Back to Journals » International Journal of Nanomedicine » Volume 20
Materials, Syntheses and Biomedical Applications of Nano-Quercetin Formulations: A Comprehensive Literature Review
Authors Wang H, Di W, Gao X, Guo Y, Tang T, Bai X, Cao H
Received 1 February 2025
Accepted for publication 7 May 2025
Published 5 July 2025 Volume 2025:20 Pages 8729—8764
DOI https://doi.org/10.2147/IJN.S517079
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Kamakhya Misra
Han Wang,* Wenli Di,* Xibao Gao,* Yuanyuan Guo,* Tian Tang, Xue Bai, Hongqian Cao
Department of Health Inspection and Quarantine, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, 250012, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongqian Cao; Xue Bai, Department of Health Inspection and Quarantine, School of Public Health, Cheeloo College of Medicine, Shandong University, 44 West Wenhua Road, Jinan, Shandong, 250012, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Quercetin (Qu), a naturally occurring flavonoid with potent antioxidant, anti-inflammatory, and anticancer properties, faces clinical limitations due to poor solubility, low stability, and suboptimal bioavailability. This review comprehensively explores nano-quercetin (nano-Qu) formulations as a transformative solution, focusing on material design, synthesis strategies, and biomedical applications. A comprehensive review of diverse nanocarriers, including lipid-based, inorganic, polymeric, and composite nanoparticles, is presented to systematically evaluate their potential in improving solubility, stability, and targeted drug delivery of Qu. Advanced synthesis techniques such as chemical conjugation, self-assembly, and physical encapsulation are evaluated for optimizing drug loading and controlled release. Preclinical studies highlight nano-Qu’s efficacy in cancer therapy, inflammatory disorders, metabolic diseases, and tissue regeneration, attributed to improved pharmacokinetics and target-specific mechanisms. Despite promising advancements, challenges in biocompatibility, long-term toxicity, and scalable production require further investigation. This work underscores the potential of nanotechnology to unlock Qu’s therapeutic versatility.
Keywords: quercetin, nanotechnology, materials, synthesis methods, biomedical applications
Graphical Abstract:

Introduction
Quercetin (Qu), chemically named 3, 3’, 4’, 5, 7-penta hydroxyl flavone, is one of the most abundant dietary flavonols widely distributed in the plant and typically exists in fruits and vegetables.1,2 The daily intake of Qu typically accounts for about 75% of the total flavonoid intake.3 It has been reported to have potent antioxidant activity by scavenging, chelating, or capturing Reactive Oxygen Species (ROS),4 and no significant toxicity or side effects have been observed in clinical trials.5 Qu is responsible for multiple functional regulations of the body, such as neuroprotective,6 immunomodulatory,7 and vascular protective,8 also has a vigorous pharmacological activity related to diabetes,9 cardiovascular diseases,10 aging,11 cancer,12 and neurodegenerative diseases.13 The use of Qu is of great significance for both disease prevention and treatment. However, due to its poor solubility in water, instability in physiological mediators, and rapid metabolism in the body (such as the first-pass effect in the liver), Qu has low oral bioavailability, meaning that most of the ingested Qu cannot effectively enter the bloodstream, which impacts its therapeutic efficacy and limits its application in pharmacology.14
Traditional approaches to address this issue include modifying the formulation to enhance permeability, such as using topical forms like ointments, creams, gels, and emulsions. Topical formulations, such as ointments and gels, depend on passive diffusion to penetrate the stratum corneum. However, the lipid structure of the skin barrier significantly impedes the permeation of hydrophilic Qu, leading to a predominant accumulation of the drug in the epidermal layer. This phenomenon restricts the efficacy of Qu in localized treatments, including deep-seated tumors and muscle inflammation. In the case of oral traditional formulations, including tablets and capsules, Qu must undergo absorption through the gastrointestinal tract.15 Nevertheless, Qu is susceptible to degradation within the intestine due to variations in pH, enzymatic hydrolysis, and interactions with gut microbiota.16 Furthermore, Qu exhibits high sensitivity to light, high temperatures, metal ions, and oxidative environments. Traditional formulations are often devoid of stabilization measures, such as antioxidant encapsulation and light barriers, which can result in degradation during storage, diminished efficacy, and potentially the formation of toxic byproducts. The lack of controlled drug release patterns and targeting capabilities further contribute to insufficient drug concentrations at the lesion sites.17
Nanotechnology, through particle size control and surface engineering, can effectively overcome many limitations of traditional formulations and provide revolutionary solutions for drug delivery.18 It has shown great potential in drug research and development, cancer treatment, vaccine development, disease diagnosis, and advanced medical devices.19 Nanotechnology has been widely applied in the encapsulation and delivery of drugs and functional foods (especially anti-inflammatory and anti-tumor drugs, as well as plant polyphenols).20 Qu, as a multifunctional plant active ingredient, has been demonstrated through numerous studies that its encapsulation into nanocarriers or combination with nanomaterials (such as nanoliposomes) can significantly improve its drawbacks, enhancing bioavailability and therapeutic efficacy.1 Specifically, by reducing the size of the carrier to the nanoscale, the penetration ability is enhanced, facilitating drug transmembrane transport and breaking through delivery barriers. The hydrophobic core protects Qu from degradation in the physiological environment. Targeted drug delivery can be achieved through ligand modification (such as folic acid and antibodies) to actively accumulate in diseased tissues. Stimuli-responsive materials (such as pH-sensitive polymers) enable on-demand drug release and prolong the duration of action.
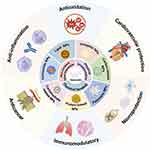 |
Figure 1 Materials, synthesis methods, and applications of nano-Qus. |
Despite the extensive research reported on the combination of Qu with nanotechnology, there is currently a lack of a comprehensive review article that thoroughly summarizes how nanomaterials can bind with Qu and enhance its properties. Against this background, our study aims to fill this gap by systematically reviewing different types of nanocarriers, synthesis methods, as well as the biological applications of nanoparticle encapsulated Qu (nano-Qu) (Figure 1). This review further explores the pivotal roles of nano-Qu in improving drug biocompatibility, stability, targeting ability, reducing adverse reactions, and enhancing bioavailability and release rates. We have comprehensively analyzed the latest research both domestically and internationally, through which we provide valuable references for future clinical applications of Qu, aiming to promote the potential of nano-Qu in the treatment of various diseases, including cancer, metabolic diseases, neurodegenerative diseases, etc., and offer new ideas and strategies for clinical treatment.
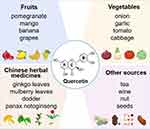 |
Figure 2 Structure and sources of Qu. |
Properties and Functions of Qu
Sources and Physicochemical Properties of Qu
Qu, one of the most abundant and widely studied flavonoids in the diet, primarily exists in various plants in the form of glycosides, such as rutin. As presented in Figure 2, its sources include fruits, vegetables, and various traditional Chinese medicinal herbs. Guo et al investigated the Qu content in commonly consumed fruits and vegetables in China. For instance, fruits with higher Qu content include pomegranates (16.78 mg/100 g), mangoes (15.72 mg/100 g), bananas (13.53 mg/100 g), and grapes (10.68 mg/100 g). Vegetables are another major source of Qu, including onions, garlic, tomatoes, and cabbage, among which onion has the highest Qu content at 8.59 mg/100 g. Traditional Chinese medicinal herbs such as ginkgo leaves, mulberry leaves, and dodder are also important sources of Qu. In addition, Qu is also abundant in other sources such as tea, wine, nuts, and seeds.
The molecular structure contains a ketone carbonyl group, a catechol type dihydroxy group on the A ring, adjacent dihydroxyls on ring B, a C2-C3 double bond, and a 4-carbonyl group. These structural features endow Qu with potential biological activities. However, the clinical application of Qu faces numerous challenges. The main challenge is its extremely low oral bioavailability, which is less than 2%, mainly attributed to its low water solubility and rapid gastrointestinal tract metabolism. Qu is a planar molecule with a tightly packed structure, generating significant intermolecular forces that hinder its dispersion in solvents or solutes. Therefore, its solubility in water is extremely low (ranging from 0.166 to 7.7 µg/mL).21
Qu is primarily metabolized by uridine diphosphate glucuronosyltransferase to form metabolites such as glucuronides, resulting in extremely low concentrations in the plasma. Experiments on rats have shown that only about 7% of Qu exists in the form of glucoside ligands in the blood one hour after oral administration of 50 mg/kg, and its bioavailability in humans is less than 1%.8 Additionally, Qu exhibits extensive first-pass metabolism, poor brain penetration, hydrophobicity, and instability at physiological pH levels. These properties collectively limit the clinical application potential of Qu. Therefore, improving the solubility and bioavailability of Qu has become the focus and challenge of current research.
Biological Functions and Mechanisms of Qu
Qu is a naturally occurring multifunctional bioactive compound that possesses a broad spectrum of biological activities, including anticancer, antioxidant, anti-inflammatory, and bone repair promotion. Additionally, it exhibits potential applications in antimicrobial, antiviral, cardiovascular protection, and blood sugar regulation, making it a highly promising multifunctional natural compound with significant developmental potential.
Qu demonstrates a broad spectrum of anticancer activity, which is achieved by inhibiting cell proliferation, inducing apoptosis, and suppressing angiogenesis. It explicitly targets key signaling pathways such as PI3K/Akt/mTOR, Wnt/β-catenin, and MAPK/ERK1/2, which are crucial in regulating cancer cell survival and metabolism.22 Furthermore, Qu enhances the sensitivity of cancer cells to apoptosis by modulating death receptors and inhibiting glycolysis.23,24 Qu is also recognized as a potent free radical scavenger that protects cells from oxidative damage. It chelates metal ions, including Cu²⁺ and Fe²⁺, and enhances the activity of antioxidant enzymes such as glutathione peroxidase and superoxide dismutase.25 By regulating pathways like Nrf2 and NF-κB, Qu helps maintain oxidative balance and mitigates diseases related to oxidative stress.26,27 Moreover, Qu exerts anti-inflammatory effects by inhibiting the production of pro-inflammatory cytokines, such as TNF-α and IL-1β, and enzymes like cyclooxygenase (COX) and lipoxygenase.28,29 It also modulates immune cell functions and suppresses oxidative stress, contributing to its effectiveness in managing inflammatory diseases.30,31 In bone health, Qu promotes osteoblast proliferation and differentiation while inhibiting osteoclast activity, accelerating bone formation, and reducing bone resorption. Qu’s antioxidant and anti-inflammatory properties further support bone healing by mitigating oxidative stress and inflammation.
Beyond these effects, Qu also exhibits antimicrobial, antiviral, and antihistamine properties, which suggest its potential applications in cardiovascular protection, anti-aging, and blood sugar regulation.32
Nano-Qu
Qu, an important flavonoid found in dietary sources, has its bioavailability influenced by various factors, including its chemical form, food matrix, processing techniques, and host factors, demonstrating significant variability. Studies indicate that Qu in natural foods primarily exists as glycosides (such as Qu-3-O-glucoside), which must be hydrolyzed into aglycones by intestinal β-glucosidase before absorption. Its absolute bioavailability ranges from 0.9% to 6.0%.33 Free Qu, due to its low solubility (< 0.01 mg/mL) and efflux by P-glycoprotein (P-gp), generally has a bioavailability of less than 2%. The food matrix effect significantly influences its absorption kinetics. For example, lipid components (such as olive oil) can promote the formation of mixed micelles, increasing the proportion of lymphatic transport to 12%.34 Meanwhile, co-existing polyphenols (such as green tea catechins) may compete for the metabolic enzyme CYP3A4, resulting in a 30%–40% decrease in bioavailability. Regarding processing techniques, moderate heat treatment (such as boiling) can increase the extraction rate of Qu glycosides by breaking down plant cell walls (+50%). Still, high-temperature baking (> 180°C) will induce oxidative degradation (loss rate> 40%).35 In addition, the metabolic transformation of Qu by the host gut microbiota (such as the generation of 3,4-dihydroxybenzoic acid) and genetic polymorphisms (such as SLCO1B1 gene variants) further lead to inter-individual differences in plasma concentration by a factor of 3.5.36
The physicochemical properties of Qu significantly influence its bioavailability and functional applications, while also offering important intervention points for nanotechnology. By controlling particle size and engineering surfaces, nanotechnology can effectively overcome many limitations of traditional formulations and provide revolutionary solutions for drug delivery. Nano-Qu refers to Qu formulations developed through nanotechnology, which exhibit distinct advantages in drug delivery systems and nutritional supplements.37 While Qu is a bioactive molecule with numerous health benefits, its poor water solubility and limited absorption in the intestine result in low bioavailability. Nanotechnology addresses this challenge by enhancing the bioavailability of Qu through increased surface area and improved solubility within the body. Nanoparticles (NPs) can be engineered to target specific cells or tissues, enabling nano-Qu to act precisely on specific cells or tissues, particularly those affected by disease, while minimizing side effects on healthy tissues. Additionally, NPs can protect Qu from environmental factors such as light, heat, and pH changes, thereby maintaining its stability and activity. Nano-Qu can be designed as a controlled-release system to regulate the rate and timing of drug release in the body, thus sustaining effective blood drug concentrations and enhancing therapeutic efficacy.38 Nano-Qu has shown potential not only in cancer therapy but also in neuroprotection, anti-inflammation, antiviral, and anti-obesity fields, indicating its promising application prospects.39 Table 1 provides a comprehensive list of the currently known types of nanocarriers capable of forming complexes with Qu.
 |
Table 1 Classification, Formulation and Related Composition of Nano-Qu |
Nanomaterials
Lipid NPs
Liposome
Liposomes encapsulate hydrophobic drugs due to their phospholipid bilayer structure, enabling targeted drug delivery. Current lipid carriers include solid lipid NPs (SLN), nanostructured lipid carriers (NLCs), and liposomal nanocapsules (LNC). NLCs and LNCs have liquid lipid cores, allowing higher drug loading compared to SLN.74 Liposome nanocarriers have unique biocompatibility compared to free Qu, which improves the water solubility of Qu, significantly enhances its stability, and provides targeting ability, increasing Qu concentration at the target organ and significantly enhancing its antioxidant and anticancer effects. Especially in brain delivery, free Qu is difficult to cross the blood-brain barrier (BBB). Qu liposome nanocarriers can improve its low bioavailability and brain permeability, making Qu a promising neuroprotective agent.
