Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Lower Free Triiodothyronine is a Risk Factor of Diabetic Peripheral Neuropathy in Patients with Type 2 Diabetes Mellitus
Authors Chen Y , Sun L, Chen M, Zhang H, Song B, Wang H, Jiang A, Zhang L, Li S, Wang J, Wang W, Zhang H
Received 30 July 2024
Accepted for publication 19 November 2024
Published 25 November 2024 Volume 2024:17 Pages 4407—4415
DOI https://doi.org/10.2147/DMSO.S489204
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rebecca Conway
Yang Chen,1,* Lijie Sun,1,* Minghui Chen,1,* Hui Zhang,2 Bing Song,3 Hongxiao Wang,3 Aijun Jiang,1,4 Li Zhang,5 Sumei Li,1 Jumei Wang,1 Wei Wang,1 Haoqiang Zhang1
1Department of Endocrinology, Centre for Leading Medicine and Advanced Technologies of IHM, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, People’s Republic of China; 2Henan Key Laboratory of Rare Diseases, Endocrinology and Metabolism Center, The First Affiliated Hospital, and College of Clinical Medicine of Henan University of Science and Technology, Luoyang, People’s Republic of China; 3Department of Endocrinology, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, People’s Republic of China; 4University of Science and Technology of China, Hefei, People’s Republic of China; 5ShuCheng People’s Hospital, Luan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei Wang; Haoqiang Zhang, Email [email protected]; [email protected]
Purpose: Our objective is to investigate the potential involvement of free triiodothyronine (FT3), a key bioactive compound found in thyroid hormones (THs) in the pathogenesis of diabetic peripheral neuropathy (DPN) in patients diagnosed with type 2 diabetes mellitus (T2DM).
Patients and Methods: A total of 121 T2DM patients were recruited. And then, they were divided into the control group and the DPN group. Clinical parameters were collected for each patient. Additionally, nerve conduction velocity was tested using neurophysiological methods. Correlation and regression analyses were employed to examine the relationship between the concentrations of FT3 and DPN.
Results: Compared to 57 patients without DPN, 64 patients with DPN showed increased HbA1c and low-density lipoprotein cholesterol (LDL-C) levels (P=0.001 and 0.042), as well as decreased concentrations of FT3 (P=0.042). Additionally, FT3 levels are positively associated with the motor and sensory fibers conduction velocity of the Ulnar nerve, as well as the motor conduction velocity of the Tibial nerve, with (R=0.205, P=0.025; R=0.191, P=0.038; R=0.220, p=0.016) or without (R=0.257, P=0.004; R=0.227, P=0.012; R=0.227, p=0.012) adjustment for HbA1c and LDL-C. Furthermore, multiple linear regression analysis suggests that decreased FT3 levels may influence the motor and sensory fibers conduction velocity of the Ulnar nerve (β=0.795, P=0.025 and β=0.909, P=0.038), as well as the motor conduction velocity of the Tibial nerve (β=0.727, P=0.016). Moreover, our study demonstrated that decreased FT3 levels are one of the risk factors for DPN in T2DM patients, as determined by binary logistic regression analysis (OR=0.542, P=0.022).
Conclusion: Lower concentrations of FT3 are one of the risk factors for DPN in patients with T2DM. Additionally, decreased FT3 levels may influence peripheral neuropathy, particularly affecting the motor and sensory fibers conduction velocity of the ulnar nerve, as well as the motor fiber conduction velocity of the tibial nerve.
Keywords: free triiodothyronine, diabetic peripheral neuropathy, type 2 diabetes mellitus, thyroid hormone, conduction velocity of nerve
Introduction
DPN is the most common cause of neuropathy worldwide, affecting approximately half of patients with diabetes.1 Numerous studies have indicated that abnormal thyroid function could affect glucose metabolism and diabetic complications in T2DM patients.2 THs are essential for fetal development, particularly for the development of the nervous system,3 due to the widespread expression of thyroid hormone receptors in most neurons.4 Triiodothyronine (T3), one of the main active ingredients of THs, is a factor that maintains the survival of neurons.5 Additionally, T3 in circulation is main derived from thyroxine (T4), another component of THs, which could be transformed into T3 in peripheral tissues.6 Although THs, including T3, T4, and thyroid-stimulating hormone (TSH), are essential for the nervous system, further investigation is needed to determine the effect of THs on DPN.
