors Wei W , Lv F , Liu S , Cao H, Lin R , Chen H, Tu M , Cao B
Received 1 August 2024
Accepted for publication 13 November 2024
Published 27 November 2024 Volume 2024:17 Pages 4511—4524
DOI https://doi.org/10.2147/DMSO.S489605
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Wen Wei,1,2 Fenyan Lv,3 Shuling Liu,3 Hui Cao,3 Ruiyu Lin,1 Hangju Chen,1 Mei Tu,1 Baozhen Cao1
1Department of Endocrinology, Fujian Longyan First Hospital, Longyan First Affiliated Hospital of Fujian Medical University, Longyan, 364000, People’s Republic of China; 2The Second School of Clinical Medicine, Southern Medical University, Guangzhou, 510280, People’s Republic of China; 3Department of Endocrinology, Fujian Longyan First Hospital, Fujian Medical University, Fuzhou, 350004, People’s Republic of China
Correspondence: Baozhen Cao; Mei Tu, Department of Endocrinology, Fujian Longyan First Hospital, Longyan First Affiliated Hospital of Fujian Medical University, Longyan, 364000, People’s Republic of China, Tel +86-0597-3082152 ; +86-0597-2958989, Fax +86-0597-2292374, Email [email protected]; [email protected]
Background: The association between lipoprotein(a) (Lp(a)) and sarcopenia in T2DM patients of general age is unclear, and whether this association differs by sex remains uncertain. We intend to analyze the association between Lp(a) and sarcopenia in patients with type 2 diabetes mellitus (T2DM) and whether this association differs by sex.
Methods: T2DM patients between December 2021 and December 2022 were consecutively enrolled. Sarcopenia was defined according to the criteria of Consensus of the Asian Working Group for Sarcopenia (AWGS) 2019.A multivariable logistic regression model was used to calculate the odds ratio of Lp(a)≥ 30 mg/dL for sarcopenia in total T2DM patients and in all sexes. Restricted cubic splines were also used to evaluate the association between Lp(a) and sarcopenia.
Results: Among the 426 patients, the mean age was 58.6 years and 56.3% were males. The prevalence of sarcopenia was 31.7% in total patients, 34.2% in male and 28.5% in female. The percentages of Lp(a)≥ 30 mg/dL were 19.0% in total patients. Compared with patients in Lp(a)< 30 mg/dL group, those in Lp(a)≥ 30 mg/dL group showed an increased risk of sarcopenia (adjusted odds ratio [aOR]: 2.19, 95% CI: 1.09 to 4.39, p = 0.027). Results from restricted cubic splines were robust. When analyzing each sex, there was also a significant association between Lp(a)≥ 30 mg/dL and sarcopenia (male: aOR: 2.59, 95% CI: 1.09 to 6.21, p = 0.032; female: aOR: 2.45, 95% CI: 1.06 to 6.03, p = 0.039).
Conclusion: In T2DM patients, elevated Lp(a) was associated with an increased risk of sarcopenia and such an association did not differ by sex. Screening for sarcopenia should be emphasized in T2DM patients with Lp(a)≥ 30 mg/dL, both men and women.
Keywords: lipoprotein(a), sarcopenia, type 2 diabetes mellitus, sex
Background
Sarcopenia is a progressive and widespread disorder associated with a reduction in muscle quantity, quality, and function.1 The prevalence of sarcopenia is much higher in different patient groups compared to the general population. The overall prevalence has reached 20% or even higher in individuals with diabetes.2,3 However, in most studies that reported sex, there is no significant association with sarcopenia prevalence.4 The multiple comorbidities associated with type 2 diabetes mellitus (T2DM) (retinopathy, renal, vascular involvement, etc.) have even been directly associated with the presence of sarcopenia in diabetic patients.5–8 Sarcopenia is also associated with poor outcomes in patients with diabetes, including cognitive impairment, infection, falls, disability, and mortality 34041847/31040938/37608605. Therefore, to find and treat modifiable risk factors of sarcopenia in diabetic patients is helpful to prevent the development and progression of sarcopenia in diabetic patients, and is of great significance to improve the quality of life of diabetic patients.