Melchior et al developed lipid-polymer nanocarriers (LPNs) with high structural integrity, stability, controlled release capabilities, as well as high biocompatibility and bioavailability. When paired with Qu, they achieved 42% encapsulation and 30 nm particle size. This improved Qu’s solubility, bioavailability, anticancer activity, and cellular uptake.44 Kamal et al used NLCs, lipid-based NPs with up to 30% liquid lipids. This oil disrupts the solid lipid structure, allowing more drug encapsulation. The optimized NLCs had 93.82% encapsulation efficiency (EE), boosting Qu delivery. Surface modifications, like phospholipids, may enhance bone healing by promoting hydroxyapatite deposition.85
Exosome
Exosomes, which also have a lipid bilayer structure like liposomes, are also considered excellent drug delivery carriers with good targeting characteristics, good biocompatibility, high delivery efficiency, and low immunogenicity. The cells that produce exosomes endow them with similar components and specific functions, such as improving glucose tolerance and increasing insulin sensitivity by the exosomes produced by human mesenchymal stem cells to alleviate T2DM.
Zhuang et al used superparamagnetic iron oxide NPs (SPION) modified exosome nanocarriers. This nanocarrier has superparamagnetic properties and can be actively targeted through an external magnetic field. After binding with Qu, the water solubility of Qu was increased by 1.97 times, enhancing its stability in vitro and in vivo. Exosomes successfully overcame the blood-pancreatic barrier between the pancreatic tissue and the blood, not only ensuring the therapeutic concentration of Qu but also effectively inhibiting cell apoptosis and significantly improving the function of pancreatic B cells.47
Inorganic NPs
Fe
Qu has a complete conjugated system of large π bonds and strong coordination oxygen atoms in its structure, which allows it to chelate stable ring complexes with metal ions. If coordinated with metal ions, Qu’s water solubility can be significantly improved, thus increasing bioavailability. The advantage of metal ions is that they often have multiple coordination sites. For example, 1 mol of Fe ions can be combined with 2 mol of Qu coordination, so Qu’s ability to carry Qu is strong.
Han et al used ultra-small Fe-Qu Natural coordination nanocomplexes (Fe-Qur NCNs) nanocarriers. Compared with Qu natural products, the synthetic Fe-Qu natural coordination NPs (Fe-Qur NCN) showed better ROS clearance, protecting cells from apoptosis, reduced pro-inflammatory macrophages, and increased the transformation of anti-inflammatory phenotype macrophages by inhibiting the activation of the intracellular NF-kB signaling pathway. In particular, the presence of Fe enables Fe-Qur NCN to move from the original joint to organs such as the heart and spleen so that Fe-Qur NCN can play a better targeting effect in the treatment of diseases in related organs.51
Au
With a hollow octahedral structure and an adjustable local surface plasmon formants (LSPR) in the near infrared (NIR) region, Qu can be loaded inside the hollow gold nanocages (AuNCs) to control drug release through photothermal therapy. Zhang used AuNCs nanocarriers in 2018. This nanocore has a hollow structure and an adjustable LSPR with photothermal effects in the NIR region, which can be used for the controlled release of drugs. When combined with Qu, the gold nanocages improved the photothermal effect of Qu and the control of drug release, achieved rapid release under near-infrared light, and showed strong cytotoxicity to MCF-7/ADR cells (IC50 of 1.5 μg/mL). In addition, the gold nanocages improve the targeting of Qu through biotin modification so that cells can take drugs up through biotin receptor-mediated endocytosis, thus improving Qu’s delivery efficiency. The AuNCs in the current study rarely released Qu at 37°C but released more than 60% at 40°C, achieving precise drug delivery.48
Others
Additionally, some inorganic NPs have a specific antioxidant effect on their own. Liu et al developed Qu-modified sulfur nanoparticles (Qc@SNPs) in a microbubble system. While sulfur NPs inherently exhibit antioxidant properties through radical scavenging, their instability is addressed by the Qu surface modification. This combination simultaneously stabilizes the NPs and creates synergistic antioxidant effects while enhancing Qu’s bioavailability.84
Wang et al used Qu-loaded nanocerium dioxide (CeO2@QU) nanocarriers. This nanocarrier has excellent antioxidant and anti-inflammatory properties. Qu was chemically bonded to nanocerium dioxide to enhance its antioxidant activity. CeO2 has a large number of surface oxygen vacancies, so cerium NPs can simulate the behavior of superoxide dismutase (SOD) and catalase (CAT), which can be used to effectively weaken and eliminate ROS production. During the anti-inflammatory process, cerium inhibits the polarization of the M1 macrophage type, and Qu drives the polarization of the M2 macrophage type. Cerium can assist Qu in completing the antioxidant and anti-inflammatory process.54
Carbohydrate-Based NPs
Chitosan
Chitosan offers exceptional advantages as a nanocarrier material, including excellent biocompatibility, modifiable surface properties, and inherent antibacterial/anticancer activity due to its -NH2 and -OH groups. While native chitosan has limited solubility at physiological pH, crosslinking modifications can significantly improve its stability. The polymer’s strong mucoadhesive properties and pH-responsive drug release (optimal at skin pH 5.4–5.9) make it particularly effective for the transdermal delivery of Qu.
Wang et al employed a multifunctional and dual-responsive nanocarrier based on chitosan oligosaccharides (CSO), referred to as GSCQ@NTB. This nanocarrier was synthesized by grafting L-cysteine and octadecylamine onto carboxymethyl chitosan, endowing it with amphiphilic properties and robust stability, maintaining minimal changes in particle size and drug concentration for 7 days. The GSCQ@NTB nanocomplexes demonstrated enhanced targeting within the ocular microenvironment through active transporter-mediated sub transport, improving the delivery efficiency of Qu to the eye. In a New Zealand rabbit model, GSCQ@NTB was found to accumulate in the corneal stromal neovascularization region within 8 h post-local administration, effectively inhibiting the progression of neovascularization with an efficacy slightly superior to that of corticosteroids, which are the frontline clinical applications.56
Cyclodextrin
Cyclodextrin is a slightly conical, hollow cylindrical low-polymer sugar with an inner cavity that is hydrophobic due to the shielding effect of C-H bonds, making it easy to bind hypericin. Combined with the hydrophilic outer edge, it can serve as a carrier for hypericin to solve the water solubility problem. The C ring of hypericin is embedded in the cavity of cyclodextrin to form a complex, which has significant progress in biocompatibility and antioxidant properties compared to free hypericin.
Studies have found that metal-organic frameworks (CMOFs) based on cyclodextrin have excellent water stability and biocompatibility due to the ester linkage. Zhao et al used CD-MOF nanocarriers, which were modified by hydrophobic cross-linking, to improve their water stability and controlled release ability. After binding with hypericin, the nanocarriers significantly improved the stability and controlled release characteristics of hypericin, resulting in a retention rate of over 90% in water environments and a sustained release effect in simulated digestion. In addition, the nanocarriers enhanced the antioxidant performance of hypericin, improved its stability and efficacy under harsh conditions. Under the condition of carrying hypericin, the Nano-CMOF retained > 90% of its structure after 14 days at 37°C, with less than 10% of γ-CD dissociation, and had one of the best stability among the many NPs of hypericin.58
Polysaccharide
Hyaluronic acid (HA) is a naturally occurring polysaccharide that is widely present in the human body’s connective, epithelial, and dermal tissues, thereby exhibiting high biocompatibility and being non-harmful to the human body upon degradation. Notably, HA demonstrates a unique affinity for specific cell surface receptors, such as CD44, which endows HA-based nanocarriers with the potential for targeted drug delivery. Sun et al synthesized an amphiphilic HA polymer (dHAD) nanocarrier with high quercetin (QT) loading capacity (75.9%). The dHAD-QT nanocomplexes improved CD44 targeting, were pH-sensitive under acidic conditions for rapid drug release, and induced cellular demise. In vivo, dHAD-QT effectively suppressed tumor growth (91.8% inhibition rate), prolonged mouse survival, and reduced drug toxicity to normal tissues.71
Although polysaccharides inherently serve as excellent nanocarriers, with the advancement of technology, researchers are seeking to optimize modified polysaccharides to achieve additional functions, such as grafting ligands with specific recognition for target sites onto natural polysaccharides. He et al created a dual-grafted dextran (ADEX) nano-micellar system for Qu. This amphiphilic nanocarrier, made by grafting L-cysteine and octadecylamine onto carboxymethyl dextran, formed stable QNMs nano-micelles with a particle size of 372 nm and zeta potential of 31.4 mV. QNMs showed pH-sensitive release behavior and enhanced Qu’s scavenging activity and cellular uptake efficiency in RAW 264.7 macrophages. Additionally, QNMs effectively modulated inflammatory cytokine expression, exhibiting better in vitro anti-inflammatory performance than free Qu.59
Protein-Based NPs
Silk Protein (SF)
The side chains of the amino acid residues in SF can interact with small molecules, and its extensive hydrogen bonds, hydrophobicity, and high crystallinity make it convenient to couple with antioxidants through simple incubation entrapment, achieving a packing efficiency of over 70%. Unlike chitosan, SF has a more minor effect on the properties of Qu itself, and it is more inclined to protect Qu from degradation in the unfavorable gastric and intestinal environment, making it easy to pass through the enterocytes or enter the colon for transportation and internalization, thus improving its pharmacokinetics and oral bioavailability. Abel et al used silk protein NPs (SFNs) as nanocarriers for Qu. These NPs improved Qu encapsulation to 70% with a loading content of 6 mg Qu/mg SFN at a 1:25 ratio. The NPs, with a uniform size of 171±1 nm, enhance cell penetration. They show bimodal release, with rapid initial release followed by sustained slow release, boosting Qu bioavailability in the gastrointestinal tract and protecting it from adverse gastric and intestinal environments, thus enhancing its oral pharmacokinetics and bioavailability.63
Zein
Corn prolamine is the primary storage protein in corn and is an insoluble alcohol-soluble protein that has the highest EE for Qu at 96%, and is usually used as an oral formulation. Campión et al used NPs and nanocapsules based on zein, a corn prolamine, as nanocarriers.64 These nanocarriers have similar physical and chemical properties, including size (230–250 nm), spherical shape, negative zeta potential, and surface hydrophobicity. After binding with Qu, these nanocarriers exhibit similar release behavior in simulated gastric and intestinal fluids and can improve the EE (about 80%) and stability of Qu. Corn prolamine nanocarriers, especially NPs, can improve the bioavailability of Qu, with relative oral bioavailability of 26% and 57%, respectively, while the relative oral bioavailability of the control formulation is 5%. This is because corn protein NPs have low mechanical strength and enter the intestine quickly, forming an amorphous state coated with a tea saponin shell for easy delivery.64
Low Molecular Weight Heparin (LMWH)
Furthermore, there are bioactive proteins with inherent special functions that, when formed into NPs, retain the unique roles of their protein matrices. Wang et al utilized an amphiphilic carboxymethyl chitosan-Qu conjugate nanocarrier with P-gp inhibitory properties. This nanocarrier, through its combination with Qu, significantly enhanced the oral delivery efficiency of Qu. LMWH, a non-immunogenic and non-toxic natural anionic polysaccharide, can suppress angiogenesis by binding to pro-angiogenic factors such as basic fibroblast growth factor and vascular endothelial growth factor. Additionally, derivatives of LMWH can reduce the anticoagulant activity and bleeding risks associated with LMWH while leveraging its bound bioactive segments, such as Qu, to block angiogenesis and inhibit the proliferation of tumor cells. These characteristics of LMWH play a significant role in nanocarriers by improving drug distribution, enhancing targeting, and augmenting therapeutic efficacy.61
Polymeric NPs
Poly (Lactic-Co-Glycolic Acid) (PLGA)
PLGA is a biodegradable polymer that has high stability, biocompatibility, and biodegradability in the cellular environment. It is typically degraded by hydrolysis of its ester bonds into low-toxic lactic acid and glycolic acid, which are then metabolized and eliminated by the cell’s natural functions. It is worth noting that PLGA NPs take advantage of the high permeability and retention effect (EPR effect) of solid tumors in tumor therapy to achieve passive targeting, which gives it a unique advantage in anticancer processes. He et al used PLGA nanocarriers, with a porous surface made by electrospinning and combined with MgO and Qu, to enhance Qu stability. These nanocarriers promote bone marrow stromal cell (BMSC) proliferation, migration, and osteogenic differentiation via the Wnt/β-Catenin pathway. Appropriate concentrations of Qu also boost endothelial progenitor cell angiogenesis. In both in vitro and in vivo models, they exhibit good biocompatibility and promote bone regeneration. Thus, this nanocarrier significantly improves the osteogenic and angiogenic effects of Qu.66
Polyvinylpyrrolidone (PVP)
PVP consists of a hydrophilic pyrrolidone part and a hydrophobic alkyl part and can be used as a surface stabilizer, growth modifier, nanoparticle dispersant, and reducing agent. Ponraj et al used PVP K30 as a crystallization and precipitation inhibitor to prepare a saturated solution of Qu, with a drug loading of up to 13% and an absolute bioavailability that was 10 times that of physically prepared Qu suspensions.
The versatility of PVP makes it possible to control the growth and shape of NPs during their synthesis. In particular, PVP can guide the growth of different directions and facet NPs by adsorbing on different crystal faces and acting as a surface inhibitor to blunt the edges when synthesizing metal NPs. This results in the formation of PVPylated-TiO2 NPs with a smooth surface in the shape of nanoballs. Such morphology enhances penetration and the EPR effect, promoting the potential passive targeting of Qu to tumors and increasing the concentration of Qu at the targeting site. Li et al utilized PVP K30 in a super-saturated drug delivery system (SDDS) nanocarrier, achieving 13% drug loading. This nanocarrier enhances Qu’s solubility by over 1700 times and maintains high super-saturated Qu concentrations, significantly improving oral bioavailability. Bioavailability increases with dose, and the Qu SDDS (QSDDS) preparation is scalable for industrial production.86
Carbon-Based NPs
Graphene Oxide (GO)
GO is a two-dimensional carbon structure with a single atomic layer thickness in general, and its bilayer plate-like structure has excellent advantages in drug delivery. The basal planes of the graphene sheets in GO are mainly surrounded by epoxy and hydroxyl groups, and the edges have carboxyl groups. These oxygen-containing functional groups make it possible for GO to have non-covalent binding with drugs in addition to physical adsorption. In practical applications, the disadvantage of GO is that it is easily prone to irreversible aggregation through strong π-π stacking and van der Waals interactions in solutions rich in salts or proteins (such as cell culture media and serum), forming multi-layer GO, which affects drug delivery.
