Back to Journals » Infection and Drug Resistance » Volume 18
Liver Function Abnormalities in Patients with Chlamydia psittaci Pneumonia: A Multicenter Retrospective Study
Authors Fang C , Xu L, Lu J, Li Y, Zhao Z
Received 18 April 2025
Accepted for publication 21 June 2025
Published 28 June 2025 Volume 2025:18 Pages 3207—3217
DOI https://doi.org/10.2147/IDR.S535247
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sandip Patil
Changquan Fang,1,2,* Limin Xu,3,* Jiancong Lu,2 Yujun Li,4,5 Ziwen Zhao1,4,5
1The First Affiliated Hospital of Jinan University, Guangzhou, Guangdong, People’s Republic of China; 2Department of Pulmonary and Critical Care Medicine, Huizhou Central People’s Hospital, Huizhou, Guangdong, People’s Republic of China; 3Department of Geriatrics, Huizhou First Hospital, Huizhou, Guangdong, People’s Republic of China; 4Department of Pulmonary and Critical Care Medicine, Guangzhou First People’s Hospital, South China University of Technology, Guangzhou, Guangdong, People’s Republic of China; 5Department of Pulmonary and Critical Care Medicine, Guangzhou First People’s Hospital, Guangzhou Medical University, Guangzhou, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yujun Li; Ziwen Zhao, Department of Pulmonary and Critical Care Medicine, Guangzhou First People’s Hospital, South China University of Technology, No. 1 Panfu Road, Yuexiu District, Guangzhou City, Guangdong Province, People’s Republic of China, Tel +86 13570574008 ; +86 13006872260, Email [email protected]; [email protected]
Background: Some patients with Chlamydia psittaci pneumonia exhibit liver function abnormalities. In this study, we aimed to elucidate the characteristics of liver function changes and the factors influencing liver injury in patients with Chlamydia psittaci pneumonia, providing a reference for clinical treatment.
Methods: The clinical data of patients with Chlamydia psittaci pneumonia admitted to three tertiary Grade A hospitals in Guangdong Province, China, from January 2020 to February 2025 were retrospectively collected. Changes in liver parameters and related influencing factors upon admission were analyzed.
Results: Overall, 120 cases were included: 100 (83.3%) exhibited liver function abnormalities and 55 (45.8%) had liver injury. The incidence of liver function abnormalities and liver injury was significantly higher in the severe group than that in the mild group. Liver function abnormalities and injury associated with Chlamydia psittaci pneumonia were characterized by elevations in alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyltransferase (GGT) levels. AST and ALT levels exceeded three times the upper limit of normal (ULN) in 43 (35.8%) and 20 (16.7%) cases, respectively, while GGT exceeded twice the ULN in 25 (20.8%) cases. ALT (70 [47– 115] vs 51 [26– 73] U/L, p = 0.002) and AST (122 [72– 252] vs 52 [30– 76] U/L, p = 0.000) were significantly different between the severe and mild groups. Hepatocellular injury was the most common type of liver injury upon admission, followed by mixed and cholestatic types. Compared to patients without liver injury, those with liver injury had a higher prevalence of alcohol consumption history, dyspnea, higher pneumonia severity index scores, and longer hospital stays.
Conclusion: Patients with Chlamydia psittaci pneumonia, particularly severe cases, are prone to concurrent liver function abnormalities and liver injury. Liver injury, predominantly hepatocellular injury, was associated with factors such as alcohol consumption history, pneumonia severity, and elevated inflammatory responses, leading to prolonged hospital stays. Monitoring liver function may aid in early identification of severe cases.
Keywords: Chlamydia psittaci, psittacosis, pneumonia, liver injury
Introduction
Chlamydia psittaci (C. psittaci), an obligate intracellular gram-negative bacterium, is a potent zoonotic pathogen capable of causing a spectrum of diseases ranging from mild, nonspecific illnesses to severe systemic conditions that primarily manifest as pneumonia and can involve damage to multiple systemic organs.1–3 Approximately 1.03% of community acquired pneumonia is caused by C. psittaci infection.2 Most patients present with typical symptoms, including high fever, chills, headache, myalgia, and dyspnea.1 Historically, this condition has been under-recognized due to diagnostic challenges with conventional methods and its low incidence.3 However, with the widespread application of high-throughput sequencing technologies in infectious diseases, the number of clinically reported C. psittaci pneumonia cases has gradually increased.