Previous evidence showed that low muscle mass was associated with higher levels of triglycerides (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), very low density lipoprotein cholesterol (VLDL-C), and remnant-like particle cholesterol (RLP-C).9,10 On the contrary, Jiang et al indicated that an increase in TG and TC may have a protective effect on sarcopenia.11 Furthermore, a cross-sectional analysis from UK Biobank demonstrated that sarcopenia was associated with higher concentration of HDL-C and lower values of apoprotein A and apoprotein B only in men.12 Apoprotein B is the main component of LDL-C and VLDL-C, so low apoprotein B means low LDL-C and VLDL-C. However, the study from UK Biobank did not found a significant association between sarcopenia and LDL-C. Previous studies have shown inconsistent relationships between clinical lipid parameters and sarcopenia, and these relationships may differ by sex. However, in sarcopenia, lipid metabolism disorders are prevalent. The relationship between sarcopenia and clinical lipid parameters needs further exploration.
Lipid disorders can be broadly divided into 4 “clinical” categories: elevated LDL-C, low HDL-C, elevated TG, and elevated lipoprotein(a) (Lp(a)). Serum Lp(a) is a special lipoprotein synthesized by the liver. It is a heterogenous glycoprotein, which is an apoB100 containing lipoprotein covalently bound to apoprotein(a).13 Convincing evidence from many studies indicated that elevated Lp(a), the fourth “clinical” category of lipid disorders, was a main driver for the risk of atherosclerotic cardiovascular disease (ASCVD), myocardial infarction, and aortic valve stenosis.14 Higher concentrations of Lp(a) have also been found to be related to sarcopenia in women.12 Recently, Li et al found that elevated Lp(a) was related to muscle loss in elderly (60 years or older) patients with T2DM.15 However, the association between Lp(a) and sarcopenia in T2DM patients of general age, and whether this association differs by sex, is still unclear.
Therefore, we aim to analyze the association between elevated Lp(a) level and sarcopenia in T2DM patients of general age, and whether this association differs by sex.
Methods
Study Population
The present study was a cross-sectional study that included adult hospitalized patients (≥18 years of age) diagnosed with T2DM according to American Diabetes Association (ADA) criteria at Longyan First Affiliated Hospital of Fujian Medical University, Fujian, China between December 2021 and December 2022. Pregnant patients and patients with other types of diabetes were excluded. Patients with ketoacidosis, hyperosmolar status, acute severe infection, kidney diseases requiring hemodialysis, severe cardiac insufficiency, autoimmune disease, neurological diseases and orthopedic diseases causing mobility impairment, and incomplete clinical parameters such as Lp(a), muscle mass, muscle strength, and physical performance were also excluded. Eventually, 426 patients were included (Figure 1). All patients in our study were not prescribed with proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor. The study was approved by the Institutional Ethics Research Committee of Longyan First Affiliated Hospital of Fujian Medical University (approval number LYREC2021-014-01) and was performed according to the Declaration of Helsinki. All patients gave written informed consent to take part in the study.
 |
Figure 1 The flow of participants through the trial. |
Data Collection and Clinical Definition
Demographic variables and health information, including age, sex, educational status and history of diagnosed diseases, were collected via a standard questionnaire by our trained staff. Clinical features and biochemical examination data were obtained from the electronic medical record system. Height, weight and blood pressure were assessed by the nurse on admission using a standardized form. Venous blood was collected early in the morning after fasting overnight. Venous blood was also taken two hours after the first bite of breakfast to detect 2 hours postprandial blood glucose (2hPBG).
Body mass index (BMI) was calculated by dividing weight (kg) by the square of height (m). Anemia was defined as hemoglobin levels of less than 13 g per deciliter in male and 12 g per deciliter in female according to diagnostic criteria set by the WHO.16 Non-high-density lipoprotein cholesterol (non-HDL-C) was calculated as TC minus HDL-C.17 The estimated glomerular filtration rate (eGFR) was calculated based on serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2009 equation.18 The diagnosis of DR was based on ophthalmologic examination and the International Clinical Diabetic Retinopathy Disease Severity Scale.19 Diabetic nephropathy (DN) was diagnosed based on the urinary albumin/creatinine ratio (ACR) ≥ 30 mg/mmol or eGFR < 60 mL/min/1.73m2, as defined by the organization Kidney Disease Improving Global Outcomes (KDIGO).20 Diabetic neuropathy was diagnosed according to the Chinese guidelines for the prevention and treatment of type 2 diabetes.21 Cardiovascular disease (CVD) was defined as stroke and/or coronary artery disease (CAD). Low education was defined as the patient’s highest level of education being at or below a high school diploma.