Rahmanian et al used nanographene oxide (GO) as a nanocarrier, and this nanocarrier has excellent biocompatibility and physiological stability. After binding with Qu, the nanographene oxide can improve the water solubility of Qu, enhance its dispersing stability in physiological solutions such as PBS, SGF, and SIF, and show no significant toxicity to A549 cells in vitro, thus improving the bioavailability and drug delivery efficiency of Qu. Furthermore, GO as a nanocarrier has considerable mechanical strength for Qu and shows excellent antibacterial activity. GO’s high aspect ratio, large specific surface area, rich surface chemical properties, and good dispersibility in aqueous solutions make it highly efficient in carrying Qu, greatly improving the water solubility and bioavailability of Qu.77
Quantum Dots (QDs)
QDs are nanoscale semiconductor crystals made of elements from the II–VI or III–V groups, which can serve as fluorescent nanoprobes to enhance the concentration and efficacy of drugs in specific areas. R. Jeyadevi used thioglycolic acid-capped cadmium telluride QDs (TGACdTe QDs) as nanocarriers. Research has shown that thioglycolic acid-capped cadmium telluride QDs as a fluorescent probe can undergo fluorescence quenching when complexed with Qu, allowing for tracking and detection during drug delivery. Additionally, QDs combined with Qu can neutralize various free radicals produced in inflamed areas and inhibit the production of COX-2 enzyme at lower concentrations, achieving anti-inflammatory effects similar to those of free Qu at higher concentrations. Like most nanocarriers, QDs can also significantly enhance the water solubility and bioavailability of Qu, addressing the most significant limitation of Qu application.79
Composite NPs
In some cases, a single nanocarrier may not be sufficient to meet the therapeutic needs, so researchers turn to develop various drug hybrids composed of two or more functional units with different mechanisms of action. For example, when encountering bacterial resistance problems, the rapid mutation of bacteria leads to the restriction of the last therapeutic option, polymyxin, in its killing effect. Therefore, researchers have combined three units, including aminoglycoside antibiotics (A domain, such as polymyxin and aminoglycosides), formylbenzoylboronic acid (B domain), and polyphenols (C domain, such as Qu and trisphenol), through reversible iminoboronic acid bonds to form A1B1C1 dynamic covalent nanoregulatory networks. In this nanocovalent network, the amino group can reduce the toxicity of antibiotics to normal cells; the introduction of formylbenzoylboronic acid enhances the water solubility of Qu, thereby having a strong effect in eliminating ROS; Qu serves as an adjuvant, enhancing the destruction function of antibiotics against biofilms. The three structures work together to display outstanding antibacterial efficacy.68
The multifunctional MIL-100 nanocarrier refers to the wrapping of 5-fluorouracil and gemcitabine hydrochloride with MIL-100, followed by coating with chitosan, and finally binding with Qu, and forming a coordination bond with Fe through chitosan. Among them, chitosan provides biocompatibility and pH sensitivity, Qu binds to MIL-100 to enhance the anticancer effect, and Fe is used to form a coordination bond with chitosan and Qu to enhance the targeting and stability of the NPs. Overall, a nanomedicine delivery system with pH sensitivity, targeting, and synergistic anticancer effects has been formed, aiming to improve the efficacy of Qu and reduce its side effects.81
In a scholarly study conducted by Song et al, a nanocarrier based on Silk Fibroin/Hydroxyapatite (SF/HAp) was investigated and combined with quercetin (Qtn) to form the Qtn/SF/HAp nanocarrier. This nanocarrier possesses osteogenic properties, particularly in enhancing the osteogenic efficacy of Qu. The Qtn/SF/HAp nanocarrier was prepared by embedding varying concentrations of Qu into the SF/HAp matrix, resulting in increased pore size and an irregular porous microstructure while maintaining good mechanical strength.
Within this composite nanocarrier, silk fibroin provides biocompatibility and tunable biodegradability, making it an ideal biomaterial for tissue regeneration, especially in bone tissue engineering. HAp, being one of the main constituents of bone, exhibits excellent osteoconductivity. When combined with Silk Fibroin, it yields a porous structural scaffold with high mechanical strength and enhanced osteoinductive properties. Qu, a bioactive phytochemical, is embedded into the SF/HAp scaffold at different concentrations to promote bone tissue generation, particularly by enhancing the activity of osteoblasts and inhibiting the activity of osteoclasts, thereby promoting bone health.62
 |
Table 2 Methods for Synthesis of Nano-Qus |
Synthesis Methods of Nano-Qus
As shown in Table 2, various methods have been developed for the synthesis of nano-Qu, including chemical bonding, non-covalent interactions, physical encapsulation, and other approaches. These methods offer different advantages and are chosen based on specific requirements such as solubility, stability, and biological activity of the resulting nano-Qu.
Chemical Bonding
Esterification Reaction
The molecular structure of Qu includes two benzene rings and a tricyclic ring, with a hydroxyl group and a carboxyl group on the tricyclic ring. When Qu binds to NPs with hydroxyl or carboxyl groups, it can undergo an esterification reaction with the NPs to enable the NPs to carry Qu into the body. After the Qu NPs reach the target site, the ester bond is broken under specific conditions, releasing Qu. Gupta et al used acryloyl chloride to acryloylate four phenolic groups of Qu, allowing it to form a cross-linked network with secondary amines through a Michael addition reaction. Qu was then fixed to the polymer matrix of the nanogel through the cross-linked ester bond, allowing the release of Qu to be controlled when the nanogel degrades.75 Similarly, Aghamohammadi et al used the Fischer esterification method to combine Qu with fatty acids (α-linolenic acid and linoleic acid) under the catalysis of an inorganic acid to form Qu fatty acid esters. Studies have shown that compared to free Qu, esterified Qu has a higher modulus of elasticity and cell-to-cell adhesion, and its cytotoxic effect is more obvious.67
Coordination Ligand Binding
Some metallic NPs, such as Fe or cadmium ions, often have multiple coordination sites, which facilitate the binding of Qu through coordination. Han et al simply mixed a methanol solution of Fe3+ and Qu to conjugate Qu’s hydroxyl and Fe ions. Further energy dispersive X-ray analysis and X-ray photoelectron spectroscopy confirmed the presence of Fe ions in the nano complex. The ratio of different valence states of Fe (Fe3+ and Fe2+) is about 5:1. Ultra-small Fe-Qu nanocomplexes (Fe-Qur NCNs) have good water solubility and biocompatibility. They can enhance the anti-inflammatory effect of macrophage polarization (Figure 3A).51 Jeyadevi et al combined appropriate concentration of Qu with cadmium-tellurium QDs modified with hydrogen sulfide acetic acid, and the hydroxyl group of Qu was anchored on the surface of the QDs to coordinate with cadmium ions on the surface of the QDs. The hydroxyl group of Qu has a stronger binding force with the surface of QDs and higher coordination efficiency than the trihydric group in hydrosulfide acetic acid. Interestingly, with the increase of Qu concentration, the recombination of electrons and holes in QDs is inhibited, resulting in fluorescence quenching.79
 |
Figure 3 Applications of synthesis methods for Qu and NPs. (A) Fe-Qur NCNs were obtained by combining iron ions with Qu through coordination. Adapted from Acta Pharm Sin, volume 13(4), Han Z, Gao X, Wang Y, et al. Ultrasmall iron-quercetin metal natural product nanocomplex with antioxidant and macrophage regulation in rheumatoid arthritis. 1726–1739. Copyright 2023, with permission from Elsevier.51 (B) Succinic anhydride activated Qu reacts with the amino group of chitosan via an amide bond. Adapted from Carbohydr Polym, volume 203, Multifunctional quercetin conjugated chitosan nano--micelles with P-gp inhibition and permeation enhancement of anticancer drug”. 10–18, copyright 2019, with permission from Elsevier.88 (C) In the synthesis of aDCN, polyamine antibiotics (domain A, such as polymyxin and aminoglycoside), formyl phenyl boric acid (domain B), and polyphenols (domain C, such as Qu and hydroquinone) are linked by borimino ester bonds. Adapted from Li Y, Piao Y-Z, Chen H, et al. Dynamic covalent nano-networks comprising antibiotics and polyphenols orchestrate bacterial drug resistance reversal and inflammation alleviation. Bioact Mater. 2023;27:288–302. Creative Commons.68 |
Amido Bond
Qu can connect with some polymers or polysaccharides, and one of the carboxyl groups on its tetra hydroxy cyclopenta[a]pyran ring is very likely to bond with an amino group through a dehydration reaction to form a stable covalent bond. El-Gogary et al bonded folic acid covalently to PLGA polymer using polyethylene glycol (PEG) as the spacer arm, where the bonding between PEG and PLGA, as well as the bonding between Qu and PEG-PLGA complex, were all completed through amide bonds.67 Similarly, examples of covalent bonding through amide bonds also include the reaction of Qu with chitosan. According to Figure 3B, Mu et al reacted the succinic anhydride-activated Qu with the amino group of chitosan using 1-ethyl-3-(3-dimethylamino propyl) carbodiimide hydrochloride and N-hydroxysuccinimide as the activators to form amide bonds.88
Complex Chemical Bond
In some cases, a single type of covalent bond may not meet the requirements of a composite drug for treating multiple diseases, and multiple effective drugs need to be linked together. A dynamic covalent bond network that can simultaneously utilize the synergistic effects of multiple components is therefore favored by researchers. Li et al constructed a dynamic covalent bond network consisting of polyamine antibiotics (A domain structure, such as colistin and aminoglycosides), flavonoids (C domain structure, such as Qu and p-coumaric acid), and acyl benzoyl boronic acids (B domain structure), as presented in Figure 3C. The network is divided into three domains, polyamine antibiotics (domains A, such as polymyxin and aminoglycosides), formylphenylboric acid (domains B), and polyphenols (domains C, such as Qu and hydroquinone), which are linked by originate linkages. The components in this covalent bond network work together to assist each other, and the boriminoborate ester bond protects the amino group of antibiotics to ensure that antibiotics can play a bactericidal role and reduce the toxicity to the human body. The synergistic effect of antibiotics and polyphenols can solve the drug resistance of bacteria.68
Non-Covalent Interactions
Self-Assembly
Self-assembly refers to the process of organizing molecular or nanoscale components into ordered structures through non-covalent interactions, such as hydrogen bonds, van der Waals forces, hydrophobic interactions, and electrostatic forces, under specific conditions. This process typically occurs at the molecular level and can form a variety of structures, ranging from micelles to vesicles, fibers, or other nanostructures. Self-assembly is spontaneous and reversible, which ensures that Qu can bind to NPs easily and quickly, and be released for action at the target site after delivery. Some nanocarriers for Qu exhibit multiple non-covalent interactions, which may involve hydrophobic forces, hydrogen bonds, and electrostatic interactions simultaneously, and cannot be summarized by a single type of interaction. The resulting Qu nano delivery carriers are often quite complex, with nanomicelles being the most common form.