As clinical case data accumulate, it has been observed that C. psittaci pneumonia is often accompanied by varying degrees of liver function abnormalities.1,4,5 A small-scale, single-center clinical study reported that liver function abnormalities and liver injury occurred in 84.8% and 50% of patients with C. psittaci pneumonia, respectively, primarily manifesting as elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and gamma-glutamyltransferase (GGT).6 However, studies on the characteristics and mechanisms of liver function abnormalities caused by C. psittaci pneumonia are limited, with small sample sizes. This study summarizes clinical data from 120 patients with C. psittaci pneumonia across multiple centers, analyzing the characteristics and extent of liver function abnormalities, differences across disease severities, and factors influencing liver injury in patients with C. psittaci pneumonia.
Methods
Study Design and Subjects
Clinical data from 120 patients with C. psittaci pneumonia, admitted to Huizhou Central People’s Hospital, Huizhou First Hospital, and Guangzhou First People’s Hospital, from January 2020 to February 2025, were retrospectively collected in this study. The inclusion criteria were: (1) meeting the diagnostic criteria for community-acquired pneumonia;7 (2) detection of C. psittaci gene sequences in bronchoalveolar lavage fluid or peripheral blood samples using metagenomic next-generation sequencing (mNGS) or targeted next-generation sequencing (tNGS); (3) age ≥ 18 years; and (4) availability of complete basic information, including sex, age, underlying diseases, and body mass index (BMI), as well as laboratory data such as complete blood count, liver and kidney function tests, and high-sensitivity C-reactive protein (CRP) and procalcitonin (PCT) levels. Patients with pre-existing liver diseases (eg, viral hepatitis, alcoholic liver disease, or cirrhosis) were excluded. The diagnosis and clinical classification of C. psittaci pneumonia adhered to the Diagnosis and Treatment of Adults with Community-Acquired Pneumonia: An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America (2019).7 The diagnosis of severe community-acquired pneumonia includes primary criteria (septic shock requiring vasoactive drugs or respiratory failure requiring mechanical ventilation) and secondary criteria (respiratory rate ≥ 30 times/minute; PaO2/FiO2 ≤ 250 mmHg; multiple lung lobe infiltrations; consciousness disorders and/or orientation disorders; blood urea nitrogen ≥ 20 mg/L; decreased white blood cells [WBC < 4 × 109/L]; thrombocytopenia [platelets <100 × 109/L]; low body temperature [body temperature < 36 °C]; hypotension requiring rapid fluid replacement for correction). If one of the primary criteria or at least three secondary criteria are met, severe pneumonia is diagnosed.7
Laboratory Assessments
Metagenomics Next-Generation Sequencing (mNGS)
Per clinical protocol, 5–10 mL of bronchoalveolar lavage fluid was collected from patients and sent to the DaAn Gene Sequencing Platform (DaAn Gene Co, Ltd., Sun Yat-sen University, Guangzhou) for mNGS testing. The process involved nucleic acid extraction, library construction, and sequencing. After obtaining sequencing data, Burrows−Wheeler Alignment (BWA; http://bio-bwa.sourceforge.net/) was used for alignment. Human reference genome sequences were removed from high-quality data, and low-complexity sequences were further excluded. The remaining data were compared against a specialized microbial database to determine the number of sequences matching specific pathogenic microorganisms.
Targeted Next-Generation Sequencing (tNGS)
Per clinical protocol, 5 mL of bronchoalveolar lavage fluid was collected and sent to the Guangzhou DaAn Clinical Testing Center for tNGS testing. The process included nucleic acid extraction, multiplex polymerase-chain reaction library construction, and sequencing. Analysis, interpretation, and report generation were completed using the independently developed DAMicrob Pathogen Analysis Reporting System.
