Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Low Muscle Mass is Independently Associated with an Increased Risk of Having Lower Limb Atherosclerosis in T2DM Patients
Authors Deng S , Lv S, Liu Y, Xu H, Yin H, Xiao B, Wang S, Lu D, Li Y, Wang X
Received 25 August 2024
Accepted for publication 24 October 2024
Published 7 November 2024 Volume 2024:17 Pages 4211—4221
DOI https://doi.org/10.2147/DMSO.S492973
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Sijie Deng, Shishi Lv, Yiying Liu, Huiwen Xu, Hanlin Yin, Bin Xiao, Sen Wang, Dan Lu, Yun Li, Xiaoqian Wang
The Center for Endocrine and Thyroid Diseases, Deyang People’s Hospital, Deyang, Sichuan Province, 618000, People’s Republic of China
Correspondence: Xiaoqian Wang, Email [email protected]
Background and Aims: Existing research suggests that low muscle mass is independently associated with carotid atherosclerosis, but its relationship with lower extremity arterial atherosclerosis in type 2 diabetes mellitus (T2DM) patients remains unclear. This study aims to investigate the association between low skeletal muscle mass and lower extremity arterial atherosclerosis in T2DM patients, in hopes of providing a scientific basis for early diagnosis and treatment.
Methods: This cross-sectional study recruited a total of 276 patients with T2DM who underwent bioelectrical impedance analysis, lower limb artery ultrasonography, brachial-ankle pulse wave velocity(baPWV) arterial stiffness measurement, and blood tests. An skeletal muscle index (SMI) < 7.0kg/m2 in men and an SMI< 5.7kg/m2 in women were defined as low skeletal muscle mass. Lower limb atherosclerosis was defined as the presence of atherosclerotic plaques in the lower extremity arteries.
Results: In our study of 276 T2DM patients, 224 (81.1%) presented with lower limb atherosclerosis: 194 (70.2%) with simple lower limb arterial plaques, 15 (5.4%) with lower limb arterial stenosis, and 15 (5.4%) with lower limb arterial occlusion. 52 (18.8%) were diagnosed with low skeletal muscle mass. Logistic regression analysis indicated the risk of having overall lower limbs atherosclerosis increased with the prevalence of low skeletal muscle (OR= 6.175,95% CI 1.328– 28.711); Patients with a low skeletal muscle mass had a higher prevalence of simple arterial plaque (OR= 6.225,95% CI 1.339– 28.935) and arterial occlusion (OR=12.345,95% CI 1.221– 124.808); after the adjustment for clinical risk factors. Spearman’s analysis showed significant negative correlations between total-P1NP and baPWV (r=− 0.166, p=0.008), N-MID and baPWV (r=− 0.163, p=0.009), and β-CTX and baPWV (r=− 0.141, p=0.024).
Conclusion: Low muscle mass is independently associated with an increased risk of having lower limb atherosclerosis in T2DM patients. And there may be some relationship between BTMs and arteriosclerosis of the lower limb atherosclerosis in T2DM.
Keywords: sarcopenia, muscle, skeletal, lower extremity, atherosclerosis, type 2 diabetes
Introduction
Sarcopenia is a geriatric syndrome, mainly characterized by reduced muscle mass, decreased muscle strength, and / or decreased somatic function, first described by Rosenberg in 1989.1 As the aging of society intensifies, sarcopenia receives increasing attention. In a systematic review of a meta-analysis published by Yuan et al2 in 2023, the prevalence of sarcopenia was expected to be 10% −16% in older adults worldwide.
Sarcopenia is more prevalent among people with some chronic diseases such as renal inadequacy,3 therioma,4 hepatocirrhosis5; and several cardiometabolic disorders, including diabetes mellitus, metabolic syndrome, and cardiovascular disease, have also been associated with sarcopenia.6
Lower extremity arterial atherosclerosis is a prevalent complication of diabetes mellitus, particularly type 2 diabetes (T2DM), which can lead to lower extremity arterial disease (LEAD), characterized by atherosclerotic narrowing of the arteries in the legs. The presence of LEAD in diabetic patients significantly increases the risk of cardiovascular events, limb amputation, and mortality.7,8 Therefore, early detection and aggressive management of lower extremity atherosclerosis are very important in the diabetic population.