 |
Figure 4 Applications of non-covalent bonding of Qu with NPs. (A) grafted L-Cys and octadecylamine on CMD, followed by a process of hydrophobic binding of ADEX and Qu. Adapted from Int J Biol Macromol, volume 224, He Z, Liu Y, Wang H, et al. Dual-grafted dextran based nanomicelles: higher antioxidant, anti-inflammatory and cellular uptake efficiency for quercetin. 1361–1372, Copyright 2023, with permission from Elsevier.59 (B) the preparation of micelles. Adapted from Int J Biol Macromol, volume 242, Sun J, Li M, Lin K, et al. Delivery of quercetin for breast cancer and targeting potentiation via hyaluronic nano-micelles.124736, copyright 2023, with permission from Elsevier.71 (C) PDA@QLipo NPs were prepared by combining Qu with liposome and PDA by thin film water method. Adapted from Yang W, Lv Y, Wang B, et al. Polydopamine synergizes with quercetin nanosystem to reshape the perifollicular microenvironment for accelerating hair regrowth in androgenetic alopecia. Nano Lett. 2024;24(20):6174–6182. Copyright © 2024 American Chemical Society.46 |
He et al prepared Qu-loaded nanocapsules by using ultrasound-assisted self-assembly with cationic-modified dextran (ADEX) as the shell material and Qu as the core material, as shown in Figure 4A. ADEX was obtained by grafting L-cysteine (L-Cys) and octadecanamine onto CMD, and the cationic-modified ADEX and Qu were dispersed in water. After the ultrasound self-assembly process, Qu-loaded NPs were formed, which may have been completed through hydrogen bonding between the -SH group in L-Cys and other molecules, as well as the hydrophobic interaction by the hydrophobic chain of ODA.59 Huang et al prepared the saponin-zein binary complex as a Qu delivery carrier via an antisolvent method. This is a core-shell structured NPs, with an inner layer serving as a hydrophobic core formed by zein binding with Qu, which are combined through hydrogen bonding and hydrophobic interactions. The outer layer consists of a hydrophilic shell composed of saponin. Since the saponin contains both hydrophilic glycosides and hydrophobic glycosides, it can encapsulate the zein-Qu complex via electrostatic interactions, transforming it into an amorphous state more suitable for drug delivery.65 According to Figure 4B, Sun et al used HA modified by dodecyl amine through grafting to form dHAD polymers, relying on the amphiphilic properties of dHAD polymers to spontaneously assemble with Qu to form drug carrier micelles. Hyaluronic acid has strong hydrophilicity, and the grafted dodecyl amine provides hydrophobicity so that the dHAD polymers can also form multiple hydrogen bonds with CD44-positive tumor cells, improving the targeting of the drug carrier to the tumor cells.71
Hydrophobic Interaction
Natural or artificially synthesized amphiphilic nanocarriers, due to their both hydrophobic and hydrophilic properties, can bind to Qu through their hydrophobic ends and bind to water in the human body through their hydrophilic ends, thus improving the water solubility and bioavailability of Qu. Gao et al manufactured Qu nanocarriers with a core-shell structure through hydrophobic and hydrophilic interactions. In this structure, PCL serves as the hydrophobic core, while PEG serves as the hydrophilic shell, and the two diblock copolymers form stable micelles. Qu, due to its hydrophobicity, tends to interact with the PCL core, while the PEG segment interacts with water through hydrophobic interactions to naturally form the core-shell structure of QU/MPEG-PCL nanomicelles.72
In many cases, Qu and nanocarriers can spontaneously form the core-shell structure in solvents through hydrophobic interactions, but there are also cases where external special treatment is needed. He et al utilized an ultrasonic self-assembly method to combine the prepared amphiphilic nano-carrier with Qu, resulting in drug-loaded nano-micelles with Qu as the hydrophobic core and ADEX as the hydrophilic shell. ADEX is synthesized by grafting L-cysteine and octadecyl amine onto dextran, possessing both hydrophilic and hydrophobic groups, which primarily bind to Qu through hydrophobic interactions.59
Hydrogen Bond
In the molecular structure of Qu, multiple hydroxyl groups can act as receptors to receive the lone pair of electrons, while the oxygen atom of the ketone group has a lone pair of electrons that can act as a donor. Additionally, benzene rings and double bonds can participate in the formation of hydrogen bonds. Zhang et al developed a “double-lock” nanoplatform that integrates Galactan coating and mesoporous poly(dopamine) to encapsulate and deliver drugs, such as dasatinib and Qu, for the purpose of inducing cell apoptosis and programmed cell death to eliminate senescent cells. The “double-lock” nanoplatform primarily uses poly(dopamine) (PDA), which has a rich supply of amino groups and is easily able to form hydrogen bonds with the hydroxyl groups on Qu to load the drugs. During the drug release process, the Galactan layer coated on the surface of the NPs can be hydrolyzed by the high expression of β-galactosidase in senescent cells, and when the NPs are engulfed by the lysosomes of senescent cells, the first “lock” is released; at this point, in the acidic environment of the lysosome of senescent cells, the amino protons are protonated and the hydrogen bonds are broken, completing the release of the drugs.76
Sadalage et al used AuNPs for Qu delivery. To facilitate the binding of AuNPs to Qu, they employed polyethylene glycol 9000 (PG9) to modify the surface of AuNPs. PG9, containing oxygen atoms with lone pairs of electrons, forms hydrogen bonds with the hydroxyl groups of Qu. In this study, Qu was co-administered with camptothecin, which was also bound to AuNPs through a similar process.83 Yang et al also used gold NPs as carriers, but unlike the previous example, they employed compressed spherical DNA nanostructures (DNS) obtained through rolling circle amplification and ligation of DNA strands (LB) to modify the AuNPs. When Qu needs to be bound, the DNA unwinds and forms hydrogen bonds with the hydroxyl groups of Qu, achieving drug loading. When Qu is delivered to the target site, the DNA on the surface of the AuNPs unwinds under 800 nm laser irradiation through the photothermal conversion effect of the AuNPs, causing the hydrogen bonds to break and triggering the disintegration of the DNA nanospheres to release Qu.91
Other Non-Covalent Bond Interactions
In addition to the common types of non-covalent interactions and self-assembly, Qu’s binding to nanocarriers occasionally occurs through some special forms, such as the thin film hydration method for preparing nanovesicles through non-covalent interactions, which mainly includes hydrogen bonds and van der Waals forces. Sayyad et al dissolved cholesterol, surfactants, and charge-inducing agents in a mixture of methanol and chloroform evaporated the organic solvent under reduced pressure to leave a thin film, and then formed nanovesicles loaded with drugs by adding water and sonicating. In this study, the delivered drugs were combined Qu and captopril, which were linked by an esterification reaction to form a binary hybrid, and then bound to the nanovesicles through hydrogen bonds and van der Waals forces.89 Yang et al encapsulated Qu in liposomes and packaged it in nanovesicles using the thin film hydration method, then combined it with PDA to prepare PDA@QLipo NPs that could simulate human melanin synthesis (Figure 4C). Since PDA has rich hydroxyl groups, strong hydrogen bonds are easily formed during the binding process, and in addition, the water-mediated hydrogen bonding and van der Waals forces also participate in the thin film hydration method when evaporating the solvent.46
Physical Encapsulation
Adsorption
In recent years, various formulations with different Qu encapsulation methods and carriers have been described. Simple adsorption stands out as one of the best silk-based immobilization strategies for improving the stability of compounds or proteins. It mainly involves simple processes such as mixing, stirring, and incubation, and it has the advantages of being mild, relatively simple, and inexpensive.
Lozano-Pérez et al prepared SFNs added Qu dissolved in ethanol to the SFN suspension, mixed it in an orbital rotator, and collected Qu-loaded SFNs (QSFNs) by centrifugation. An EE of ~ 70% was achieved when the Q/SFN ratio was 1:250 (w/w), and a drug loading capacity of 6 μg Qu/mg QSFN was obtained when the Q/SFN ratio was 1:25 (w/w). QSFNs possess many advantages, including nanosize, effective Qu encapsulation capacity, the ability to protect drugs from degradation in adverse gastrointestinal environments, and improved bioavailability.63
Rahmanian et al loaded Qu onto nano-GO by simply mixing a Qu solution with a nano-GO aqueous suspension and incubating it at room temperature. Under optimal conditions, the loading efficiency of Qu on nano-GO sheets was approximately 44%, with dispersible stability in various physiological solutions and negligible toxicity.77 Similarly, Islami et al designed an efficient drug delivery system based on GO by covalently grafting hyperbranched polyglycerol (HPG) onto the GO surface to control the release of Qu. Firstly, HPG-GO was synthesized and dispersed in deionized water, then mixed with a Qu solution dissolved in ethanol, and drug loading was achieved by vigorous shaking at 4°C for 12 to 24 hours. HPG-GO exhibited a high drug loading capacity (up to 185%) and excellent EE (up to 93%). This encapsulation method achieved controlled and slow sustained release of Qu, with good biocompatibility and no cell toxicity observed.78
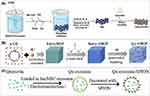 |
Figure 5 Applications of physical method for synthesis of nano-Qus: (A) scheme of preparation protocol for MBG and Quercetin/MBG. Adapted from Yang S-Y, Hu Y, Zhao R, et al. Quercetin-loaded mesoporous nano-delivery system remodels osteoimmune microenvironment to regenerate alveolar bone in periodontitis via the miR-21a-5p/PDCD4/NF-κB pathway. J Nanobiotechnol. 2024;22(1):94. Creative Commons.52 (B) the synthesis method of Qu-CMOF. Adapted from Food Chem, volume 448, Zhao R, Chen T, Li Y, et al. Biocompatible hydrophobic cross-linked cyclodextrin-based metal-organic framework as quercetin nanocarrier for enhancing stability and controlled release. 139167, copyright 2024, with permission from Elsevier.58 (C) The synthesis of Qu-exosome-SPION. Adapted from with permission from Dove Medical Press. Zhuang M, Rao L, Chen Y, et al. Controlled SPION-Exosomes loaded with quercetin preserves pancreatic beta cell survival and function in type 2 diabetes mellitus. Int J Nanomed. 2023;18:5733–5748.47 |
Mesoporous bioactive glass (MBG) NPs are considered potential drug carriers for bone defects due to their high porosity, specific surface area, and potent ability to stimulate bone tissue regeneration. In a study by Yang et al, Qu dissolved in ethanol was mixed with MBG and stirred in a rotary incubator for 24 hours. Quercetin-loaded MBG (quercetin/MBG) was then obtained by centrifugation (Figure 5A). Qu was loaded into the core of MBG, with a loading capacity and EE of 320.25 mg/g and 8.54%, respectively. The release profile showed an initial burst release within 3 hours, followed by sustained release over 21 days. The treatment effect of quercetin/MBG on periodontitis was superior to that of pure MBG treatment.52
Electrospinning
Fibers produced through electrospinning technology possess interconnected pores capable of loading various therapeutic agents, making them highly suitable for drug delivery systems. Bioactive molecules such as drugs, proteins, and nucleic acids can be encapsulated within or attached to the fibers to achieve precise drug release for biomedical applications such as tumor treatment and regenerative medicine.
Hudecki et al first synthesized polymer materials PLA and PEO for the preparation of Qu-loaded fibers. PLA and PEO were dissolved in a mixed solvent of acetone and chloroform, with 9% Qu added as the active ingredient to ensure uniform dispersion within the polymer matrix. Using electrospinning technology, the polymer solution is stretched into fibers under the action of an electrostatic field, while Qu molecules are physically encapsulated within or on the surface of the fibers along with the arrangement of polymer chains, thereby preparing Qu-loaded fibers for delivering Qu to cancer cells. The incorporation efficiencies (IE) of PLA/Q and PLA/PEO/Q were 84±2% and 91±2%, respectively. Qu enhances the stability of the fibers, and the anticancer activity of Qu-loaded fibers is more pronounced than that of free Qu.73
Furthermore, nanofibrous membranes prepared by electrospinning technology have the potential to become artificial bone membranes due to their similarity to the extracellular matrix and periosteum structure. Xi et al used PLGA as the main substrate to prepare nano-artificial bone membranes through electrospinning. PLGA, MgO, and Qu were dissolved in a mixed solvent of DMF and acetone. This electrospinning solution was loaded into a syringe and fixed onto a plunger to prepare PLGA/Qu electrospun membranes with different mass ratios. The membranes featured a highly porous surface and microstructure, providing the necessary space for neovascularization. They exhibited good biocompatibility, and an appropriate concentration of Qu, in cooperation with MgO, exerted an osteogenic-angiogenic coupling effect.66
Solvent-Based Method
The SDDS can rapidly form a supersaturated solution of the drug in the gastrointestinal environment and maintain it without precipitation for some time, thereby enhancing oral absorption. Various polymers have been utilized as inhibitors to prevent drug precipitation in supersaturated solutions. Li et al prepared a QSDDS using a solvent method, in which PVP K30 was employed as a crystallization and precipitation inhibitor to maintain the supersaturated state of Qu in an aqueous system. Specifically, Qu and PVP K30 were co-dissolved in ethanol and mixed with an aqueous xylitol solution. After evaporation and vacuum drying, the mixture was milled to obtain QSDDS powder. The resulting QSDDS exhibited a relatively high drug loading capacity of 13%, could rapidly disperse in water to form a colloidal system with an average particle size of approximately 200 nm, and induced the formation of a supersaturated Qu solution for over 12 hours. The QSDDS achieved a high absolute bioavailability of 36.05%, which is 10 times that of a physical Qu suspension.86
HAp, as an important mineral phase of bone, exhibits excellent osteoconductivity. Various studies have reported the fabrication of silk fibroin/HAp composites/scaffolds through different bone tissue engineering approaches. Zheng et al designed a silk fibroin/HAp scaffold embedded with different concentrations of Qu (Qtn/SF/HAp) to promote osteogenesis. To synthesize Qtn/SF/HAp scaffold, Qu dissolved in DMSO was mixed with the SF aqueous solution. The mixture was then freeze-dried and treated with methanol to achieve physical crosslinking. The scaffold was further coated with HAp by stirring in simulated body fluid. The Qtn/SF/HAp scaffolds demonstrated increased pore size and an irregular porous microstructure, possessed good mechanical strength, promoted cell growth and osteogenic differentiation, and exhibited excellent cytocompatibility.62
Zhao et al synthesized nano-CMOF dissolved Qu in ethanol and mixed it with nano-CMOF. The mixture was stirred in the dark at 45°C for 6 hours. The solvent was removed by centrifugation, and the precipitate was washed with methanol and ethanol before being vacuum-dried to obtain the Qu-CMOF sample (Figure 5B). Loading Qu into nano-CMOF enhanced the stability, controlled release, and biocompatibility of Qu.58
Desolvation
Campión et al prepared and evaluated two distinct zein-based NPs: zein nanospheres (NS) and zein nanocapsules (NC). Zein nanospheres were fabricated via desolvation by mixing zein and L-lysine in a 70% (v/v) ethanol-water solution and stirring until dissolution. Pure water was slowly added to this mixture to induce the formation of nanospheres. An aqueous mannitol solution was then added to stabilize the dried nanospheres. Qu-loaded nanospheres (Q-NS) were prepared by incorporating Qu into an initial zein and lysine ethanol-water solution, without the addition of mannitol during drying. The preparation of nanocapsules was similar to that of nanospheres but with a crucial step involving the addition of an oily compound. Similarly, Qu was added to the initial zein, oil, and lysine mixture before forming the nanocapsules to prepare Qu-loaded nanocapsules (Q-NC). Both Q-NS and Q-NC exhibited an average size of approximately 230–250 nm, with a Qu payload of about 75 μg per milligram of NPs and an EE of around 80%. Notably, the relative oral bioavailability of Q-NS and Q-NC was significantly enhanced, at 26% and 57%, respectively, compared to the control formulation (5%).64
Kamal et al dissolved Qu and SPC in methanol, subjected the mixture to magnetic stirring for 2 hours, and then evaporated the solvent using a hot plate at 40°C with a stirring speed of 600 rpm to obtain Qu phospholipid complex (QT-pc). Subsequently, NLCs loaded with Qu and Qu-phospholipid complex (QT-NLCs, QT-pc NLCs) were prepared. Incorporating Qu-pc into the NLCs increased the EE to above 95%. Loading the lipid NPs (QT-NLC/CPC) into calcium phosphate cement (CPC) resulted in appropriate setting time, adequate porosity, mechanical strength, and controlled release of Qu.85
Other Methods
Electroporation
Electroporation is a technique that utilizes brief high-voltage electric fields to create micropores in cell membranes. Electroporation allows exogenous molecules such as Qu to be loaded into exosomes, thereby endowing the exosomes with specific therapeutic effects. The advantages of this method lie in its relatively simple operation and its ability to maintain the integrity and biological activity of exosomes to a certain extent. Superparamagnetic Fe oxide NPs (SPIONs) have shown great potential in targeted drug delivery by enabling the targeting of specific tissues or organs through the application of an external magnetic field (MF). Zhuang et al mixed exosomes with a Qu solution and subjected them to electroporation in an electroporator to load Qu into the exosomes. Unloaded Qu was removed through methods such as ultrafiltration, and Qu-loaded exosomes (Qu-exosomes) were harvested. The Qu-exosomes were then incubated with Tf-SPIONs to allow the Tf-SPIONs to bind to the exosomes, forming Qu-exosome SPIONs, as shown in Figure 5C. The exosomes provide a barrier to protect Qu from degradation, overcoming its pH instability. The Qu loaded in exosome-SPIONs exhibited higher water solubility (1.97 times) than free Qu. The application of SPIONs/MF endowed the Qu-exosomes with good targeting ability.47
Microemulsion Method
Kumar et al prepared Qu-loaded NLCs using biocompatible components such as phospholipids and tocopheryl acetate to enhance brain delivery. Specifically, Qu was dissolved in anhydrous ethanol, while phospholipids were dispersed in 2 mL of water and 7 g of Tween 80 and heated to 70°C. Additionally, Compritol was melted and tocopheryl acetate was added to it. The ethanol solution containing Qu, the lipid phase, and the aqueous solution of phospholipids and Tween 80 were mixed to form a transparent microemulsion. The hot microemulsion was rapidly poured into ice-cold water and stirred for 30 minutes using a mechanical stirrer. Subsequently, the dispersion was left at room temperature until the foam disappeared. Both types of nanocolloids provided better drug loading and controlled drug release, exhibiting significant stability. The retention of the drug was markedly increased, and SLNs and NLCs were able to enhance the relative bioavailability of Qu by 3.5 times and 5.4 times, respectively.42
Sol-Gel Route
Researchers synthesized SiO2-based hybrid materials containing the antioxidant Qu and PEG through a Sol-gel method. Firstly, an inorganic silica sol was prepared, and PEG was dissolved in ethanol and uniformly mixed with the silica sol. An ethanol-dissolved Qu solution was added to the system, and the mixture was thoroughly stirred to gelate the system. The gel material was dried at 50°C for 24 hours to obtain SiO2/PEG/Qu hybrid material. This material exhibited excellent biocompatibility.90
Biomedical Applications of Nano-Qus
Nano-Qu exhibits broad application potential in the treatment of various diseases, with specific applications referred to in Table 3.