Measurement of Lp(a)
Lipid assessment was performed at admission. Lp(a) mass was evaluated by an auto immunoturbidimetry assay on a chemistry analyzer (AU5800 Analyzer, Beckman Coulter, Brea, California). The intraassay coefficient of variation was ≤ 4% and the interassay coefficient of variation was ≤ 10%. Based on prior report22 and the instructions for our hospital’s Lp (a) kit, serum level of Lp(a)<30 mg/dL was considered as the normal range.
Muscle Mass, Grip Strength, and Physical Performance Assessment
Methods of assessing muscle mass, grip strength, and physical performance were described in our previous manuscript.23 The Appendicular Skeletal Muscle Mass Index (ASMI) was calculated using the following formula: ASMI = extremity muscle mass (kg) / height squared (m2). Low muscle mass was defined as ASMI <7.0 kg/m2 for men and <5.4 kg/m2 for women. Low muscle strength was defined as handgrip strength <28 kg for men and <18 kg for women. Low physical performance was defined as 6-meter walk speed <1.0 m/s. The diagnosis of sarcopenia include low muscle mass, low muscle strength, or poor physical performance following the guidelines from the Asian Working Group for Sarcopenia (AWGS) 2019. Severe sarcopenia was defined as having both low muscle mass and low muscle strength, as well as poor physical performance.
Statistical Analysis
Continuous variables were reported as mean ± standard deviation (SD) or median (interquartile range, IQR) and compared by the two-sample independent t-test or Wilcoxon-rank test as appropriate. Categorical variables were displayed as percentage (number) and compared by the Chi-square test. Correlations of Lp(a) with other lipid parameters were assessed by the Spearman correlation coefficient analysis.
Restricted cubic splines were used to detect the association between Lp(a) and sarcopenia. The association between Lp(a) and sarcopenia was assessed by univariable and multivariable logistic regression. Variables that were entered into the model were carefully selected on the basis of variables associated with known poor prognosis or variables with p-value <0.05 in the baseline or univariable regression analysis. We also performed subsequent analysis to assess the effect of sex on the association between Lp(a) and sarcopenia.
Statistical analyses were performed using R, version 4.0.3 software (R Foundation for Statistical Computing, Vienna, Austria). Two-sided p values <0.05 were considered statistically significant.
Results
Patient Characteristics
The mean age of the 426 enrolled patients was 58.6 years, and 240 (56.3%) were males. Totally, the patients with low education were 359 (84.3%), and the mean BMI was 24.56 kg/m2. The median duration of diabetes was 6.0 years, and the glycosylated hemoglobin (HbA1c) was 9.8% ± 2.3. 12.9% (n = 55) of the patients had diabetic nephropathy (DN), 29.0% (n = 113) had diabetic retinopathy (DR), and 43.9% (n =187) had diabetic neuropathy. Almost half of the patients had hypertension (46.9%; n = 200), 10.8% (n = 46) had CVD, and 19.9% (n = 84) had anemia (Table 1).
