Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 20
Low-Density Lipoprotein Cholesterol as a Protective Factor in COPD and Implications for Statin Therapy: A Multi-Omics Genetic Epidemiology Study
Authors Du T , Cao J, Dai Z, Xie X, Zhang G, Li Y, Chen B, Xu T, Feng J
Received 10 January 2025
Accepted for publication 29 June 2025
Published 14 July 2025 Volume 2025:20 Pages 2409—2422
DOI https://doi.org/10.2147/COPD.S516906
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Tiantao Du,1,* Jianye Cao,1,* Ziyao Dai,1 Xianting Xie,1 Guoshu Zhang,1 Yulin Li,1 Baiyu Chen,1 Tao Xu,2 Jia Feng3
1Clinical Medical College, Southwest Medical University, Luzhou, People’s Republic of China; 2Department of Thoracic Surgery, The Affiliated Hospital of Southwest Medical University, Luzhou, People’s Republic of China; 3Department of Laboratory Medicine, The Affiliated Hospital of Southwest Medical University, Sichuan Province Engineering Technology Research Center of Molecular Diagnosis of Clinical Diseases, Molecular Diagnosis of Clinical Diseases Key Laboratory of Luzhou, Luzhou, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tao Xu, Email [email protected] Jia Feng, Email [email protected]
Background: Chronic obstructive pulmonary disease (COPD) is a complex lung condition primarily affecting the airways and alveoli, characterized by persistent progressive airflow limitation. COPD ranks as the third leading cause of death worldwide, with its incidence and mortality rates escalating annually due to an aging population. This study aimed to explore the association between low-density lipoprotein cholesterol (LDL-C) and COPD, as well as the impact of statin drugs on the progression of COPD.
Methods: Employing an integrated approach that encompasses observational studies, genetic epidemiology, and molecular biology, this research investigated the link between LDL-C and COPD using clinical survey data, genome-wide association study (GWAS) data, and transcriptomic data. Additionally, it assessed the potential role of statin drugs in the treatment of COPD.
Results: The study discovered that LDL-C serves as a protective factor for COPD, and statin drugs may promote the progression of COPD by reducing LDL-C levels. This finding provides a new perspective on the metabolic disruptions in COPD and offers significant guidance for future therapeutic strategies.
Conclusion: This research confirms the inverse correlation between LDL-C and COPD and reveals that statin drugs might influence the progression of COPD by affecting LDL-C levels. These findings underscore the importance of considering metabolic factors in COPD management and suggest new directions for therapeutic strategies.
Keywords: COPD, LDL-C, statins, NHANES, Mendelian Randomization, multicohomology
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a multifaceted pulmonary disorder that manifests as airway pathology, encompassing conditions such as bronchitis and bronchiectasis, alongside alveolar abnormalities, notably emphysema. This disease is distinguished by its persistent and progressive airflow limitation, which is accompanied by chronic respiratory symptoms including, but not limited to, dyspnea, cough, and expectoration of sputum.1–4 COPD stands as the third leading cause of mortality and the seventh leading cause of morbidity worldwide, imposing a substantial health burden on global populations.5,6 Amidst the demographic shift towards an aging global population, there is a concomitant escalation in the incidence and mortality rates associated with COPD.7,8 Projections for the year 2060 anticipate that COPD and its comorbidities will claim the lives of over 5.4 million individuals annually.9 The escalating disease burden underscores the imperative for heightened focus on the preventative strategies and therapeutic management of COPD.10–12
COPD is a multifaceted condition that implicates dysfunction across various metabolic pathways, including amino acid metabolism, lipid metabolism, mitochondrial dysfunction, and oxidative stress.13–16 Particularly noteworthy is the aberrant lipid metabolism, which exerts a significant influence on the pathogenesis of COPD. Lipid metabolism is intricately involved not only in energy production and the construction of cell membranes but also in the modulation of inflammatory responses and oxidative stress.17,18 The biological role of low-density lipoprotein cholesterol (LDL-C), a pivotal component of lipid metabolism, extends beyond its association with cardiovascular disease to include connections with inflammation and oxidative stress.19,20 This suggests a potentially intimate link between LDL-C and the development of COPD. However, research exploring the relationship between LDL-C and COPD is rather scarce, and the findings have been somewhat inconsistent. For instance, a study conducted by Beti Zafirova-Ivanovska et al reported that LDL-C levels were elevated in COPD patients when compared to a control group.21 In contrast, a separate study led by Ivona Markelić et al arrived at an opposing conclusion.22
Furthermore, the interplay between statins and COPD has garnered significant interest within the intricate framework of COPD pathophysiology. A multitude of studies have demonstrated a strong correlation between statin use and COPD, suggesting that statins may offer therapeutic benefits by modulating inflammatory responses and enhancing pulmonary function.23,24 One of the primary mechanisms by which statins exert their effects is through the reduction of serum LDL-C levels.25–27 However, the impact of statins on COPD outcomes is a subject of debate, with some research indicating that statins can ameliorate the frequency and severity of COPD exacerbations,28 while other studies have reported contradictory findings.29,30 These divergent results underscore the necessity for additional investigation into the role of LDL-C and the potential therapeutic role of statins in the management of COPD.