 |
Table 3 The Biological Applications of Nano-Qus |
Cancer
Qu demonstrates potent anticancer activity against various malignancies, including breast cancer, colorectal cancer (CRC), bladder carcinoma, ovarian, gliomas, and neuroblastoma, primarily by inhibiting cancer cell growth, proliferation, and migration, as well as inducing apoptosis.
While Qu demonstrates promising therapeutic properties, its clinical translation has been constrained by inherent challenges such as physicochemical instability, extensive first-pass metabolism, and poor tumor-specific accumulation. Emerging nanotechnology approaches, spanning lipid-based systems, polymeric architectures, inorganic carriers, and bioengineered exosome mimics, are revolutionizing Qu delivery through advanced encapsulation methodologies and surface engineering. These engineered platforms enhance the flavonoid’s solubility, pharmacokinetic stability, and tumor-targeting precision. As monotherapy, nanoformulated Qu operates through dual epigenetic mechanisms, modulating DNA methylation patterns and non-coding RNA networks to restore apoptotic sensitivity in malignant cells while selectively inducing oxidative stress-mediated cytotoxicity in tumors with minimal off-target effects.
The true therapeutic potential unfolds in combinatorial regimens where Qu’s polypharmacology synergizes with conventional therapies. Experimental models reveal its capacity to amplify chemotherapeutic efficacy through concurrent pathway modulation-disrupting PI3K/AKT/mTOR survival signaling while suppressing P-gp-mediated drug efflux, thereby enhancing DNA damage retention and reversing chemoresistance. When paired with immunotherapies, the nanoformulation acts as an immune microenvironment modulator, downregulating Treg/MDSC immunosuppressive networks while epigenetically enhancing tumor antigen presentation to potentiate T-cell responses. In radiation oncology, it functions as a biological response modifier, counteracting HIF-1α-mediated radioresistance in neoplasms while utilizing its antioxidant properties to shield healthy tissues from ionizing radiation damage. These multidimensional synergies stem from the nanocarriers’ ability to temporally coordinate drug release patterns and spatially colocalize therapeutic agents within tumors, establishing a new paradigm for overcoming nanotherapeutic limitations through engineered combination approaches.
Breast Cancer
Breast cancer is one of the most common malignancies among women, and its incidence has significantly increased in recent years. Qu demonstrates therapeutic efficacy against breast cancer through pleiotropic mechanisms that modulate critical oncogenic pathways governing tumor cell proliferation, apoptosis regulation, and metastatic competence. Sun et al grafted dodecylamine onto HA to synthesize the amphiphilic hyaluronic acid derivative dHAD, which self-assembles with Qu to form drug-loaded micelles, dHAD-QT. These pH-sensitive micelles rapidly release Qu under low pH conditions, exhibiting excellent drug-loading capacity, high cytotoxicity, and the ability to induce apoptosis. The dHAD-QT effectively inhibits tumor growth, extends the survival time of tumor-bearing mice, and reduces toxicity to normal tissues. Images of resected tumors show that the tumor volume in the dHAD-QT micelles group is more significantly suppressed, indicating enhanced anti-tumor activity of Qu (Figure 6A).
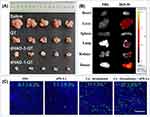 |
Figure 6 The anti-tumor application of nano-Qu. (A) anticancer study of dHAD-QT micelles. Images of tumors treated with free Qu and dHAD-QT micelles (n = 5, mean ± SD). Adapted from Int J Biol Macromol, volume 242, Sun J, Li M, Lin K, et al. Delivery of quercetin for breast cancer and targeting potentiation via hyaluronic nano-micelles. 124736, copyright 2023, with permission from Elsevier.71 (B) Micelle distribution in CT26-FL3 tumor-bearing mice at 24 after injection with DiD-loaded micelles (150 μg/kg) and observed by IVIS imaging. Adapted from Zhang J, Shen L, Li X, Song W, Liu Y, Huang L. Nanoformulated codelivery of quercetin and alantolactone promotes an antitumor response through synergistic immunogenic cell death for microsatellite-stable colorectal cancer. ACS Nano. 2019;13(11):12511–12524. Copyright © 2019 American Chemical Society.82 (C) Immunofluorescent staining assay (green = DNA fragments and blue = nuclei) on Day 20 to assess apoptosis in the tumor (scale bar = 50 3). *P < 0.05 and **P < 0.01, relative to PBS. Adapted from Acta Pharm Sin B, volume 12(1), Sun D, Zou Y, Song L, et al. A cyclodextrin-based nanoformulation achieves co-delivery of ginsenoside Rg3 and quercetin for chemo-immunotherapy in colorectal cancer. 378–393, copyright 2022, with permission from Elsevier.57 |
Although its single-drug use is limited by pharmacokinetic defects, as a natural low-toxic compound, it can improve its efficacy through structural modification (such as Qu derivatives) or binding with nanocallers (such as liposomes and polymer micelles) and is expected to become an adjuvant means for comprehensive treatment of breast cancer in the future. The combination of anti-angiogenic therapy and chemotherapy has emerged as an effective strategy for cancer treatment. Tian et al developed an amphiphilic LyP-1-LMWH-Qu conjugate capable of delivering the hydrophobic chemotherapeutic drug GA while simultaneously possessing dual functions of targeted chemotherapy and angiogenesis inhibition. Near-infrared optical imaging revealed that following intravenous administration of green fluorescent-labeled dHAD-QT micelles, detectable fluorescence signals emerged within tumor tissue within 4 hours post-injection, intensifying robust emission levels by 24-hour monitoring. The nanocarrier system demonstrated preferential accumulation in the predicted target organs (hepatic and renal systems), exhibiting superior targeting specificity compared to conventional monotherapeutic formulations.61
CRC
CRC is another significant cause of cancer-related deaths globally, and immunotherapy, especially immunogenic cell death (ICD) induction, has emerged as a new strategy for treating aggressive cancers. ICD activates the immune response by triggering cancer cell death. Qu has garnered attention due to its potential in cancer prevention and treatment, especially when combined with other drugs.
Andrea et al discovered in vivo experiments that supplementation with Qu in SPF-grade BALB/C nude mice significantly inhibited tumor growth. The tumor volume in the Qu-treated group was reduced by approximately 30% compared to the control group. In an AOM/DSS-treated wild-type C57BL/6J mouse model, Qu supplementation significantly reduced the number of tumor nodules. The Qu-treated group showed about 40% fewer tumor nodules than the control group. This indicates that Qu can reduce the risk of tumor formation and spread. However, more studies have shown that Qu combined with other drugs exhibits synergistic effects, enhancing the efficacy and efficiency of cancer treatment.92 Zhang et al found that the combination of Qu and alaninolactone (A) can induce ICD effects characterized by CRT translocation and HMGB1 release. The prepared QA-M exhibits ICD synergy in vivo, and a nanodrug delivery system enhances the accumulation of Qu and A in tumors. DiD, used as a probe, demonstrated that DiD-M micelles mainly accumulate in tumors (Figure 6B). In this study, the administration of 0.33 μM Qu showed undetectable HMGB1 release. However, the combination of Qu and alantolactone at the same concentration increased the percentage of HMGB1-releasing cells. Therefore, we conclude that Q and A can synergistically induce apoptosis at low concentrations. QA-M treatment enhances anti-tumor immunity, alters the tumor microenvironment, and promotes anti-tumor responses.82 In another study, the potential of Rg3 as an ICD inducer was confirmed. When combined with a Qu derivative (QTN), it generates ROS and enhances Rg3-mediated ICD activity. Folate-targeted pegylated cyclodextrin NPs were developed for the co-delivery of Rg3 and QTN, achieving synergistic anticancer effects in a CRC mouse model and improving pharmacokinetics and biodistribution. As shown in Figure 6C, the targeted co-formulation significantly induced tumor cell apoptosis (11%), and the combined strategy further enhanced apoptosis to 27%, altering the immunosuppressive nature of the tumor microenvironment and significantly extending the survival time of mice when used in combination with anti-PD-L1 therapy.57
Other Cancer
For most cancers, a single drug often has adverse effects, such as insufficient pharmacokinetic power and excessive side effects, so researchers are more inclined to carry out a combination of drugs and treatment methods.
In terms of drug combinations, Qu NPs have demonstrated advantages in the treatment of various cancers. The Qu-loaded thermosensitive hydrogel system (Qu-M-hydrogel complex) developed by the Xu team enhances the anticancer effect of Qu, achieving high local accumulation and sustained release, and exhibits enhanced cytotoxicity and anti-tumor effects on the ovarian cancer model SKOV-3.93 The co-delivery of Qu and doxorubicin using tumor-targeting hyaluronic acid-modified silica NPs demonstrated enhanced therapeutic efficacy in SGC7901/ADR tumor xenograft mouse models. The fluorescence intensity in tumor tissues of the experimental group was observed to be 7.96-fold and 2.39-fold higher than that in the blank control group and the HA pretreatment group, respectively. This significantly improved tumor-targeting capability ultimately enhanced anticancer efficacy.94 The combination of Qu and anhydroicaritin exerts synergistic multi-target mechanisms by stably binding to key target proteins (such as GSK3B and PPARG), thereby modulating critical cellular processes, including cell cycle regulation, proliferation, and survival. This multi-target intervention significantly inhibits the growth and proliferation of pancreatic cancer cells. In vitro experiments demonstrated a dose-dependent increase in inhibitory effects on PANC-1 cell proliferation, with markedly enhanced suppression observed at 40 µg/mL drug concentration. Furthermore, the combined treatment downregulated the expression of both GSK3B and PPARG in pancreatic cancer cells, validating its antitumor mechanism by inhibiting these pivotal molecular targets.95
A particularly notable example involves emerging photothermal therapy (PTT), which eliminates tumor cells through photothermal conversion but faces limitations due to the immunosuppressive microenvironment. Nanophotosensitizers fabricated from Qu and Fe ions address this challenge by releasing Qu, which suppresses the phosphorylation of JAK2 and STAT3, consequently downregulating programmed death-ligand 1 (PD-L1) in tumor cells. This dual mechanism effectively reshapes the immunosuppressive landscape while overcoming the therapeutic constraints of conventional PTT.96 In short, Qu as a natural polyphenol still has many possibilities waiting for us to explore.
Inflammation and Tissue Fibrosis
Qu shows multi-functional anti-inflammatory and anti-fibrotic effects, working alone or with other therapies. Its nanotechnology-enhanced strategies address bioavailability challenges while boosting therapeutic effects through synergistic targeting of disease mechanisms, offering precise treatment for inflammatory conditions.