Researches on metabolic syndrome or T2DM populations all indicates that the low muscle mass is independently associated with an increased risk of carotid atherosclerosis, as well as with the severity of carotid atherosclerosis.9,10 We are interested in investigating whether this relationship extends to lower extremity atherosclerosis in individuals with T2DM. Therefore, we conducted a clinical cross-sectional study to explore the association between lower extremity atherosclerosis and low skeletal muscle mass.
Given the pivotal role of vitamin D deficiency and inflammation in sarcopenia development,11,12 and their established association with atherosclerosis,13,14 this study also explored levels of 25-hydroxyvitamin D(25(OH)D), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6).
Furthermore, previous research suggests that individuals with sarcopenia are at an increased risk of bone loss and osteoporosis.15,16 Bone turnover markers (BTMs) are biochemical or cellular compounds produced during the continuum of bone resorption or formation, have been implicated in the assessment of skeletal health. There is also evidence of a link between the reduction in skeletal muscle mass and BTMs.17 Consequently, this investigation also included an assessment of BTMs within its scope.
Subjects and Methods
Subjects
Participants with T2DM for this investigation (n = 276) aged 40–83 years were recruited from the Endocrine ward of Deyang People’s Hospital. We excluded patients with acute diabetes complications and serious chronic complications such as diabetic ketoacidosis, hyperglycemic hyperosmolar status, diabetic hypoglycemic coma, diabetic foot. Additionally, individuals with a history of myocardial infarction, cerebral infarction and, presence of active infection, autoimmune disease, therioma, severe hepatic insufficiency (defined by Alanine aminotransferase or Aspartate aminotransferase levels exceeding three times the upper limit of normal), severe kidney dysfunction (defined by estimated glomerular filtration rate (eGFR)<30mL/min/1.73m2), severe respiratory and circulatory disease, thyroid disease, and parathyroid disease were not included in the study. Patients taking sex hormones, vitamin D supplements, and glucocorticoids were also excluded from our study.
All participants provided written informed consent, and the study protocol was approved by the medical ethics committee of Deyang People’s Hospital. The ethics approval was given in compliance with the Declaration of Helsinki.
Based on previous research,18,19 the prevalence of lower limb atherosclerosis in patients with T2DM is estimated to be approximately 70%. Considering an acceptable margin of error of 6%, we initially calculated the sample size to be 225 participants. To further enhance the robustness and representativeness of our study, we increased this sample size by 20%. As a result of this adjustment, the final sample size was determined to be 276 participants.
Measurements of Clinical and Laboratory Indices
Anthropometric indices, including height, weight, waist circumference and hip circumference were measured by nurses. BMI was calculated as weight divided by the square of height. Systolic and diastolic blood pressures were consecutively measured on three occasions using an blood pressure monitor (HEM-7124; OMRON,Japan), with the mean values being calculated for analysis.
All patients underwent blood tests after having fasted for at least 8 hours. Glycosylated hemoglobin (HbA1c) levels were determined through high-performance liquid chromatography using the Automatic Glycohemoglobin Analyzer (ADAMS-HA8160;Arkray, Japan). Fasting blood glucose, triglycerides, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), serum creatinine, and uric acid were measured using an Automatic Biochemical Detector (Atellica CH 930;Siemens,Germany). The eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) study equation.25 (OH)D, total procollagen type 1 aminoterminal propeptide (total-P1NP), N-terminal mid-fragment of osteocalcin (N-MID), and cross-linked C-terminal telopeptide of type I collagen (β-CTX) were measured using an electrochemiluminescence immunoassay analyzer (Roche,Cobas e601). IL-6 and TNF-α were measured using an electrochemiluminescence immunoassay analyzer (Wan200+;UMIC,China).
Each patient’s smoking and drinking history was collected by a self-questionnaire. Smoking History Criteria: In this study, individuals with a smoking history were defined as either current smokers or former smokers. Current smokers are those who are actively smoking at the time of the study, while former smokers are individuals who have previously smoked but have since quit. Drinking History Criteria: Similarly, individuals classified as drinkers were defined as either current drinkers or former drinkers. Current drinkers are those who consume alcoholic beverages at the time of the study, whereas former drinkers are those who have consumed alcohol in the past but do not currently drink.
Measurements of Body Composition Using Bioelectrical Impedance
Body composition of each patient was evaluated utilizing a segmental multifrequency bioelectrical impedance analysis (BIA) system (InBodys 10; Biospace, Korea).