Given the conflicting evidence regarding the relationship between LDL-C and COPD, as well as the effects of statins on COPD, our study sought to elucidate the connection between LDL-C and COPD from various angles. We investigated both the direct associations between LDL-C and COPD, and the indirect associations mediated by statins. Employing an integrated methodology, we analyzed clinical survey data, genome-wide association study (GWAS) data, and transcriptomic data, harnessing the power of multiple databases in a synergistic approach to bolster our findings. Our research confirms that LDL-C acts as a protective factor against COPD, while statins may exacerbate the disease by reducing LDL-C levels. This discovery not only sheds new light on the metabolic underpinnings of COPD but also offers crucial insights for the development of future therapeutic strategies. It underscores the imperative to consider metabolic factors in the comprehensive management of COPD.
Method
Study Design
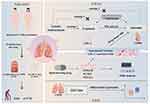
The study, as depicted in Figure 1, is meticulously designed and conducted in three distinct phases. Initially, we leveraged the National Health and Nutrition Examination Survey (NHANES) database to conduct an observational study aimed at establishing whether there is a correlation between LDL-C levels and COPD. In the second phase, we utilized GWAS data and advanced our investigation by employing Mendelian Randomization (MR) and Summary Mendelian Randomization (SMR) methods to delve into the specific causal relationships between low-density lipoprotein cholesterol and chronic obstructive pulmonary disease across different populations, particularly European and East Asian cohorts, and to further confirm the impact of statin drugs on COPD, thereby substantiating the role of LDL-C in modulating the risk of chronic obstructive pulmonary disease. The final stage involved the use of the Gene Expression Omnibus (GEO) database to confirm the differential expression patterns of genes associated with LDL-C metabolism between COPD patients and healthy controls.
Data Sources
Data Sources and Study Population for the NHANES Analysis
NHANES utilizes a sophisticated, multistage sampling and weighting strategy aimed at capturing a sample that accurately reflects the US noninstitutionalized civilian population. This comprehensive survey is dedicated to evaluating the health and nutritional status of this demographic. In our quest to explore the correlation between LDL-C and COPD, we included participants from the NHANES cycles of 2011/2012, 2013/2014, and 2015/2016. Initially, we identified a cohort of 11,184 individuals aged 40 years or older. We then excluded 25 participants due to the absence of data on COPD prevalence. Following this, 6449 participants were further excluded for lacking information on LDL-C. After these exclusions, our study cohort was comprised of 4710 subjects, including 242 with COPD and 4468 without COPD. All NHANES procedures are ethically reviewed and approved by the NCHS Research Ethics Review Board, with written informed consent obtained from each participant to confirm their voluntary agreement to participate.
COPD was diagnosed based on affirmative responses to either of the following questions: (1) ‘Has a doctor or other health professional ever told you that you have emphysema?’ (2) “Has a doctor or other health professional ever told you that you have COPD?”.31
Additionally, the study incorporated relevant covariates such as age, gender, race/ethnicity (divided into five categories), education level (categorized as less than high school or more than high school), BMI (categorized into four groups: underweight, normal weight, overweight, and obese), smoking status (current, former, or never), alcohol consumption status, history of hypertension, and history of diabetes. The NHANES protocol was approved by the Institutional Review Board of the National Center for Health Statistics at the Centers for Disease Control and Prevention (CDC). Informed consent was obtained from all participants involved in the study.32
Primary Exposure Data Sources
The LDL-C data we analyzed were extracted from a substantial prospective cohort study of European populations, as reported by Armstrong et al in the prestigious journal Nature Genetics in 2021.33 This study boasts an extensive dataset, encompassing 2.1 million single nucleotide polymorphism (SNP) loci and 35 blood/urine metabolites, which were utilized for a comprehensive genome-wide association analysis. We selected the LDL-C data from this study for our discovery set. For the replication set, we sourced data from another publicly accessible GWAS dataset, comprising 343,621 individuals of European descent. The LDL-C data specific to Asian populations were derived from the pooled GWAS data of the Asian Genetic Epidemiology Network (AGEN) (https://blog.nus.edu.sg/agen/), encompassing a sample size of 34,421 East Asians. Lastly, the LDL-C data employed for drug target analysis were procured from the UK Biobank, encompassing a robust participant pool of 440,546 individuals, both male and female.
Expression Quantitative Trait Loci (eQTLs) linked to the drug target genes HMGCR, PCSK9, and NPC1L1 were utilized as surrogate markers to assess exposure to lipid-lowering therapies. The eQTLs for HMGCR were sourced from the eQTLGen Consortium database, which can be accessed at https://www.eqtlgen.org/.34 Meanwhile, the eQTLs for PCSK9 and NPC1L1 were derived from the GTEx Consortium Version 8, available at https://gtexportal.org/.35 Detailed data information is shown in Supplementary Table S1.
Primary Outcome Data Sources
The primary outcome data for chronic COPD were sourced from FinnGen Release 11 (R11), a comprehensive genotype dataset derived from the Finnish Biobank. This dataset encompasses 21,617 individuals with COPD and 372,627 control subjects. For the Asian population, COPD data were extracted from the IEU openGWAS database, which includes a substantial cohort of 166,670 East Asians, comprising 4017 cases and 162,653 controls.
Transcriptome Data Sources
Transcriptomic and clinical data pertaining to COPD patients were retrieved from the GEO database, which is accessible at https://www.ncbi.nlm.nih.gov/geo/. Our analysis included the GEO COPD cohort found within the GSE38974 dataset, comprising 23 samples from COPD patients and 9 control samples. For replication purposes, we utilized the GSE10006 dataset, which contains 27 COPD samples and 60 control samples.