In a recent clinical study, Zhang et al demonstrated that the nomogram of several factors, including FT3, exhibited a good predictive ability for DPN in patients with T2DM.7 Another study investigated the association between subclinical hypothyroidism and DPN, indicating that an elevated TSH concentration is related to DPN in patients with T2DM.8 He et al demonstrated that low T3 syndrome is also related to a higher risk and increase the severity of DPN in T2DM patients.9 Apart from patients with thyroid disease, it is suggested that lower FT3 concentration (in the normal range) is independently related to DPN in T2DM patients with euthyroid in China.10 A study evaluating the relationship between vascular complications and thyroid-related hormones claimed that not only FT4 but also the FT3/FT4 ratio is negatively correlated with DPN in T2DM patients with euthyroidism.11 However, a cross-sectional study did not find any significant statistical difference in T3 levels between T2DM patients with mild peripheral neuropathy and those without DPN.12
In general, the role of T3 in the development of DPN, especially its relationship with specific nerve fiber injury, remains undiscovered in patients with T2DM. The present work aims to investigate the effect of FT3 on DPN, particularly on the conduction velocity of nerve fibers in T2DM patients without thyroid disease (except for thyroid nodules).
Material and Methods
Experiment Design
This cross-sectional study was conducted at Department of Endocrinology, The First Affiliated Hospital of USTC. A total of 121 patients with T2DM were recruited for this present cross-sectional study. All of them met the standard for T2DM. Of these volunteers, 64 were diagnosed with DPN while 57 were not diagnosed with DPN despite having T2DM. All patients were diagnosed with T2DM according to the World Health Organization’s 1999 criteria and were recruited for this research. The exclusion criteria were previously described in our study, briefly defined as any clinically evident causes of neuropathy apart from diabetes. DPN patients were diagnosed according to the Toronto consensus on diabetic neuropathy,13 while those without DPN were defined as controls.
Inclusion and Exclusion Criteria
Patients with T2DM were included in this study. The exclusion criteria were similar with a previous study14 and defined specially described as follows: (a) other types of diabetes (including type 1 diabetes mellitus, gestational diabetes, and specific types of diabetes); (b) diabetes with acute complications; (c) neuropathy caused by other diseases or drugs; (d) severe vascular disease (eg venous embolism, lymphangitis); (e) neurotoxicity caused by drugs; (f) other undefined disease or drugs may influence neuropathy; (f) any amputation; (g) diagnosed thyroid disease (except for thyroid nodules); (g) any other undefined condition influence the performance of neurophysiological examinations or measurement of THs.
Ethics
This study complied with the Declaration of Helsinki. All patients signed statements upon admission informing them that their medical records may be used for research purposes. Additionally, this present work was approved by the Research Ethics Committee of The First Affiliated Hospital of USTC (Approval No.: 2023-RE-013).
Clinical Data Collection and Calculation
The information of age, gender and the diabetes mellitus (DM) duration were collected from the medical histories of patients with T2DM. All their medical histories were self-reported. Additionally, levels of fasting C peptide (FCP) (Roche Group, Basel, Switzerland); triglyceride (TG) (Roche Group, Basel, Switzerland), total cholesterol (TC) (Roche Group, Basel, Switzerland), LDL-C (Ningbo Ruiyuan Biotechnology Co., Ltd., Ningbo, China), high-density lipoprotein cholesterol (HDL-C) (Roche Group, Basel, Switzerland), FT3 (Roche Group, Basel, Switzerland), FT4 (Roche Group, Basel, Switzerland) and TSH (Roche Group, Basel, Switzerland;) were determined from their fasting blood samples for medical use. All these measurements were performed by Laboratory Center of The First Affiliated Hospital of USTC. Additionally, information of thyroid nodule was also collected from the medical history. Weight (kg)/height (m)2 was used for the calculation of Body mass index (BMI). HOMA-IR was also calculated by FCP: fasting plasma glucose (FBG) (mmol/L) x FCP (nmol/L)/22.5.