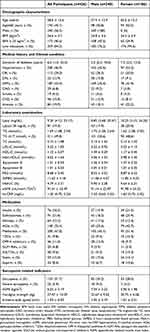 |
Table 1 Baseline Characteristics of All Participants |
The prevalence of sarcopenia was 31.7%, of which 25 cases were severe sarcopenia among 135 patients, accounting for 18.5%. Patients with sarcopenia were older, had lower BMI, lower eGFR, lower TG, higher Lp(a) levels, and had longer duration of diabetes than those without sarcopenia (all p < 0.05). Patients with sarcopenia also had lower educational level and higher prevalence of DR, CVD and anemia than patients without sarcopenia. The use of insulin was higher in patients with sarcopenia compared to those without sarcopenia, while the use of other hypoglycemic and lipid-lowering drugs did not differ between the two groups. Study population characteristics were summarized in Table 2.
 |
Table 2 Baseline Characteristics of Patients with and without Sarcopenia |
The percentages of Lp(a)≥30 mg/dL were 19.0% (n = 81). Patients were also divided into groups with Lp(a)<30 and ≥30 mg/dL. Compared with patients in Lp(a)<30 mg/dL group, those in Lp(a)≥30 mg/dL group had lower educational level, higher prevalence of sarcopenia, and higher LDL-C and apoprotein B levels. The use of hypoglycemic and lipid-lowering drugs did not differ between the two groups (Supplementary Table 1 and Figure 2).
 |
Figure 2 Prevalence of sarcopenia in total, male, and female patients with Lp(a)< 30 and ≥30 mg/dL. |
Correlations Between Lp(a) and Other Lipid Measurements
Figure 3 showed all correlations between Lp(a) and other lipid measurements. Except apoprotein A, Lp(a) showed a significantly positive correlation with TC (r = 0.13, p < 0.01), LDL-C (r = 0.18, p < 0.001), HDL-C (r = 0.12, p < 0.05), non-HDL-C (r = 0.12, p < 0.05) and apoprotein B(r = 0.18, p < 0.01). By contrast, there was a negative correlation between Lp(a) and TG (r = −0.14, p < 0.01). In a word, the absolute values of the r index of Lp(a) associated with other lipid parameters were all less than 0.20.
Correlations Between Lp(a) and Clinical Indicators of Sarcopenia
Lp(a) showed a significantly positive correlation with sarcopenia (r = 0.14, p < 0.01), and a negative correlation with ASMI (r = −0.11, p < 0.05), Handgrip strength (r = −0.10) and 6-metre walk speed (r = −0.03). The correlation between Lp(a) and Handgrip strength and 6-metre walk speed was not significant (Table 3).
 |
Table 3 Correlation Between Lp(a) and Clinical Indicators of Sarcopenia |
Association Between Lp(a) and Sarcopenia
There was a linear correlation between Lp(a) and sarcopenia in our study population. We found that the higher the Lp(a) level, the higher the incidence of sarcopenia (Nonlinear: p = 0.185) (Figure 4A).
In univariable logistic regression analysis, a statistically significant positive correlation was found between Lp(a) as a continuous variable and sarcopenia (odds ratio [OR]: 1.01, 95% confidence interval [CI]: 1.00 to 1.02, p = 0.016). Patients in Lp(a)≥30 mg/dL group showed an increased risk of sarcopenia than those in Lp(a)< 30mg/dL group (OR: 1.86, 95% CI: 1.13 to 3.06, p = 0.014) in univariable model (Supplementary Table 2). After careful selection, multivariable logistic regression analyses with stepwise adjustment for clinical variables showed that the association between Lp(a) as a categorical variable and sarcopenia was robust (fully adjusted odds ratio [aOR]: 2.19, 95% CI: 1.09 to 4.39, p = 0.027) (Table 4).
 |
Table 4 Multivariable Logistic Regression Analysis for Lp(a) and Sarcopenia in Total Patients |
Restricted cubic spline which was developed based on the same covariates of the multivariable logistic regression model, showed that the higher the Lp(a) level, the higher increased incidence of sarcopenia (Nonlinear: p = 0.545) (Figure 4B).
In terms of other lipid parameters, only TG was negatively correlated with sarcopenia when not adjusting for covariates. Multivariable logistic regression showed that hypertriglyceridemia (TG≥1.7 mmol/L) was negatively correlated with sarcopenia after adjusting for covariates (Supplementary Table 3).