Genetic Instrument Selection
To establish a causal link between LDL-C and COPD, we applied stringent inclusion criteria for selecting single nucleotide polymorphisms (SNPs). We chose SNPs with a P-value less than 5 × 10^-8 and a minor allele frequency (MAF) greater than 0.01 as instrumental variables. To mitigate the impact of linkage disequilibrium (LD) in the two-sample MR analysis, we implemented LD exclusion criteria, setting an R2 threshold of 0.001 and a maximum distance threshold of 10,000 kb. For SNPs associated with lipid-lowering drug exposure, we selected those within 100 kb of each drug target gene, with an R2 threshold of 0.3. Furthermore, to ensure the validity of each SNP, we calculated its F-statistic and excluded those with F-statistics below 10, thereby avoiding bias that could arise from weak instrumental variables. In the SMR analyses, only cis-eQTLs were considered for generating genetic instruments, defined as eQTLs located within 1 Mb on either side of the encoding gene.36
Statistics
In the analysis of NHANES data, we employed weighted analyses to ensure accurate representation of different groups.37 Continuous variables are presented as mean ± standard deviation (SD), while categorical variables are expressed in percentages. To assess the relationship between LDL levels and COPD, we conducted multivariate logistic regression analyses, yielding odds ratios (ORs) and 95% confidence intervals. This analysis was performed across three models: Model 1 was unadjusted, Model 2 was adjusted for sex, age, and race, and Model 3 included adjustments for all covariates. Furthermore, a restricted cubic spline (RCS) with three knots at the 10th, 50th, and 90th percentiles was utilized to investigate the dose-response relationship between LDL and COPD. Nonlinear trends were assessed using analysis of variance (ANOVA). To identify potential linear threshold inflection points, segmented regression was applied to model the segmented linear relationship between LDL levels and COPD.
For MR analysis, we deployed five analytical methods, including inverse variance weighted (IVW), MR-Egger, weighted median, weighted mode, and sample mode, with IVW being the primary method. In sensitivity analyses, Cochrane’s Q-value was calculated to evaluate heterogeneity; heterogeneity was not assumed when the p-value was greater than 0.05. Horizontal multidimensionality was assessed via Egger regression, and it was not considered present when the p-value exceeded 0.05. Positive controls for coronary heart disease (CHD) were analyzed following the previously described methods.
In the context of SMR analyses, a Heterogeneity in Instrumental Dependence (HEIDI) test was conducted to detect any potential chaining in the observed associations. HEIDI tests with p-values less than 0.01 indicated the presence of pleiotropy, suggesting that the observed associations might be due to linkage disequilibrium.38
For transcriptome analysis, we used the “ggplot” R package to visualize the differential expression of key genes.All analyses were performed on R 4.3.1 (https://www.r-project.org).
Results
LDL-C as a Protective Factor in COPD
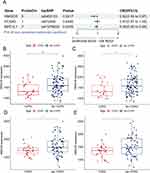
In this study, a cohort of 4710 individuals over the age of 40, with an average age of 57.83 years, was carefully selected based on predefined inclusion and exclusion criteria. The COPD patient group, in comparison to those without COPD, exhibited a tendency towards older age, lower levels of education, higher prevalence of smoking and alcohol consumption, increased rates of hypertension and diabetes, and notably, lower levels of LDL-C. Specifically, the mean LDL-C level in the COPD group was significantly lower at 2.71 mmol/L, contrasted with 3.03 mmol/L in the control group, with a statistically significant p-value of less than 0.001, as detailed in Table 1.
 |
Table 1 Demographic and Clinical Characteristics of the Participants with and without COPD |
Table 2 presents the findings from the multiple regression analysis. In the crude model, LDL-C demonstrated a robust association with COPD, with an odds ratio of 0.66 (95% confidence interval 0.56–0.79), achieving statistical significance at the p<0.001 level. After adjusting for gender, age, and race, the association between LDL-C and COPD persisted, with an odds ratio of 0.76 (95% CI 0.62–0.92), and the association was still significant at the p=0.007 level. Upon further adjustment for all covariates, LDL-C continued to show a significant association with COPD, with an odds ratio of 0.8 (95% CI 0.65–0.98), and the p-value was 0.035. These results collectively suggest that LDL-C may exert a protective effect against COPD. As shown in Figure 2, the RCS analysis revealed a nonlinear dose-response relationship between LDL-C levels and COPD, with the p-value for nonlinearity being less than 0.001, and an inflection point observed at 3.63 mmol/L.