Neurophysiological Examinations
Neurophysiological examinations were performed on patients with T2DM in our hospital by trained staff in the Electrophysiology room, using an electromyographic evoked potential meter according to the protocol of the manufacturer (Natus Neurology, USA).15–17 The examination room should be maintained at a temperature between 20°C and 25°C. The room should provide comfortable lighting and remain quiet throughout the examination to ensure there are no disturbing noises. The motor fiber conduction velocity of the Ulnar nerve, Radial nerve, Median nerve, Tibial nerve, and Common peroneal nerve, as well as the sensory fiber conduction velocity of the Ulnar nerve, Radial nerve, Median nerve, and Sural nerve, were collected from the medical histories of the recruited T2DM patients. The average values of the bilateral nerve conduction velocity were calculated for further analysis.
Statistical Methods
All data were analyzed using SPSS 22.0 (IBM, USA). Z-Scores were used to exclude outliers. Kolmogorov–Smirnov tests were used to detect the normality of data. T-tests were performed to compare the differences in normally distributed variables (LDL-C, motor conduction fiber velocity of the Tibial nerve, as well as sensory fiber conduction velocity of the Ulnar nerve, Radial nerve, Median nerve, and Sural nerve) between patients with and without DPN. Mann–Whitney U-Tests were carried out to compare the differences in asymmetrically distributed variables (Age, BMI, Duration of DM, HbA1c, C-peptide, HOMA-IR, TG, TC, HDL-C, LDL-C, FT3, FT4, and TSH, as well as motor conduction fiber velocity of the Ulnar nerve, Radial nerve, Median nerve, Tibial nerve, and Common peroneal nerve) between the DPN group and the control group. Chi-squared tests were applied to compare the differences in the binary variables (gender and thyroid nodule) between the two groups. Partial correlation and Pearson correlation analyses were employed to observe the association between FT3 levels and nerve conduction fiber velocity, with or without adjustment for HbA1c and LDL-C levels. Binary logistic regression analysis was carried out to investigate the risk factors for DPN in T2DM patients. Multiple linear regression analyses were performed to further explore factors influencing nerve conduction fiber velocity. A significance level of P<0.05 was defined.
Results
Clinical Data Difference Between T2DM Patients with and without DPN
To determine the characteristics of patients with DPN and T2DM, we compared the baseline data of the control group with that of the DPN group. Firstly, there were no differences in age and gender between patients with and without DPN in the patients recruited for this study. Additionally, patients in the DPN group showed significant increases in HbA1c (P=0.001) and LDL-C (P=0.042) levels, while FT3 (P=0.042) levels decreased. However, there were no significant differences in BMI, DM duration, C-peptide, HOMA-IR, TG, TC, HDL-C, FT4, and TSH between T2DM patients in the DPN group and the control group (All P>0.05). Moreover, patients in both groups showed similar incidences of thyroid nodules (P>0.05) (Table 1).
 |
Table 1 Comparison of Clinical Parameters and Neurophysiological Test Results Between Control and DPN Group in T2DM Patients |
Conduction Velocity of Nerve Fibers Between T2DM Patients with and without DPN
To confirm nerve damage in patients with T2DM, the conduction velocity of nerve fibers was measured. We found that not only the conduction velocity of motor fibers in the Ulnar nerve, Radial nerve, Median nerve, Tibial nerve, and Common peroneal nerve, but also the conduction velocity of sensory fibers in the Ulnar nerve, Radial nerve, Median nerve, and Sural nerve were significantly decreased in patients with T2DM and DPN, compared to those without DPN (All P<0.05) (Table 1).