Inflammation
Rheumatoid Arthritis (RA)
RA is an autoimmune inflammation related to ROS induced by intra-articular inflammation. Qu has shown potential in treating RA due to its antioxidant and anti-inflammatory properties. Han et al created Fe-Qur-NCN NPs that combat oxidative stress and inflammation in RA. These NPs alleviated joint damage in mice by modulating macrophage polarization (reducing M1/increasing M2) and balancing pro-/anti-inflammatory cytokines (eg, lowering IL-6/TNF-α while elevating IL-10), demonstrating dual therapeutic action through immune regulation.51 Jeyadevi et al used thioglycolic acid-capped cadmium telluride quantum dots (TGA-CdTe QD) to deliver Qu to arthritic rats. Histopathological evaluation via H&E staining revealed pronounced joint degeneration in complete Freund’s adjuvant (CFA)-treated controls, characterized by thinned cartilage plates, severe bone erosion, extensive inflammatory cell infiltration within synovial cavities, and pathological pannus formation. In stark contrast, the 0.4 mg/kg QDs-QE nanocomposite-treated group exhibited complete cartilage regeneration with normalized synovial architecture, demonstrating the therapeutic capacity of this nanohybrid system to reverse CFA-induced inflammatory osteolysis and promote structural restoration. They found significant improvements in oxidative stress markers, including lipid peroxidation, SOD, GSH, GPx, and CAT.79
Periodontal Inflammation
Periodontitis is an inflammatory disease affecting the tissues surrounding and supporting the teeth, often destroying bone and soft tissue. Research has found that a nanocomposite consisting of Qu and nano-octahedral cerium dioxide can effectively regulate the host’s immunity to periodontal disease. This material can control the polarization of macrophages, inhibiting M1 macrophages to reduce damage and promoting M2 macrophages during the repair phase to enhance tissue regeneration. It also increases the M2/M1 ratio and demonstrates therapeutic potential by modulating cytokine levels. According to Figure 7A, in animal models of periodontitis, after four consecutive days of administration, the inflamed areas treated with nanomedicine exhibited colors and characteristics similar to normal gingival tissue, while the gums of untreated rats showed significant redness and swelling.54
 |
Figure 7 The biological applications of nano-Qu. (A) anti-inflammatory activities of CeO2@QU and CeO2 nanoparticles on P. gingivalis-caused rat periodontitis model, gingival photos of rats with periodontitis after different nanoparticles treatment. Adapted from Wang Y, Li C, Wan Y, et al. Quercetin-loaded ceria nanocomposite potentiate dual-directional immunoregulation via macrophage polarization against periodontal inflammation. Small. 2021;17(41):e2101505. © 2021 Wiley-VCH GmbH.54 (B) in vivo targeting capacity of Qu-exosome-SPIONs/MF, NIRF imaging of Cy5.5-labeled Qu and Qu-exosome-SPIONs in the pancreas of Kunming mice 10 or 30 min after venous injection in the absence or presence of an external MF (MF density: 1 T). Adapted from Mater Today Bio, volume 16, He X, Liu W, Liu Y, et al. Nano artificial periosteum PLGA/MgO/Quercetin accelerates repair of bone defects through promoting osteogenic − angiogenic coupling effect via Wnt/ β-catenin pathway. 100348, copyright 2022, with permission from Elsevier.66 (C) by H&E, Masson staining and histochemistry staining of CD31 and OCN, we observed better repairing of bone in 0.1% Qu membrane group (N = 3). Adapted with permission from Dove Medical Press. Zhuang M, Rao L, Chen Y, et al. Controlled SPION-Exosomes loaded with quercetin preserves pancreatic beta cell survival and function in type 2 diabetes mellitus. Int J Nanomed. 2023;18:5733–5748.47 |
Tissue Fibrosis
Fibrosis, the final stage of chronic inflammation, commonly affects multiple tissues such as the liver, lungs, skin, and others, potentially leading to organ failure. Qu can inhibit fibrosis by regulating signaling pathways to reduce inflammation and apoptosis.
For instance, HA-coated Qu/fennel oil lipid nanocapsules (HA-QE-FE-LNCs) exhibit anti-fibrotic effects by reducing oxidative and inflammatory markers, improving drug release time, and enhancing cellular uptake through CD44 targeting. Furthermore, this nano-formulation decreases oxidative stress indicators like ROS and inflammatory markers such as TNF-α and IL-6, significantly improving liver fibrosis.97 Additionally, an inhalable Qu-alginate nanogel (Qu nanogel) has been developed as a treatment option for acute lung injury. Compared to free Qu, the Qu nanogel demonstrates superior biocompatibility, antioxidant, and anti-inflammatory properties in vitro, providing protective effects on damaged A549-GFP cells. In animal experiments, compared to the model group, treatment with 150 mg/kg QU-Nanogel significantly reduced IL-1β secretion relative to the positive control drug ulinastatin, suggesting that QU-Nanogel can activate NF-κB, induce pro-inflammatory cytokines, and thereby trigger a cascade of immune responses.98
Metabolism Diseases
Qu nanomedicine has pioneered new therapeutic pathways for metabolic diseases through the “precision design” of delivery systems and “functional synergy”, expanding Qu’s metabolic regulatory dimensions but also provide intelligent multi-target intervention strategies via the “carrier-as-drug” design philosophy, redefining therapeutic paradigms for metabolic disorders.
Diabetes
Diabetes is a chronic metabolic disease characterized by hyperglycemia and has become one of the most significant non-communicable diseases threatening human health. Qu demonstrates antidiabetic effects by reducing oxidative damage, increasing β-cell regeneration, enhancing insulin release, and regulating glucose and lipid metabolism. To overcome the defects of poor water solubility in Qu, researchers have developed Qu-based nano-formulations to enhance their efficacy and targeted delivery.
Singh et al encapsulated Qu in Soluplus® micelles (SM), which exhibited low hemolytic activity and a slow release rate. In diabetic rat models, Qu SM was more effective in lowering blood glucose levels and improving antioxidant activity than free Qu, showing sound antidiabetic effects.99 Zhuang et al synthesized transferrin-modified SPION and enhanced the loading of Qu in exosomes through electroporation. The resulting Qu-loaded exosomes (Qu exosomes) exhibited enhanced solubility and targeting ability under a magnetic field. In vitro experiments showed that Qu exosomes SPION/MF could effectively inhibit β-cell apoptosis and promote insulin secretion, with better effects than Qu alone. In vivo studies also confirmed the ability of Qu exosomes SPION/MF to target the islets of Langerhans, improving islet function recovery. Under the influence of an MF, Qu exosomes SPION demonstrated ideal targeting to the islets, with fluorescence intensity around the islets enhanced at 10 minutes and significantly increased after 30 minutes (Figure 7B).47
Qu-loaded nanoparticle composites have also effectively alleviated diabetic complications, including diabetic nephropathy. For instance, this study developed pH-sensitive Qu-succinyl chitosan-alginate core-shell corona NPs (QNT) to enhance Qu efficacy. Using a streptozotocin-induced diabetic rat model, four groups were evaluated over 28 days: NC, DC, QT, and QNT. Results demonstrated that QNT significantly outperformed QT in reducing blood glucose (eg, QNT: ~150 mg/dL vs QT: ~200 mg/dL), improving glucose tolerance, lowering lipid levels (including cholesterol and triglycerides), and reducing liver injury markers (ALT, AST, ALP). Histopathological analysis revealed superior structural restoration of the pancreas, liver, kidneys, and heart in the QNT group compared to QT, confirming that the nanocarrier markedly enhances Qu’s therapeutic effects by improving bioavailability and targeted delivery.88 Furthermore, Qu-conjugated superparamagnetic Fe oxide NPs can ameliorate learning and memory impairments in type 1 diabetic rats, with superior efficacy compared to free Qu.100
Hyperlipidemia
Hyperlipidemia, characterized by abnormally elevated levels of lipids such as cholesterol and triglycerides in the blood, is a condition that can lead to serious health issues. Qu possesses anti-hyperlipidemic activity. Campión et al prepared zein-based NPs, including NS and NC, to enhance the oral bioavailability of Qu and evaluate its anti-hyperlipidemic effects. These NPs could effectively encapsulate Qu with a loading capacity of approximately 7.5%. Compared to the control formulation, the relative oral bioavailability of Q-NS and Q-NC in Wistar rats was significantly increased to 26% and 57%, respectively.64
Other Diseases
Beyond its established therapeutic applications, Qu-based nanoformulations exhibit multifaceted efficacy across diverse pathologies. Xi et al developed PLGA/MgO/Quercetin electrospun membranes that dose-dependently stimulated BMSC proliferation, migration, and osteogenesis through Wnt/β-Catenin pathway activation. Low-dose Qu (< 1 wt%) additionally promoted EPC angiogenesis. The 0.1% Qu formulation demonstrated superior bone regeneration in rat cranial defects compared to PLGA and PLGA/MgO controls (Figure 7C).66 For neurodegenerative challenges like Alzheimer’s disease, lipid-based vectors and magnetic mesoporous silica carriers achieve targeted delivery across the blood-brain barrier, counteracting Tau hyperphosphorylation and Aβ plaque accumulation while alleviating oxidative neurodegeneration and cognitive decline.101 Cardiovascular applications leverage nanoemulsions and silica-PLGA hybrids to stabilize Qu’s bioactivity, where synergistic antioxidant and anti-inflammatory actions restore vascular homeostasis, offering dual protection against atherosclerotic progression and hypertension-mediated complications.102 These disease-tailored nanoplatforms exemplify Qu’s capacity to transcend biological barriers through intelligent engineering, coupling precise biodistribution with multi-mechanistic interventions for enhanced therapeutic outcomes.
Furthermore, Qu, combined with nanocomplex, also has applications in many other diseases, such as chronic obstructive pulmonary disease,103 androgenic alopecia,104 aging and age-related diseases,105 bacterial infections,106 neurological disorders,107 skin damage, and skin aging.108 For example, Qu decorated Nano-gold (Qu-AuNPs) was used against Escherichia coli through cell lysis induced by the interaction of Qu-AuNPs with bacterial membrane. The antioxidant activity of Qu-AuNPs was also demonstrated.109
Although significant progress has been made, current research still faces challenges such as in vivo metabolic heterogeneity of nanomedicines, long-term toxicity evaluation, and stability in scaled production. Future directions may focus on intelligent delivery system design (eg, AI-assisted carrier optimization) and precision combination therapies based on patient stratification to accelerate the clinical translation of Qu-based nanomedicines.
Conclusions and Future Perspectives
The transformative potential of nanotechnology in overcoming Qu’s pharmacokinetic limitations has propelled its clinical prospects across diverse therapeutic areas. Innovations in nanocarrier design, including lipid-based NPs, biodegradable polymers (PLGA/PEG), and inorganic frameworks, have enhanced Qu’s solubility and bioavailability by 5–8 times while enabling targeted delivery and controlled release. These advancements have demonstrated significant therapeutic efficacy, particularly in oncology, where pH-responsive co-delivery systems combining Qu with chemotherapeutics like paclitaxel exhibit synergistic tumor suppression (CI=0.62) in breast and colorectal cancer models. Beyond cancer, nano-Qu formulations mitigate inflammation, insulin resistance, and neurodegeneration through mechanisms such as macrophage polarization modulation and oxidative stress regulation. However, critical challenges persist, including biocompatibility risks from synthetic carrier degradation byproducts, manufacturing complexities that lead to batch variability, and insufficient targeting precision in chronic disease microenvironments, such as fibrotic tissues or the blood-brain barrier.
To accelerate clinical translation, priority should be given to developing biodegradable platforms using GRAS-certified materials, such as chitosan and cyclodextrins, and scalable synthesis methods, including microfluidic nanoprecipitation. Implantable depot systems, such as silk fibroin hydrogels, for sustained release in chronic diseases, combined with patient-specific microbiome profiling, could address long-term therapeutic needs. Furthermore, synergistic strategies integrating Qu with immune checkpoint inhibitors (anti-PD-1) or antidiabetic agents (metformin) show promise in priming tumor immunity and amplifying metabolic benefits. Formulations leveraging dual-stimuli responsiveness (pH/ROS-sensitive carriers) or magnetic guidance are particularly poised for regulatory success, provided they align with standardized evaluation frameworks assessing nanocarrier degradation kinetics and organ-specific biodistribution. By harmonizing innovative manufacturing technologies with rigorous preclinical validation, nano-Qu stands to bridge the gap between preclinical innovation and clinical impact, offering transformative solutions for complex chronic diseases while prioritizing safety, scalability, and patient-centric design.