 |
Table 2 Association Between LDL-C and COPD |
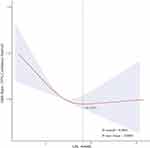 |
Figure 2 RCS curves of the relationship between LDL-C levels and COPD risk. |
Assessing the Causal Relationship Between LDL-C and COPD Using MR Analysis
As a significant negative association of LDL-C with COPD was observed in the previous cross-sectional study, we further performed MR analysis to infer the causal effect of LDL-C on COPD risk. Detailed information on the relevant SNPs for LDL-C is shown in Supplementary Table S2. As shown in Figure 3A, the IVW results indicated that LDL-C was associated with a reduced risk of COPD in the discovery set (OR 0.90; 95% CI 0.85 to 0.95; P = 0.0001). We validated this using a replication set and the results also held (OR 0.87; 95% CI 0.80 to 0.94; P = 0.0002). In addition, we repeated the MR analysis in the East Asian population to enhance the reliability of the above results. Consistent with the results in the European population, there was a significant negative association between LDL-C and COPD in the Asian population (OR 0.83; 95% CI 0.71 to 0.97; P = 0.0176). MR-Egger regression analyses did not confirm the presence of horizontal pleiotropy for the genetic instrumental variables in any of the causality relationships (P < 0.05). In addition, heterogeneity was detected in the results of Discovery Set and Replication Set, and no heterogeneity in the results of Asian set, as shown in Supplementary Table S3. Therefore, we used the randomized model IVW approach for re-validation. We found that LDL-C is a protective factor for COPD, which is consistent with our findings in the cross-sectional study.
Mendelian Randomization Analysis of Statin Targets
To further validate the causal relationship between statins and COPD, we performed a drug-target Mendelian randomization analysis to explore the indirect effect of statins on COPD by lowering LDL-C. We identified 18 SNPs in HMGCR, 29 SNPs in NPC1L1, and 6 SNPs in PCSK9, as shown in Supplementary Table S4. Significant associations between genetically proxied drug targets and CAD risk reduction were identified in positive control analyses, ensuring the validity of the genetic instrumentation, which is consistent with previous studies. As shown in Figure 3B, the results of the MR analyses demonstrated that HMGCR gene-mediated reduction in LDL-C levels significantly increased the risk of COPD (OR 1.67; 95% CI 1.39 to 2.01; P = 3.7e-08). The PCSK9 drug target (OR 1.22; 95% CI 1.10 to 1.36; P = 2.0e-04) and the NPC1L1 drug target (OR 1.89; 95% CI 1.25 to 2.87; P = 2.8e-03) showed the same results, but there was directional inconsistency in the ORs for NPC1L1. Cochran’s Q-test showed no heterogeneity in all results (all p > 0.05). In addition, no significant overall pleiotropy was detected according to the MR-Egger regression intercept term, as shown in Supplementary Table S5. We found that statins promote COPD by lowering LDL-C levels, especially HMGCR inhibitors and PCSK9 inhibitors.
SMR Analysis
As shown in Figure 4A, there was an association between the HMGCR drug target (OR 0.82; 95% CI 0.69 to 0.97; P = 0.0217) and the NPC1L1 drug target (OR 0.95; 95% CI 0.90 to 0.99; P = 0.0435) and the risk of COPD as suggested by the results of the SMR analysis. The HEIDI test for assessing pleiotropy showed no pleiotropy in the analysis of these three drug targets, as shown in Supplementary Table S6. This finding suggests that HMGCR inhibitors and NPC1L1 inhibitors have the potential to increase the risk of COPD.
Differential Expression of the HMGCR Gene
Based on the results of these analyses, we focused on exploring the HMGCR gene that showed better results in both MR and SMR of drug targets. Therefore, we utilized GEO transcriptome data to verify the differential expression of HMGCR gene in COPD patients and healthy controls. As shown in Figure 4B, significantly lower expression of HMGCR was observed in COPD patients than in the healthy group (p<0.05). The same result was observed in the replication set, as shown in Figure 4C–E). This suggests that HMGCR may play an important role in the promotion of COPD development by statins, and subsequent studies should explore the association that exists between them in more depth.
Discussion
In this investigation, we set out to elucidate the intricate relationship between LDL-C and COPD, with particular emphasis on exploring potential causal associations. LDL-C, commonly referred to as “bad cholesterol”, has been traditionally implicated in the pathogenesis of various diseases.19,20,39,40 Counterintuitively, our study has consistently demonstrated that LDL-C serves as a significant protective factor against the development of COPD when assessed through diverse methodologies. This paradoxical role of LDL-C - exhibiting detrimental effects in the systemic vasculature while demonstrating protective functions within the pulmonary environment - highlights the organ-specific complexity of lipid metabolism. This novel finding carries substantial implications for the clinical management, diagnosis, and therapeutic strategies for COPD.
While elevated LDL-C is unequivocally associated with atherosclerosis and coronary artery disease through endothelial dysfunction and plaque formation, its role in pulmonary health demonstrates marked divergence. For instance, in asthma, certain observational studies suggest that elevated LDL-C levels may be associated with improved lung function and lower exacerbation risk. This paradoxical association could be partially attributed to LDL subfraction-specific effects (eg, potential antioxidant properties linked to vitamin E transport within LDL particles) and immunomodulatory pathways, such as suppression of Th2-driven airway inflammation mediated through the apoE-LDLR axis.41,42 In contrast, in idiopathic pulmonary fibrosis (IPF), elevated LDL-C levels are associated with accelerated disease progression, potentially attributable to dysregulated lipid signaling within alveolar epithelial repair processes and fibrotic pathways.43 These discordant findings underscore the imperative to refine our understanding of lipid metabolism in respiratory diseases—LDL-C may exhibit dichotomous roles (protective or deleterious) contingent on distinct underlying pathophysiological mechanisms.