Liver Test Parameters and Abnormalities
Liver function abnormalities were defined as any of the following exceeding the upper limit of normal (ULN): ALT > 1× ULN (40 U/L), AST > 1× ULN (35 U/L), ALP > 1× ULN (125 U/L), GGT > 1× ULN (60 U/L), or total bilirubin (TBIL) > 1× ULN (23 μmol/L). Liver injury was defined as meeting one or more of the following: ALT > 3× ULN, AST > 3× ULN, ALP > 2× ULN, GGT > 2× ULN, or TBIL > 2× ULN. Liver function abnormalities were classified as hepatocellular (ALT and/or AST > 3× ULN), cholestatic (ALP and/or GGT > 2× ULN), or mixed (ALT and/or AST > 3× ULN and ALP and/or GGT > 2× ULN).6,8
Data Collection
A case report form was established to record basic patient information, including age, sex, time from symptom onset to admission, hospital stay duration, BMI, alcohol consumption history, and underlying disease history. Clinical manifestations upon admission, routine laboratory tests post-admission (eg, complete blood count, high-sensitivity CRP, PCT, creatine kinase [CK], lactate dehydrogenase [LDH], liver and kidney function, coagulation function, and cardiac function parameters), disease severity, and prognosis were also documented.
Statistical Analysis
All data were analyzed using SPSS 25.0 software. Normally distributed continuous data were expressed as mean ± standard deviation (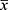 ± s), with between-group differences compared using the t-test and correlations analyzed using Pearson correlation analysis. Non-normally distributed data were expressed as median (interquartile range) [M (P25–P75)], with between-group differences compared using the Mann−Whitney U-test and correlations analyzed using Spearman correlation analysis. Categorical data were expressed as counts (percentages) and analyzed using the chi-square test. P < 0.05 was considered statistically significant.
± s), with between-group differences compared using the t-test and correlations analyzed using Pearson correlation analysis. Non-normally distributed data were expressed as median (interquartile range) [M (P25–P75)], with between-group differences compared using the Mann−Whitney U-test and correlations analyzed using Spearman correlation analysis. Categorical data were expressed as counts (percentages) and analyzed using the chi-square test. P < 0.05 was considered statistically significant.
Results
Clinical Characteristics of Patients with C. Psittaci Pneumonia
The data of 132 patients with C. psittaci pneumonia were initially collected; 8 with chronic liver diseases and 4 with incomplete clinical data were excluded, leaving the data of 120 patients for analysis. There were 69 males (57.5%) and 51 females (42.5%), with a mean age of 58.9±11.6 years. The median time from symptom onset to admission was 5 (4–7) days, and the total hospital stay was 10 (8–14) days. Sixty-four patients had underlying diseases, including hypertension (32 cases), diabetes (25 cases), and coronary heart disease (10 cases). Four patients died, while the rest recovered and were discharged (Table 1).
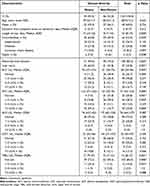 |
Table 1 Abnormal Liver Function in Patients with Chlamydia psittaci Pneumonia |
Among the 120 patients, 100 (83.3%) had liver function abnormalities, and 55 (45.8%) had liver injury (see Figure 1). The severe group included 54 patients, and the mild group included 66 patients. The incidence of liver function abnormalities and liver injury was significantly higher in the severe group than that in the mild group. The incidences of elevated AST, ALT, GGT, ALP, and TBIL levels in the severe group were 92.6%, 88.9%, 42.6%, 29.6%, and 27.8%, respectively, compared to 68.2%, 60.6%, 39.4%, 21.2%, and 4.5% in the mild group. There were significant differences in ALT (70 [47–115] vs 51 [26–73] U/L, p = 0.002), AST (122 [72–252] vs 52 [30–76] U/L, p = 0.000), and TBIL (17 [9–27] vs 10 [7–15] U/L, p = 0.000) levels between the severe and mild groups (Table 1).