Pearson Correlation Between FT3 Levels and Nerve Conduction Velocity
To investigate the association between FT3 levels and DPN in diabetic patients, Pearson correlation analysis was conducted. Herein, we demonstrate that FT3 levels are not only positively related to the motor fiber conduction velocity of the Ulnar nerve (R=0.257, P=0.004), Tibial nerve (R=0.276, P=0.002), and Common peroneal nerve (R=0.214, P=0.008), but also positively associated with the sensory fiber conduction velocity of the Ulnar nerve (R=0.227, P=0.012) and Sural nerve (R=0.226, P=0.013) (Table 2).
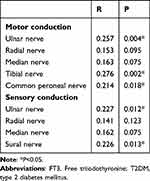 |
Table 2 Pearson Correlation Between FT3 and Nerve Conduction Velocity in T2DM Patients |
Partial Correlation Between FT3 and Nerve Conduction Velocity Adjusted for HbA1c and LDL-C
To further investigate the correlation between FT3 and DPN in T2DM patients, partial correlation analysis was performed. In this present work, it is suggested that FT3 levels are positively related to the motor fiber conduction velocity of the Ulnar (R=0.205, P=0.025) and Tibial (R=0.220, P=0.016) nerves, adjusted for HbA1c and LDL-C. Additionally, there is a positive correlation between FT3 and the Ulnar nerve sensory fiber conduction velocity (R=0.191, P=0.038), adjusted for HbA1c and LDL-C (Table 3).
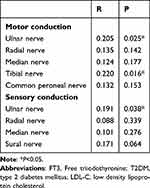 |
Table 3 Partial Correlation Between FT3 and Nerve Conduction Velocity Adjusted for HbA1c and LDL-C in T2DM Patients |
Analysis of Risk Factors of DPN in T2DM Patients
To clarify risk factors for DPN in patients with T2DM, binary logistic regression analysis was carried out. In this section, we found that FT3 is a risk factor for DPN in T2DM patients (OR=0.542, P=0.022) (Supplementary Table 1). However, FT3 was not found to be a risk factor for DPN in T2DM patients when HbA1c and LDL-C were included in the analysis (OR=0.637, P=0.101) (Table 4).
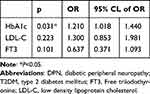 |
Table 4 Binary Logistic Regression Analysis for Influencing Factors for DPN in T2DM Patients |
Analysis of the Factors Influencing the Nerve Conduction Velocity of T2DM Patients
Since HbA1c, LDL-C, and FT3 were included in the analysis of multiple linear regression, the results indicate that FT3 is a factor that influences the conduction velocity of motor fibers in the Tibial nerve (β=0.727, P=0.016) and Ulnar nerve (β=0.795, P=0.025), as well as the conduction velocity of sensory fibers in the Ulnar nerve (β=0.909, P=0.038) (Table 5).
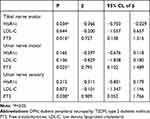 |
Table 5 Multiple Linear Regression Analysis for Factors Influencing the Nerve Conduction Velocity of T2DM Patients |
Discussion
To the best of our knowledge, there were 451 million adults with diabetes worldwide in 2017, and this figure was expected to be 693 million in 2045.18 There are approximately 1.09 billion adults in mainland China. Additionally, the overall prevalence of diabetes of adults in China was 10.9%.19 Additionally, DPN affects approximately half of patients with diabetes,1 and its frequency rises with age until it is seen in more than half of T2DM patients with the age over 60.20 Uncontrolled hyperglycemia is a risk factor for DPN, and HbA1c could be a predictor for complications following DPN, as our study has confirmed.21 We found elevated HbA1c levels in diabetic patients with DPN in this present work. Moreover, increased HbA1c variability is closely associated with DPN22,23 and could be considered as an indicator for DPN in T2DM patients.24 Therefore, it is estimated that anti-diabetic treatment may exhibit a protective effect in DPN, given the molecular mechanism of DPN described above. However, the result may not always be consistent with this assumption. Although metformin has been shown to have neuroprotective effect, its treatment did not affect DPN and even showed an adverse effect on diabetic autonomic neuropathy.25 There is an association between inflammation and BMI among patients with DPN.26 Another study demonstrated that obesity is related to DPN,27 and lipids disorder is involved in progressive diabetic neuropathy.28 In our study, we found increased LDL-C levels, but did not observe increased BMI in patients with DPN, compared with those diabetic patients without DPN. However, statin utilization was positively associated with peripheral neuropathy. In other words, the prevalence of peripheral neuropathy was lower among the statin-free group than among statin users.29
Except for the elevated levels of HbA1c and LDL-C, FT3 levels are decreased in patients with DPN, compared to those patients without DPN. This result is similar to that of a recent cross-sectional study.10 The authors demonstrated that FT3 levels are lower in DPN patients than in those without DPN. Additionally, the prevalence of DPN is higher in patients with decreased FT3, for both males and females. Another study did not observe any significant difference in T3 levels between patients without DPN and those with mild peripheral neuropathy. However, further studies measured decreased T3 levels in patients with moderate-severe peripheral neuropathy.12