In the context of chronic obstructive pulmonary disease (COPD), although previous studies have investigated the relationship between LDL-C levels and disease manifestations, their conclusions remain equivocal due to methodological heterogeneity across study cohorts.21,44 To reconcile these conflicting reports, we conducted a nationally representative cross-sectional analysis using the NHANES dataset. Our investigation demonstrated significantly lower LDL-C levels in COPD patients compared to healthy controls (2.71 vs 3.03 mmol/L, p < 0.001), a finding consistent with population-based cohort studies by Reed et al.22 From a mechanistic perspective, LDL particles serve as the primary transport vehicle for vitamin E, delivering this lipid-soluble antioxidant to pulmonary tissues through systemic circulation—a process critical for maintaining redox homeostasis in alveolar epithelial cells.45 During COPD pathogenesis, reduced LDL particle quantity or functional impairment may diminish pulmonary bioavailability of vitamin E. This deficiency could subsequently upregulate the EGFR/MAPK pathway to enhance COX2 expression, while promoting phosphorylated STAT3 nuclear translocation, thereby exacerbating COPD pathogenesis.46 Concurrently, vitamin E depletion amplifies lipid peroxidation reactions, generating toxic products such as malondialdehyde (MDA) that further potentiate the vicious cycle of oxidative stress and chronic inflammation.47 These findings not only provide a theoretical foundation for metabolically targeted COPD therapies but also open new avenues for investigating lipid metabolism-immune interaction networks.
Notably, restricted cubic spline (RCS) modeling identified a nonlinear dose-response relationship between LDL-C and COPD risk: at LDL-C levels below 3.63 mmol/L, each 1 mmol/L increase was associated with a 22% reduction in COPD risk (95% CI: 0.65–0.93); beyond this threshold, however, risk escalated with rising LDL-C concentrations. This biphasic effect may reconcile conflicting results from prior observational studies—the protective role of LDL-C at lower concentrations could be obscured by its pro-inflammatory effects at supraphysiological levels in analyses failing to account for threshold effects.
The role of statins in COPD management presents a complex duality that warrants careful examination. Numerous observational studies48–50 have demonstrated their potential benefits in reducing acute exacerbations, decreasing disease-related hospitalizations, and improving lung function parameters – effects primarily attributed to statins’ pleiotropic properties, including anti-inflammatory, antithrombotic, and immunomodulatory mechanisms.24,51,52 Our drug-target Mendelian randomization analysis revealed a significant positive association between statin use and COPD risk, aligning with the counterintuitive finding that LDL-C reduction (a primary pharmacological effect of statins) may paradoxically increase COPD susceptibility. This discovery fundamentally challenges the traditional paradigm of lipid management in respiratory diseases.
Clinicians now face the critical task of balancing statins’ established cardiopulmonary benefits against their newly identified pulmonary risks. Particularly in COPD patients with concurrent cardiovascular disease, therapeutic decisions must integrate quantitative assessments of both systemic inflammation levels and baseline lipid profiles. Future research should prioritize large-scale longitudinal studies evaluating COPD progression trajectories in statin-treated versus non-treated cohorts, with stratification by lipid subfractions and inflammatory markers. Concurrently, pharmacological strategies to decouple statins’ beneficial pleiotropic effects from their lipid-lowering actions warrant exploration, potentially through targeted antioxidant delivery systems or localized anti-inflammatory formulations. Ultimately, these findings advocate for a precision medicine framework in COPD management. Rather than contraindicating statin use, our analysis calls for optimized therapeutic algorithms that simultaneously leverage statins’ anti-inflammatory properties while preserving pulmonary LDL-C homeostasis. This approach may involve combined therapies with lipid-modulating agents or timed intervention strategies based on disease stage-specific lipid requirements. Such paradigm refinement could unlock novel therapeutic synergies, ultimately advancing toward personalized COPD care that harmonizes cardiovascular and pulmonary outcomes.
In terms of the generalizability of our study, we leveraged GWAS data from both representative European and Asian populations for cross-validation, yielding significant results in both cohorts. This finding corroborates with previous research that has established an inverse relationship between lower LDL-C levels and an elevated risk of COPD, as demonstrated in a comprehensive study of the Danish general population.53 These observations suggest that the protective role of LDL-C against COPD risk may be a phenomenon of global relevance, which is particularly significant given the widespread impact of COPD as a leading global health challenge. Future research endeavors should therefore prioritize the investigation of LDL-C’s influence on COPD susceptibility across diverse racial and ethnic groups to further elucidate the global implications of this relationship.
This study boasts several pivotal strengths. Firstly, the employment of Mendelian randomization analysis has enabled us to leverage genetic data as a bridge to explore the relationship between LDL-C and COPD, thereby mitigating the impact of potential confounding factors. Secondly, by including diverse ethnic populations as exposure factors, we have expanded the global applicability of our research, which holds significant positive implications for COPD, a disease with a high global incidence rate. Lastly, we have further validated our findings using transcriptomic data, thereby enhancing the reliability of our experimental conclusions. However, the study is not without its limitations. Firstly, due to the incompleteness of data in the NHANES cross-sectional analysis, we have relied solely on questionnaire survey results as the diagnostic basis for COPD, which may deviate from the actual prevalence of COPD. Secondly, the limited sample size in the NHANES database necessitates the validation of our results in larger clinical cohort studies. Thirdly, Mendelian randomization analysis may be subject to certain confounding influences and pleiotropy issues. Additionally, the limited sample size of the transcriptomic data included in our study may exert a certain impact on the experimental outcomes, and further validation with high-quality transcriptomic data is warranted in subsequent studies. Finally, while our findings highlight associations between LDL-C, statins, and COPD, the exact biological mechanisms (eg, inflammatory or oxidative pathways) remain unexplored. Future experimental studies using in vitro or animal models are critical to elucidate these mechanisms.