Skeletal muscle mass was measured in kilograms, and the skeletal muscle index (SMI) was derived by dividing skeletal muscle mass (kg) by height squared (m²). An SMI< 7.0kg/m2 in men and an SMI< 5.7kg/m2 in women were defined as low skeletal muscle mass.20
Lower Limb Arteries Ultrasonography
Each patient underwent lower limb arteries ultrasonography. The measurement were handled by certified sonographers using a high-resolution ultrasonographic system (EPIQ 7C;PHILIPS,Holland), with a probe frequency of 7.5–12 MHz.
Patients were positioned in a supine position with both lower limbs fully exposed, allowing for a comprehensive examination of the femoral, popliteal, anterior tibial, posterior tibial, and dorsal arteries of the foot. During the examination, we meticulously observed and documented the inner vessel diameter, intimal-medial thickness (IMT), presence of vascular plaques, degree of stenosis and occlusion, as well as peak flow velocity.
According to the Mannheim consensus,21 atherosclerotic plaque was defined as a focal structure encroaching into the arterial lumen of 0.5 mm or 50% of the surrounding IMT value or by a thickness of 1.5 mm as measured from the media–adventitia interface to the intima– lumen interface. Atherosclerosis was defined as the presence of lower extremity arterial atherosclerotic plaques in any of the above-mentioned artery segments. Furthermore, we applied the Cossman criteria22 to assess the presence of arterial stenosis and occlusion in the lower extremities.
Measurement of Brachial-Ankle Pulse-Wave Velocity
Pulse-Wave Velocity (PWV) is a classic method for measuring arterial stiffness, widely used in the field of cardiovascular disease. The faster the pulse wave propagation speed, the higher the arterial stiffness, which may increase the risk of cardiovascular diseases. Brachial-ankle Pulse-Wave Velocity (baPWV) is a simple, convenient, and repeatable method for measuring PWV, and it is widely used in clinical practice to determine arterial stiffness.23 In our study, baPWV was measured in a quiet environment by trained nurses using arterial stiffness detector (BP-203RPE III;OMRON,Japan). The highest values in the baPWV on the left and right sides was used in the analysis.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics (Version 22.0). Normality tests were conducted on continuous variables. Normally distributed data were presented as X±s, while non-normally distributed data were presented as M (Q1, Q3). Categorical variables were expressed as numbers and percentages. The participants’ baseline characteristics were compared using t-tests or Mann Whitney U-test for continuous variables and the Chi-square test or Fisher’s exact test for categorical variables as appropriate. The factors affecting lower limb atherosclerosis was assessed binary and multiple logistic regression. The relationship of serum BTMs and inflammatory factors and baPWV were analyzed by Spearman correlation. P < 0.05 was considered significant.
Results
The Comparison of Baseline Characteristics of the T2DM Patients According to the Presence of Lower Limb Atherosclerosis
The baseline characteristics of the study patients according to the presence of lower limb atherosclerosis are shown in Table 1. A total of 276 patients with T2DM, 182 males and 94 females were included in this study. The mean age was 60.0±9.1 years. The median duration of diabetes mellitus was 7.0 (2.0,10.0) years. Of all participants, lower limb atherosclerosis was observed in 224 patients (81.1%), with 194 cases (70.2%) classified as simple lower limb plaque, 15 cases (5.4%) as lower limb stenosis, and 15 cases (5.4%) as lower limb occlusion. Additionally, 52 cases (18.8%) were diagnosed with low skeletal muscle mass. The incidence of lower limb atherosclerosis was higher in males. Diabetes individuals with lower limb atherosclerosis tend to be older; had a longer duration of diabetes; had a higher systolic blood pressure, baPWV, serum creatinine, uric acid, 25(OH)D, and had a lower LDL-C, eGFR, total-P1NP, N-MID, than diabetes without lower limb atherosclerosis; The prevalence of the smoking history and hypertension was more pronounced among diabetic individuals with lower limb atherosclerosis compared to those without. The proportion of patients with lower limb atherosclerosis using antihypertensive drugs and antiplatelet agents was also higher than that of patients without lower limb atherosclerosis. Additionally, in particular, the occurrence of low skeletal muscle mass was significantly elevated in diabetics suffering from lower limb atherosclerosis.
 |
Table 1 The Comparison of Baseline Characteristics of the T2DM Patients According to the Presence of Lower Limb Arteriosclerosis |
Binary Logistic Regression Analysis of Factors Affecting Lower Limb Atherosclerosis in Patients with T2DM
A binary logistic regression analysis was conducted to examine the factors affecting lower limb atherosclerosis. Male gender, age, and low skeletal muscle mass were all influencing factors. The risk of having overall lower limbs atherosclerosis increased with the prevalence of low skeletal muscle, after adjustment for gender, age, duration of diabetes, percent body fat, LDL-C, serum creatinine, uric acid, 25(OH)D, total-P1NP, hypertension and smoking (Table 2).