The relationship between FT3 levels and DPN was analyzed by Pearson correlation to investigate the increased FT3 level in DPN patients. It is suggested that FT3 levels are positively associated with the motor fibers conduction velocity of the Ulnar nerve, Tibial nerve, and Common peroneal nerve, as well as the sensory fibers conduction velocity of the Ulnar nerve and Sural nerve. As described earlier, HbA1c and LDL-C levels are higher in diabetic patients with DPN than in those without DPN. We performed further partial correlation analysis to investigate the relationship between FT3 and nerve fibers conduction velocity. Interestingly, FT3 levels are not only positively associated with the motor fibers conduction velocity of the Ulnar nerve and Tibial nerve but also positively correlated with the sensory fiber conduction velocity of the Ulnar nerve, adjusted for HbA1c and LDL-C. This is the first study to demonstrate the association between FT3 levels and conduction velocity of nerve fibers in patients with T2DM. Several studies have observed the association between FT3 and DPN in T2DM, but none of them explored the relationship between FT3 and specific nerve fibers injury,9,11,12,30 except for a study that demonstrated associations between specific nerve fibers conduction velocity and thyroid hormones in type 1 diabetes.31 As the diagnosis of DPN is subjective and based on the skills of the implementer, this objective measurement of nerve fibers conduction velocity is more suitable for the analysis of the relationship between FT3 levels and DPN. In our study, we not only explored the relationship between FT3 levels and DPN in patients with T2DM but also analyzed the correlation between FT3 levels and nerve conduction velocity. This has two main implications. On the one hand, it is a quantitative study, and the extent of decline in nerve conduction velocity could potentially reflect the severity of the disease. On the other hand, the correlational analysis may help identify which specific nerve is more likely to be affected when FT3 levels decline, providing insights into the potential impact of FT3 reduction on nerve susceptibility. Although the effect of thyroid nodules on DPN is unclear, we compared the difference in the prevalence of thyroid nodules between patients with or without DPN, but no significant difference was found.
It was suggested that decreased FT3 is a risk factor for DPN, analyzed by binary logistic regression. However, this conclusion was overturned when HbA1c and LDL-C were entered as correction factors. Although a lower level of FT3 is not a risk factor for DPN after adjusting for HbA1c and LDL-C, decreased FT3 could still influence the motor fiber conduction velocity of the Tibial nerve and Ulnar nerve, as well as the sensory fiber conduction velocity of the Ulnar nerve. All these results demonstrate that FT3 is associated DPN. However, the mechanism remains need further exploration. One possibility is that lower T3 is associated with a decrease in peripheral insulin sensitivity, with it not only associated with diabetes but also related to its complications.32 Indeed, our previous study found that lower T3 is associated with mild cognitive impairments, related to insulin resistance in patients with T2DM.33 Additionally, thyroid is important for maintaining the survival of neurons.5
Although we have made some new findings in this study, there are still several limitations that need to be discussed. Some of our data come from self-reported medical histories, which may introduce potential biases. Since thyroid function was a key aspect of this study, we recorded the prevalence of thyroid nodules. However, the severity of these nodules was not considered in our subgroup analysis in order to avoid compromising the statistical outcomes due to the limited sample size. Although reduced FT3 levels have been identified as a risk factor for DPN in patients with T2DM, the underlying mechanisms remain unclear and warrant further research. Additionally, this cross-sectional study involved unmatched participants, and while we adjusted for various variables, these adjustments may have contributed to small correlation coefficients. Furthermore, our study population did not include healthy volunteers, nor did it compare nerve conduction velocities between T2DM patients (with or without DPN) and healthy controls. Finally, while we observed associations between different FT3 levels and DPN, well-designed cohort studies and basic research are needed to establish a causal relationship.