Through these studies, we have gained a deeper understanding that COPD is not only a respiratory disease but also a complex disease involving multi-system metabolic disorders. Therefore, future research directions should include in-depth exploration of metabolic pathways in COPD patients, as well as the development of novel therapeutic strategies targeting these metabolic abnormalities, in hopes of alleviating this global health burden.
Conclusion
The findings of this study suggest that LDL-C serves as a protective factor for COPD, and that statin medications, by reducing LDL-C levels, may facilitate the progression of COPD. This discovery holds significant clinical implications for the treatment of COPD, indicating that metabolic factors should be considered in COPD management, particularly when statin medications are employed. Future research should further investigate the mechanistic role of LDL-C in COPD and assess the benefits and risks of statin therapy in the treatment of COPD.
Data Sharing Statement
NHANES data are publicly available through the Center for Disease Control. (https://wwwn.cdc.gov/nchs/nhanes/). GWAS data are available through the MRC IEU Open GWAS database (http://gwas.mrcieu.ac.uk/). The datasets generated and/or analysed during the current study are available in the GEO database (https://www.ncbi.nlm.nih.gov/geo/).
Ethics Approval and Informed Consent
The data used in this study were obtained from public databases. The data are fully anonymized, containing no personally identifiable information. In accordance with China’s Measures for Ethical Review of Life Sciences and Medical Research Involving Human Subjects (2023, Article 32, Sections 1-2). Since the data do not involve identifiable personal information, the study avoids direct human subject research and meets the requirements for ethical exemption.
Author Contributions
All authors made substantial contributions to the conception, experimental design, data acquisition and analysis, and interpretation of this study; participated in drafting the manuscript, multiple rounds of critical revision, and scholarly editing; provided final approval of the version to be published; collectively selected this journal as the publication venue; and agree to be accountable for all aspects of the work, including academic integrity and reliability of the findings.
Funding
This work was supported by the Sichuan Science and Technology Program, China (2024YFFK0356); the Luzhou City Southwest Medical University Joint Project Program, China (2023LZXNYDJ007); the Luzhou City Science and Technology Plan Project Program, China (2024JYJ030); the 2024 Open Research Project of the Sichuan Provincial Engineering Technology Research Center of Molecular Diagnosis of Clinical Diseases (24GCZXZD03) and the 2023 Open Fund Project of the Luzhou Key Laboratory of Molecular Diagnosis of Clinical Diseases (FZZD2023-01); the Scientific Research Program of Southwest Medical University, China (2022QN071); the Southwest Medical University 2023 Undergraduate Innovation and Entrepreneurship Training Program (Project Numbers: S202310632181; 2023378) and the Southwest Medical University 2024 Undergraduate Innovation and Entrepreneurship Training Program (Project Numbers: 202410632007; S202410632131; 2024386; 2024390).
Disclosure
Tiantao Du and Jianye Cao are co-first authors for this study. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease 2017 report GOLD executive summary. Respirology. 2017;22(3):575–601. doi:10.1111/resp.13012
2. Stratton MA, McCabe ML. Chronic obstructive pulmonary disease. Primary Care. 1990;17(3):667–684. doi:10.1016/S0095-4543(21)00889-7
3. Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet. 2004;364(9434):613–620. doi:10.1016/S0140-6736(04)16855-4
4. Yang IA, Jenkins CR, Salvi SS. Chronic obstructive pulmonary disease in never-smokers: risk factors, pathogenesis, and implications for prevention and treatment. Lancet Resp Med. 2022;10(5):497–511. doi:10.1016/S2213-2600(21)00506-3
5. Patel B, Priefer R. Impact of chronic obstructive pulmonary disease, lung infection, and/or inhaled corticosteroids use on potential risk of lung cancer. Life Sci. 2022;294:7. doi:10.1016/j.lfs.2022.120374