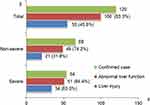 |
Figure 1 Liver test abnormality at admission in patients with Chlamydia psittaci pneumonia by severity of disease. (Bars represent number of patients). |
Clinical Characteristics of Patients with C. Psittaci Pneumonia and Abnormal Liver Function Results
As shown in Table 2, the incidence of elevated AST, ALT, GGT, ALP, and TBIL levels in patients with C. psittaci pneumonia was 78.3%, 72.5%, 40.8%, 25.0%, and 15.0%, respectively.
 |
Table 2 Liver Function Parameters of Patients with Chlamydia psittaci Pneumonia |
As shown in Table 3, among the 100 patients with liver function abnormalities, 51 were severe cases and 49 were mild; no patient progressed to liver failure. The primary manifestations were mild elevations in AST, ALT, and GGT levels, with peak values of 1233 U/L, 617 U/L, and 624 U/L, respectively. Significant elevations (AST and ALT > 3× ULN) occurred in 43 and 20 cases, respectively, while marked elevations (GGT, TBIL, and ALP > 2× ULN) occurred in 25, 6, and 5 cases, respectively. The mild group predominantly showed mild AST elevation, whereas the severe group exhibited significantly higher AST and TBIL levels and a higher proportion of AST > 3× ULN, with no significant differences in ALT, ALP, or GGT between groups.
 |
Table 3 Clinical Characteristics of 100 Patients with Chlamydia psittaci Pneumonia and Abnormal Liver Test Results |
Clinical Characteristics of Patients with C. Psittaci Pneumonia and Liver Injury
As shown in Table 2, the incidences of markedly elevated AST, GGT, ALT, TBIL, and ALP levels in patients with C. psittaci pneumonia were 35.8%, 20.8%, 16.7%, 5.0%, and 4.2%, respectively.
As shown in Table 4, among the 55 patients with liver injury, severe cases accounted for 61.8%; 31 (56.4%) were hepatocellular, 10 (18.2%) were cholestatic, and 14 (25.5%) were mixed. Severe patients were more prone to hepatocellular injury, with significantly higher AST, GGT, and TBIL levels in the severe group compared to the mild group.
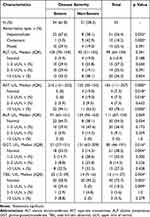 |
Table 4 Clinical Characteristics of Patients with Chlamydia psittaci Pneumonia and Liver Injury |
As shown in Table 5, patients with liver injury had a higher prevalence of alcohol consumption history, dyspnea, and higher pneumonia severity index (PSI) scores, as well as longer hospital stays. Compared to those without liver injury, the liver injury group showed significantly elevated WBC, neutrophil–lymphocyte ratio (NLR), CRP, PCT, CK, LDH, and D-dimer levels, and significantly reduced serum albumin levels.
 |
Table 5 Clinical Characteristics of 120 Patients with Chlamydia psittaci Pneumonia |
Correlation analysis revealed that ALT and AST levels were significantly positively correlated with PSI scores (r = 0.298, p = 0.001; r = 0.514, p = 0.000), NLR (r = 0.2, p = 0.028; r = 0.421, p = 0.000), CRP (r = 0.182, p = 0.047; r = 0.347, p = 0.000), PCT (r = 0.283, p = 0.002; r = 0.549, p = 0.000), CK (r = 0.404, p = 0.000; r = 0.565, p = 0.000), LDH (r = 0.595, p = 0.000; r = 0.779, p = 0.000), and D-dimer (r = 0.345, p = 0.000; r = 0.583, p = 0.000), while AST was significantly negatively correlated with albumin (r = −0.283, p = 0.002) (see Figure 2).
Discussion
This study elucidated the characteristics of liver function changes and the factors influencing liver injury in patients with C. psittaci pneumonia. The incidence of liver function abnormalities and liver injury was significantly higher in the severe group compared to that in the mild group. Hepatocellular injury was the most common type of liver injury upon admission. Compared to patients without liver injury, those with liver injury had a higher prevalence of alcohol consumption history, dyspnea, higher PSI scores and longer hospital stays, though mortality rates did not increase.