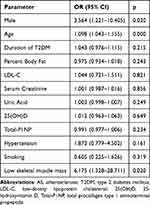 |
Table 2 Binary Logistic Regression Analysis of Factors Affecting Lower Limb Arteriosclerosis in Patients with T2DM Patients |
Multivariate Logistic Regression Analysis of Factors Influencing Varying Degrees of Lower Limb Arteriosclerosis in Patients With T2DM
Lower limb arterial lesions were classified into three categories: simple arterial plaque, arterial stenosis, and arterial occlusion. A multiple logistic regression analysis was used to examine the factors affecting different degrees of lower limb arteriosclerosis. The absence of lower limb arteriosclerosis was used as a reference. The influencing factors for simple arterial plaque were found to be male gender, age, and low skeletal muscle mass. Age and hypertension were identified as the influencing factors for arterial stenosis. The influencing factors for arterial occlusion were male gender, age, hypertension, and low skeletal muscle mass.
T2DM patients with low skeletal muscle mass had a higher prevalence of simple arterial plaque (OR= 6.225,95% CI 1.339–28.935) and arterial occlusion (OR=12.345,95% CI 1.221–124.808), after adjusting for for age, duration of diabetes, percent body fat, LDL-C, serum creatinine, uric acid, 25(OH)D, total-P1NP, hypertension and smoking (Table 3).
 |
Table 3 Multivariate Logistic Regression Analysis of Factors Influencing Varying Degrees of Lower Limb Arteriosclerosis in Patients with T2DM Patients |
Correlation Analysis Between Serum BTMs and Inflammatory Factors and baPWV
As depicted in Table 4, a Spearman correlation analysis was conducted to explore the relationship between BTMs and inflammatory factors and atherosclerosis. Total-P1NP was negatively associated with baPWV (r = −0.166, p = 0.008). Similarly, N-MID was found to be negatively associated with baPWV (r=−0.163,p=0.009). β-CTX was also negatively associated with baPWV (r=−0.141,p=0.024). However, no significant associations were found between 25(OH)D, IL-6, TNF-α, and baPWV.
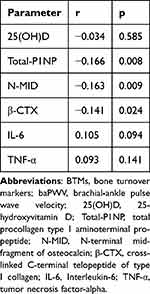 |
Table 4 Correlation Analysis Between Serum BTMs and Inflammatory Factors and baPWV |
Discussion
The present study showed that low skeletal muscle mass was associated with an increased risk of lower limb atherosclerosis in T2DM patients. Moreover, low skeletal muscle significantly increased the risk of developing simple lower limb arterial plaques and lower limb arterial occlusion in varying degrees of lower limb arterial disease after adjustment for mixed factors.
The research findings24 have shown that compared to patients with normal muscle mass, individuals with low skeletal muscle mass in T2DM exhibit a significant decrease in ankle-brachial index(ABI), which suggests that low skeletal muscle mass may serve as an early predictor of peripheral arterial disease. A recent study25 revealed that low skeletal muscle mass is associated with an increased overall risk of multi-vessel disease in diabetic foot patients (OR=3.230, 95% CI 1.069–9.758), particularly with an increased risk of involvement of four vascular sites (OR=7.024, 95% CI 1.711-28.826). However, both of the aforementioned studies only employed ABI assessments to evaluate lower limb arterial lesions, thus limiting the precision of the results. Therefore, this study conducted more accurate lower limb arterial Doppler ultrasound examinations on patients with T2DM and analyzed the impact of low skeletal muscle mass on various degrees of lower limb arterial lesions. We found that the overall risks of lower limb atherosclerosis, simple lower limb arterial plaque, and lower limb arterial occlusion in patients with low skeletal muscle mass were 6.175 times, 6.225 times, and 12.345 times higher, respectively, compared to those without low skeletal muscle mass. However, the influence of low skeletal muscle mass on lower limb arterial stenosis was not statistically significant. Considering the aforementioned discrepancies may be related to insufficient sample size, but overall, our study better elucidates the close relationship between low skeletal muscle mass and lower limb arteries in T2DM.