6. Viegi G. GLOBAL BURDEN OF CHRONIC RESPIRATORY DISEASES. J Aerosol Med Pulm Drug Deliv. 2019;32(3):A5–A.
7. Momtazmanesh S, Moghaddam SS, Ghamari SH, et al. Global burden of chronic respiratory diseases and risk factors, 1990-2019: an update from the global burden of disease study 2019. EClinicalMedicine. 2023;59:22. doi:10.1016/j.eclinm.2023.101936
8. Stolz D, Mkorombindo T, Schumann DM, et al. Towards the elimination of chronic obstructive pulmonary disease: a lancet commission. Lancet. 2022;400(10356):921–972. doi:10.1016/S0140-6736(22)01273-9
9. Adeloye D, Song PG, Zhu YJ, et al. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Resp Med. 2022;10(5):447–458. doi:10.1016/S2213-2600(21)00511-7
10. Meghji J, Mortimer K, Agusti A, et al. Improving lung health in low-income and middle-income countries: from challenges to solutions. Lancet. 2021;397(10277):928–940. doi:10.1016/S0140-6736(21)00458-X
11. Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370(9589):765–773. doi:10.1016/S0140-6736(07)61380-4
12. Zou JH, Sun T, Song XH, et al. Distributions and trends of the global burden of COPD attributable to risk factors by SDI, age, and sex from 1990 to 2019: a systematic analysis of GBD 2019 data. Respir Res. 2022;23(1):17. doi:10.1186/s12931-022-02011-y
13. Wang CX, Zhou JD, Wang JQ, et al. Progress in the mechanism and targeted drug therapy for COPD. Signal Transduct Target Ther. 2020;5(1):20. doi:10.1038/s41392-020-0122-1
14. Henrot P, Dupin I, Schilfarth P, et al. Main pathogenic mechanisms and recent advances in COPD peripheral skeletal muscle wasting. Int J Mol Sci. 2023;24(7):28. doi:10.3390/ijms24076454
15. Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138(1):16–27. doi:10.1016/j.jaci.2016.05.011
16. Novotna B, Abdel-Hamid M, Koblizek V, et al. A pilot data analysis of a metabolomic HPLC-MS/MS study of patients with COPD. Adv Clin Exp Med. 2018;27(4):531–539. doi:10.17219/acem/68763
17. Chen HP, Li ZY, Dong LL, Wu YF, Shen HH, Chen ZH. Lipid metabolism in chronic obstructive pulmonary disease. Int J Chronic Obstr Pulm Dis. 2019;14:1009–1018. doi:10.2147/COPD.S196210
18. Kilk K, Aug A, Ottas A, Soomets U, Altraja S, Altraja A. Phenotyping of chronic obstructive pulmonary disease based on the integration of metabolomes and clinical characteristics. Int J Mol Sci. 2018;19(3):17. doi:10.3390/ijms19030666
19. Zhang YY, Vittinghoff E, Pletcher MJ, et al. Associations of blood pressure and cholesterol levels during young adulthood with later cardiovascular events. J Am Coll Cardiol. 2019;74(3):330–341. doi:10.1016/j.jacc.2019.03.529
20. Mortensen MB, Dzaye O, Botker HE, et al. Low-density lipoprotein cholesterol is predominantly associated with atherosclerotic cardiovascular disease events in patients with evidence of coronary atherosclerosis: the Western Denmark heart registry. Circulation. 2023;147(14):1053–1063. doi:10.1161/CIRCULATIONAHA.122.061010
21. Zafirova-Ivanovska B, Stojkovikj J, Dokikj D, et al. The level of cholesterol in COPD patients with severe and very severe stage of the disease. Open Access Maced J Med Sci. 2016;4(2):277–282. doi:10.3889/oamjms.2016.063
22. Markelic I, Hlapcic I, Rogic D, et al. Lipid profile and atherogenic indices in patients with stable chronic obstructive pulmonary disease. Nutr Metab Carbiovasc Dis. 2021;31(1):153–161. doi:10.1016/j.numecd.2020.07.039
23. Zhang W, Zhang Y, Li CW, Jones P, Wang C, Fan Y. Effect of statins on COPD A meta-analysis of randomized controlled trials. Chest. 2017;152(6):1159–1168. doi:10.1016/j.chest.2017.08.015
24. Cao C, Wu YF, Xu ZW, et al. The effect of statins on chronic obstructive pulmonary disease exacerbation and mortality: a systematic review and meta-analysis of observational research. Sci Rep. 2015;5:8. doi:10.1038/srep16461
25. Li Z, Zhang B, Liu QR, et al. Genetic association of lipids and lipid-lowering drug target genes with non-alcoholic fatty liver disease. EBioMedicine. 2023;90:12. doi:10.1016/j.ebiom.2023.104543
26. Bi YD, Zhu YC, Tang S, Huang YG. Lipids, lipid-modifying drug target genes and migraine: a Mendelian randomization study. J Headache Pain. 2023;24(1):14. doi:10.1186/s10194-023-01633-x
27. Tao HQ, Yu Z, Dong YQ, Liu LG, Peng L, Chen XQ. Lipids, lipid-lowering agents, and inflammatory bowel disease: a Mendelian randomization study. Front Immunol. 2023;14:8. doi:10.3389/fimmu.2023.1160312
28. Schenk P, Spiel AO, Hüttinger F, et al. Can simvastatin reduce COPD exacerbations? A randomised double-blind controlled study. Eur Resp J. 2021;58(1):10. doi:10.1183/13993003.01798-2020
29. Criner GJ, Connett JE, Aaron SD, et al. Simvastatin for the prevention of exacerbations in moderate-to-severe COPD. N Engl J Med. 2014;370(23):2201–2210. doi:10.1056/NEJMoa1403086
30. Carlson AA, Smith EA, Reid DJ. The stats are in: an update on statin use in COPD. Int J Chronic Obstr Pulm Dis. 2015;10:2277–2284.