Conclusion
In general, this study is the first to report a link between FT3 and DPN, particularly in terms of the specific nerve fibers injury in T2DM patients. A lower FT3 concentration is a risk factor for DPN in T2DM patients without diagnosed thyroid diseases (except for thyroid nodule). Furthermore, reduced FT3 levels may affect peripheral neuropathy, particularly the motor and sensory fibers conduction velocity of the Ulnar nerve, as well as the motor conduction velocity of the Tibial nerve in T2DM with euthyroidism. Additionally, we highlighted the possibility of interactive effects of FT3 and DPN in T2DM patients with euthyroidism. We strongly call for more probes to suitable reference ranges of thyroid hormones (including FT3 levels) in the perspective of DPN.
Data Sharing Statement
All data are available on reasonable request from corresponding authors.
Acknowledgments
We are grateful to all staffs and participants involved in the study.
Author Contributions
Yang Chen, Lijie Sun, and Minghui Chen contribute equally to this work and considered as co-first authors for this manuscript. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (Haoqiang Zhang, 82400950; Wei Wang, 32271176 and 81971264), China Postdoctoral Science Foundation (Haoqiang Zhang, 2024M753132), China Endocrine Metabolism Talent Research Project from China International Medical Foundation (Haoqiang Zhang, 2023-N-03-12), Research Funds of Center for Leading Medicine and Advanced Technologies of IHM (Haoqiang Zhang, 2023IHM02006), and Scientific Research Start-up Funds of The First Affiliated Hospital of USTC (Haoqiang Zhang, RC2021178).
Disclosure
There is no potential conflict of interest for all authors.
References
1. Iqbal Z, Azmi S, Yadav R. et al. Diabetic Peripheral Neuropathy: epidemiology, Diagnosis, and Pharmacotherapy. Clin Ther. 2018;40(6):828–849. doi:10.1016/j.clinthera.2018.04.001
2. Wang C. The Relationship between Type 2 Diabetes Mellitus and Related Thyroid Diseases. J Diabetes Res. 2013;2013:390534. doi:10.1155/2013/390534
3. Chen Z, Meima ME, Peeters RP, Visser WE. Thyroid Hormone Transporters in Pregnancy and Fetal Development. Int J Mol Sci. 2022;23(23):15113. doi:10.3390/ijms232315113
4. Wallis K, Dudazy S, van Hogerlinden M, Nordstrom K, Mittag J, Vennstrom B. The thyroid hormone receptor alpha 1 protein is expressed in embryonic postmitotic neurons and persists in most adult neurons. Mol Endocrinol. 2010;24(10):1904–1916. doi:10.1210/me.2010-0175
5. Oyanagi K, Negishi T, Tashiro T. Action of thyroxine on the survival and neurite maintenance of cerebellar granule neurons in culture. J Neurosci Res. 2015;93(4):592–603. doi:10.1002/jnr.23519
6. Salas-Lucia F, Bianco AC. T3 levels and thyroid hormone signaling. Front Endocrinol. 2022;13:1044691. doi:10.3389/fendo.2022.1044691
7. Zhang W, Chen L. A Nomogram for Predicting the Possibility of Peripheral Neuropathy in Patients with Type 2 Diabetes Mellitus. Brain Sci. 2022;12(10):1328. doi:10.3390/brainsci12101328
8. Zhao W, Zeng H, Zhang X, et al. A high thyroid stimulating hormone level is associated with diabetic peripheral neuropathy in type 2 diabetes patients. Diabet Res Clin Pract. 2016;115:122–129. doi:10.1016/j.diabres.2016.01.018