Previous studies have shown a certain correlation between sarcopenia and arteriosclerosis. Both conditions share common risk factors, but the specific mechanisms underlying their relationship remain unclear. Aging may be the most significant factor; it leads to a decrease in muscle mass and strength, as well as a reduction in vascular elasticity. Unhealthy lifestyles, such as diets high in fats and sugars, low in fiber, lack of exercise, and smoking, all exacerbate the risk of muscle loss and arteriosclerosis. Additionally, insulin resistance,26,27 myokines,28–30 oxidative stress and inflammation,31,32 and changes in sex hormone levels33–35 might also play roles. In our study, the prevalence of sarcopenia in patients with T2DM was 18.8%, and the prevalence of lower limb arteriosclerosis was 81.1%, both higher than those found in studies conducted on the general population.6,36 This may be related to factors associated with T2DM, such as insulin resistance and increased release of oxidative stress and inflammatory factors due to persistent hyperglycemia. However, although our data showed a trend of increased levels of TNF-α in patients with lower limb arteriosclerosis compared to those with normal lower limbs, the differences in IL-6 and TNF-α between the two groups were not statistically significant. This uncertainty could also be related to the small sample size. Future research should expand the sample size to improve the reliability and accuracy of statistical results. Moreover, introducing more biomarkers related to inflammation and oxidative stress may help deepen our understanding of the relationship between sarcopenia and arteriosclerosis in patients with T2DM.
Dyslipidemia is a major risk factor for atherosclerosis, with elevated levels of oxidized low-density lipoprotein (ox-LDL) in the bloodstream playing a critical role in the disease’s pathogenesis. It promotes the accumulation of lipids in the arterial wall and the formation of atheromatous plaques by triggering inflammatory responses, damaging endothelial function, and activating immune cells.37 However, in this study, we found that LDL-C levels in patients with lower limb atherosclerosis were significantly lower than those in patients without lower limb atherosclerosis. Moreover, logistic regression analysis indicated that LDL-C had no correlation with lower limb atherosclerosis, which seems to contradict our general understanding. While analyzing this phenomenon, we noted an important clinical trend: the usage rate of statins in patients with lower limb atherosclerosis in this study (26.3%) was higher than that in patients without lower limb atherosclerosis (13.5%). Although the difference between the two groups did not reach statistical significance (P=0.05), it still reveals a noticeable tendency: statin use is more prevalent among the population of patients with T2DM and lower limb atherosclerosis. Considering the role of statins in lowering lipid levels, particularly their clear effect in reducing LDL-C levels, the widespread use of these medications may partially explain the unexpected relationship observed between lipid levels and atherosclerosis in our study.
A large body of experimental and clinical research now shows that a deficiency in vitamin D is closely linked with arteriosclerosis and cardiovascular disease.38 Vitamin D can participate in the regulation of blood vessels through many mechanisms. These mechanisms include acting as a regulatory factor affecting the renin-angiotensin-aldosterone system (RAAS), regulating the proliferation and growth of vascular smooth muscle cells, and increasing levels of endothelin and nitric oxide in endothelial cells. Additionally, vitamin D can inhibit arteriosclerosis by promoting the expression of monocyte/macrophage subtypes, inhibiting the transformation of macrophages to foam cells, reducing the intake of low-density lipoprotein cholesterol, and also involves in the regulation of the immune system, anti-inflammatory actions, and epigenetic regulation. However, there is still controversy in current clinical studies regarding whether supplementing vitamin D can bring definite cardiovascular health benefits.39 In contrast to the previous studies, In this study, patients with T2DM who had combined lower limb arteriosclerosis had higher levels of vitamin D compared to the group without lower limb arteriosclerosis. However, the median 25(OH)D was below 20 ng / mL in both groups, which means most of patients in our study are in vitamin D deficiency. This could explain why there is no statistically significant difference in the prevalence of vitamin D deficiency between the two groups. Our multivariate regression analysis did not show an independent correlation between vitamin D and lower limb arteriosclerosis in T2DM, and Spearman correlation analysis also did not suggest a correlation between vitamin D and baPWV. Similarly, Wang40 et al also found that there was no difference of the incidence deficiency of vitamin D between T2DM with peripheral artery disease and those without. Their multivariate logistic regression further verified that 25(OH)D was irrelevant to peripheral artery disease in T2DM patients. Different from our study, their research population had an average vitamin D level of 20.86 ± 0.486 ng/mL, which is higher than the median level in our study population of 17.81 ng/mL (interquartile range: 12.825–23.180 ng/mL), and their overall prevalence of vitamin D deficiency was lower than in our study (44.3% vs 60.9%). This could be largely related to differences in sunlight exposure. In summary, these results highlight the complexity of the relationship between vitamin D and lower extremity arterial atherosclerosis in patients with T2DM, necessitating further investigation in the future.