Subgroup analysis also revealed that Lp(a)≥30 mg/dL had a relatively consistent risk of sarcopenia across dichotomized subgroups (Age≥60 years or <60 years, BMI≥ 25 kg/m2 or <25 kg/m2, and TG ≥1.7 mmol/L or <1.7 mmol/L). No significant interaction between Lp(a)≥30 mg/dL and dichotomized subgroups was observed (all p-interaction > 0.05) (Figure 5).
 |
Figure 5 Odds ratio for sarcopenia in different subgroups. |
Association Between Lp(a) and Sarcopenia in Different Sexes
Subsequent analysis was performed for individuals of different sexes. The prevalence of sarcopenia was 34.2% in male, while 28.5% in female. Patients with sarcopenia were older, had lower BMI, lower eGFR, lower TG, higher Lp(a) levels, and had longer duration of diabetes than those without sarcopenia in both male and female (all p < 0.05)(Table 2).
Compared with patients in Lp(a) <30 mg/dL group, patients in Lp(a) ≥30 mg/dL group tended to had higher prevalence of sarcopenia in both male and female (Figure 2). After adjusting for confounding variables, multivariable logistic regression analyses showed that patients in Lp(a)≥30 mg/dL group had an increased risk of sarcopenia than those in Lp(a)<30 mg/dL group, regardless of the different sexes (male: aOR: 2.59, 95% CI: 1.09 to 6.21, p = 0.032; female: aOR: 2.45, 95% CI: 1.06 to 6.03, p = 0.039) (Table 5). In total patients, the interaction between Lp(a) groups and sexes was not statistically significant (p = 0.422).
 |
Table 5 Multivariable Logistic Regression Analysis for Lp(a) and Sarcopenia in Male and Female |
Discussion
In this cross-sectional study of hospitalized T2DM patients aged 18 years and older, we found that patients with sarcopenia had higher Lp(a) levels than those without sarcopenia, and patients in Lp(a)≥ 30mg/dL group had higher prevalence of sarcopenia than those in Lp(a)<30 mg/dL group. Patients in Lp(a)≥30 mg/dL group showed an increased risk of sarcopenia than those in Lp(a)<30 mg/dL group, and this association did not differ by sex.
The association between serum Lp(a) level and sarcopenia has been little studied in previous research. A cross-sectional study of 461 elderly T2DM patients showed that Lp(a) levels were higher in sarcopenia group than in control group, and elevated Lp(a) was a risk factor for muscle loss.15 Consistent with the above study, patients with sarcopenia had higher Lp(a) levels than those without sarcopenia in our study. We also found that T2DM patients in the elevated Lp(a) group had higher prevalence and increased risk of sarcopenia than T2DM patients in the normal Lp(a) group when serum Lp(a) less than 30 mg/dL was defined as the normal range. In addition, our subjects were about 10 years younger than the patients in the above study, so our subjects were middle-aged and elderly patients with T2DM. Therefore, our study complements the above study and provides new evidence for the association between Lp(a) and sarcopenia in middle-aged and elderly patients with T2DM.
A cross-sectional analysis from UK Biobank indicated that the association between Lp(a) and sarcopenia varied by sex in general population.12 Our study also explored whether the association between Lp(a) and sarcopenia in T2DM patients differed by sex. The results showed that in both male and female, T2DM patients in Lp(a)≥30 mg/dL group had an increased risk of sarcopenia than those in Lp(a)<30 mg/dL group. Larger-sample-sized studies are needed to confirm our results. The potential mechanisms underlying the association between Lp(a) and sarcopenia may be related to chronic inflammation. Lp(a) can lead to muscle loss by regulating inflammation. Chronic inflammation reduces muscle primarily by accelerating catabolism, decreasing appetite, increasing insulin resistance, and decreasing levels of growth factors and insulin-like growth factor-1.15,24
In previous studies, the association between lipid metabolism-related parameters, such as TG, TC, HDL-C, LDL-C, apoprotein A and apoprotein B, and sarcopenia exhibited inconsistencies.9–11 In our study, we found that the levels of TC, LDL-C and apoprotein B tended to decrease in patients with sarcopenia, while the levels of HDL-C and apolipoprotein A tended to increase than patients without sarcopenia. Only the TG level in patients with sarcopenia was significantly lower than those without sarcopenia. TG was negatively correlated with sarcopenia in logistic regression analyses. After adjusting for TG, there was also a significant association between Lp(a) as a categorical variable and sarcopenia in patients with T2DM.