Upon entering the human body via the respiratory tract, C. psittaci first invades epithelial cells and then proliferates within inclusions in mononuclear macrophages, evading host immune defenses and lysosomal phagocytosis. Due to the widespread presence of the mononuclear phagocytic system, human infection with C. psittaci can lead to multisystem symptoms, including pneumonia, hepatitis, and myocardial injury.9–11 As a systemic disease primarily affecting the respiratory system, the pathogenesis and clinical features of C. psittaci pneumonia are incompletely understood. Several studies have reported varying degrees of liver function abnormalities in patients with C. psittaci pneumonia,1,4,5 yet targeted research remains limited. Our study found that among 120 patients with C. psittaci pneumonia, 83.3% exhibited liver function abnormalities and 45.8% developed liver injury, results that closely align with those by Guo X et al.6 Guo et al ‘s single-center study included only 46 patients.6 Unlike prior studies, we conducted a multi-center study with a larger sample size, and we performed stratified comparisons on liver biochemical parameters. We compared liver biochemical parameters across different disease severities, revealing that severe cases are more prone to liver function abnormalities and injury. Collectively, these findings suggest that C. psittaci pneumonia, particularly in severe cases, is frequently complicated by liver dysfunction and injury. In clinical practice, this highlights the need for awareness regarding possible C. psittaci infection in patients with pneumonia presenting with extrapulmonary complications, especially liver function abnormalities.
To systematically analyze the liver function abnormalities associated with C. psittaci pneumonia, we examined and stratified liver biochemical parameters upon admission. These included ALT and AST (reflecting hepatocyte injury), ALP and GGT (indicating bile duct damage), and TBIL (assessing liver clearance and bile secretion capacity).8,12 These markers exhibited varying degrees of abnormality in patients with C. psittaci pneumonia, predominantly with elevations in ALT, AST, and GGT levels, consistent with previous research.6,13 Further analysis showed that the severe group had significantly higher levels and abnormality rates of ALT, AST, and TBIL compared to those in the mild group. While prior studies rarely stratified these parameters, our comparative analysis revealed that mild cases predominantly exhibited mild AST elevations, whereas severe cases showed significantly higher AST and TBIL levels, with a greater proportion of AST values exceeding three times the ULN. These findings indicate that the abnormality rates of AST, ALT, and TBIL are closely linked to disease severity.
Given the clinical rarity of C. psittaci pneumonia,1,13 studies on its associated liver injury are sparse, and uncertainties persist regarding its definition, clinical presentation, diagnosis, and treatment. Referencing definitions of liver injury related to novel coronavirus infection,14 we termed liver damage occurring during the progression and treatment of C. psittaci pneumonia as C. psittaci pneumonia-related liver injury. We found that hepatocellular injury was the most common type, followed by mixed and cholestatic patterns. The mechanisms underlying C. psittaci-induced liver injury are still unclear, but several possibilities arise. First, direct damage to liver tissue by the pathogen is conceivable. After infecting the host, C. psittaci proliferates in the mononuclear phagocytic system of the liver and spleen, then disseminates hematogenously to organs such as the liver, kidneys, and nervous system, resulting in systemic disease.10,11 Our data showed elevated ALT, AST, ALP, and GGT (indicative of hepatocyte and bile duct injury), alongside significant increases in CK and LDH, suggesting that C. psittaci may also affect other tissues and organs, such as the heart and muscles, beyond the liver. Second, stress and systemic inflammatory responses triggered by the infection may contribute. The strong pathogenicity by C. psittaci can trigger a robust systemic inflammatory response, with progressive lymphopenia and rising inflammatory