This study indicates that there may be a significant relationship BTMs and arteriosclerosis of the lower extremities in T2DM. In previous research, Li W41 et al analyzed the relationship between carotid arteriosclerosis and BTMs in patients with T2DM, finding that serum levels of N-MID, P1NP, and β-CTX were significantly lower in the carotid plaque (CAP) group. Multivariate logistic regression analysis showed that reduced levels of N-MID were a significant independent risk factor, while the levels of P1NP and β-CTX were not related to the risk of CAP. Similar to these findings, our study found that compared to the group without lower extremity arteriosclerosis, the lower extremity arteriosclerosis group had significantly lower total-P1NP and N-MID levels, and β-CTX also showed a decreasing trend, although the difference was not statistically significant. Although our study’s multivariate regression analysis did not show BTMs as independent risk factors for lower extremity arteriosclerosis, further Spearman correlation analysis indicated that total-P1NP, N-MID, and β-CTX were all negatively correlated with baPWV. These correlations suggest that BTMs have an undeniable relationship with vascular lesions associated with T2DM. More research is needed in the future to explore the potential relationship and mechanisms between bone turnover markers and arteriosclerosis of the lower extremities in diabetes.
There are several limitations in our study. First, the sample size was not large enough to make definite conclusions. Second, this was a cross-sectional study;Therefore, the causal association between low muscle mass and lower limb atherosclerosis is uncertain. Third, we did not employ highly accurate methods like dual-energy X-ray absorptiometry (DEXA) or computed tomography to estimate skeletal muscle mass in our study. However, BIA is a non-invasive method for assessing skeletal muscle mass, which is more portable and easily accepted. Previous studies have indicated a strong correlation between the findings obtained from BIA and DEXA when estimating skeletal muscle mass.42
In conclusion, the present study showed that the low muscle mass is independently associated with an increased risk of having lower limb atherosclerosis in T2DM patients. Additionally, there may be some relationship between BTMs and arteriosclerosis of the lower limb atherosclerosis in T2DM. The findings suggest that the that interventions aimed at improving muscle mass may serve as a beneficial strategy for mitigating the risk of lower limb atherosclerosis among patients with T2DM. Further prospective trials are warranted to confirm that the relationship between reduced muscle mass and lower limb atherosclerosis. More clinical and experimental research is also needed to explore the potential relationship and mechanisms between BTMs and arteriosclerosis of the lower extremities in diabetes.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the 2021 Deyang City Science and Technology Plan Key Research and Development Projects (Grant No. 2021SZZ076).
Disclosure
The authors state that they have no conflicts of interest to disclose in relation to this manuscript.
References
1. Garry PJ, Chumlea WC. Epidemiologic and methodologic problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19-21, 1988. Am J Clin Nutr. 1989;50(5 Suppl):1121–1235.
2. Yuan S, Larsson SC. Epidemiology of sarcopenia: prevalence, risk factors, and consequences. Metabolism. 2023;144:155533. doi:10.1016/j.metabol.2023.155533
3. Duarte MP, Almeida LS, Neri S, et al. Prevalence of sarcopenia in patients with chronic kidney disease: a global systematic review and meta-analysis. J Cachexia, Sarcopenia Muscle. 2024;15(2):501–512. doi:10.1002/jcsm.13425
4. Surov A, Wienke A. Prevalence of sarcopenia in patients with solid tumors: a meta-analysis based on 81,814 patients. JPEN J Parenter Enteral Nutr. 2022;46(8):1761–1768. doi:10.1002/jpen.2415