Abbreviations
Qu, quercetin; ROS, Reactive Oxygen Species; nano-Qu, nano-quercetin; LDL, low-density lipoprotein; TNF-α, tumor necrosis factor α; P-gp, P-glycoprotein; NPs, Nanoparticles; COX, cyclooxygenase; SLN, solid lipid NPs; NLCs, nanostructured lipid carriers; LNC, liposomal nanocapsules; BBB, blood-brain barrier; LPNs, lipid-polymer nanocarriers; SPION, superparamagnetic iron oxide NPs; Fe-Qur NCNs, iron-Qu Natural coordination nanocomplexes; LSPR, local surface plasmon formants; AuNCs, gold nanocages; NIR, near infrared; Qc@SNPs, Qu-modified sulfur NPs; CeO2@QU, Qu-loaded nanocerium dioxide; SOD, superoxide dismutase; CAT, catalase; CSO, chitosan oligosaccharide; HA, hyaluronic acid; QT, quercetin; ADEX, a dual-grafted dextran; SFNs, silk protein NPs; LMWH, low molecular weight heparin; PLGA, poly (lactic-co-glycolic acid); BMSCs, bone marrow stromal cells; PVP, polyvinylpyrrolidone; GO, graphene oxide; QDs, quantum dots; Qtn, quercetin; PEG, polyethylene glycol; L-Cys, L-cysteine; PG9, polyethylene glycol 9000; DNS, DNA nanostructures; HPG, hyperbranched polyglycerol; MBG, mesoporous bioactive glass; IE, incorporation efficiencies; SDDS, Supersaturated Drug Delivery System; NS, nanospheres; NC, nanocapsules; Q-NS, Qu-loaded nanospheres; Q-NC, Qu-loaded nanocapsules; CPC, calcium phosphate cement; MF, magnetic field; CRC, colorectal cancer; ICD, immunogenic cell death; PTT, photothermal therapy; LCP, lipid/calcium/phosphate; RA, rheumatoid arthritis; CFA, complete Freund’s adjuvant; QNT, Qu-succinyl chitosan-alginate core-shell corona nanoparticles.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (22102091, 82304193, and 22106089), Future Program for Young Scholars of Shandong University (21320082364135), China Postdoctoral Science Foundation (2023M732052), and Natural Science Foundation of Shandong Province (ZR2023QH329).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mirza MA, Mahmood S, Hilles AR. et al. Quercetin as a therapeutic product: evaluation of its pharmacological action and clinical applications—a review. Pharmaceuticals. 2023;16(11):1631. doi:10.3390/ph16111631
2. Wang S, Su R, Nie S, et al. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J Nutr Biochem. 2014;25(4):363–376. doi:10.1016/j.jnutbio.2013.10.002
3. Suzuki T, Morishita T, Kim S-J, et al. Physiological roles of rutin in the buckwheat plant. Jpn Agric Res Q. 2015;49(1):37–43. doi:10.6090/JARQ.49.37
4. Yang Y, Cai X, Yang J, et al. Chemoprevention of dietary digitoflavone on colitis-associated colon tumorigenesis through inducing Nrf2 signaling pathway and inhibition of inflammation. Mol Cancer. 2014;13(4):48. doi:10.1186/1476-4598-13-48
5. Xu D, Hu MJ, Wang YQ, Cui YL. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules. 2019;24(6):1123. doi:10.3390/molecules24061123
6. Kao TK, Ou YC, Raung SL, Lai CY, Liao SL, Chen CJ. Inhibition of nitric oxide production by quercetin in endotoxin/cytokine-stimulated microglia. Life Sci. 2010;86(9–10):315–321. doi:10.1016/j.lfs.2009.12.014
7. Sternberg Z, Chadha K, Lieberman A, et al. Quercetin and interferon-β modulate immune response(s) in peripheral blood mononuclear cells isolated from multiple sclerosis patients. J Neuroimmunol. 2008;205(1–2):142–147. doi:10.1016/j.jneuroim.2008.09.008
8. Mahmoud MF, Hassan NA, El Bassossy HM, Fahmy A. Quercetin protects against diabetes-induced exaggerated vasoconstriction in rats: effect on low grade inflammation. PLoS One. 2013;8(5):e63784. doi:10.1371/journal.pone.0063784
9. Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. OxidMed Cell Longevity. 2009;2(5):270–278. doi:10.4161/oxim.2.5.9498
10. Wu XJ, Zhou XB, Chen C, Mao W. Systematic investigation of quercetin for treating cardiovascular disease based on network pharmacology. Comb Chem High Throughput Screen. 2019;22(6):411–420. doi:10.2174/1386207322666190717124507
11. Geng F, Xu M, Zhao L, et al. Quercetin alleviates pulmonary fibrosis in mice exposed to silica by inhibiting macrophage senescence. Front Pharmacol. 2022;13:912029. doi:10.3389/fphar.2022.912029
12. Cao LH, Jia XY, He HJ, et al. Using a system pharmacology method to search for the potential targets and pathways of Yinqiaosan against COVID-19. J Healthc Eng. 2022;2022:9248674. doi:10.1155/2022/9248674
13. Michala AS, Pritsa A. Quercetin: a molecule of great biochemical and clinical value and its beneficial effect on diabetes and cancer. Diseases. 2022;10(3):37. doi:10.3390/diseases10030037
14. Batiha GE, Beshbishy AM, Ikram M, et al. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: quercetin. Foods. 2020;9(3):374. doi:10.3390/foods9030374
15. Lopes L. Overcoming the cutaneous barrier with microemulsions. Pharmaceutics. 2014;6(1):52–77. doi:10.3390/pharmaceutics6010052
16. Sorrenti V, Buriani A, Fortinguerra S, et al. Cell survival, death, and proliferation in senescent and cancer cells: the role of (poly)phenols. Adv Nutr. 2023;14(5):1111–1130. doi:10.1016/j.advnut.2023.05.014
17. Hatahet T, Morille M, Hommoss A, Devoisselle JM, Müller RH, Bégu S. Quercetin topical application, from conventional dosage forms to nanodosage forms. Eur J Pharm Biopharm. 2016;108:41–53. doi:10.1016/j.ejpb.2016.08.011
18. International BR. Retracted: big data analysis of manufacturing and preclinical studies of nanodrug‐targeted delivery systems: a literature review. Biomed Res Int. 2023;2023(1):9759424. doi:10.1155/2023/9759424
19. Wang G, Wang Y, Yao L, et al. Pharmacological activity of quercetin: an updated review. Evid Based Complement Altern Med. 2022;2022:1–12. doi:10.1155/2022/3997190
20. Chowdhury S, Kar K, Mazumder R. Exploration of different strategies of nanoencapsulation of bioactive compounds and their ensuing approaches. Futur J Pharm Sci. 2024;10(1). doi:10.1186/s43094-024-00644-y
21. Cheshmehnoor P, Bolourchian N, Abdollahizad E, Derakhshi A, Dadashzadeh S, Haeri A. Particle size tailoring of quercetin nanosuspensions by wet media milling technique: a study on processing and formulation parameters. Iran J Pharm Res. 2023;21(1):e130626. doi:10.5812/ijpr-130626
22. He M, Xia L, Li J. Potential mechanisms of plant-derived natural products in the treatment of cervical cancer. Biomolecules. 2021;11(10):1539. doi:10.3390/biom11101539
23. Hu Y, Yang L, Lu Y, et al. Systems network pharmacology-based prediction and analysis of potential targets and pharmacological mechanism of Actinidia chinensis planch. Root extract for application in hepatocellular carcinoma. Evid-Based Complement Altern Med. 2022;2022:2116006. doi:10.1155/2022/2116006
24. Sun J, Tian Z, Wu J, et al. Pristimerin exerts pharmacological effects through multiple signaling pathways: a comprehensive review. Drug Des Dev Ther. 2024;18:1673–1694. doi:10.2147/DDDT.S460093
25. Amararathna M, Johnston M, Rupasinghe H. Plant polyphenols as chemopreventive agents for lung cancer. Int J Mol Sci. 2016;17(8):1352. doi:10.3390/ijms17081352
26. Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic Biol Med. 1994;16(6):845–850. doi:10.1016/0891-5849(94)90202-x
27. Tang Y, Gao C, Xing M, et al. Quercetin prevents ethanol-induced dyslipidemia and mitochondrial oxidative damage. Food Chem Toxicol. 2012;50(5):1194–1200. doi:10.1016/j.fct.2012.02.008
28. Li Y, Yao J, Han C, et al. Quercetin, inflammation and immunity. Nutrients. 2016;8(3):167. doi:10.3390/nu8030167
29. Tavakoli Z, Tahmasebi Dehkordi H, Lorigooini Z, Rahimi-Madiseh M, Korani MS, Amini-Khoei H. Anticonvulsant effect of quercetin in pentylenetetrazole (PTZ)-induced seizures in male mice: the role of anti-neuroinflammatory and anti-oxidative stress. Int Immunopharmacol. 2023;116:109772. doi:10.1016/j.intimp.2023.109772
30. Shorobi FM, Nisa FY, Saha S, et al. Quercetin: a functional food-flavonoid incredibly attenuates emerging and re-emerging viral infections through immunomodulatory actions. Molecules. 2023;28(3):938. doi:10.3390/molecules28030938
31. Ye X, Wu K, Xu L, et al. Methanol extract of Inonotus obliquus improves type 2 diabetes mellitus through modifying intestinal flora. Front Endocrinol. 2023;13:1103972. doi:10.3389/fendo.2022.1103972
32. Kim JK, Park SU. Quercetin and its role in biological functions: an updated review. EXCLI J. 2018;856:1611–2156. doi:10.17179/excli2018-1538
33. Zhu X, Ding G, Ren S, Xi J, Liu K. The bioavailability, absorption, metabolism, and regulation of glucolipid metabolism disorders by quercetin and its important glycosides: a review. Food Chem. 2024;458:140262. doi:10.1016/j.foodchem.2024.140262
34. Nguyen TT, Duong VA, Maeng HJ. Pharmaceutical formulations with P-Glycoprotein inhibitory effect as promising approaches for enhancing oral drug absorption and bioavailability. Pharmaceutics. 2021;13(7):1103. doi:10.3390/pharmaceutics13071103
35. Basheer L, Kerem Z. Interactions between CYP3A4 and dietary polyphenols. Oxid Med Cell Longev. 2015;2015:1–15. doi:10.1155/2015/854015
36. Zhou M, Ma J, Kang M, et al. Flavonoids, gut microbiota, and host lipid metabolism. Eng Life Sci. 2023;24(5):2300065. doi:10.1002/elsc.202300065
37. Ma C, Cheng Z, Tan H, et al. Nanomaterials: leading immunogenic cell death-based cancer therapies. Front Immunol. 2024;15:1447817. doi:10.3389/fimmu.2024.1447817
38. Liu N, Ruan J, Li H, Fu J. Nanoparticles loaded with natural medicines for the treatment of Alzheimer’s disease. Front Neurosci. 2023;17:1112435. doi:10.3389/fnins.2023.1112435
39. Joshi H, Gupta DS, Kaur G, et al. Nanoformulations of quercetin for controlled delivery: a review of preclinical anticancer studies. Naunyn Schmiedebergs Arch Pharmacol. 2023;396(12):3443–3458. doi:10.1007/s00210-023-02625-z
40. Hu K, Miao L, Goodwin TJ, Li J, Liu Q, Huang L. Quercetin remodels the tumor microenvironment to improve the permeation, retention, and antitumor effects of nanoparticles. ACS Nano. 2017;11(5):4916–4925. doi:10.1021/acsnano.7b01522
41. Zhu B, Yu L, Yue Q. Co-delivery of vincristine and quercetin by nanocarriers for lymphoma combination chemotherapy. Biomed Pharmacother. 2017;91:287–294. doi:10.1016/j.biopha.2017.02.112
42. Kumar P, Sharma G, Kumar R, et al. Promises of a biocompatible nanocarrier in improved brain delivery of quercetin: biochemical, pharmacokinetic and biodistribution evidences. Int J Pharm. 2016;515(1–2):307–314. doi:10.1016/j.ijpharm.2016.10.024
43. Sun M, Nie S, Pan X, Zhang R, Fan Z, Wang S. Quercetin-nanostructured lipid carriers: characteristics and anti-breast cancer activities in vitro. Colloid Surf B-Biointerfaces. 2014;113:15–24. doi:10.1016/j.colsurfb.2013.08.032
44. Melchior S, Codrich M, Gorassini A, et al. Design and advanced characterization of quercetin-loaded nano-liposomes prepared by high-pressure homogenization. Food Chem. 2023;428:136680. doi:10.1016/j.foodchem.2023.136680
45. Fan W, He Y, Hu P, et al. A novel acceptor–donor–acceptor structured molecule-based nanosystem for tumor mild photothermal therapy. J Colloid Interface Sci. 2024;670:762–773. doi:10.1016/j.jcis.2024.05.143
46. Yang W, Lv Y, Wang B, et al. Polydopamine synergizes with quercetin nanosystem to reshape the perifollicular microenvironment for accelerating hair regrowth in androgenetic alopecia. Nano Lett. 2024;24(20):6174–6182. doi:10.1021/acs.nanolett.4c01843
47. Zhuang M, Rao L, Chen Y, et al. Controlled SPION-Exosomes loaded with quercetin preserves pancreatic beta cell survival and function in type 2 diabetes mellitus. Int J Nanomed. 2023;18:5733–5748. doi:10.2147/ijn.S422416
48. Zhang Z, Xu S, Wang Y, et al. Near-infrared triggered co-delivery of doxorubicin and quercetin by using gold nanocages with tetradecanol to maximize anti-tumor effects on MCF-7/ADR cells. J Colloid Interface Sci. 2018;509:47–57. doi:10.1016/j.jcis.2017.08.097
49. Yang Y, Cai X, Shi M, et al. Biomimetic retractable DNA nanocarrier with sensitive responsivity for efficient drug delivery and enhanced photothermal therapy. J Nanobiotechnol. 2023;21(1):46. doi:10.1186/s12951-023-01806-5
50. Ren M, Wang X, Hu M, et al. Enhanced bone formation in rat critical-size tibia defect by a novel quercetin-containing alpha-calcium sulphate hemihydrate/nano-hydroxyapatite composite. Biomed Pharmacother. 2022;146:112570. doi:10.1016/j.biopha.2021.112570
51. Han Z, Gao X, Wang Y, et al. Ultrasmall iron-quercetin metal natural product nanocomplex with antioxidant and macrophage regulation in rheumatoid arthritis. Acta Pharm Sin. 2023;13(4):1726–1739. doi:10.1016/j.apsb.2022.11.020
52. Yang S-Y, Hu Y, Zhao R, et al. Quercetin-loaded mesoporous nano-delivery system remodels osteoimmune microenvironment to regenerate alveolar bone in periodontitis via the miR-21a-5p/PDCD4/NF-κB pathway. J Nanobiotechnol. 2024;22(1):94. doi:10.1186/s12951-024-02352-4
53. Huang X, Chen X, Chen Q, Yu Q, Sun D, Liu J. Investigation of functional selenium nanoparticles as potent antimicrobial agents against superbugs. Acta Biomater. 2016;30:397–407. doi:10.1016/j.actbio.2015.10.041
54. Wang Y, Li C, Wan Y, et al. Quercetin-loaded ceria nanocomposite potentiate dual-directional immunoregulation via macrophage polarization against periodontal inflammation. Small. 2021;17(41):e2101505. doi:10.1002/smll.202101505