cytokines (eg, interleukin-6 and tumor necrosis factor-alpha) in some severe patients,15–17 potentially leading to a cytokine storm. This uncontrolled inflammation may exacerbate nonspecific immune-inflammatory responses in the liver, causing secondary injury. Our results support this, showing significantly higher WBC, NLR, CRP, and PCT levels in patients with liver injury compared to those without, with NLR, CRP, and PCT positively correlated with liver injury. These findings suggest that immune-mediated inflammation following C. psittaci infection may drive or exacerbate liver damage. Third, pneumonia-related hypoxia may play a role. Severe C. psittaci pneumonia is prone to complications such as respiratory failure, sepsis, and multiorgan failure,17,18 reducing hepatic blood perfusion and inducing hypoxic liver injury. We found a higher prevalence of dyspnea and elevated PSI scores in patients with liver injury, with PSI scores positively correlated with liver damage, indicating that pneumonia-associated hypoxia may promote the onset and progression of liver injury. Fourth, medications used clinically may have contributed to the findings. Many patients, particularly those with severe cases, received multiple drugs, including antipyretics/analgesics (eg, acetaminophen), antibiotics (eg, tetracyclines, quinolones, macrolides), and steroids, which may potentially be hepatotoxic.19–21 To minimize the influence of drugs and pre-existing liver diseases, we analyzed liver biochemical parameters upon admission and excluded patients with prior liver conditions. Notably, we found a higher prevalence of alcohol consumption history among patients with liver injury, suggesting that those with underlying hepatic vulnerability may be more susceptible to C. psittaci-related liver damage. Patients with liver injury had longer hospital stays but no increase in mortality. This may be attributed, on the one hand, to greater pneumonia severity necessitating extended hospitalization and, on the other, to the absence of progression to liver failure, with significant liver function improvement following targeted anti-infective and hepatoprotective treatments.
This study had some limitations. As a retrospective analysis, some cases lacked complete current medical history data. Additionally, many patients had used antipyretics, analgesics, or antibiotics prior to admission, with unspecified types, doses, and durations, potentially affecting statistical outcomes.
Conclusion
C. psittaci pneumonia, particularly in severe cases, is associated with a high incidence of liver function abnormalities and liver injury. Among liver biochemical parameters, elevated AST and ALT predominate, with more pronounced increases in severe patients. Liver injury in C. psittaci pneumonia is likely related to factors such as alcohol consumption history, pneumonia severity, and elevated inflammatory responses. In clinical practice, monitoring liver function may aid in early identification of severe cases.
Abbreviations
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; CRP, C-reactive protein; GGT, gamma-glutamyltransferase; LDH, lactate dehydrogenase; NLR, neutrophil-lymphocyte ratio; PCT, procalcitonin; PSI, pneumonia severity index; ULN, upper limit of normal; WBC, white blood cell.
Data Sharing Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
The Ethics Committees of the Huizhou Central People’s Hospital, Huizhou First Hospital, and Guangzhou First People’s Hospital jointly approved this study. This study follows the Helsinki Declaration. All patients and legal guardians provided informed consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Medical Scientific Research Foundation of Guangdong Province of China (No. B2024131).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Liu K, Wu L, Chen G. et al. Clinical characteristics of Chlamydia psittaci infection diagnosed by metagenomic next-generation sequencing: a retrospective multi-center study in Fujian, China. Infect Drug Resist. 2024;17:697–708. doi:10.2147/IDR.S443953
2. Hogerwerf L, Gier DE, Baan B, et al. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect. 2017;145(15):3096–3105. doi:10.1017/S0950268817002060