9. He W, Pang C, Chen L, et al. Low T3 syndrome is associated with peripheral neuropathy in patients with type 2 diabetes mellitus. Muscle Nerve. 2022;66(6):723–729. doi:10.1002/mus.27719
10. Li MF, Ke JF, Li S, Wang JW, Zhu ZH, Li JB. Serum free triiodothyronine is inversely associated with diabetic peripheral neuropathy but not with carotid atherosclerotic lesions in euthyroid patients with type 2 diabetes. Diabetol Metab Syndr. 2021;13(1):142. doi:10.1186/s13098-021-00760-2
11. Lin J, Xiang X, Qin Y, Gui J, Wan Q. Correlation of thyroid-related hormones with vascular complications in type 2 diabetes patients with euthyroid. Front Endocrinol. 2022;13:1037969. doi:10.3389/fendo.2022.1037969
12. Zheng YH, Ren CY, Shen Y, Li JB, Chen MW. A Cross-Sectional Study on the Correlation Between Inflammatory Cytokines, Negative Emotions, and Onset of Peripheral Neuropathy in Type 2 Diabetes. Neuropsychiatr Dis Treat. 2020;16:2881–2890. doi:10.2147/NDT.S278439
13. Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi:10.2337/dc10-1303
14. Zhang X, Shen X, Zhou W, et al. The association of elevated serum lipocalin 2 levels with diabetic peripheral neuropathy in type 2 diabetes. Endocr Connect. 2021;10(11):1403–1409. doi:10.1530/EC-21-0290
15. Zhang H, Chen Y, Zhu W, et al. The mediating role of HbA1c in the association between elevated low-density lipoprotein cholesterol levels and diabetic peripheral neuropathy in patients with type 2 diabetes mellitus. Lipids Health Dis. 2023;22(1):102. doi:10.1186/s12944-023-01865-5
16. Zhang H, Vladmir C, Zhang Z, et al. Serum Uric Acid Levels Are Related to Diabetic Peripheral Neuropathy, Especially for Motor Conduction Velocity of Tibial Nerve in Type 2 Diabetes Mellitus Patients. J Diabetes Res. 2023;2023:3060013. doi:10.1155/2023/3060013
17. Zhang H, Yang S, Wang H, et al. Assessing the diagnostic utility of urinary albumin-to-creatinine ratio as a potential biomarker for diabetic peripheral neuropathy in type 2 diabetes mellitus patients. Sci Rep. 2024;14(1):27198. doi:10.1038/s41598-024-78828-y
18. Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabet Res Clin Pract. 2018;138:271–281. doi:10.1016/j.diabres.2018.02.023
19. Wang L, Gao P, Zhang M, et al. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA. 2017;317(24):2515–2523. doi:10.1001/jama.2017.7596
20. Pathak R, Sachan N, Chandra P. Mechanistic approach towards diabetic neuropathy screening techniques and future challenges: a review. Biomed Pharmacother. 2022;150:113025. doi:10.1016/j.biopha.2022.113025
21. Maiya AG, Parameshwar A, Hande M, Nandalike V. Relationship Between Glycated Hemoglobin and Vibration Perception Threshold in Diabetic Peripheral Neuropathy. Int J Low Extrem Wounds. 2020;19(2):120–124. doi:10.1177/1534734619882173
22. Allam MA, Nassar YA, Shabana HS, et al. Prevalence and Clinical Significance of Subclinical Hypothyroidism in Diabetic Peripheral Neuropathy. Int J Gen Med. 2021;14:7755–7761. doi:10.2147/IJGM.S337779
23. Nozawa K, Ikeda M, Kikuchi S. Association Between HbA1c Levels and Diabetic Peripheral Neuropathy: a Case-Control Study of Patients with Type 2 Diabetes Using Claims Data. Drugs Real World Outcomes. 2022;9(3):403–414. doi:10.1007/s40801-022-00309-3