5. Tantai X, Liu Y, Yeo YH, et al. Effect of sarcopenia on survival in patients with cirrhosis: a meta-analysis. J Hepatol. 2022;76(3):588–599. doi:10.1016/j.jhep.2021.11.006
6. Pacifico J, Geerlings M, Reijnierse EM, Phassouliotis C, Lim WK, Maier AB. Prevalence of sarcopenia as a comorbid disease: a systematic review and meta-analysis. Exp Gerontol. 2020;131:
7. Pang XH, Han J, Ye WL, et al. Lower extremity peripheral arterial disease is an independent predictor of coronary heart disease and stroke risks in patients with type 2 diabetes mellitus in China. Int J Endocrinol. 2017;2017:9620513. doi:10.1155/2017/9620513
8. Huang CL, Wu IH, Wu YW, et al. Association of lower extremity arterial calcification with amputation and mortality in patients with symptomatic peripheral artery disease. PLoS One. 2014;9(2):e90201. doi:10.1371/journal.pone.0090201
9. Cao Y, Zhong M, Zhang Y, et al. Presarcopenia is an independent risk factor for carotid atherosclerosis in Chinese population with metabolic syndrome. Diabetes Metab Syndr Obes. 2020;13:81–88. doi:10.2147/DMSO.S235335
10. Seo DH, Lee YH, Suh YJ, et al. Low muscle mass is associated with carotid atherosclerosis in patients with type 2 diabetes. Atherosclerosis. 2020;305:19–25. doi:10.1016/j.atherosclerosis.2020.05.021
11. Remelli F, Vitali A, Zurlo A, Volpato S. Vitamin D deficiency and sarcopenia in older persons. Nutrients. 2019;11(12):2861. doi:10.3390/nu11122861
12. Antuña E, Cachán-Vega C, Bermejo-Millo JC, et al. Inflammaging: implications in Sarcopenia. Int J Mol Sci. 2022;23(23):15039. doi:10.3390/ijms232315039
13. Anilkumar SA, Dutta S, Aboo S, Ismail A. Vitamin D as a modulator of molecular pathways involved in CVDs: evidence from preclinical studies. Life Sci. 2024;357:123062. doi:10.1016/j.lfs.2024.123062
14. Attiq A, Afzal S, Ahmad W, Kandeel M. Hegemony of inflammation in atherosclerosis and coronary artery disease. Eur J Pharmacol. 2024;966:176338. doi:10.1016/j.ejphar.2024.176338
15. Ontan MS, Dokuzlar O, Ates Bulut E, Soysal P, Isik AT. The relationship between osteoporosis and sarcopenia, according to EWGSOP-2 criteria, in outpatient elderly. J BONE MINERAL METAB. 2021;39(4):684–692. doi:10.1007/s00774-021-01213-6
16. Xu K, Feng X, Xu Z, Pan Y, Zhang P, Zhu H. Association of sarcopenia with osteoporosis in Chinese patients with type 2 diabetes. BMC Musculoskelet Disord. 2024;25(1):226. doi:10.1186/s12891-024-07323-2
17. Fathi M, Heshmat R, Ebrahimi M, et al. Association between biomarkers of bone health and osteosarcopenia among Iranian older people: the Bushehr Elderly Health (BEH) program. BMC Geriatr. 2021;21(1):654. doi:10.1186/s12877-021-02608-w
18. Li LX, Wu X, Lu JX, et al. Comparison of carotid and lower limb atherosclerotic lesions in both previously known and newly diagnosed type 2 diabetes mellitus. J Diabetes Investig. 2014;5(6):734–742. doi:10.1111/jdi.12204
19. Li L, Yu H, Zhu J, et al. The combination of carotid and lower extremity ultrasonography increases the detection of atherosclerosis in type 2 diabetes patients. J Diabetes Complications. 2012;26(1):23–28. doi:10.1016/j.jdiacomp.2011.11.006
20. Chen LK, Woo J, Assantachai P, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–307.e2. doi:10.1016/j.jamda.2019.12.012
21. Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim intima-media thickness consensus. Cerebrovasc Dis. 2004;18(4):346–349. doi:10.1159/000081812
22. Cossman DV, Ellison JE, Wagner WH, et al. Comparison of contrast arteriography to arterial mapping with color-flow duplex imaging in the lower extremities. J Vasc Surg. 1989;10(5):522–528. doi:10.1016/0741-5214(89)90133-X
23. Munakata M. Brachial-ankle pulse wave velocity: background, method, and clinical evidence. Pulse (Basel). 2016;3(3–4):195–204. doi:10.1159/000443740
24. Nakanishi S, Iwamoto M, Shinohara H, Iwamoto H, Kaneto H. Impact of sarcopenia on glycemic control and atherosclerosis in Japanese patients with type 2 diabetes: cross-sectional study using outpatient clinical data. Geriatr Gerontol Int. 2020;20(12):1196–1201. doi:10.1111/ggi.14063