The last European and American cholesterol management guidelines recommend the use of Lp(a) for risk stratification in patients at high risk for atherosclerotic cardiovascular disease. The guidelines state that Lp(a) concentrations above 50 mg/dL are considered a significant risk factor for prognosis.25,26 Our study provides a basis for risk stratification of sarcopenia using Lp(a) in patients with T2DM. Our findings suggest that screening for sarcopenia should be emphasized in patients with T2DM with Lp(a)≥30 mg/dL. Some studies have found that PCSK9 inhibitors could lower Lp(a) by 20–30%.27 However, further research is needed to confirm whether Lp(a) lowering measures, such as PCSK9 inhibitors use, can prevent sarcopenia in patients with T2DM. In addition, elevated Lp(a) are consequently associated with an increased risk of adverse clinical events because of the atherogenic and thrombogenic properties of Lp(a).28,29 Therefore, patients with T2DM complicated with sarcopenia should pay attention to the detection of serum Lp(a) level and reduce the high Lp(a) level to reduce the risk of adverse clinical events.
There are several limitations meriting careful consideration. First, this is a cross-sectional study so that the causality between Lp(a) and sarcopenia cannot be definitively established. Second, because the patients in our study came from the same medical facility, the generalizability of our findings might be restricted. Third, Our study did not incorporate significant risk factors such as nutrition and physical activity, potentially limiting our comprehensive understanding of sarcopenia. Finally, the sample size of this study is relatively small. Future studies with larger sample sizes are required.
Conclusion
In hospitalized T2DM patients aged 18 years and older, elevated Lp(a) (≥30 mg/dL) was associated with an increased risk of sarcopenia, and such an association did not differ by sex. The results are of great significance to the risk stratification of screening for sarcopenia in clinical practice. Prospective studies are needed to clarify the causal relationship between elevated Lp(a) and sarcopenia, and to confirm whether lowering Lp(a) can prevent sarcopenia in patients with T2DM.
Abbreviations
ASMI, appendicular skeletal muscle mass index; AWGS, Asian Working Group for Sarcopenia; aOR, adjusted odds ratio; ASCVD, atherosclerotic cardiovascular disease; AGIs, alpha-glucosidase inhibitors; BMI, body mass index; CAD, coronary artery disease; CVD, cardiovascular disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; DR, diabetic retinopathy; DN, diabetic nephropathy; DPN, diabetic peripheral neuropathy; DPP-4, Dipeptidyl peptidase-4; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; GLP1-RAs, glucagon-like peptide-1 receptor agonists; HDL-C, high-density lipoprotein cholesterol; hs-CRP, hypersensitive C-reactive protein; HbA1c, glycosylated hemoglobin; Lp(a), lipoprotein(a); LDL-C, low-density lipoprotein cholesterol; non-HDL-C, non-high-density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9; RLP-C, remnant-like particle cholesterol; SGLT-2is, sodium-glucose cotransporter-2 inhibitors; T2DM, type 2 diabetes mellitus; TG, triglyceride; TC, total cholesterol; TZDs, thiazolidinediones; VLDL-C, very low density lipoprotein cholesterol; 2hPBG, 2 hours postprandial blood glucose.
Data Sharing Statement
The data used to support the findings of this study have not been made available due to patient privacy concerns.
Ethics Approval and Consent to Participate
The study was approved by the Institutional Ethics Research Committee of Longyan First Affiliated Hospital of Fujian Medical University. All participants provided written informed consent prior to enrolment.
Consent for Publication
All authors support the submission to this journal.