55. Gupta A, Kumar Mehta S, Qayoom I, Gupta S, Singh S, Kumar A. Biofunctionalization with Cissus quadrangularis phytobioactives accentuates Nano-Hydroxyapatite based ceramic Nano-Cement for Neo-Bone formation in critical sized bone defect. Int J Pharm. 2023;642:123110. doi:10.1016/j.ijpharm.2023.123110
56. Wang R, Li Y, Gao S, et al. An active transport dual adaptive nanocarrier designed to overcome the corneal microenvironment for neovascularization therapy. Biomater Sci. 2024;12(2):361–374. doi:10.1039/d3bm01349a
57. Sun D, Zou Y, Song L, et al. A cyclodextrin-based nanoformulation achieves co-delivery of ginsenoside Rg3 and quercetin for chemo-immunotherapy in colorectal cancer. Acta Pharm Sin B. 2022;12(1):378–393. doi:10.1016/j.apsb.2021.06.005
58. Zhao R, Chen T, Li Y, et al. Biocompatible hydrophobic cross-linked cyclodextrin-based metal-organic framework as quercetin nanocarrier for enhancing stability and controlled release. Food Chem. 2024;448:139167. doi:10.1016/j.foodchem.2024.139167
59. He Z, Liu Y, Wang H, et al. Dual-grafted dextran based nanomicelles: higher antioxidant, anti-inflammatory and cellular uptake efficiency for quercetin. Int J Biol Macromol. 2023;224:1361–1372. doi:10.1016/j.ijbiomac.2022.10.222
60. Wang B, Guo C, Liu Y, et al. Novel nano-pomegranates based on astragalus polysaccharides for targeting ERα-positive breast cancer and multidrug resistance. Drug Deliv. 2020;27(1):607–621. doi:10.1080/10717544.2020.1754529
61. Tian F, Dahmani FZ, Qiao J, et al. A targeted nanoplatform co-delivering chemotherapeutic and antiangiogenic drugs as a tool to reverse multidrug resistance in breast cancer. Acta Biomater. 2018;75:398–412. doi:10.1016/j.actbio.2018.05.050
62. Song JE, Tripathy N, Lee DH, Park JH, Khang G. Quercetin inlaid silk fibroin/hydroxyapatite scaffold promotes enhanced osteogenesis. ACS Appl Mater Interfaces. 2018;10(39):32955–32964. doi:10.1021/acsami.8b08119
63. Lozano-Pérez AA, Rivero HC, Hernández MdC P, et al. Silk fibroin nanoparticles: efficient vehicles for the natural antioxidant quercetin. Int J Pharm. 2017;518(1–2):11–19. doi:10.1016/j.ijpharm.2016.12.046
64. Campión R, Gonzalez-Navarro CJ, Luisa Martínez López A, et al. Zein-based nanospheres and nanocapsules for the encapsulation and oral delivery of quercetin. Int J Pharm. 2023;643:123216. doi:10.1016/j.ijpharm.2023.123216
65. Huang J, Liao J, Li X, et al. Tea saponin-Zein binary complex as a quercetin delivery vehicle: preparation, characterization, and functional evaluation. Int J Biol Macromol. 2024;279(Pt 4):135485. doi:10.1016/j.ijbiomac.2024.135485
66. He X, Liu W, Liu Y, et al. Nano artificial periosteum PLGA/MgO/Quercetin accelerates repair of bone defects through promoting osteogenic − angiogenic coupling effect via Wnt/ β-catenin pathway. Mater Today Bio. 2022;16:100348. doi:10.1016/j.mtbio.2022.100348
67. El-Gogary RI, Rubio N, Wang JT-W, et al. Polyethylene glycol conjugated polymeric nanocapsules for targeted delivery of quercetin to folate-expressing cancer cells in vitro and in vivo. ACS Nano. 2014;8(2):1384–1401. doi:10.1021/nn405155b
68. Li Y, Piao Y-Z, Chen H, et al. Dynamic covalent nano-networks comprising antibiotics and polyphenols orchestrate bacterial drug resistance reversal and inflammation alleviation. Bioact Mater. 2023;27:288–302. doi:10.1016/j.bioactmat.2023.04.014
69. Rezvani M, Mohammadnejad J, Narmani A, Bidaki K. Synthesis and in vitro study of modified chitosan-polycaprolactam nanocomplex as delivery system. Int J Biol Macromol. 2018;113:1287–1293. doi:10.1016/j.ijbiomac.2018.02.141
70. Ponraj T, Vivek R, Paulpandi M, et al. Mitochondrial dysfunction-induced apoptosis in breast carcinoma cells through a pH-dependent intracellular quercetin NDDS of PVPylated-TiO2NPs. J Mater Chem B. 2018;6(21):3555–3570. doi:10.1039/c8tb00769a
71. Sun J, Li M, Lin K, et al. Delivery of quercetin for breast cancer and targeting potentiation via hyaluronic nano-micelles. Int J Biol Macromol. 2023;242:124736. doi:10.1016/j.ijbiomac.2023.124736
72. Gao X, Wang B, Wei X, et al. Anticancer effect and mechanism of polymer micelle-encapsulated quercetin on ovarian cancer. Nanoscale. 2012;4(22):7021–7030. doi:10.1039/c2nr32181e
73. Hudecki A, Rzeszutek I, Lewińska A, et al. Electrospun fiber-based micro- and nano-system for delivery of high concentrated quercetin to cancer cells. Biomater Adv. 2023;153:213582. doi:10.1016/j.bioadv.2023.213582
74. Simon L, Lapinte V, Lionnard L, et al. Polyoxazolines based lipid nanocapsules for topical delivery of antioxidants. Int J Pharm. 2020;579:119126. doi:10.1016/j.ijpharm.2020.119126
75. Gupta P, Authimoolam SP, Hilt JZ, Dziubla TD. Quercetin conjugated poly(β-amino esters) nanogels for the treatment of cellular oxidative stress. Acta Biomater. 2015;27:194–204. doi:10.1016/j.actbio.2015.08.039
76. Zhang H, Xu X, Shou X, et al. Senolytic therapy enabled by senescent cell‐sensitive biomimetic melanin nano‐senolytics. Adv Healthc Mater. 2024;13(23):e2401085. doi:10.1002/adhm.202401085
77. Rahmanian N, Hamishehkar H, Dolatabadi JEN, Arsalani N. Nano graphene oxide: a novel carrier for oral delivery of flavonoids. Colloid Surf B-Biointerfaces. 2014;123:331–338. doi:10.1016/j.colsurfb.2014.09.036
78. Islami M, Zarrabi A, Tada S, Kawamoto M, Isoshima T, Ito Y. Controlled quercetin release from high-capacity-loading hyperbranched polyglycerol-functionalized graphene oxide. Int J Nanomed. 2018;13:6059–6071. doi:10.2147/ijn.S178374
79. Jeyadevi R, Sivasudha T, Rameshkumar A, et al. Enhancement of anti arthritic effect of quercetin using thioglycolic acid-capped cadmium telluride quantum dots as nanocarrier in adjuvant induced arthritic Wistar rats. Colloid Surf B-Biointerfaces. 2013;112:255–263. doi:10.1016/j.colsurfb.2013.07.065
80. Elsayed AM, Sherif NM, Hassan NS, Althobaiti F, Hanafy NAN, Sahyon HA. Novel quercetin encapsulated chitosan functionalized copper oxide nanoparticles as anti-breast cancer agent via regulating p53 in rat model. Int J Biol Macromol. 2021;185:134–152. doi:10.1016/j.ijbiomac.2021.06.085
81. Resen AK, Atiroğlu A, Atiroğlu V, et al. Effectiveness of 5-Fluorouracil and gemcitabine hydrochloride loaded iron‑based chitosan-coated MIL-100 composite as an advanced, biocompatible, pH-sensitive and smart drug delivery system on breast cancer therapy. Int J Biol Macromol. 2022;198:175–186. doi:10.1016/j.ijbiomac.2021.12.130
82. Zhang J, Shen L, Li X, Song W, Liu Y, Huang L. Nanoformulated codelivery of quercetin and alantolactone promotes an antitumor response through synergistic immunogenic cell death for microsatellite-stable colorectal cancer. ACS Nano. 2019;13(11):12511–12524. doi:10.1021/acsnano.9b02875
83. Sadalage PS, Patil RV, Havaldar DV, Gavade SS, Santos AC, Pawar KD. Optimally biosynthesized, PEGylated gold nanoparticles functionalized with quercetin and camptothecin enhance potential anti-inflammatory, anti-cancer and anti-angiogenic activities. J Nanobiotechnol. 2021;19(1):84. doi:10.1186/s12951-021-00836-1
84. Liu Y, Gong Y, Xie W, et al. Microbubbles in combination with focused ultrasound for the delivery of quercetin-modified sulfur nanoparticles through the blood brain barrier into the brain parenchyma and relief of endoplasmic reticulum stress to treat Alzheimer’s disease. Nanoscale. 2020;12(11):6498–6511. doi:10.1039/c9nr09713a
85. Kamal NH, Heikal LA, Ali MM, Aly RG, Abdallah OY. Development and evaluation of local regenerative biomimetic bone-extracellular matrix scaffold loaded with nano-formulated quercetin for orthopedic fractures. Biomater Adv. 2023;145:213249. doi:10.1016/j.bioadv.2022.213249
86. Li M, Li H, Lu L, et al. Simple preparation and greatly improved oral bioavailability: the supersaturated drug delivery system of quercetin based on PVP K30. Drug Deliv Transl Res. 2024;14(11):3225–3238. doi:10.1007/s13346-024-01544-7
87. Aghamohammadi M, Zolghadr L, Nezhad NS, Ahmadpour Yazdi H, Esfahani AJ, Gheibi N. Investigating the effects of quercetin fatty acid esters on apoptosis, mechanical properties, and expression of ERK in melanoma cell line (A375). Life Sci. 2022;310:121007. doi:10.1016/j.lfs.2022.121007
88. Mu Y, Fu Y, Li J, et al. Multifunctional quercetin conjugated chitosan nano-micelles with P-gp inhibition and permeation enhancement of anticancer drug. Carbohydr Polym. 2019;203:10–18. doi:10.1016/j.carbpol.2018.09.020
89. Sayyad N, Maji R, Omolo CA, et al. Development of niosomes for encapsulating captopril-quercetin prodrug to combat hypertension. Int J Pharm. 2021;609:121191. doi:10.1016/j.ijpharm.2021.121191
90. Catauro M, Bollino F, Nocera P, Piccolella S, Pacifico S. Entrapping quercetin in silica/polyethylene glycol hybrid materials: chemical characterization and biocompatibility. Mater Sci Eng C. 2016;68:205–212. doi:10.1016/j.msec.2016.05.082
91. Zhu C, Yang Y, Li X, Chen X, Lin X, Wu X. Develop potential multi-target drugs by self-assembly of quercetin with amino acids and metal ion to achieve significant efficacy in anti-Alzheimer’s disease. Nano Res. 2022;15(6):5173–5182. doi:10.1007/s12274-021-4066-8
92. Neamtu AA, Maghiar TA, Alaya A, et al. A comprehensive view on the quercetin impact on colorectal cancer. Molecules. 2022;27(6):1873. doi:10.3390/molecules27061873
93. Xu G, Li B, Wang T, et al. Enhancing the anti-ovarian cancer activity of quercetin using a self-assembling micelle and thermosensitive hydrogel drug delivery system. RSC Adv. 2018;8(38):21229–21242. doi:10.1039/c8ra03274b
94. Fang J, Zhang S, Xue X, et al. Quercetin and doxorubicin co-delivery using mesoporous silica nanoparticles enhance the efficacy of gastric carcinoma chemotherapy. Int J Nanomed. 2018;13:5113–5126. doi:10.2147/ijn.S170862
95. Liu X, Ou J. Revealing the multi-target compounds of Sarcandra glabra identification and inhibition of novel target genes for the treatment of pancreatic cancer. BMC Complement Med Ther. 2025;25(1):106. doi:10.1186/s12906-025-04839-5
96. Li L, Zhang M, Liu T, et al. Quercetin-ferrum nanoparticles enhance photothermal therapy by modulating the tumor immunosuppressive microenvironment. Acta Biomater. 2022;154:454–466. doi:10.1016/j.actbio.2022.10.008
97. Hafez DA, Abdelmonsif DA, Aly RG, Samy WM, Elkhodairy KA, Abo Aasy NK. Role of fennel oil/ quercetin dual nano-phytopharmaceuticals in hampering liver fibrosis: comprehensive optimization and in vivo assessment. Drug Deliv Sci Technol. 2022;69:103177. doi:10.1016/j.jddst.2022.103177
98. Chen YB, Zhang YB, Wang YL, et al. A novel inhalable quercetin-alginate nanogel as a promising therapy for acute lung injury. J Nanobiotechnol. 2022;20(1):272. doi:10.1186/s12951-022-01452-3
99. Singh J, Mittal P, Vasant Bonde G, et al. Design, optimization, characterization and in-vivo evaluation of Quercetin enveloped Soluplus®/P407 micelles in diabetes treatment. Artif Cell Nanomed Biotechnol. 2018;46(sup3):S546–S555. doi:10.1080/21691401.2018.1501379
100. Ebrahimpour S, Shahidi SB, Abbasi M, Tavakoli Z, Esmaeili A. Quercetin-conjugated superparamagnetic iron oxide nanoparticles (QCSPIONs) increases Nrf2 expression via miR-27a mediation to prevent memory dysfunction in diabetic rats. Sci Rep. 2020;10(1):15957. doi:10.1038/s41598-020-71971-2
101. Halevas E, Mavroidi B, Nday CM, et al. Modified magnetic core-shell mesoporous silica nano-formulations with encapsulated quercetin exhibit anti-amyloid and antioxidant activity. J Inorg Biochem. 2020;213:111271. doi:10.1016/j.jinorgbio.2020.111271
102. Buya AB, Beloqui A, Memvanga PB, Préat V. Self-nano-emulsifying drug-delivery systems: from the development to the current applications and challenges in oral drug delivery. Pharmaceutics. 2020;12(12):1194. doi:10.3390/pharmaceutics12121194
103. Ding K, Jiang W, Zhan W, et al. The therapeutic potential of quercetin for cigarette smoking–induced chronic obstructive pulmonary disease: a narrative review. Ther Adv Respir Dis. 2023;17:17534666231170800. doi:10.1177/17534666231170800
104. Zhang Z, Li W, Chang D, et al. A combination therapy for androgenic alopecia based on quercetin and zinc/copper dual-doped mesoporous silica nanocomposite microneedle patch. Bioact Mater. 2023;24:81–95. doi:10.1016/j.bioactmat.2022.12.007
105. Alharbi KS, Afzal O, Altamimi ASA, et al. A study of the molecular mechanism of quercetin and dasatinib combination as senolytic in alleviating age-related and kidney diseases. J Food Biochem. 2022;46(12):e14471. doi:10.1111/jfbc.14471
106. Al Hagbani T, Rizvi SMD, Shakil S, Lila ASA. Nano-formulating besifloxacin and employing quercetin as a synergizer to enhance the potency of besifloxacin against pathogenic bacterial strains: a nano-synergistic approach. Nanomaterials. 2023;13(14):2083. doi:10.3390/nano13142083
107. Amanzadeh E, Esmaeili A, Rahgozar S, Nourbakhshnia M. Application of quercetin in neurological disorders: from nutrition to nanomedicine. Rev Neurosci. 2019;30(5):555–572. doi:10.1515/revneuro-2018-0080
108. Boo YC. Emerging strategies to protect the skin from ultraviolet rays using plant-derived materials. Antioxidants. 2020;9(7):637. doi:10.3390/antiox9070637
109. Alhadrami HA, Orfali R, Hamed AA, et al. Flavonoid-coated gold nanoparticles as efficient antibiotics against gram-negative bacteria—evidence from in silico-supported in vitro studies. J Antibiot. 2021;10(8):968. doi:10.3390/antibiotics
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