25. Xia N, Jiahui C, Qiuhong H, Zhipeng D, Ziwei T, Qingfeng C. Relationship between sarcopenia and polyvascular disease in diabetic foot patients. Chin J Diabetes Mellitus. 2023;15(01):26–31. doi:10.3760/cma.j.cn115791-20220613-00271
26. Liu ZJ, Zhu CF. Causal relationship between insulin resistance and sarcopenia. Diabetol Metab Syndr. 2023;15(1):46. doi:10.1186/s13098-023-01022-z
27. Di Pino A, DeFronzo RA. Insulin resistance and atherosclerosis: implications for insulin-sensitizing agents. Endocr Rev. 2019;40(6):1447–1467. doi:10.1210/er.2018-00141
28. Pan JA, Zhang H, Yu Q, et al. Association of circulating irisin levels and the characteristics and prognosis of coronary artery disease. Am J Med Sci. 2021;362(1):63–71. doi:10.1016/j.amjms.2021.02.020
29. Shokoohi Nahrkhalaji A, Ahmadi R, Fadaei R, Panahi G, Razzaghi M, Fallah S. Higher serum level of CTRP15 in patients with coronary artery disease is associated with disease severity, body mass index and insulin resistance. Arch Physiol Biochem. 2022;128(1):276–280. doi:10.1080/13813455.2019.1675713
30. Yin B, Wang YB, Li X, Hou XW. β‑aminoisobutyric acid ameliorates hypertensive vascular remodeling via activating the AMPK/SIRT1 pathway in VSMCs. Bioengineered. 2022;13(6):14382–14401. doi:10.1080/21655979.2022.2085583
31. Chen M, Wang Y, Deng S, Lian Z, Yu K. Skeletal muscle oxidative stress and inflammation in aging: focus on antioxidant and anti-inflammatory therapy. Front Cell Dev Biol. 2022;10:964130. doi:10.3389/fcell.2022.964130
32. Yoo JI, Kim MJ, Na JB, et al. Relationship between endothelial function and skeletal muscle strength in community dwelling elderly women. J Cachexia, Sarcopenia Muscle. 2018;9(6):1034–1041. doi:10.1002/jcsm.12340
33. Priego T, Martín AI, González-Hedström D, Granado M, López-Calderón A. Role of hormones in sarcopenia. Vitam Horm. 2021;115:535–570. doi:10.1016/bs.vh.2020.12.021
34. Meng Q, Li Y, Ji T, et al. Estrogen prevent atherosclerosis by attenuating endothelial cell pyroptosis via activation of estrogen receptor α-mediated autophagy. J Adv Res. 2021;28:149–164. doi:10.1016/j.jare.2020.08.010
35. Lorigo M, Mariana M, Oliveira N, Lemos MC, Cairrao E. Vascular pathways of testosterone: clinical implications. J Cardiovasc Transl Res. 2020;13(1):55–72. doi:10.1007/s12265-019-09939-5
36. Aday AW, Matsushita K. Epidemiology of peripheral artery disease and polyvascular disease. Circ Res. 2021;128(12):1818–1832. doi:10.1161/CIRCRESAHA.121.318535
37. Gaggini M, Gorini F, Vassalle C. Lipids in atherosclerosis: pathophysiology and the role of calculated lipid indices in assessing cardiovascular risk in patients with hyperlipidemia. Int J Mol Sci. 2022;24(1):75. doi:10.3390/ijms24010075
38. Carbone F, Liberale L, Libby P, Montecucco F. Vitamin D in atherosclerosis and cardiovascular events. Eur Heart J. 2023;44(23):2078–2094. doi:10.1093/eurheartj/ehad165
39. Krishna SM. Vitamin D as A protector of arterial health: potential role in peripheral arterial disease formation. Int J Mol Sci. 2019;20(19):4907. doi:10.3390/ijms20194907
40. Wang Y, Feng T, Zhou H, Lu K, Bai Y, Zhang P. Vitamin D deficiency may not be an independent risk factor for peripheral arterial disease in middle-aged and elderly patients with type 2 diabetes in China. Dis Markers. 2020;2020:1–7. doi:10.1155/2020/8854717
41. Li W, Liu X, Liu L, et al. Relationships of serum bone turnover markers with metabolic syndrome components and carotid atherosclerosis in patients with type 2 diabetes mellitus. Front Cardiovasc Med. 2022;9:824561. doi:10.3389/fcvm.2022.824561
42. Stewart SP, Bramley PN, Heighton R, et al. Estimation of body composition from bioelectrical impedance of body segments: comparison with dual-energy X-ray absorptiometry. BRIT J NUTR. 1993;69(3):645–655. doi:10.1079/BJN19930066
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