Author Contributions
Wen Wei, Fenyan Lv and Shuling Liu contributed equally to this work and share first authorship. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. PubMed PMID: 30312372; PubMed Central PMCID: PMCPMC6322506. doi:10.1093/ageing/afy169
2. Chung SM, Moon JS, Chang MC. Prevalence of sarcopenia and its association with diabetes: a meta-analysis of community-dwelling Asian population. Front Med. 2021;8:681232. PubMed PMID: 34095184; PubMed Central PMCID: PMCPMC8174659. doi:10.3389/fmed.2021.681232
3. Ai Y, Xu R, Liu L. The prevalence and risk factors of sarcopenia in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr. 2021;13(1):93. PubMed PMID: 34479652; PubMed Central PMCID: PMCPMC8414692. doi:10.1186/s13098-021-00707-7
4. Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43(6):748–759. PubMed PMID: 25241753; PubMed Central PMCID: PMCPMC4204661. doi:10.1093/ageing/afu115
5. Fukuda T, Bouchi R, Takeuchi T, et al. Association of diabetic retinopathy with both sarcopenia and muscle quality in patients with type 2 diabetes: a cross-sectional study. BMJ Open Diabetes Res Care. 2017;5(1):e000404. PubMed PMID: 28761661; PubMed Central PMCID: PMCPMC5530250. doi:10.1136/bmjdrc-2017-000404
6. Yang Q, Zhang Y, Zeng Q, et al. Correlation between diabetic peripheral neuropathy and sarcopenia in patients with type 2 diabetes mellitus and diabetic foot disease: a cross-sectional study. Diabetes Metab Syndr Obes. 2020;13:377–386. PubMed PMID: 32104034; PubMed Central PMCID: PMCPMC7025667. doi:10.2147/DMSO.S237362
7. Nakanishi S, Iwamoto M, Shinohara H, Iwamoto H, Kaneto H. Impact of sarcopenia on glycemic control and atherosclerosis in Japanese patients with type 2 diabetes: cross-sectional study using outpatient clinical data. Geriatr Gerontol Int. 2020;20(12):1196–1201. PubMed PMID: 33084163. doi:10.1111/ggi.14063
8. Zhang Y, Weng S, Huang L, Shen X, Zhao F, Yan S. Association of sarcopenia with a higher risk of infection in patients with type 2 diabetes. Diabetes Metab Res Rev. 2022;38(1):e3478. PubMed PMID: 34041847. doi:10.1002/dmrr.3478
9. Vella CA, Nelson MC, Unkart JT, Miljkovic I, Allison MA. Skeletal muscle area and density are associated with lipid and lipoprotein cholesterol levels: the multi-ethnic study of atherosclerosis. J Clin Lipidol. 2020;14(1):143–153. PubMed PMID: 32061531; PubMed Central PMCID: PMCPMC7085431. doi:10.1016/j.jacl.2020.01.002
10. Gong H, Liu Y, Lyu X, Dong L, Zhang X. Lipoprotein subfractions in patients with sarcopenia and their relevance to skeletal muscle mass and function. Exp Gerontol. 2022;159:111668. PubMed PMID: 34954281. doi:10.1016/j.exger.2021.111668
11. Jiang Y, Xu B, Zhang K, et al. The association of lipid metabolism and sarcopenia among older patients: a cross-sectional study. Sci Rep. 2023;13(1):17538. PubMed PMID: 37845303; PubMed Central PMCID: PMCPMC10579328. doi:10.1038/s41598-023-44704-4
12. Petermann-Rocha F, Gray SR, Pell JP, Celis-Morales C, Ho FK. Biomarkers profile of people with sarcopenia: a cross-sectional analysis from UK Biobank. J Am Med Dir Assoc. 2020;21(12):2017e1–e9. PubMed PMID: 32641273. doi:10.1016/j.jamda.2020.05.005
13. Nicholls SJ, Tang WH, Scoffone H, et al. Lipoprotein(a) levels and long-term cardiovascular risk in the contemporary era of statin therapy. J Lipid Res. 2010;51(10):3055–3061. PubMed PMID: 20601648; PubMed Central PMCID: PMCPMC2936758. doi:10.1194/jlr.M008961
14. Thomas PE, Vedel-Krogh S, Kamstrup PR, Nordestgaard BG. Lipoprotein(a) is linked to atherothrombosis and aortic valve stenosis independent of C-reactive protein. Eur Heart J. 2023;44(16):1449–1460. PubMed PMID: 36805188. doi:10.1093/eurheartj/ehad055