31. Xu YF, Yan ZQ, Li KK, Liu L. The association between systemic immune-inflammation index and chronic obstructive pulmonary disease in adults aged 40 years and above in the United States: a cross-sectional study based on the NHANES 2013-2020. Front Med. 2023;10:9. doi:10.3389/fmed.2023.1270368
32. Jayanama K, Theou O, Blodgett JM, Cahill L, Rockwood K. Frailty, nutrition-related parameters, and mortality across the adult age spectrum. BMC Med. 2018;16:23. doi:10.1186/s12916-018-1009-7
33. Sinnott-Armstrong N, Tanigawa Y, Amar D, et al. Genetics of 35 blood and urine biomarkers in the UK Biobank. Nature Genet. 2021;53(2):185–+. doi:10.1038/s41588-020-00757-z
34. Vosa U, Claringbould A, Westra HJ, et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nature Genet. 2021;53(9):1300–+. doi:10.1038/s41588-021-00913-z
35. Aguet F, Barbeira AN, Bonazzola R, et al. The GTEx consortium atlas of genetic regulatory effects across human tissues. Science. 2020;369(6509):1318–1330. doi:10.1126/science.aaz1776
36. Bottigliengo D, Foco L, Seibler P, Klein C, König IR, Del Greco MF. A Mendelian randomization study investigating the causal role of inflammation on parkinson’s disease. Brain. 2022;145(10):3444–3453. doi:10.1093/brain/awac193
37. Ko F, Vitale S, Chou CF, Cotch MF, Saaddine J, Friedman DS. Prevalence of nonrefractive visual impairment in US adults and associated risk factors, 1999-2002 and 2005-2008. JAMA. 2012;308(22):2361–2368. doi:10.1001/jama.2012.85685
38. Chauquet S, Zhu ZH, O’Donovan MC, Walters JTR, Wray NR, Shah S. Association of antihypertensive drug target genes with psychiatric disorders A Mendelian randomization study. JAMA Psychiatry. 2021;78(6):623–631. doi:10.1001/jamapsychiatry.2021.0005
39. Ntaios G, Milionis H. Low-density lipoprotein cholesterol lowering for the prevention of cardiovascular outcomes in patients with ischemic stroke. Int J Stroke. 2019;14(5):476–482. doi:10.1177/1747493019851283
40. Ferhatbegovic L, Mrsic D, Kusljugic S, Pojskic BLDL-C. The only causal risk factor for ASCVD. why is it still overlooked and underestimated? Curr Atheroscleros Rep. 2022;24(8):635–642. doi:10.1007/s11883-022-01037-3
41. Zhang Y, Jiang Z, Chen L, Lei T, Zheng X. Repurposing lipid-lowering drugs on asthma and lung function: evidence from a genetic association analysis. J Transl Med. 2024;22(1):615. doi:10.1186/s12967-024-05359-5
42. Gong Z, Wu D, Ku Y, et al. Lipid-lowering drug targets associated with risk of respiratory disease: a Mendelian randomization study. BMC Pulm Med. 2025;25(1):71. doi:10.1186/s12890-025-03527-x
43. Cai G, Liu J, Cai M, Shao L. Exploring the causal effect between lipid-modifying drugs and idiopathic pulmonary fibrosis: a drug-target Mendelian randomization study. Lipids Health Dis. 2024;23(1):237. doi:10.1186/s12944-024-02218-6
44. Xuan LL, Han FF, Gong LL, et al. Association between chronic obstructive pulmonary disease and serum lipid levels: a meta-analysis. Lipids Health Dis. 2018;17:8. doi:10.1186/s12944-018-0904-4
45. Kiokias S, Proestos C, Oreopoulou V. Effect of natural food antioxidants against LDL and DNA oxidative changes. Antioxidants. 2018;7(10):133. doi:10.3390/antiox7100133
46. Zhao H, Gong J, Li L, et al. Vitamin E relieves chronic obstructive pulmonary disease by inhibiting COX2-mediated p-STAT3 nuclear translocation through the EGFR/MAPK signaling pathway. Lab Invest. 2022;102(3):272–280. doi:10.1038/s41374-021-00652-z
47. Bezerra FS, Lanzetti M, Nesi RT, et al. Oxidative stress and inflammation in acute and chronic lung injuries. Antioxidants. 2023;12(3). doi:10.3390/antiox12030548
48. Ajmera M, Shen C, Sambamoorthi U. Association between statin medications and COPD-specific outcomes: a real-world observational study. Drugs. 2017;4(1):9–19. doi:10.1007/s40801-016-0101-6
49. Lin CM, Yang TM, Yang Yh, et al. Statin use and the risk of subsequent hospitalized exacerbations in COPD patients with frequent exacerbations. Int J Chronic Obstr Pulm Dis. 2020;15:289–299. doi:10.2147/COPD.S229047
50. Keddissi JI, Younis WG, Chbeir EA, Daher NN, Dernaika TA, Kinasewitz GT. The use of statins and lung function in current and former smokers. Chest. 2007;132(6):1764–1771. doi:10.1378/chest.07-0298
51. Lee TM, Lin MS, Chang NC. Usefulness of C-reactive protein and interleukin-6 as predictors of outcomes in patients with chronic obstructive pulmonary disease receiving pravastatin. Am J Cardiol. 2008;101(4):530–535. doi:10.1016/j.amjcard.2007.09.102
52. Undas A, Kaczmarek P, Sladek K, et al. Fibrin clot properties are altered in patients with chronic obstructive pulmonary disease beneficial effects of simvastatin treatment. Thromb Haemost. 2009;102(6):1176–1182. doi:10.1160/TH09-02-0118
53. Freyberg J, Landt EM, Afzal S, Nordestgaard BG, Dahl M. Low-density lipoprotein cholesterol and risk of COPD: Copenhagen General Population Study. ERJ Open Res. 2023;9(2):9. doi:10.1183/23120541.00496-2022
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.