3. Jia Q, Sun J, Wang D, et al. Clinical features and prognostic predictors of C. Psittaci Pneumonia: a systematic review and individual patient meta-analysis. BMC Pulm Med. 2025;25(1):55. doi:10.1186/s12890-025-03511-5
4. Chen J, Wang J, Deng Z, et al. Clinical features of 50 cases of Chlamydia psittaci pneumonia identified through metagenomic next-generation sequencing. Infect Drug Resist. 2024;17:5775–5784. doi:10.2147/IDR.S493927
5. Lu Y, Gai W, Li M, et al. Psittacosis pneumonia features, distinguishing characteristics, and outcomes: a retrospective study. Infect Drug Resist. 2024;17:5523–5533. doi:10.2147/IDR.S482471
6. Guo X, Zhu D, Chen H. Clinical features and risk factors of liver injury in patients with Chlamydia psittaci pneumonia- a retrospective analysis. Front Cell Infect Microbiol. 2024;13:1320758. doi:10.3389/fcimb.2023.1320758
7. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. doi:10.1164/rccm.201908-1581ST
8. Cai Q, Huang D, Yu H, et al. COVID-19: abnormal liver function tests. J Hepatol. 2020;73(3):566–574. doi:10.1016/j.jhep.2020.04.006
9. Yang M, Yang DH, Yang H, et al. Clinical Characteristics of Chlamydia psittaci Pneumonia Infection in Central South China. Infect Dis Ther. 2022;11(4):1631–1647. doi:10.1007/s40121-022-00662
10. Radomski N, Einenkel R, Müller A, et al. Chlamydia–host cell interaction not only from a bird’s eye view: some lessons from Chlamydia psittaci. FEBS Lett. 2016;590(21):3920–3940. doi:10.1002/1873-3468.12295
11. Knittler MR, Sachse K. Chlamydia psittaci: update on an underestimated zoonotic agent. Pathog Dis. 2015;73(1):1–15. doi:10.1093/femspd/ftu007
12. Fan Z, Chen L, Li J, et al. Clinical Features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18(7):1561–1566. doi:10.1016/j.cgh.2020.04.002
13. Li H, Hao B, Wang Y, et al. Metagenomic next-generation sequencing for the diagnosis of Chlamydia psittaci pneumonia. Clin Respir J. 2022;16(7):513–521. doi:10.1111/crj.13519
14. Wu X, Li Y, Zhang M, et al. Etiology of severe community-acquired pneumonia in adults based on metagenomic next-generation sequencing: a prospective multi-center study. Infect Dis Ther. 2020;9(4):1003–1015. doi:10.1007/s40121-020-00353-y
15. Zhang Z, Wang P, Ma C, et al. Host inflammatory response is the major factor in the progression of Chlamydia psittaci pneumonia. Front Immunol. 2022;13:929213. doi:10.3389/fimmu.2022.929213
16. Huang M, Wang Y, Lu Y, et al. Clinical characteristics and predicting disease severity in Chlamydia psittaci infection based on metagenomic next-generation sequencing. Infect Drug Resist. 2025;18:1171–1181. doi:10.2147/IDR.S509879
17. Tang X, Wang N, Liu G, et al. Psittacosis caused severe community-acquired pneumonia accompanied by acute hypoxic respiratory failure: a multi-center retrospective cohort study from China. BMC Infect Dis. 2023;23(1):532. doi:10.1186/s12879-023-08283-z
18. Ni Y, Zhong H, Gu Y, et al. Clinical features, treatment, and outcome of psittacosis pneumonia: a multi-center Study. Open Forum Infect Dis. 2023;10(2):ofac518. doi:10.1093/ofid/ofac518
19. Ramachandran A, Jaeschke H. Acetaminophen hepatotoxicity. Semin Liver Dis. 2019;39(2):221–234. doi:10.1055/s-0039-1679919
20. Low EXS, Zheng Q, Chan E, et al. Drug induced liver injury: east versus West - a systematic review and meta-analysis. Clin Mol Hepatol. 2020;26(2):142–154. doi:10.3350/cmh.2019.1003
21. Wicherski J, Peltner J, Becker C, et al. High risk for life-threatening adverse events of fluoroquinolones in young adults: a large German population-based cohort study. BMC Med. 2025;23(1):76. doi:10.1186/s12916-025-03919-0
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Omadacycline for the Treatment of Severe Chlamydia psittaci Pneumonia Complicated with Multiple Organ Failure: A Case Report
Fang C, Xu L, Tan J, Tan H, Lin J, Zhao Z
Infection and Drug Resistance 2022, 15:5831-5838
Published Date: 4 October 2022
Psittacosis Pneumonia: Diagnosis, Treatment and Interhuman Transmission
Cui Z, Meng L
International Journal of General Medicine 2023, 16:1-6
Published Date: 4 January 2023
Chlamydia Psittaci Pneumonia-Induced Myocarditis: A Case Report
Yang X, Liu Z, Liu X, Li Q, Huang H, Li R, He M
Infection and Drug Resistance 2023, 16:4259-4264
Published Date: 29 June 2023