15. Li X, Kong X, Li R. Correlation between lipoprotein(a), albuminuria, myostatin and sarcopenia in elderly patients with type 2 diabetes. J Diabetes Complications. 2023;37(1):108382. PubMed PMID: 36535110. doi:10.1016/j.jdiacomp.2022.108382
16. Jager U, Barcellini W, Broome CM, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the First International Consensus Meeting. Blood Rev. 2020;41:100648. PubMed PMID: 31839434. doi:10.1016/j.blre.2019.100648
17. Wilkins JT, Li RC, Sniderman A, Chan C, Lloyd-Jones DM. Discordance between apolipoprotein B and LDL-cholesterol in young adults predicts coronary artery calcification: the CARDIA study. J Am Coll Cardiol. 2016;67(2):193–201. PubMed PMID: 26791067; PubMed Central PMCID: PMCPMC6613392. doi:10.1016/j.jacc.2015.10.055
18. Seidu S, Barrat J, Khunti K. Clinical update: the important role of dual kidney function testing (ACR and eGFR) in primary care: identification of risk and management in type 2 diabetes. Prim Care Diabetes. 2020;14(4):370–375. PubMed PMID: 32139245. doi:10.1016/j.pcd.2020.02.006
19. Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–1682. PubMed PMID: 13129861. doi:10.1016/S0161-6420(03)00475-5
20. de Boer IH, Khunti K, Sadusky T, et al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: improving Global Outcomes (KDIGO). Diabetes Care. 2022;45(12):3075–3090. PubMed PMID: 36189689; PubMed Central PMCID: PMCPMC9870667. doi:10.2337/dci22-0027
21. Jia W, Weng J, Zhu D, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. 2019;35(6):e3158. PubMed PMID: 30908791. doi:10.1002/dmrr.3158
22. Chen Z, Jiang C, Qu H, et al. Association of lipoprotein(a) and major adverse cardiovascular events in patients with percutaneous coronary intervention. Arch Med Sci. 2019;15(6):1375–1380. PubMed PMID: 31749864; PubMed Central PMCID: PMCPMC6855154. doi:10.5114/aoms.2018.79401
23. Wei W, Xie C, Cao R, et al. Ultrasound assessment of the gastrocnemius muscle as a potential tool for identifying sarcopenia in patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2023;16:3435–3444. PubMed PMID: 37929058; PubMed Central PMCID: PMCPMC10624255. doi:10.2147/DMSO.S435517
24. Nishikawa H, Asai A, Fukunishi S, Nishiguchi S, Higuchi K. Metabolic Syndrome and Sarcopenia. Nutrients. 2021;13(10):3519. PubMed PMID: 34684520; PubMed Central PMCID: PMCPMC8541622. doi:10.3390/nu13103519
25. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):3168–3209. PubMed PMID: 30423391. doi:10.1016/j.jacc.2018.11.002
26. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. PubMed PMID: 31504418. doi:10.1093/eurheartj/ehz455
27. Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69(6):692–711. PubMed PMID: 28183512. doi:10.1016/j.jacc.2016.11.042
28. Wu MF, Xu KZ, Guo YG, Yu J, Wu Y, Lin LM. Lipoprotein(a) and atherosclerotic cardiovascular disease: current understanding and future perspectives. Cardiovasc Drugs Ther. 2019;33(6):739–748. PubMed PMID: 31655942. doi:10.1007/s10557-019-06906-9
29. Jin JL, Cao YX, Zhang HW, et al. Lipoprotein(a) and cardiovascular outcomes in patients with coronary artery disease and prediabetes or diabetes. Diabetes Care. 2019;42(7):1312–1318. PubMed PMID: 31076417. doi:10.2337/dc19-0274
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
The Association Between Sarcopenia and Diabetes: From Pathophysiology Mechanism to Therapeutic Strategy
Chen H, Huang X, Dong M, Wen S, Zhou L, Yuan X
Diabetes, Metabolic Syndrome and Obesity 2023, 16:1541-1554
Published Date: 30 May 2023
Imaging of Sarcopenia in Type 2 Diabetes Mellitus
Wang D, Zhang G, Yu Y, Zhang Z
Clinical Interventions in Aging 2024, 19:141-151
Published Date: 26 January 2024