24. Su JB, Zhao LH, Zhang XL, et al. HbA1c variability and diabetic peripheral neuropathy in type 2 diabetic patients. Cardiovasc Diabetol. 2018;17(1):47. doi:10.1186/s12933-018-0693-0
25. Hansen CS, Lundby-Christiansen L, Tarnow L, et al. Metformin may adversely affect orthostatic blood pressure recovery in patients with type 2 diabetes: substudy from the placebo-controlled Copenhagen Insulin and Metformin Therapy (CIMT) trial. Cardiovasc Diabetol. 2020;19(1):150. doi:10.1186/s12933-020-01131-3
26. Mottaghi T, Khorvash F, Khorvash F, Maracy M, Kheirrollahi M, Askari G. Association Between BMI and Inflammation Among Diabetic Polyneuropathy Patients. Int J Prev Med. 2019;10(1):212. doi:10.4103/ijpvm.IJPVM_48_18
27. Katsiki N, Anagnostis P, Kotsa K, Goulis DG, Mikhailidis DP. Obesity, Metabolic Syndrome and the Risk of Microvascular Complications in Patients with Diabetes mellitus. Curr Pharm Des. 2019;25(18):2051–2059. doi:10.2174/1381612825666190708192134
28. Mohapatra D, Damodar KS. Glycaemia Status, Lipid Profile and Renal Parameters in Progressive Diabetic Neuropathy. J Clin Diagn Res. 2016;10(9):CC14–CC17. doi:10.7860/JCDR/2016/20004.8515
29. Hammad MA, Syed Sulaiman SA, Alghamdi S, Mangi AA, Aziz NA, Mohamed Noor DA. Statins-related peripheral neuropathy among diabetic patients. Diabetes Metab Syndr. 2020;14(4):341–346. doi:10.1016/j.dsx.2020.04.005
30. Hu Y, Hu Z, Tang W, Liu W, Wu X, Pan C. Association of Thyroid Hormone Levels with Microvascular Complications in Euthyroid Type 2 Diabetes Mellitus Patients. Diabetes Metab Syndr Obes. 2022;15:2467–2477. doi:10.2147/DMSO.S354872
31. Wysocka-Mincewicz M, Baszynska-Wilk M, Mazur M, Byczynska A, Nowacka-Gotowiec M. Thyroid Hormones, Peripheral White Blood Count, and Dose of Basal Insulin Are Associated with Changes in Nerve Conduction Studies in Adolescents with Type 1 Diabetes. Metabolites. 2021;11(11):795. doi:10.3390/metabo11110795
32. Rochon C, Tauveron I, Dejax C, et al. Response of glucose disposal to hyperinsulinaemia in human hypothyroidism and hyperthyroidism. Clin Sci. 2003;104(1):7–15. doi:10.1042/cs1040007
33. Zhang H, Yang S, Zhu W, et al. Free Triiodothyronine Levels are Related to Executive Function and Scene Memory in Type 2 Diabetes Mellitus Patients Without Diagnosed Thyroid Diseases. Diabetes Metab Syndr Obes. 2022;15:1041–1050. doi:10.2147/DMSO.S355656
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Increased Systemic Immune-Inflammation Index Was Associated with Type 2 Diabetic Peripheral Neuropathy: A Cross-Sectional Study in the Chinese Population
Li J, Zhang X, Zhang Y, Dan X, Wu X, Yang Y, Chen X, Li S, Xu Y, Wan Q, Yan P
Journal of Inflammation Research 2023, 16:6039-6053
Published Date: 11 December 2023
Association of HbA1c Variability with Vibrating Perception Threshold in Middle-Aged and Elderly Patients with Type 2 Diabetes Mellitus: A Retrospective Cohort Study
Ding J, Shi Q, Dong L, Su H, Du Y, Pan T, Zhong X
Diabetes, Metabolic Syndrome and Obesity 2024, 17:193-202
Published Date: 10 January 2024
Correlation Between Thyroid-Related Hormones and Diabetic Retinopathy in Type 2 Diabetes Mellitus Patients with Normal Thyroid Function: A Retrospective Study
Xiao M, Luo G, Zhang Z, Liu Y, Gong R, Ke J
Diabetes, Metabolic Syndrome and Obesity 2024, 17:1481-1490
Published Date: 26 March 2024

